Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
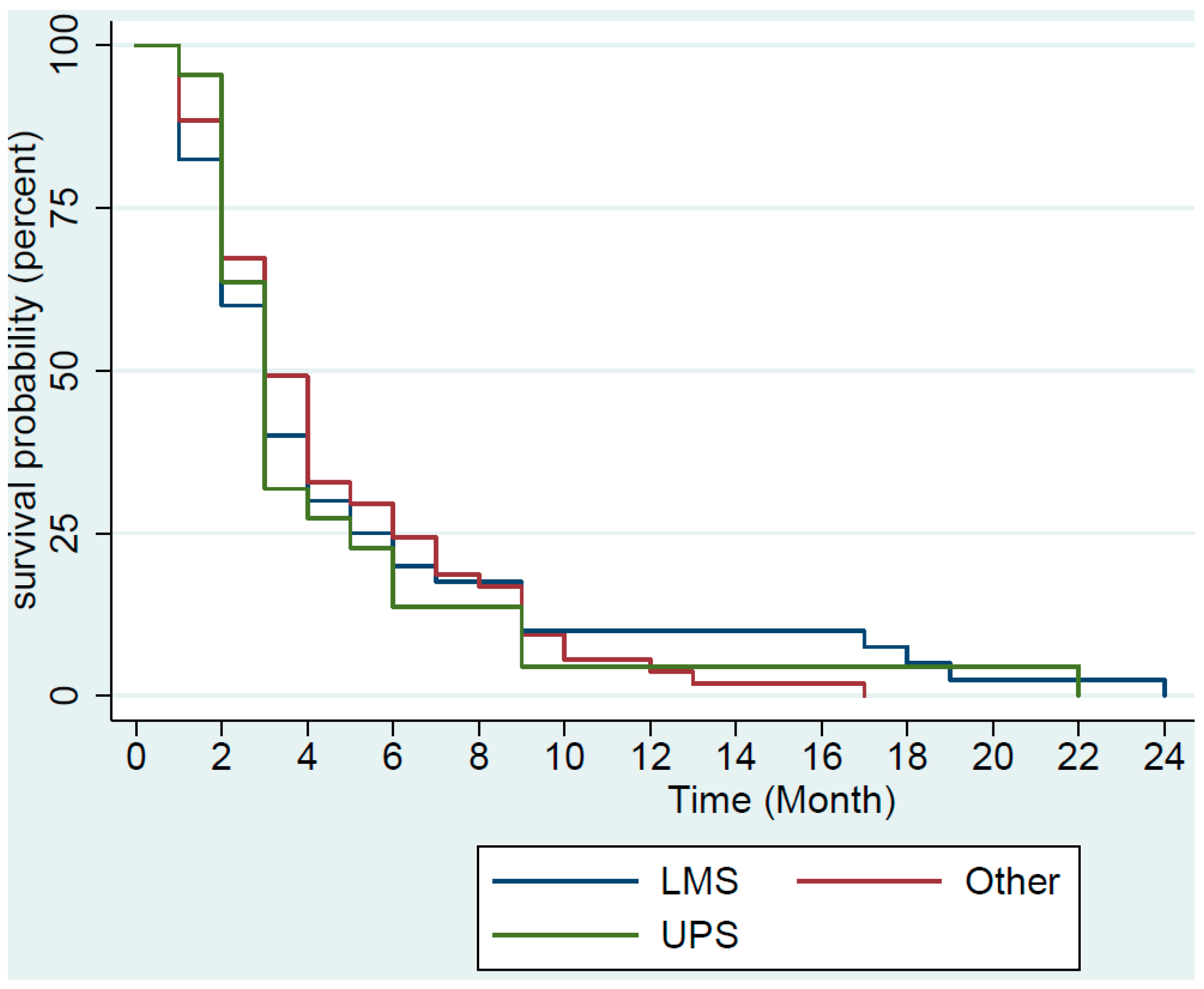
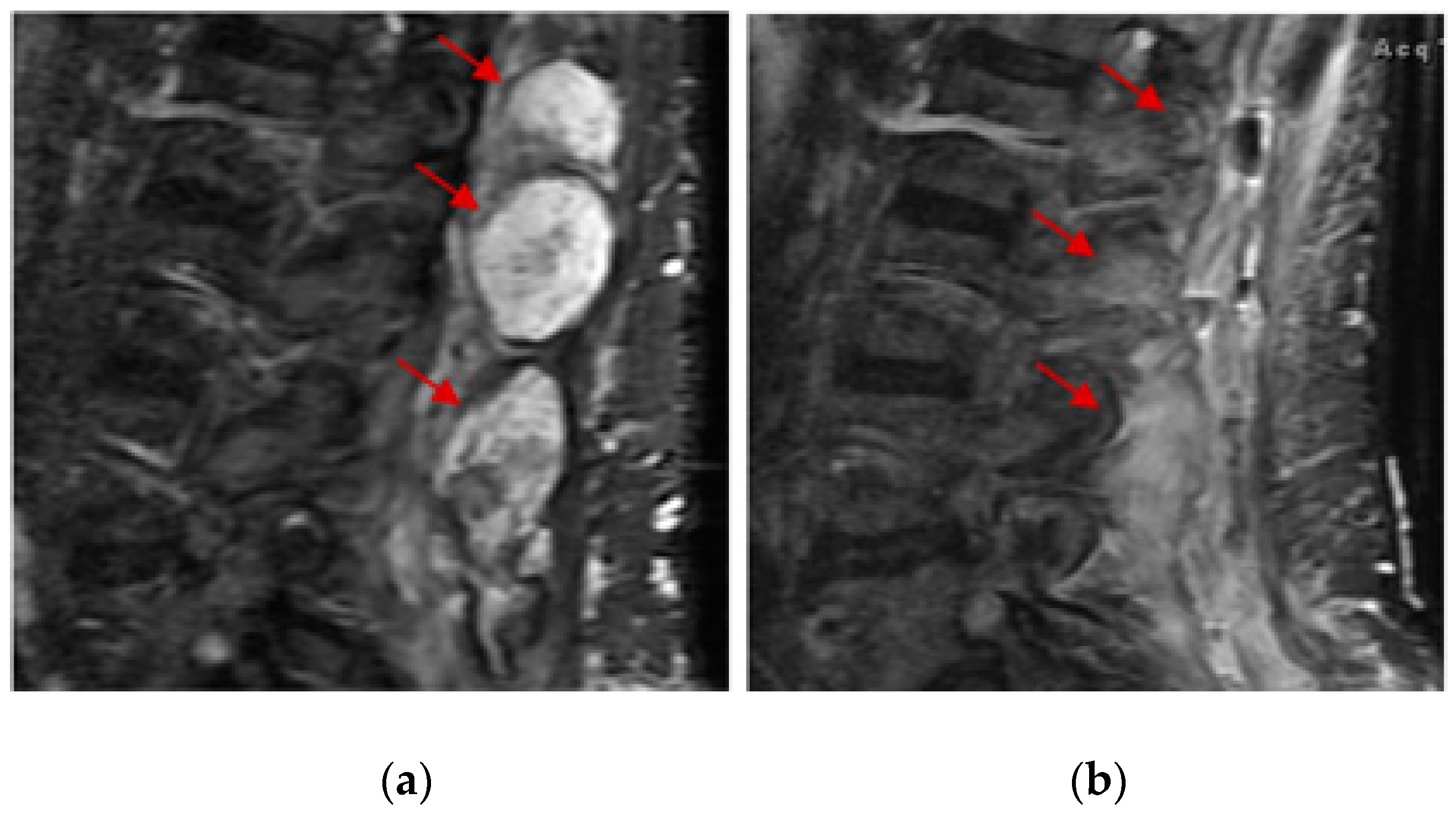
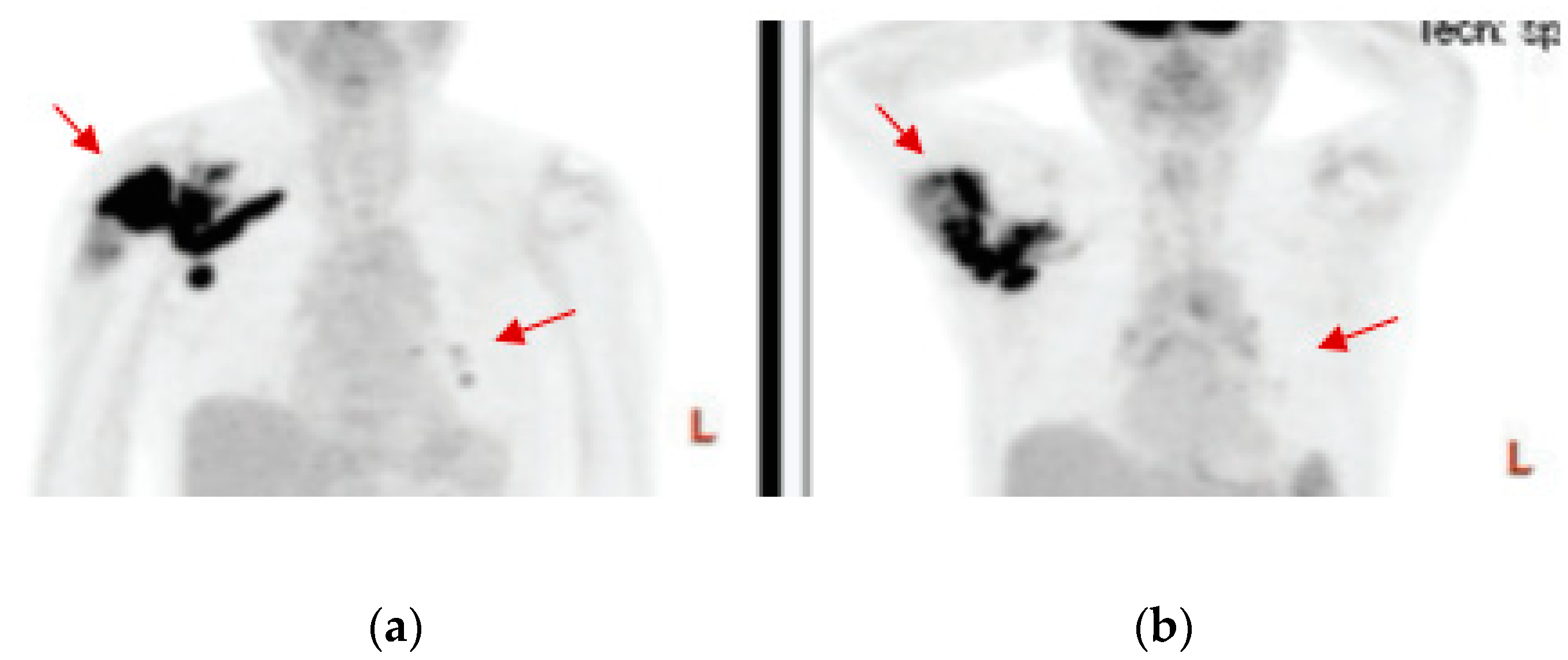
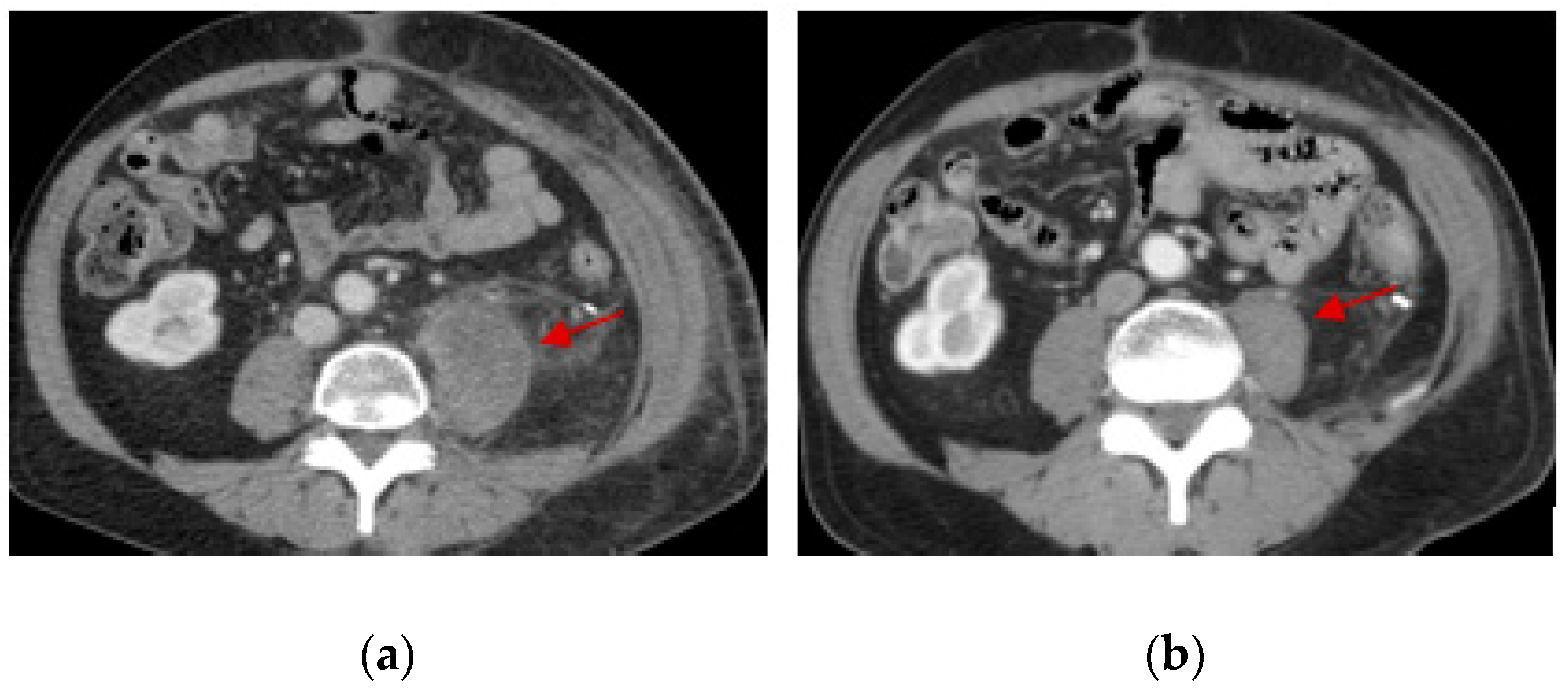
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Versleijen-Jonkers, Y.M.; Vlenterie, M.; van de Luijtgaarden, A.C.; van der Graaf, W.T. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit. Rev. Oncol. Hematol. 2014, 91, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A.; Meyer, C.F.; Trent, J.C. Pazopanib in sarcomas: Expanding the PALETTE. Curr. Opin. Oncol. 2013, 25, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Okuno, S.H.; Robinson, S.I.; Weber, N.P.; Indelicato, D.J.; Jones, R.L; Bagaria, S.P.; Sherman, C.; Kozak, K.R.; Cortese, C.M.; et al. Clinical Activity of Pazopanib in Metastatic Extraosseous Ewing Sarcoma. Rare Tumors 2015, 7, 5992. [Google Scholar] [CrossRef] [PubMed]
- Alcindor, T. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol. 2015, 54, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Safwat, A.; Boysen, A.; Lucke, A.; Rossen, P. Pazopanib in metastatic osteosarcoma: Significant clinical response in three consecutive patients. Acta Oncol. 2014, 53, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; ItalianO, A.; Collard, O.; et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef]
- Sloan, B.; Scheinfeld, N.S. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr. Opin. Investig. Drugs 2008, 9, 1324–1335. [Google Scholar]
- Keisner, S.V.; Shah, S.R. Pazopanib: The newest tyrosine kinase inhibitor for the treatment of advanced or metastatic renal cell carcinoma. Drugs 2011, 71, 443–454. [Google Scholar] [CrossRef]
- Potti, A.; Ganti, A.K.; Tendulkar, K.; Sholes, K.; Chitajallu, S.; Koch, M.; Kargas, S. Determination of vascular endothelial growth factor (VEGF) overexpression in soft tissue sarcomas and the role of overexpression in leiomyosarcoma. J. Cancer Res. Clin. Oncol. 2004, 130, 52–56. [Google Scholar] [CrossRef]
- Yudoh, K.; Kanamori, M.; Ohmori, K.; Yasuda, T.; Aoki, M.; Kimura, T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br. J. Cancer 2001, 84, 1610–1615. [Google Scholar] [CrossRef]
- Pakos, E.E.; Goussia, A.C.; Tsekeris, P.G.; Papachristou, D.J.; Stefanou, D.; Agnantis, N.J. Expression of vascular endothelial growth factor and its receptor, KDR/Flk-1, in soft tissue sarcomas. Anticancer Res. 2005, 25, 3591–3596. [Google Scholar] [PubMed]
- Hayes, A.J.; Mostyn-Jones, A.; Koban, M.U.; A’Hern, R.; Burton, P.; Thomas, J.M. Serum vascular endothelial growth factor as a tumour marker in soft tissue sarcoma. Br. J. Surg. 2004, 91, 242–247. [Google Scholar] [CrossRef]
- Yoon, S.S.; Segal, N.H.; Olshen, A.B.; Brennan, M.F.; Singer, S. Circulating angiogenic factor levels correlate with extent of disease and risk of recurrence in patients with soft tissue sarcoma. Ann. Oncol. 2004, 15, 1261–1266. [Google Scholar] [CrossRef]
- Schutz, F.A.; Choueiri, T.K.; Sternberg, C.N. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit. Rev. Oncol. Hematol. 2011, 77, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Menefee, M.E.; Smallridge, RC.; Molina, JR.; Maples, WJ.; Karlin, NJ.; Traynor, AM.; Kumar, P.; Goh, B.C.; et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Sideras, K.; Morris, J.C., 3rd; McIver, B.; et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol. 2010, 11, 962–972. [Google Scholar] [CrossRef]
- Jonasch, E.; McCutcheon, I.E.; Gombos, D.S.; Ahrar, K.; Perrier, N.D.; Liu, D.; Robichaux, C.C.; Villarreal, M.F.; Weldon, J.A.; Woodson, A.H.; et al. Pazopanib in patients with von Hippel-Lindau disease: A single-arm, single-centre, phase 2 trial. Lancet Oncol. 2018, 19, 1351–1359. [Google Scholar] [CrossRef]
- Mir, O.; Cropet, C.; Toulmonde, M.; Cesne, AL.; Molimard, M.; Bompas, E.; Cassier, P.; Ray-Coquard, I.; Rios, M.; Adenis, A.; et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): A randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016, 17, 632–641. [Google Scholar] [CrossRef]
- Ganjoo, K.N.; Villalobos, V.M.; Kamaya, A.; Fisher, G.A.; Butrynski, J.E.; Morgan, J.A.; Wagner, A.J.; D’Adamo, D.; McMillan, A.; Demetri, G.D.; et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann. Oncol. 2014, 25, 236–240. [Google Scholar] [CrossRef]
- Spira, A.I.; Ettinger, D.S. The use of chemotherapy in soft-tissue sarcomas. Oncologist 2002, 7, 348–359. [Google Scholar] [CrossRef]
- Frezza, A.M.; Stacchiotti, S.; Gronchi, A. Systemic treatment in advanced soft tissue sarcoma: What is standard, what is new. BMC Med. 2017, 15, 109. [Google Scholar] [CrossRef]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Bauer, R.; Biagini, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; Brodowicz, T.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litiere, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar] [PubMed]
- Nakamura, T.; Matsumine, A.; Kawai, A.; Araki, N.; Goto, T.; Yonemoto, T.; Sugiura, H.; Nishida, Y.; Hiraga, H.; Honoki, K.; et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer 2016, 122, 1408–1416. [Google Scholar] [CrossRef]
- Samuels, B.L.; Chawla, S.P.; Somaiah, N.; Staddon, A.P.; Skubitz, K.M.; Milhem, M.M.; Kaiser, P.E.; Portnoy, D.C.; Priebat, D.A.; Walker, M.S.; et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer 2017, 123, 4640–4647. [Google Scholar] [CrossRef]
- Mori, Y.; Kinoshita, S.; Kanamori, T.; Kataoka, H.; Joh, T.; Iida, S.; Takemoto, M.; Kondo, M.; Kuroda, J.; Komatsu, H. The Successful Treatment of Metastatic Extraosseous Ewing Sarcoma with Pazopanib. Intern. Med. 2018, 57, 2753–2757. [Google Scholar] [CrossRef]
- Longhi, A.; Paioli, A.; Palmerini, E.; Cesari, M.; Abate, M.E.; Setola, E.; Spinnato, P.; Donati, D.; Hompland, I.; Boye, K. Pazopanib in relapsed osteosarcoma patients: Report on 15 cases. Acta Oncol. 2018, 1–4. [Google Scholar] [CrossRef]
- Dembla, V.; Groisberg, R.; Hess, K.; Fu, S.; Wheler, J.; Hong, D.S.; Janku, F.; Zinner, R.; Piha-Paul, S.A.; Ravi, V.; et al. Outcomes of patients with sarcoma enrolled in clinical trials of pazopanib combined with histone deacetylase, mTOR, Her2, or MEK inhibitors. Sci. Rep. 2017, 7, 15963. [Google Scholar] [CrossRef] [PubMed]
- Berry, V.; Basson, L.; Bogart, E.; Mir, O.; Blay, J.Y.; Italiano, A.; Bertucci, F.; Chevreau, C.; Clisant-Delaine, S.; Liegl-Antzager, B.; et al. REGOSARC: Regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma-A quality-adjusted time without symptoms of progression or toxicity analysis. Cancer 2017, 123, 2294–2302. [Google Scholar] [CrossRef] [PubMed]

| Median Age (Year) | Sex Distribution | Ethnicity | Median Lines of Prior Therapy | ||||
|---|---|---|---|---|---|---|---|
| Female | Male | White | Asian | Latino | African American | ||
| 60 | 52.8% | 47.2% | 60% | 12% | 19% | 8% | 3 |
| Response | All Cases (N = 123) | At 8 Weeks (N = 65) | At 12 Weeks (N = 104) | Cases Assessed after 12 Weeks (N = 19) |
|---|---|---|---|---|
| RR (PR + CR) | 13 (12 +1) (10.6%) | 9 (13.8%) | 12 (11.5%) | 1 (5.3%) |
| SD | 34 (27.6%) | 10 (15.4%) | 26 (25%) | 8 (42.1%) |
| PD | 76 (61.8%) | 46 (70.1%) | 66 (63.5%) | 10 (52.6%) |
| DCR (RR + SD) | 47 (38.2%) | 19 (29.2%) | 38 (36.5%) | 9 (47.4%) |
| Histology | CR | PR | SD | PD | Total |
|---|---|---|---|---|---|
| Alveolar rhabdomyosarcoma | 1 | 1 | |||
| ASPS | 1 | 1 | |||
| Angiomatoid histiocytoma | 1 | 1 | |||
| Angiosarcoma | 1 | 1 | 2 | 4 | |
| Chondrosarcoma | 3 | 5 | 8 | ||
| Chordoma | 1 | 1 | |||
| Dedifferentiated liposarcoma | 1 | 4 | 5 | ||
| Desmoplastic small round cell tumor | 3 | 3 | |||
| Epithelioid hemangioendothelioma | 1 | 1 | |||
| Ewing’s sarcoma | 2 | 1 | 3 | ||
| Endometrial stromal tumor | 1 | 1 | |||
| Hemangiopericytoma | 1 | 1 | 2 | ||
| Leiomyosarcoma | 4 (10%) | 9 (22.5%) | 27 (67.5%) | 40 | |
| Low grade fibromyxoid sarcoma | 1 | 1 | |||
| Malignant peripheral nerve sheath tumor | 4 | 3 | 7 | ||
| Malignant phyllodes | 1 | 1 | |||
| Myxoid liposarcoma | 1 | 1 | |||
| Osteosarcoma | 2 | 4 | 6 | ||
| Pleomorphic liposarcoma | 1 | 1 | 1 | 3 | |
| Pleomorphic rhabdomyosarcoma | 2 | 1 | 3 | ||
| PeComa | 1 | 1 | |||
| Synovial sarcoma | 4 | 3 | 7 | ||
| UPS | 3 (13.6%) | 5 (22.7%) | 14 (63.6%) | 22 | |
| Total cases | N = 1 (0.8%) | N = 12 (9.7%) | N = 34 (27.6%) | N = 76 (61.8%) | N = 123 (100%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seto, T.; Song, M.-N.; Trieu, M.; Yu, J.; Sidhu, M.; Liu, C.-M.; Sam, D.; Pan, M. Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California. Med. Sci. 2019, 7, 48. https://doi.org/10.3390/medsci7030048
Seto T, Song M-N, Trieu M, Yu J, Sidhu M, Liu C-M, Sam D, Pan M. Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California. Medical Sciences. 2019; 7(3):48. https://doi.org/10.3390/medsci7030048
Chicago/Turabian StyleSeto, Tiffany, Mee-Na Song, Maily Trieu, Jeanette Yu, Manpreet Sidhu, Chi-Mei Liu, Danny Sam, and Minggui Pan. 2019. "Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California" Medical Sciences 7, no. 3: 48. https://doi.org/10.3390/medsci7030048
APA StyleSeto, T., Song, M.-N., Trieu, M., Yu, J., Sidhu, M., Liu, C.-M., Sam, D., & Pan, M. (2019). Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California. Medical Sciences, 7(3), 48. https://doi.org/10.3390/medsci7030048





