Occurrence of Surgical Site Infections at a Tertiary Healthcare Facility in Abuja, Nigeria: A Prospective Observational Study
Abstract
1. Introduction
2. Methods
2.1. Description of Study Centre
2.2. Determination of Sample Size and Sampling Method
2.3. Study Design and Patient Selection Criteria
2.4. Monitoring Patients for Development of Surgical Site Infections
2.5. Data Analysis
2.6. Ethical Consideration and Recruitment of Patients for the Study
3. Results
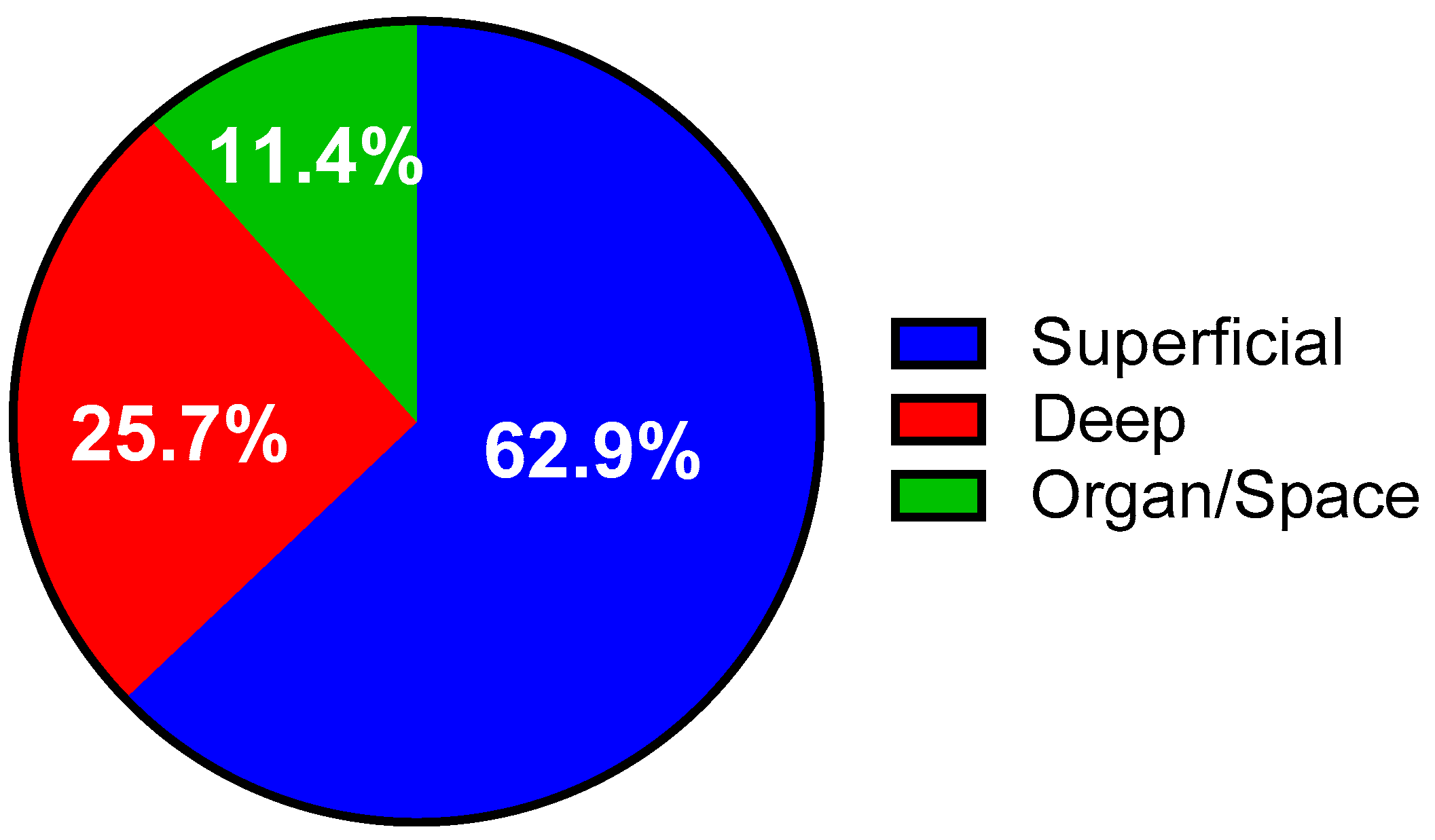
3.1. Incidence of Surgical Site Infections
3.2. Patients Related Factors
3.3. Procedure Related Factors
3.4. Predictors of Surgical Site Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyal, R.; Singh Sandhu, H.P.; Kumar, A.; Kosey, S.; Mehra, N. Surveillance Method for Surgical Site Infection. Indian J. Pharm. Pract. 2015, 8, 54–60. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. The Hospital Infection Control Practices Advisory Committee. Guideline for the prevention of surgical site infection, 1999. Infect. Control Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef] [PubMed]
- Reichman, D.E.; Greenberg, J.A. Reducing Surgical Site Infections: A Review. Rev. Obstet. Gyneacol. 2009, 2, 212–221. [Google Scholar]
- Jenks, P.J.; Laurent, M.; Mcquarry, S.; Watkins, R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J. Hosp. Infect. 2014, 86, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Broex, E.C.J.; van Asselt, A.D.; Bruggeman, C.A.; van Tiel, F.H. Surgical site infections: how high are the costs? J. Hosp. Infect. 2009, 72, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Poggio, J.L. Perioperative Strategies to Prevent Surgical-Site Infection. Clin. Colon. Rectal Surg. 2013, 26, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singla, P.; Chaudhary, U. Surgical Site Infections: Classification, Risk factors, Pathogenesis and Preventive Management. Int. J. Pharm. Res. Heal. Sci. 2014, 2, 203–214. [Google Scholar]
- Gottrup, F.; Melling, A.; Hollander, D.A. An Overview of Surgical Site Infections: Aetiology, Incidence and Risk Factors. EWMA J. 2005, 5, 11–15. [Google Scholar]
- Manyahi, J. Bacteriological Spectrum of Post-Operative Wound Infections and Their Antibiogram in a Tertiary Hospital, Dar Es Salaam, Tanzania. Mater’s Thesis, Muhimbili University of Health and Allied Sciences, Muhimbili, Dar es Salaam, Tanzania, 2012. [Google Scholar]
- Nejad, S.B.; Allegranzi, B.; Syed, S.B.; Pittet, D. Health-care-associated infection in Africa: A systematic review. Bull. World Heal Organ. 2011, 89, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.M.; Karuri, D.M.; Wanyoro, A.K.; Macleod, J. Interventional studies for preventing surgical site infections in sub-Saharan Africa—A systematic review. Int. J. Surg. 2012, 10, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mpogoro, F.J.; Mshana, S.E.; Mirambo, M.M.; Kidenya, B.R.; Gumodoka, B.; Imirzalioglu, C. Incidence and predictors of surgical site infections following caesarean sections at Bugando Medical Centre, Mwanza, Tanzania. Antimicrob. Resist. Infect. Control 2014, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Scherbaum, M.; Kösters, K.; Mürbeth, R.E.; Ngoa, U.A.; Kremsner, P.G.; Lell, B.; Alabi, A. Incidence, pathogens and resistance patterns of nosocomial infections at a rural hospital in Gabon. BMC Infect. Dis. 2014, 14, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Thrushfield, M. Veterinary Epidemiology, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2005; pp. 228–242. [Google Scholar]
- Osakwe, J.O.; Nnaji, G.A.; Osakwe, R.C.; Agu, U.; Chineke, H.N. Role of premorbid status and wound related factors in surgical site infection in a tertiary hospital in sub-Saharan Africa. Fam. Pract. Rep. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Olowo-Okere, A.; Ibrahim, Y.K.E.; Sani, A.S.; Atata, R.F.; Olayinka, B.O. Prevalence of Surgical Site Infection in a Nigerian University Teaching Hospital. J. Pharm. Allied Sci. 2017, 14, 2430–2438. [Google Scholar]
- Atata, R.F.; Ibrahim, Y.K.E.; Olurinola, P.F.; Adigun, I.A.; Giwa, A.; Ii, A. Prevalence of Surgical Site Nosocomial Infection in A Tertiary Health Care Institution in Nigeria. Int. J. Epidemiol. Infect. 2013, 1, 52–57. [Google Scholar] [CrossRef]
- Shuaibu, A.S.; Ibrahim, Y.K.E.; Olayinka, B.O.; Atata, R.F. Aerobic Bacteria from Surgical Wound Infections in Obstetrics and Gynecology Ward in Specialist Hospital Sokoto—North West Nigeria. Asian J. Med. Heal. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.K.; Adesanmi, A.A.; Asuzu, M.C. Hospital-acquired infections in a Nigerian tertiary health facility: An audit of surveillance reports. Niger. Med. J. 2011, 52, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, A.A.; Nuhu, M.; Afolaranmi, T. Incidence of and risk factors for abdominal surgical site infection in a Nigerian tertiary care centre. Int. J. Infect. Control 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Etonyeaku, A.; Talabi, A.; Akinkuolie, A.; Olasehinde, O.; Omotola, C.; Mosanya, A.; Agbakwuru, E.A. Surgical Abdomen in School Age Children: A Prospective Review from Two Centers in South- Western Nigeria. East Cent. Afr. J. Surg. 2016, 21, 133–139. [Google Scholar] [CrossRef]
- Legesse Laloto, T.; Hiko Gemeda, D.; Abdella, S.H. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect. Dis. 2017, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Mawalla, B.; Mshana, S.E.; Chalya, P.L.; Imirzalioglu, C.; Mahalu, W. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surg. 2011, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Borg, T.F.; Aly, K.K.; Salahaldeen, A.S.; Alkallaf, A.S. Incidence and risk factors for surgical site infections following cesarean section in a tertiary care university hospital versus a rural hospital: A prospective, two-armed parallel cohort study. J. Evid.-Based Women Health J. Soc. 2016, 6, 51–57. [Google Scholar] [CrossRef]
- Tabiri, S.; Yenli, E.; Kyere, M.; Anyomih, T.T.K. Surgical Site Infections in Emergency Abdominal Surgery at Tamale Teaching Hospital, Ghana. World J. Surg. 2017, 42, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Surveillance of Surgical Site Infections in NHS Hospitals in England, 2016 to 2017. Public Health England, December 2017. Available online: www.gov.uk/phe (accessed on 4 April 2018).
- Centre for Disease Control. Surgical Site Infection (SSI) Event. Procedure-Associated Module SSI; Centre for Disease Control: Atlanta, GA, USA, 2017. Available online: www.cdc.gov (accessed on 21 July 2017).
- Makanjuola, O.B.; Olowe, O.A.; Adeyankinnu, A.F. Bacterial Agents of Surgical Site Infections in South-Western Nigeria. Am. J. Biomed. Sci. 2013, 5, 217–225. [Google Scholar] [CrossRef]
- Kolmos, H.J. Health Care Associated Infections: Sources and Routes of Transmission. In Infection Control–Updates; Sudhakar, C., Ed.; Intech: Rijeka, Croatia, 2012; Chapter 2; pp. 21–38. [Google Scholar]
- Ameh, E.A.; Mshelbwala, P.M.; Nasir, A.A.; Lukong, C.S.; Jabo, B.A.; Anumah, M.A.; Nmadu, P.T. Surgical Site Infection in children: Prospective analysis of the burden and risk factors in a sub-Saharan African setting. Surg. Infect. (Larchmt) 2009, 10, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, E. Isolation of pathogenic bacteria from fomites in the operating rooms of a specialist hospital in Kano, North-western Nigeria. Pak. J. Pharm. Sci. 2012, 12, 90–96. [Google Scholar]
- Olowo-okere, A.; Ibrahim, Y.K.E.; Olayinka, B.O. Molecular characterisation of extended-spectrum β-lactamase-producing Gram-negative bacterial isolates from surgical wounds of patients at a hospital in North Central Nigeria. J. Glob. Antimicrobial. Resist. 2018, 14, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.J.; Snow, G.A. Biochemical mechanisms of resistance to antimicrobial drugs. In Biochemistry and Molecular Biology of Antimicrobial Drug Action, 6th ed.; Springer Science: New York, NY, USA, 2005; pp. 151–170. [Google Scholar]
- Mcconoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Jason, H. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Metcalf, D.; Parsons, D.; Bowler, P. A real-life clinical evaluation of a next-generation antimicrobial dressing on acute and chronic wounds. J. Wound Care 2015, 24, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kathju, S.; Nistico, L.; Hall-Stoodley, L.; Post, J.C.; Ehrlich, G.D.; Stoodley, P. Chronic Surgical Site Infection Due to Suture-Associated Polymicrobial Biofilm. Surg. Infect. 2009, 10, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Atata, R.F.; Ibrahim, Y.K.E.; Olurinola, P.F.; Sani, A.; Akanbi, A. Prevalence of Surgical Site Nosocomial Infection in Nigeria Tertiary Health Facility: A Retrospective study. J. Appl. Environ. Sci. 2006, 2, 212–215. [Google Scholar]
- Pathak, A.; Mahadik, K.; Swami, M.B.; Roy, P.K.; Sharma, M.; Mahadik, V.K.; Lundborg, C.S. Incidence and risk factors for surgical site infections in obstetric and gynecological surgeries from a teaching hospital in rural India. Antimicrob. Resist. Infect. Control 2017, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Korol, E.; Johnston, K.; Waser, N.; Sifakis, F.; Jafri, H.S.; Lo, M.; Kyaw, M.H. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS ONE 2013, 8, e83743. [Google Scholar] [CrossRef] [PubMed]
- Ikeanyi, U.O.E.; Chukwuka, C.N.; Chukwuanukwu, T.O.G. Risk factors for surgical site infections following clean orthopaedic operations. Niger. J. Clin. Pract. 2013, 16, 443–447. [Google Scholar] [PubMed]

| Parameters | SSI Present Frequency (%) | SSI Absent Frequency (%) | Χ2 | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 19 (29.2) | 46 (70.8) | 0.186 | 0.666 |
| Male | 16 (25.8) | 46 (74.2) | ||
| Age Categories | ||||
| 0–18 Years | 15 (30.6) | 34 (69.4) | 1.387 | 0.500 |
| 19–50 Years | 14 (23.0) | 47 (77.0) | ||
| >50 Years | 6 (35.3) | 11 (64.7) | ||
| Types of Surgery | ||||
| Elective | 17 (33.3) | 34 (66.7) | 1.423 | 0.233 |
| Emergency | 18 (23.7) | 58 (76.3) | ||
| Nature of Wound | ||||
| Clean | 3 (6.5) | 43 (93.5) | 23.1 | <0.0001 |
| Clean-Contaminated | 11 (30.6) | 25 (69.4) | ||
| Contaminated | 11 (36.7) | 19 (63.3) | ||
| Dirty | 10 (66.7) | 5 (33.3) | ||
| Co-Morbid Factor | ||||
| Alcohol Consumption | ||||
| Yes | 2 (13.33) | 13 (86.67) | 1.724 | 0.189 |
| No | 33 (29.46) | 79 (70.54) | ||
| Cigarette Smoking | ||||
| Yes | 2 (50.0) | 2 (50.0) | 1.042 | 0.307 |
| No | 33 (26.8) | 90 (73.2) | ||
| Pre-Existing Infection | ||||
| Yes | 5 (31.25%) | 11 (68.75%) | 0.125 | 0.724 |
| No | 30 (27.03%) | 81 (72.97%) | ||
| Anaemia | ||||
| Yes | 3 (37.50%) | 5 (62.50%) | 0.4230 | 0.516 |
| No | 32 (26.89%) | 87 (73.11%) | ||
| Diabetes mellitus | ||||
| Yes | 2 (33.33%) | 4 (66.67%) | 0.105 | 0.746 |
| No | 33 (27.27%) | 88 (72.73%) |
| Variables | B | S.E. | Wald | Sig. | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.023 | 0.015 | 2.344 | 0.126 | 1.024 | 0.993–1.055 |
| Post-operative hospital stays | 0.067 | 0.029 | 5.402 | 0.020 | 1.069 | 1.011–1.131 |
| Nature of operation (Emergency) | −1.037 | 0.601 | 2.970 | 0.085 | 0.355 | 0.109–1.153 |
| Diabetes mellitus | 0.186 | 1.032 | 0.033 | 0.857 | 1.205 | 0.159–9.109 |
| Anaemia | −0.045 | 1.022 | 0.002 | 0.965 | 0.956 | 0.129–7.084 |
| Pre-existing Infection | −0.186 | 0.811 | 0.053 | 0.818 | 0.830 | 0.169–4.071 |
| Cigarette smoking | 1.830 | 1.595 | 1.316 | 0.251 | 6.236 | 0.274–142.154 |
| Alcohol Consumption | −1.666 | 1.228 | 1.843 | 0.175 | 0.189 | 0.017–2.095 |
| Constant | −1.216 | 0.731 | 2.762 | 0.097 | 0.297 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olowo-okere, A.; Ibrahim, Y.K.E.; Sani, A.S.; Olayinka, B.O. Occurrence of Surgical Site Infections at a Tertiary Healthcare Facility in Abuja, Nigeria: A Prospective Observational Study. Med. Sci. 2018, 6, 60. https://doi.org/10.3390/medsci6030060
Olowo-okere A, Ibrahim YKE, Sani AS, Olayinka BO. Occurrence of Surgical Site Infections at a Tertiary Healthcare Facility in Abuja, Nigeria: A Prospective Observational Study. Medical Sciences. 2018; 6(3):60. https://doi.org/10.3390/medsci6030060
Chicago/Turabian StyleOlowo-okere, Ahmed, Yakubu Kokori Enevene Ibrahim, Ali Samuel Sani, and Busayo Olalekan Olayinka. 2018. "Occurrence of Surgical Site Infections at a Tertiary Healthcare Facility in Abuja, Nigeria: A Prospective Observational Study" Medical Sciences 6, no. 3: 60. https://doi.org/10.3390/medsci6030060
APA StyleOlowo-okere, A., Ibrahim, Y. K. E., Sani, A. S., & Olayinka, B. O. (2018). Occurrence of Surgical Site Infections at a Tertiary Healthcare Facility in Abuja, Nigeria: A Prospective Observational Study. Medical Sciences, 6(3), 60. https://doi.org/10.3390/medsci6030060





