A Short Empathy Paradigm to Assess Empathic Deficits in Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Tasks
2.2.1. Emotion Recognition
2.2.2. Emotional Perspective Taking
2.2.3. Affective Responsiveness
2.2.4. Empathy Questionnaire
2.3. Statistical Analyses
3. Results
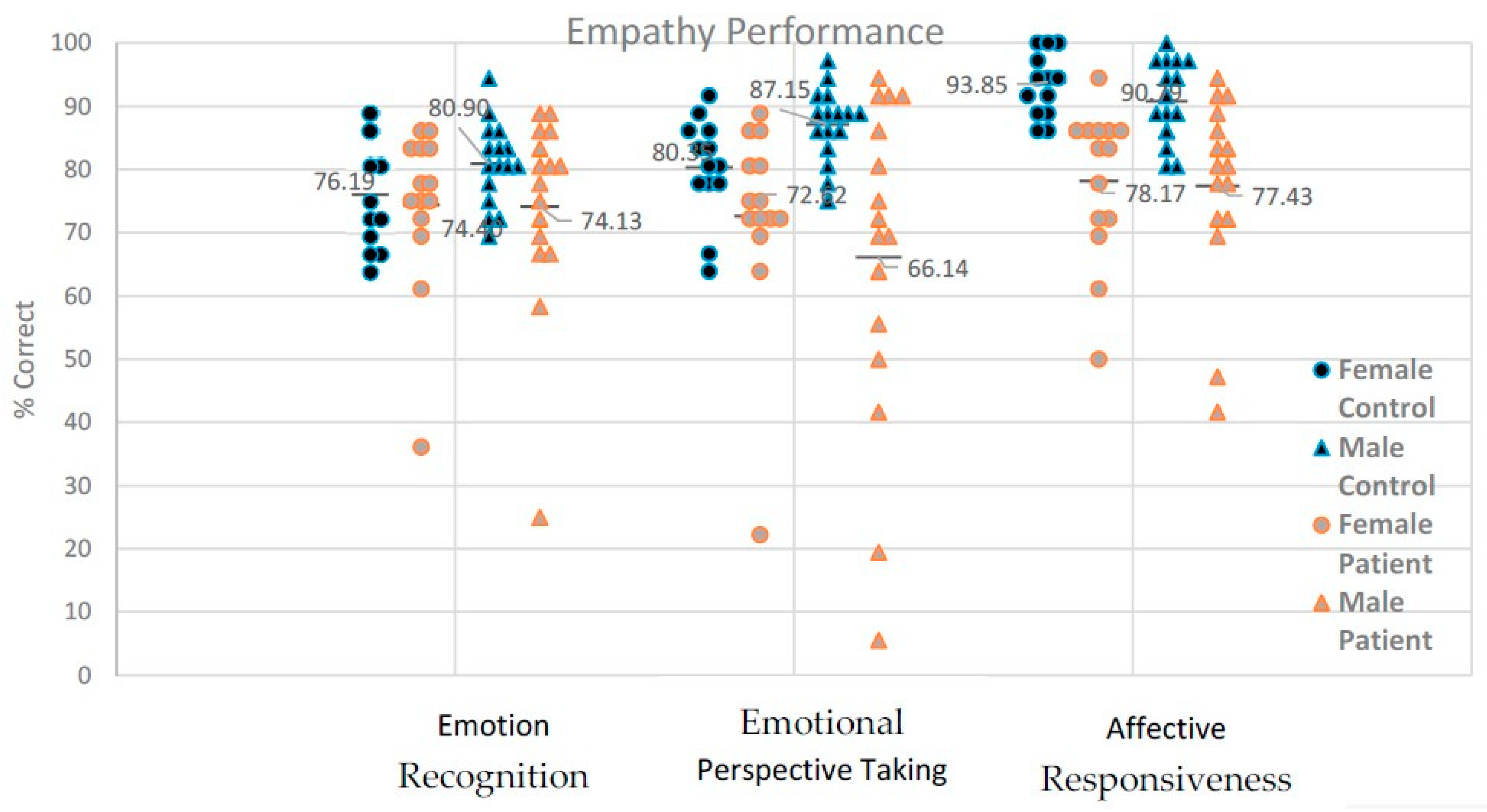
3.1. Emotion Recognition: Accuracy
3.1.1. Emotion Recognition: Speed
3.1.2. Perspective Taking: Accuracy
3.1.3. Perspective Taking: Speed
3.1.4. Affective Responsiveness: Accuracy
3.1.5. Affective Responsiveness: Speed
3.1.6. Inter-Task Relations
3.1.7. Psychopathology and Performance
3.1.8. Self-Reported Empathy
4. Discussion
4.1. Empathy Tasks
4.2. Reaction Times and Emotions
4.3. Influence of Illness Characteristics
4.4. Empathy Questionnaires and Gender
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eisenberg, N.; Miller, P.A. The relation of empathy to prosocial and related behaviors. Psychol. Bull. 1987, 101, 91–119. [Google Scholar] [CrossRef]
- Riess, H. The science of empathy. J. Patient Exp. 2017, 4, 74–77. [Google Scholar] [CrossRef]
- Segrin, C. Social skills deficits associated with depression. Clin. Psychol. Rev. 2000, 20, 379–403. [Google Scholar] [CrossRef]
- Blair, R.J. Responding to the emotions of others: Dissociating forms of empathy through the study of typical and psychiatric populations. Conscious. Cogn. 2005, 14, 698–718. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015, 16, 620–631. [Google Scholar] [CrossRef]
- Premack, D.; Woodruff, G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978, 4, 515–526. [Google Scholar] [CrossRef]
- Derntl, B.; Habel, U. Deficits in social cognition: A marker for psychiatric disorders? Eur. Arch. Psychiatr. Clin. Neurosci. 2011, 261 (Suppl. 2), S145–S149. [Google Scholar] [CrossRef]
- Frith, U.; Frith, C.D. The biological basis of social interaction. Psychol. Sci. 2001, 10, 151–155. [Google Scholar] [CrossRef]
- Fett, A.K.; Viechtbauer, W.; Dominguez, M.D.; Penn, D.L.; van Os, J.; Krabbendam, L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 573–588. [Google Scholar] [CrossRef]
- Derntl, B.; Finkelmeyer, A.; Toygar, T.K.; Hülsmann, A.; Schneider, F.; Falkenberg, D.I.; Habel, U. Generalized deficit in all core components of empathy in schizophrenia. Schizophr. Res. 2009, 108, 197–206. [Google Scholar] [CrossRef]
- Derntl, B.; Seidel, E.M.; Schneider, F.; Habel, U. How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr. Res. 2012, 142, 58–64. [Google Scholar] [CrossRef]
- Kohler, C.G.; Walker, J.B.; Martin, E.A.; Healey, K.M.; Moberg, P.J. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr. Bull. 2010, 36, 1009–1019. [Google Scholar] [CrossRef]
- Lee, J.; Zaki, J.; Harvey, P.O.; Ochsner, K.; Green, M.F. Schizophrenia patients are impaired in empathic accuracy. Psychol. Med. 2011, 41, 2297–2304. [Google Scholar] [CrossRef]
- Achim, A.M.; Ouellet, R.; Roy, M.A.; Jackson, P.L. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatr. Res. 2011, 190, 3–8. [Google Scholar] [CrossRef]
- Haker, H.; Rössler, W. Empathy in schizophrenia: Impaired resonance. Eur. Arch. Psychiatr. Clin. Neurosci. 2009, 259, 352–361. [Google Scholar] [CrossRef]
- Bonfils, K.A.; Lysaker, P.H.; Minor, K.S.; Salyers, M.P. Empathy in schizophrenia: A meta-analysis of the Interpersonal Reactivity Index. Psychiatr. Res. 2017, 249, 293–303. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1998, 13, 261–276. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatr. 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Gur, R.C.; Radim, S.; Hagendoorn, M.; Marom, O.; Hughett, P.; Macy, L.; Turner, T.; Bajcsy, R.; Posner, A.; Gur, R.E. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods 2002, 115, 137–143. [Google Scholar] [CrossRef]
- Derntl, B.; Kryspin-Exner, I.; Fernbach, E.M.; Moser, E.; Habel, U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm. Behav. 2008, 53, 90–95. [Google Scholar] [CrossRef]
- Derntl, B.; Michel, T.M.; Prempeh, P.; Backes, V.; Finkelmeyer, A.; Schneider, F.; Habel, U. Empathy in individuals clinically at risk for psychosis: Brain and behaviour. Br. J. Psychiatr. 2015, 207, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Habel, U.; Windischberger, C.; Derntl, B.; Robinson, S.; Kryspin-Exner, I.; Gur, R.C.; Moser, E. Amygdala activation and facial expressions: Explicit emotion discrimination versus implicit emotion processing. Neuropsychologia 2007, 45, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.H. The effects of dispositional empathy on emotional reactions and helping: A multidimensional approach. J. Pers. 1983, 51, 167–184. [Google Scholar] [CrossRef]
- Decety, J.; Jackson, P.L. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004, 3, 71–100. [Google Scholar] [CrossRef]
- Schneider, F.; Gur, R.C.; Gur, R.E.; Shtasel, D.L. Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr. Res. 1995, 17, 67–75. [Google Scholar] [CrossRef]
- Schneider, F.; Gur, R.C.; Koch, K.; Backes, V.; Amunts, K.; Shah, N.J.; Bilker, W.; Gur, R.E.; Habel, U. Impairment in the specificity of emotion processing in schizophrenia. Am. J. Psychiatr. 2006, 163, 442–447. [Google Scholar] [CrossRef]
- Mandal, M.K.; Pandey, R.; Prasad, A.B. Facial expressions of emotions and schizophrenia: A review. Schizophr. Bull. 1998, 24, 399–412. [Google Scholar] [CrossRef]
- Sachs, G.; Steger-Wuchse, D.; Kryspin-Exner, I.; Gur, R.C.; Katschnig, H. Facial recognition deficits and cognition in schizophrenia. Schizophr. Res. 2004, 68, 27–35. [Google Scholar] [CrossRef]
- Chan, R.C.; Li, H.; Cheung, E.F.; Gong, Q.Y. Impaired facial emotion perception in schizophrenia: A meta-analysis. Psychiatr. Res. 2010, 178, 381–390. [Google Scholar] [CrossRef]
- Barkl, S.J.; Lah, S.; Harris, A.W.; Williams, L.M. Facial emotion identification in early-onset and first-episode psychosis: A systematic review with meta-analysis. Schizophr. Res. 2014, 159, 62–69. [Google Scholar] [CrossRef]
- Comparelli, A.; Corigliano, V.; De Carolis, A.; Mancinelli, I.; Trovini, G.; Ottavi, G.; Dehning, J.; Tatarelli, R.; Brugnoli, R.; Girardi, P. Emotion recognition impairment is present early and is stable throughout the course of schizophrenia. Schizophr. Res. 2013, 143, 65–69. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schäfer, M.R.; Papageorgiou, K.; Klier, C.M.; Schlögelhofer, M.; Mossaheb, N.; Werneck-Rohrer, S.; Nelson, B.; McGorry, P.D. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr. Bull. 2012, 38, 1030–1039. [Google Scholar] [CrossRef]
- Bediou, B.; Asri, F.; Brunelin, J.; Krolak-Salmon, P.; D’Amato, T.; Saoud, M.; Tazi, I. Emotion recognition and genetic vulnerability to schizophrenia. Br. J. Psychiatr. 2007, 191, 126–130. [Google Scholar] [CrossRef]
- Schmidt, S.J.; Mueller, D.R.; Roder, V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: Empirical review and new results by structural equation modeling. Schizophr. Bull. 2011, 37 (Suppl. 2), S41–S54. [Google Scholar] [CrossRef]
- Langdon, R.; Coltheart, M.; Ward, P.B. Empathetic perspective-taking is impaired in schizophrenia: Evidence from a study of emotion attribution and theory of mind. Cogn. Neuropsychiatr. 2006, 11, 133–155. [Google Scholar] [CrossRef]
- Imura, O. Schizophrenia and perspective taking: A comparison of schizophrenic and transient psychotic disorder patients. Jpn. J. Psychol. 2002, 73, 383–390. [Google Scholar] [CrossRef][Green Version]
- Regenbogen, C.; Kellermann, T.; Seubert, J.; Schneider, D.A.; Gur, R.E.; Derntl, B.; Schneider, F.; Habel, U. Neural responses to dynamic multimodal stimuli and pathology-specific impairments of social cognition in schizophrenia and depression. Br. J. Psychiatr. 2015, 206, 198–205. [Google Scholar] [CrossRef]
- Schiffman, J.; Lam, C.W.; Jiwatram, T.; Ekstrom, M.; Sorensen, H.; Mednick, S. Perspective-taking deficits in people with schizophrenia spectrum disorders: A prospective investigation. Psychol. Med. 2004, 34, 1581–1586. [Google Scholar] [CrossRef]
- Ramos-Loyo, J.; Mora-Reynoso, L.; Sánchez-Loyo, L.M.; Medina-Hernández, V. Sex differences in facial, prosodic, and social context emotional recognition in early-onset schizophrenia. Schizophr. Res. Treat. 2012, 2012, 584725. [Google Scholar] [CrossRef]
- Barkhof, E.; de Sonneville, L.M.J.; Meijer, C.J.; de Haan, L. Specificity of facial emotion recognition impairments in patients with multi-episode schizophrenia. Schizophr. Res. Cogn. 2015, 2, 12–19. [Google Scholar] [CrossRef]
- Montag, C.; Heinz, A.; Kunz, D.; Gallinat, J. Self-reported empathic abilities in schizophrenia. Schizophr. Res. 2007, 92, 85–89. [Google Scholar] [CrossRef]
- Sasson, N.; Tsuchiya, N.; Hurley, R.; Couture, S.M.; Penn, D.L.; Adolphs, R.; Piven, J. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia 2007, 45, 2580–2588. [Google Scholar] [CrossRef]
- Marcos-Pablos, S.; González-Pablos, E.; Martín-Lorenzo, C.; Flores, L.A.; Gómez-García-Bermejo, J.; Zalama, E. Virtual Avatar for Emotion Recognition in Patients with Schizophrenia: A Pilot Study. Front. Hum. Neurosci. 2016, 10, 421. [Google Scholar] [CrossRef]
- Bonfils, K.A.; Haas, G.L.; Salyers, M.P. Emotion-specific performance across empathy tasks in schizophrenia: Influence of metacognitive capacity. Schizophr. Res. Cogn. 2020, 19, 100139. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Shur, S.; Barcai-Goodman, L.; Medlovich, S.; Harari, H.; Levkovitz, Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatr. Res. 2007, 149, 11–23. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Shur, S.; Harari, H.; Levkovitz, Y. Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology 2007, 21, 431–438. [Google Scholar] [CrossRef]
- Sparks, A.; McDonald, S.; Lino, B.; O’Donnell, M.; Green, M.J. Social cognition, empathy and functional outcome in schizophrenia. Schizophr. Res. 2010, 122, 172–178. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Mehl, S.; Kesting, M.L.; Rief, W. Negative symptoms and social cognition: Identifying targets for psychological interventions. Schizophr. Bull. 2011, 37 (Suppl. 2), S23–S32. [Google Scholar] [CrossRef]
- Thirioux, B.; Tandonnet, L.; Jaafari, N.; Berthoz, A. Disturbances of spontaneous empathic processing relate with the severity of the negative symptoms in patients with schizophrenia: A behavioural pilot-study using virtual reality technology. Brain Cogn. 2014, 90, 87–99. [Google Scholar] [CrossRef]
- Konstantakopoulos, G.; Ploumpidis, D.; Oulis, P.; Patrikelis, P.; Nikitopoulou, S.; Papadimitriou, G.N.; David, A.S. The relationship between insight and theory of mind in schizophrenia. Schizophr. Res. 2014, 152, 217–222. [Google Scholar] [CrossRef]
- Lysaker, P.H.; Hasson-Ohayon, I.; Kravetz, S.; Kent, J.S.; Roe, D. Self perception of empathy in schizophrenia: Emotion recognition, insight, and symptoms predict degree of self and interviewer agreement. Psychiatr. Res. 2013, 206, 146–150. [Google Scholar] [CrossRef]
- Abu-Akel, A. A neurological mapping of theory mind. Brain Res. Rev. 2003, 43, 29–40. [Google Scholar] [CrossRef]
- Habel, U.; Klein, M.; Shah, N.J.; Toni, I.; Zilles, K.; Falkai, P.; Schneider, F. Genetic load on amygdala hypofunction during sadness in non-affected brothers of schizophrenia patients. Am. J. Psychiatr. 2004, 161, 1806–1813. [Google Scholar] [CrossRef]
- Habel, U.; Klein, M.; Kellermann, T.; Shah, N.J.; Schneider, F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage 2005, 26, 206–214. [Google Scholar] [CrossRef]
- Schneider, F.; Grodd, W.; Weiss, U.; Klose, U.; Mayer, K.R.; Nägele, T.; Gur, R.C. Functional MRI reveals left amygdala activation during emotion. Psychiatr. Res. 1997, 76, 75–82. [Google Scholar] [CrossRef]
- Brüne, M. Emotion recognition, ‘theory of mind’, and social behavior in schizophrenia. Psychiatr. Res. 2005, 133, 135–147. [Google Scholar] [CrossRef]
- Bell, M.D.; Corbera, S.; Johannesen, J.K.; Fiszdon, J.M.; Wexler, B.E. Social cognitive impairments and negative symptoms in schizophrenia: Are there subtypes with distinct functional correlates? Schizophr. Bull. 2013, 39, 186–196. [Google Scholar] [CrossRef]
- Mestre, M.V.; Samper, P.; Frías, M.D.; Tur, A.M. Are women more empathetic than men? A longitudinal study in adolescence. Span. J. Psychol. 2009, 12, 76–83. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004, 34, 163–175. [Google Scholar] [CrossRef]
- Derntl, B.; Finkelmeyer, A.; Eickhoff, S.; Kellermann, T.; Falkenberg, D.I.; Schneider, F.; Habel, U. Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology 2010, 35, 67–82. [Google Scholar] [CrossRef]
- Michalska, K.J.; Kinzler, K.D.; Decety, J. Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Dev. Cogn. Neurosci. 2013, 3, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Christov-Moore, L.; Simpson, E.A.; Coudé, G.; Grigaityte, K.; Iacoboni, M.; Ferrari, P.F. Empathy: Gender effects in brain and behavior. Neurosci. Biobehav. Rev. 2014, 46, 604–627. [Google Scholar] [CrossRef] [PubMed]
- Vongas, J.G.; Al Hajj, R. The Evolution of Empathy and Women’s Precarious Leadership Appointments. Front. Psychol. 2015, 6, 1751. [Google Scholar] [CrossRef] [PubMed]
- Baez, S.; Flichtentrei, D.; Prats, M.; Mastandueno, R.; García, A.M.; Cetkovich, M.; Ibáñez, A. Men, women…who cares? A population-based study on sex differences and gender roles in empathy and moral cognition. PLoS ONE 2017, 12, e0179336. [Google Scholar] [CrossRef]
- Mote, J.; Kring, A.M. Facial emotion perception in schizophrenia: Does sex matter? World J. Psychiatr. 2016, 6, 257–268. [Google Scholar] [CrossRef]
- Javed, A.; Charles, A. The Importance of Social Cognition in Improving Functional Outcomes in Schizophrenia. Front. Psychiatr. 2018, 9, 157. [Google Scholar] [CrossRef]
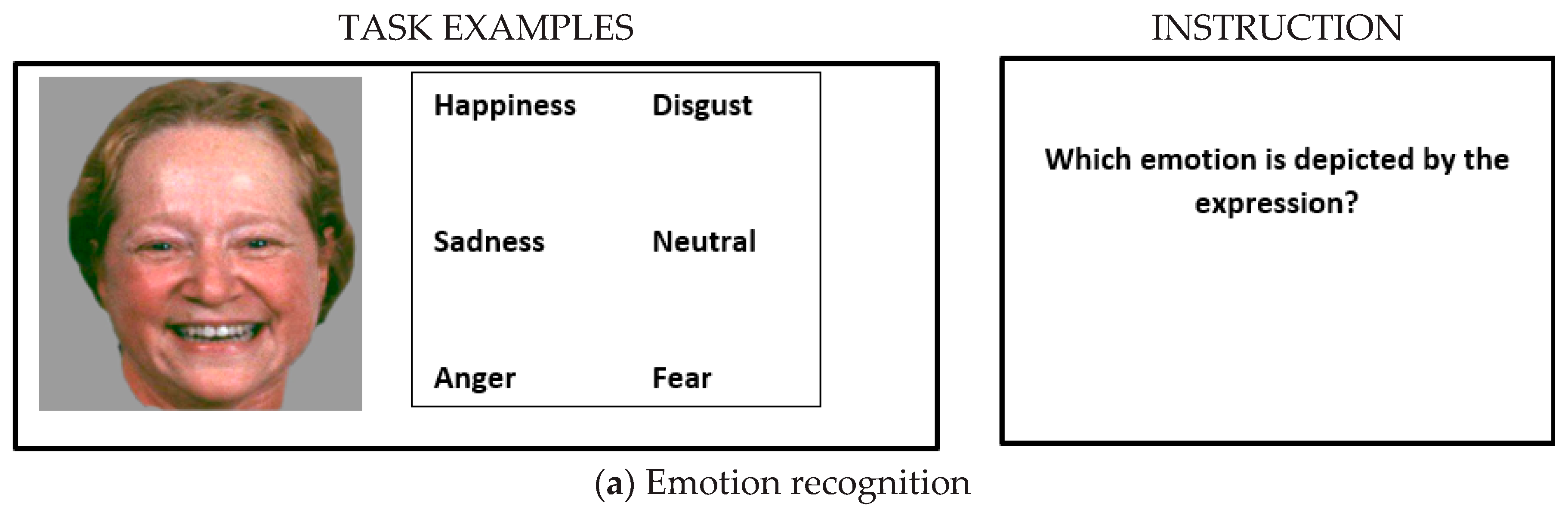
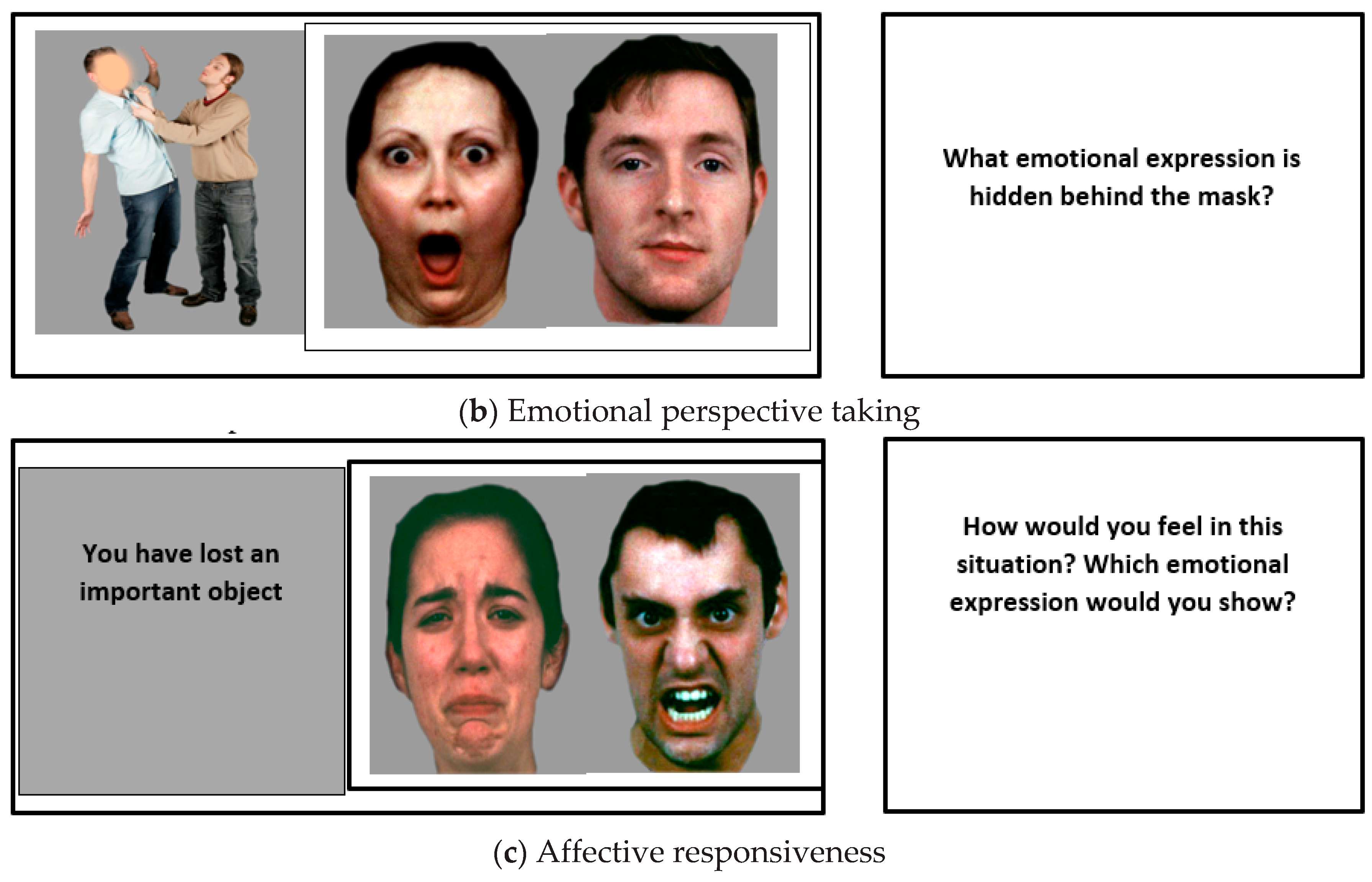
| Patients (n = 30) | Controls (n = 30) | p-Values | |
|---|---|---|---|
| Gender f:m | 14:16 | 14:16 | |
| Mean years of age | 39.3 | 39.5 | 0.951 |
| Duration of illness (yrs) | 11.9 (Range: 2 months-37 yrs) | ||
| Education (yrs) | 10.9 | 11.6 | 0.327 |
| PANSS positive score | 12.56 | ||
| PANSS negative score | 14.66 | ||
| PANSS general score | 26.46 | ||
| PANSS global score | 53.7 |
| Whole Sample (n = 60) | HC (n = 30) | SZP (n = 30) | |||||
|---|---|---|---|---|---|---|---|
| Emotion recognition | F | M | S | F | M | S | |
| Happiness | 91.7 | 95.0 | 95.0 | 95.0 | 90.0 | 86.7 | 88.3 |
| Sadness | 60.0 | 60.0 | 60.0 | 60.0 | 65.5 | 51.7 | 56.7 |
| Anger | 85.0 | 85.0 | 90.0 | 86.7 | 80.0 | 85.0 | 82.8 |
| Fear | 76.7 | 80.0 | 71.7 | 75.0 | 75.0 | 78.3 | 74.4 |
| Disgust | 63.3 | 56.7 | 75.0 | 66.2 | 55.0 | 66.7 | 58.9 |
| Neutral | 88.3 | 86.7 | 95.0 | 91.7 | 85.0 | 83.3 | 84.4 |
| Perspective taking | |||||||
| Happiness | 86.7 | 91.7 | 96.7 | 93.3 | 81.7 | 78.3 | 80.0 |
| Sadness | 80.0 | 90.0 | 86.7 | 88.3 | 78.3 | 65.0 | 71.7 |
| Anger | 66.7 | 63.3 | 80.0 | 71.7 | 61.7 | 61.7 | 61.7 |
| Fear | 75.0 | 75.0 | 81.7 | 88.3 | 76.7 | 66.7 | 71.7 |
| Disgust | 75.0 | 75.0 | 85.0 | 80.0 | 73.3 | 68.3 | 70.0 |
| Neutral | 81.7 | 88.3 | 93.3 | 91.7 | 68.3 | 73.3 | 71.7 |
| Affective responsiveness | |||||||
| Happiness | 90.0 | 93.3 | 98.3 | 96.7 | 81.7 | 85.0 | 83.3 |
| Sadness | 78.3 | 93.3 | 85.0 | 90.0 | 68.3 | 68.3 | 68.3 |
| Anger | 86.7 | 93.3 | 95.0 | 95.0 | 81.7 | 76.7 | 78.3 |
| Fear | 90.0 | 100 | 88.3 | 93.3 | 88.3 | 83.3 | 85.0 |
| Disgust | 90.0 | 98.3 | 93.3 | 95.0 | 86.7 | 81.7 | 85.0 |
| Neutral | 75.0 | 83.3 | 85.0 | 83.3 | 63.3 | 68.3 | 66.1 |
| Whole Sample | HC | SZP | |
|---|---|---|---|
| Emotion recognition | (n = 56) | (n = 29) | (n = 27) |
| Happiness | 4310 | 3243 | 5376 |
| Sadness | 5942 | 5207 | 6676 |
| Anger | 5316 | 4692 | 5940 |
| Fear | 7014 | 5666 | 8361 |
| Disgust | 5816 | 3886 | 7745 |
| Neutral | 5419 | 4204 | 6633 |
| Perspective taking | (n = 58) | (n = 30) | (n = 28) |
| Happiness | 1531 | 1344 | 1718 |
| Sadness | 1745 | 1532 | 1957 |
| Anger | 1891 | 1761 | 2021 |
| Fear | 1825 | 1787 | 1863 |
| Disgust | 1741 | 1667 | 1814 |
| Neutral | 1691 | 1589 | 1792 |
| Affective responsiveness | (n = 60) | (n = 30) | (n = 30) |
| Happiness | 1450 | 1291 | 1609 |
| Sadness | 1661 | 1526 | 1795 |
| Anger | 1701 | 1500 | 1902 |
| Fear | 1599 | 1432 | 1766 |
| Disgust | 1512 | 1364 | 1661 |
| Neutral | 1720 | 1569 | 1871 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peveretou, F.; Radke, S.; Derntl, B.; Habel, U. A Short Empathy Paradigm to Assess Empathic Deficits in Schizophrenia. Behav. Sci. 2020, 10, 41. https://doi.org/10.3390/bs10020041
Peveretou F, Radke S, Derntl B, Habel U. A Short Empathy Paradigm to Assess Empathic Deficits in Schizophrenia. Behavioral Sciences. 2020; 10(2):41. https://doi.org/10.3390/bs10020041
Chicago/Turabian StylePeveretou, Foteini, Sina Radke, Birgit Derntl, and Ute Habel. 2020. "A Short Empathy Paradigm to Assess Empathic Deficits in Schizophrenia" Behavioral Sciences 10, no. 2: 41. https://doi.org/10.3390/bs10020041
APA StylePeveretou, F., Radke, S., Derntl, B., & Habel, U. (2020). A Short Empathy Paradigm to Assess Empathic Deficits in Schizophrenia. Behavioral Sciences, 10(2), 41. https://doi.org/10.3390/bs10020041




