The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS)
Abstract
1. Introduction
2. Preclinical Models of PCOS
2.1. Need for Preclinical Animal Models
2.2. Prenatally Androgenized Animals
2.3. Prenatal Treatment with AMH
2.4. Postnatally Androgenized Animals
2.5. Aromatase Inhibition Models
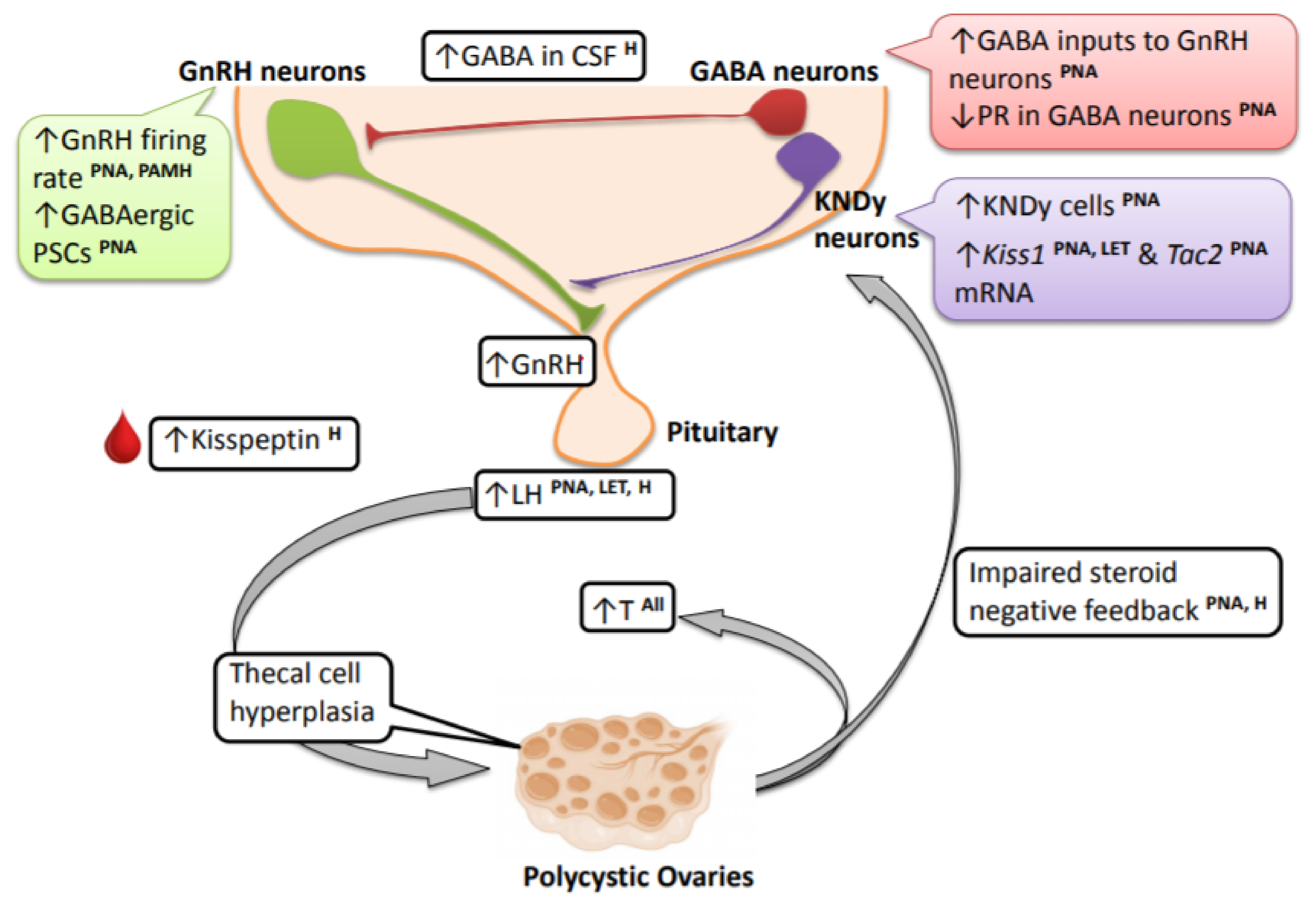
3. Cellular and Molecular Mechanisms Contributing to the PCOS Neuroendocrine Phenotype
3.1. Implications of GnRH and LH Pulse Frequency vs. Amplitude
3.2. GnRH Neurons
3.3. GABAergic Neurons
3.4. Kisspeptin Neurons
3.5. Contributions and Effects of Androgen Signaling in the Brain
4. Concluding Remarks and Perspectives
Funding
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L.; Yen, A.S.S.C. Opioidergic regulation of lh pulsatility in women with polycystic ovary syndrome. Clin. Endocrinol. 1989, 30, 177–184. [Google Scholar] [CrossRef]
- De Vane, G.W.; Czekala, N.M.; Judd, H.L.; Yen, S.S.C. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am. J. Obstet. Gynecol. 1975, 121, 496–500. [Google Scholar] [CrossRef]
- Yen, S.S.C.; Vela, P.; Rankin, J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. J. Clin. Endocrinol. Metab. 1970, 30, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kazer, R.R.; Kessel, B.; Yen, S.S.C. Circulating luteinizing hormone pulse frequency in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1987, 65, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Carmel, P.W.; Araki, S.; Ferin, M. Pituitary stalk portal blood collection in rhesus monkeys: Evidence for pulsatile release of gonadotropin-releasing hormone (gnrh). Endocrinology 1976, 99, 243–248. [Google Scholar] [CrossRef]
- Wildt, L.; Häusler, A.; Marshall, G.; Hutchison, J.S.; Plant, T.M.; Belchetz, P.E.; Knobil, E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 1981, 109, 376–385. [Google Scholar] [CrossRef]
- Caldwell, A.S.L.; Edwards, M.C.; Desai, R.; Jimenez, M.; Gilchrist, R.B.; Handelsman, D.J.; Walters, K.A. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E3334–E3343. [Google Scholar] [CrossRef]
- Deswal, R.; Nanda, S.; Dang, A.S. Association of luteinizing hormone and lh receptor gene polymorphism with susceptibility of polycystic ovary syndrome. Syst. Biol. Reprod. Med. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Day, F.R.; Hinds, D.A.; Tung, J.Y.; Stolk, L.; Styrkarsdottir, U.; Saxena, R.; Bjonnes, A.; Broer, L.; Dunger, D.B.; Halldorsson, B.V.; et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 2015, 6, 8464. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, W.; Guo, L.; Zhang, Z.; Shi, H.; Wang, J.; Zhang, H.; Gao, L.; Feng, G.; He, L. Two fshr variants, haplotypes and meta-analysis in chinese women with premature ovarian failure and polycystic ovary syndrome. Mol. Genet. Metab. 2010, 100, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Mutharasan, P.; Galdones, E.; Peñalver Bernabé, B.; Garcia, O.A.; Jafari, N.; Shea, L.D.; Woodruff, T.K.; Legro, R.S.; Dunaif, A.; Urbanek, M. Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of european ancestry. J. Clin. Endocrinol. Metab. 2013, 98, E185–E190. [Google Scholar] [CrossRef] [PubMed]
- Layman, L.C.; Lee, E.-J.; Peak, D.B.; Namnoum, A.B.; Vu, K.V.; van Lingen, B.L.; Gray, M.R.; McDonough, P.G.; Reindollar, R.H.; Jameson, J.L. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N. Engl. J. Med. 1997, 337, 607–611. [Google Scholar] [CrossRef]
- Qadri, S.; Hussain, A.; Bhat, M.; Baba, A. Polycystic ovary syndrome in bipolar affective disorder: A hospital-based study. Indian J. Psychol. Med. 2018, 40, 121–128. [Google Scholar] [PubMed]
- Herzog, A.G.; Seibel, M.M.; Schomer, D.; Vaitukaitis, J.; Geschwind, N. Temporal lobe epilepsy: An extrahypothalamic pathogenesis for polycystic ovarian syndrome? Neurology 1984, 34, 1389–1393. [Google Scholar] [CrossRef]
- Herzog, A.G.; Seibel, M.M.; Schomer, D.L.; Vaitukaitis, J.L.; Geschwind, N. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch. Neurol. 1986, 43, 341–346. [Google Scholar] [CrossRef]
- Dana-Haeri, J.; Trimble, M.; Oxley, J. Prolactin and gonadotrophin changes following generalised and partial seizures. J. Neurol. Neurosurg. Psychiatry 1983, 46, 331–335. [Google Scholar] [CrossRef]
- Joffe, H.; Taylor, A.E.; Hall, J.E. Editorial: Polycystic ovarian syndrome—Relationship to epilepsy and antiepileptic drug therapy. J. Clin. Endocrinol. Metab. 2001, 86, 2946–2949. [Google Scholar]
- Viswanathan, L.; Satishchandra, P.; Bhimani, B.; Reddy, J.; Rama Murthy, B.; Subbakrishna, D.; Sinha, S. Polycystic ovary syndrome in patients on antiepileptic drugs. Ann. Indian Acad. Neurol. 2016, 19, 339–343. [Google Scholar] [PubMed]
- Zhang, L.; Li, H.; Li, S.; Zou, X. Reproductive and metabolic abnormalities in women taking valproate for bipolar disorder: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 202, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic acid pathway: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2013, 23, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Xita, N.; Tsatsoulis, A. Review: Fetal programming of polycystic ovary syndrome by androgen excess: Evidence from experimental, clinical, and genetic association studies. J. Clin. Endocrinol. Metab. 2006, 91, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Falbo, A.; Rocca, M.; Russo, T.; D’Ettore, A.; Tolino, A.; Zullo, F.; Orio, F.; Palomba, S. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): Relationships with adverse outcomes. J. Ovarian Res. 2010, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Sir-Petermann, T.; Maliqueo, M.; Angel, B.; Lara, H.E.; Pérez-Bravo, F.; Recabarren, S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: Possible implications in prenatal androgenization. Hum. Reprod. 2002, 17, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Roland, A.V.; Nunemaker, C.S.; Keller, S.R.; Moenter, S.M. Prenatal androgen exposure programs metabolic dysfunction in female mice. J. Endocrinol. 2010, 207, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.D.; Moenter, S.M. Prenatal androgens alter gabaergic drive to gonadotropin-releasing hormone neurons: Implications for a common fertility disorder. Proc. Natl. Acad. Sci. USA 2004, 101, 7129–7134. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Prescott, M.; Campbell, R.E. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology 2013, 154, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Prescott, M.; Marshall, C.J.; Yip, S.H.; Campbell, R.E. Enhancement of a robust arcuate gabaergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 596–601. [Google Scholar] [CrossRef]
- Abbott, D.H.; Barnett, D.K.; Bruns, C.M.; Dumesic, D.A. Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Hum. Reprod. Update 2005, 11, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Abbott, D.H.; Eisner, J.R.; Goy, R.W. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil. Steril. 1997, 67, 155–163. [Google Scholar] [CrossRef]
- Robinson, J.E.; Forsdike, R.A.; Taylor, J.A. In utero exposure of female lambs to testosterone reduces the sensitivity of the gnrh neuronal network to inhibition by progesterone. Endocrinology 1999, 140, 5797–5805. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.N.; Manikkam, M.; Herkimer, C.; Dell’Orco, J.; Welch, K.B.; Foster, D.L.; Padmanabhan, V. Fetal programming: Excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology 2005, 146, 4281–4291. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Li, Z.-L.; Wu, C.-Y.; Liu, Y.-M.; Lin, H.; Wang, S.-H.; Xiao, W.-F. Endocrine traits of polycystic ovary syndrome in prenatally androgenized female sprague-dawley rats. Endocr. J. 2010, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yuan, C.; Zhao, N.; Cui, Y.; Liu, J. Prenatal androgen excess enhances stimulation of the gnrh pulse in pubertal female rats. J. Endocrinol. 2014, 222, 73. [Google Scholar] [CrossRef]
- Manikkam, M.; Thompson, R.C.; Herkimer, C.; Welch, K.B.; Flak, J.; Karsch, F.J.; Padmanabhan, V. Developmental programming: Impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep1. Biol. Reprod. 2008, 78, 648–660. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.-L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-müllerian hormone in the regulation of gnrh neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef]
- Caldwell, A.S.L.; Middleton, L.J.; Jimenez, M.; Desai, R.; McMahon, A.C.; Allan, C.M.; Handelsman, D.J.; Walters, K.A. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014, 155, 3146–3159. [Google Scholar] [CrossRef]
- Manneras, L.; Cajander, S.; Holmang, A.; Seleskovic, Z.; Lystig, T.; Lonn, M.; Stener-Victorin, E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef]
- Osuka, S.; Iwase, A.; Nakahara, T.; Kondo, M.; Saito, A.; Nakamura, B.T.; Takikawa, S.; Goto, M.; Kotani, T.; Kikkawa, F. Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinology 2016, 158, 367–377. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Tungalagsuvd, A.; Munkhzaya, M.; Yiliyasi, M.; Kato, T.; Kuwahara, A.; Irahara, M. Effects of chronic dhea treatment on central and peripheral reproductive parameters, the onset of vaginal opening and the estrous cycle in female rats. Gynecol. Endocrinol. 2016, 32, 752–755. [Google Scholar] [CrossRef]
- Abramovich, D.; Irusta, G.; Bas, D.; Cataldi, N.I.; Parborell, F.; Tesone, M. Angiopoietins/tie2 system and vegf are involved in ovarian function in a dhea rat model of polycystic ovary syndrome. Endocrinology 2012, 153, 3446–3456. [Google Scholar] [CrossRef]
- Kafali, H.; Iriadam, M.; Ozardalı, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Tungalagsuvd, A.; Iwasa, T.; Munkhzaya, M.; Yanagihara, R.; Tokui, T.; Yano, K.; Mayila, Y.; Kato, T.; Kuwahara, A.; et al. Kisspeptin mrna expression is increased in the posterior hypothalamus in the rat model of polycystic ovary syndrome. Endocr. J. 2017, 64, 7–14. [Google Scholar] [CrossRef]
- Chaudhari, N.; Dawalbhakta, M.; Nampoothiri, L. Gnrh dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod. Biol. Endocrinol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Thackray, V.G.; Ryan, G.E.; Tolson, K.P.; Glidewell-Kenney, C.A.; Semaan, S.J.; Poling, M.C.; Iwata, N.; Breen, K.M.; Duleba, A.J.; et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol. Reprod. 2015, 93, 69. [Google Scholar] [CrossRef]
- Masek, K.S.; Wood, R.I.; Foster, D.L. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology 1999, 140, 3459–3466. [Google Scholar] [CrossRef]
- Tehrani, F.R.; Noroozzadeh, M.; Zahediasl, S.; Piryaei, A.; Azizi, F. Introducing a rat model of prenatal androgen-induced polycystic ovary syndrome in adulthood. Exp. Phys. 2014, 99, 792–801. [Google Scholar] [CrossRef]
- Maliqueo, M.; Sun, M.; Johansson, J.; Benrick, A.; Labrie, F.; Svensson, H.; Lönn, M.; Duleba, A.J.; Stener-Victorin, E. Continuous administration of a p450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. Endocrinology 2013, 154, 434–445. [Google Scholar] [CrossRef]
- Clarkson, J.; Han, S.Y.; Piet, R.; McLennan, T.; Kane, G.M.; Ng, J.; Porteous, R.W.; Kim, J.S.; Colledge, W.H.; Iremonger, K.J.; et al. Definition of the hypothalamic gnrh pulse generator in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10216–E10223. [Google Scholar] [CrossRef]
- Haisenleder, D.J.; Khoury, S.; Zmeili, S.M.; Papavasiliou, S.; Ortolano, G.A.; Dee, C.; Duncan, J.A.; Marshall, J.C. The frequency of gonadotropin-releasing hormone secretion regulates expression of α and luteinizing hormone β-subunit messenger ribonucleic acids in male rats. Mol. Endocrinol. 1987, 1, 834–838. [Google Scholar] [CrossRef]
- Dalkin, A.C.; Haisenleder, D.J.; Ortolano, G.A.; Ellis, T.R.; Marshall, J.C. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology 1989, 125, 917–923. [Google Scholar] [CrossRef]
- Pralong, F.P.; Boepple, P.A.; Conn, P.M.; Whitcomb, R.W.; Butler, J.P.; Schoenfeld, D.; Crowley, J.W.F. Contour of the gnrh pulse independently modulates gonadotropin secretion in the human male. Neuroendocrinology 1996, 64, 247–256. [Google Scholar] [CrossRef]
- Roland, A.V.; Moenter, S.M. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (gnrh) neurons that is reversed by metformin treatment in adulthood. Endocrinology 2011, 152, 618–628. [Google Scholar] [CrossRef]
- DeFazio, R.A.; Heger, S.; Ojeda, S.R.; Moenter, S.M. Activation of a-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol. Endocrinol. 2002, 16, 2872–2891. [Google Scholar] [CrossRef]
- Pastor, C.L.; Griffin-Korf, M.L.; Aloi, J.A.; Evans, W.S.; Marshall, J.C. Polycystic ovary syndrome: Evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone1. J. Clin. Endocrinol. Metab. 1998, 83, 582–590. [Google Scholar] [CrossRef][Green Version]
- Hrabovszky, E.; Steinhauser, A.R.; Barabás, K.; Shughrue, P.J.; Petersen, S.L.; Merchenthaler, I.N.; Liposits, Z. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2001, 142, 3261–3264. [Google Scholar] [CrossRef]
- Herbison, A.; Skynner, M.; Sim, J. Lack of detection of estrogen receptor-α transcripts in mouse gonadotropin-releasing hormone neurons. Endocrinology 2001, 142, 493. [Google Scholar]
- Huang, X.; Harlan, R.E. Absence of androgen receptors in lhrh immunoreactive neurons. Brain Res. 1993, 624, 309–311. [Google Scholar] [CrossRef]
- Kawwass, J.F.; Sanders, K.M.; Loucks, T.L.; Rohan, L.C.; Berga, S.L. Increased cerebrospinal fluid levels of gaba, testosterone and estradiol in women with polycystic ovary syndrome. Hum. Reprod. 2017, 32, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; McCartney, C.R.; Yoo, R.Y.; Eagleson, C.A.; Chang, R.J.; Marshall, J.C. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: Evidence for varied effects in hyperandrogenemic adolescent girls. J. Clin. Endocrinol. Metab. 2005, 90, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Jahan, S.; Razak, S.; Pirzada, M.; Ullah, H.; Almajwal, A.; Rauf, N.; Afsar, T. Protective effects of gaba against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. J. Ovarian Res. 2017, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspepeptin-gpr54 signaling in the neuroendocrine reproductive axis. Mol. Cell. Endocrinol. 2006, 254, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via g protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Katulski, K.; Podfigurna, A.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Kisspeptin and lh pulsatile temporal coupling in PCOS patients. Endocrine 2018, 61, 149–157. [Google Scholar] [CrossRef]
- Wang, T.; Han, S.; Tian, W.; Zhao, M.; Zhang, H. Effects of kisspeptin on pathogenesis and energy metabolism in polycystic ovarian syndrome (PCOS). Gynecol. Endocrinol. 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- D’Anglemont de Tassigny, X.; Fagg, L.A.; Dixon, J.P.C.; Day, K.; Leitch, H.G.; Hendrick, A.G.; Zahn, D.; Franceschini, I.; Caraty, A.; Carlton, M.B.L.; et al. Hypogonadotropic hypogonadism in mice lacking a functional kiss1 gene. Proc. Natl. Acad. Sci. USA 2007, 104, 10714–10719. [Google Scholar] [CrossRef]
- Lapatto, R.; Pallais, J.C.; Zhang, D.; Chan, Y.-M.; Mahan, A.; Cerrato, F.; Le, W.W.; Hoffman, G.E.; Seminara, S.B. Kiss1−/−mice exhibit more variable hypogonadism than Gpr54−/−mice. Endocrinology 2007, 148, 4927–4936. [Google Scholar] [CrossRef]
- Kirilov, M.; Clarkson, J.; Liu, X.; Roa, J.; Campos, P.; Porteous, R.; Schütz, G.; Herbison, A.E. Dependence of fertility on kisspeptin–gpr54 signaling at the gnrh neuron. Nat. Commun. 2013, 4, 2492. [Google Scholar] [CrossRef] [PubMed]
- Novaira, H.J.; Sonko, M.L.; Hoffman, G.; Koo, Y.; Ko, C.; Wolfe, A.; Radovick, S. Disrupted kisspeptin signaling in gnrh neurons leads to hypogonadotrophic hypogonadism. Mol. Endocrinol. 2014, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Seminara, S.; Clifton, D.K.; Steiner, R.A. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004, 145, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.; Chahal, N.; Munaganuru, N.; Parra, R.A.; Kauffman, A.S. Estrogen stimulation of kiss1 expression in the medial amygdala involves estrogen receptor-alpha but not estrogen receptor-beta. Endocrinology 2016, 157, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.Z.; Di Giorgio, N.P.; Liaw, R.B.; Parra, R.A.; Yang, J.A.; Chahal, N.; Lux-Lantos, V.A.; Kauffman, A.S. Estradiol-dependent and -independent stimulation of kiss1 expression in the amygdala, bnst, and lateral septum of mice. Endocrinology 2018, 159, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Lehman, M.N.; Smith, J.T.; Coolen, L.M.; de Oliveira, C.V.R.; Jafarzadehshirazi, M.R.; Pereira, A.; Iqbal, J.; Caraty, A.; Ciofi, P.; et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin a and neurokinin b. Endocrinology 2007, 148, 5752–5760. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin b neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M. Interactions between kisspeptins and neurokinin b. In Kisspeptin Signaling in Reproductive Biology; Kauffman, A.S., Smith, J.T., Eds.; Springer: New York, NY, USA, 2013; pp. 325–347. [Google Scholar]
- Topaloglu, A.K.; Reimann, F.; Guclu, M.; Yalin, A.S.; Kotan, L.D.; Porter, K.M.; Serin, A.; Mungan, N.O.; Cook, J.R.; Ozbek, M.N.; et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin b in the central control of reproduction. Nat. Genet. 2008, 41, 354. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Wilhelm, A.; Pirke, K.M.; Gramsch, C.; Herz, A. Beta-endorphin and dynorphin control serum luteinizing hormone level in immature female rats. Nature 1981, 294, 757–759. [Google Scholar] [CrossRef]
- Terasawa, E.; Garcia, J.P.; Seminara, S.B.; Keen, K.L. Role of kisspeptin and neurokinin b in puberty in female non-human primates. Front. Endocrinol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Smith, J.T.; Cunningham, M.J.; Rissman, E.F.; Clifton, D.K.; Steiner, R.A. Regulation of kiss1 gene expression in the brain of the female mouse. Endocrinology 2005, 146, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, A.S.; Gottsch, M.L.; Roa, J.; Byquist, A.C.; Crown, A.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A.; Tena-Sempere, M. Sexual differentiation of kiss1 gene expression in the brain of the rat. Endocrinology 2007, 148, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.; Tolson, K.P.; Rouse, M.L., Jr.; Poling, M.C.; Hashimoto-Partyka, M.K.; Mellon, P.L.; Kauffman, A.S. Absent progesterone signaling in kisspeptin neurons disrupts the lh surge and impairs fertility in female mice. Endocrinology 2015, 156, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Rometo, A.M.; Krajewski, S.J.; Lou Voytko, M.; Rance, N.E. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab. 2007, 92, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Hrabovszky, E.; Ciofi, P.; Vida, B.; Horvath, M.C.; Keller, E.; Caraty, A.; Bloom, S.R.; Ghatei, M.A.; Dhillo, W.S.; Liposits, Z.; et al. The kisspeptin system of the human hypothalamus: Sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin b neurons. Eur. J. Neurosci. 2010, 31, 1984–1998. [Google Scholar] [CrossRef] [PubMed]
- Cernea, M.; Padmanabhan, V.; Goodman, R.L.; Coolen, L.M.; Lehman, M.N. Prenatal testosterone treatment leads to changes in the morphology of kndy neurons, their inputs, and projections to gnrh cells in female sheep. Endocrinology 2015, 156, 3277–3291. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Wilkinson, D.A.; Imran, S.A.; Caraty, A.; Wilkinson, M. Hypothalamic kiss1 mrna and kisspeptin immunoreactivity are reduced in a rat model of polycystic ovary syndrome (PCOS). Brain Res. 2012, 1467, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, E.; Namavar, M.R.; Mortezaee, K.; Toolee, H.; Keshtgar, S.; Mirkhani, H.; Akbari, M.; Rastegar, T.; Solhjoo, S. Kisspeptin expression features in the arcuate and anteroventral periventricular nuclei of hypothalamus of letrozole-induced polycystic ovarian syndrome in rats. Arch. Gynecol. Obstet. 2017, 296, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Ruka, K.A.; Burger, L.L.; Moenter, S.M. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin b, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology 2013, 154, 2761–2771. [Google Scholar] [CrossRef]
- George, J.T.; Kakkar, R.; Marshall, J.; Scott, M.L.; Finkelman, R.D.; Ho, T.W.; Veldhuis, J.; Skorupskaite, K.; Anderson, R.A.; McIntosh, S.; et al. Neurokinin b receptor antagonism in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2016, 101, 4313–4321. [Google Scholar] [CrossRef]
- Eagleson, C.A.; Gingrich, M.B.; Pastor, C.L.; Arora, T.K.; Burt, C.M.; Evans, W.S.; Marshall, J.C. Polycystic ovarian syndrome: Evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone1. J. Clin. Endocrinol. Metab. 2000, 85, 4047–4052. [Google Scholar] [PubMed]
- Silva, M.S.; Prescott, M.; Campbell, R.E. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Semaan, S.J.; Clifton, D.K.; Steiner, R.A.; Dhamija, S.; Kauffman, A.S. Regulation of kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 2011, 152, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Prenatally Androgenized Animals (PNA) | Postnatally Androgenized Animals | Letrozole Treated Animals | Clinical Data |
|---|---|---|---|---|
| Hyperandrogenemia | Mouse—Yes | Mouse, Rat—High dihydrotestosterone (DHT) due to exogenous DHT treatment, but no increase in testosterone (T) | Mouse—Yes | Yes (60% according to the Rotterdam criteria) |
| Rat—Yes | Rat—Yes | |||
| Sheep—Yes | - | - | ||
| Monkey—Yes | - | - | ||
| Luteinizing hormone (LH) levels | Mouse—High + ↑pulse frequency | Mouse—No change | Mouse—High | High LH pulse frequency and amplitude |
| Rat—High + ↑pulse frequency | Rat—No data | Rat—High | ||
| Sheep—↑↓ (Conflicting reports) | - | - | - | |
| Monkey—High | - | - | - | |
| Negative feedback | Mouse—Impaired | Mouse—No data | Mouse—No data | Need higher doses of Estradiol and progesterone to decrease LH to similar levels in healthy women |
| Rat—No data | Rat—No data | Rat—No data | ||
| Sheep—Impaired | - | - | ||
| Monkey—Impaired | - | - | ||
| GnRH/GnRH neurons | Mouse (PNA)—↑GnRH firing rate; ↑GABAergic inputs to GnRH neurons; ↑GABAergic postsynaptic currents Mouse (PAMH)—↑GnRH firing rate; ↑GABAergic inputs to GnRH neurons | Mouse—No data | Mouse—No data | No data |
| Rat—No data | Rat—No data | Rat—No data | ||
| Sheep—No data | - | - | ||
| Monkey—No data | - | - | ||
| Kisspeptin/Kisspeptin neurons | Mouse—No data | Mouse—No data | Mouse—No data | Positive correlation between kisspeptin and LH levels; NK3R antagonist treatment decreased LH and T levels in women with PCOS |
| Rat—↑Kiss and NKB positive cells in ARN; ↑Kiss1 and Tac2 mRNA levels | Rat—↓Kiss1 mRNA in the hypothalamus ↓Kiss-ir cells in ARN | Rat—↑ARN Kiss cells | ||
| Sheep—No change Kiss cells, but ↑ in cell size of ARN Kiss cells; ↓excitatory glutamatergic inputs to Kiss cells | - | - | ||
| Monkey—No data | - | - | ||
| GABA/GABA neurons | Mouse—↑GABAergic inputs from ARN to GnRH neurons; ↓Progesterone receptor in ARN GABA neurons | Mouse—No data | Mouse—No data | Increased GABA in CSF of PCOS women |
| Rat—No data | Rat—No data | Rat (LET by oral gavage)—↓GABA mRNA levels in several brain regions including the hypothalamus; Co-administration of GABA with LET decreased T levels and body weight | ||
| Sheep—No data | - | - | ||
| Monkey—No data | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutinho, E.A.; Kauffman, A.S. The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS). Med. Sci. 2019, 7, 84. https://doi.org/10.3390/medsci7080084
Coutinho EA, Kauffman AS. The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS). Medical Sciences. 2019; 7(8):84. https://doi.org/10.3390/medsci7080084
Chicago/Turabian StyleCoutinho, Eulalia A., and Alexander S. Kauffman. 2019. "The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS)" Medical Sciences 7, no. 8: 84. https://doi.org/10.3390/medsci7080084
APA StyleCoutinho, E. A., & Kauffman, A. S. (2019). The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS). Medical Sciences, 7(8), 84. https://doi.org/10.3390/medsci7080084






