Contribution of Inhibitor of Differentiation and Estrogenic Endocrine Disruptors to Neurocognitive Disorders
Abstract
1. Introduction
2. Inhibitor of Differentiation
2.1. Structure and Function
2.2. Inhibitor of Differentiation Proteins and Neurocognitive Disorders
2.3. Inhibitor of Differentiation Proteins and Estrogenic Endocrine Disruptors
3. Influence of Estrogenic Endocrine Disruptors Exposure on Neurocognitive Disorders
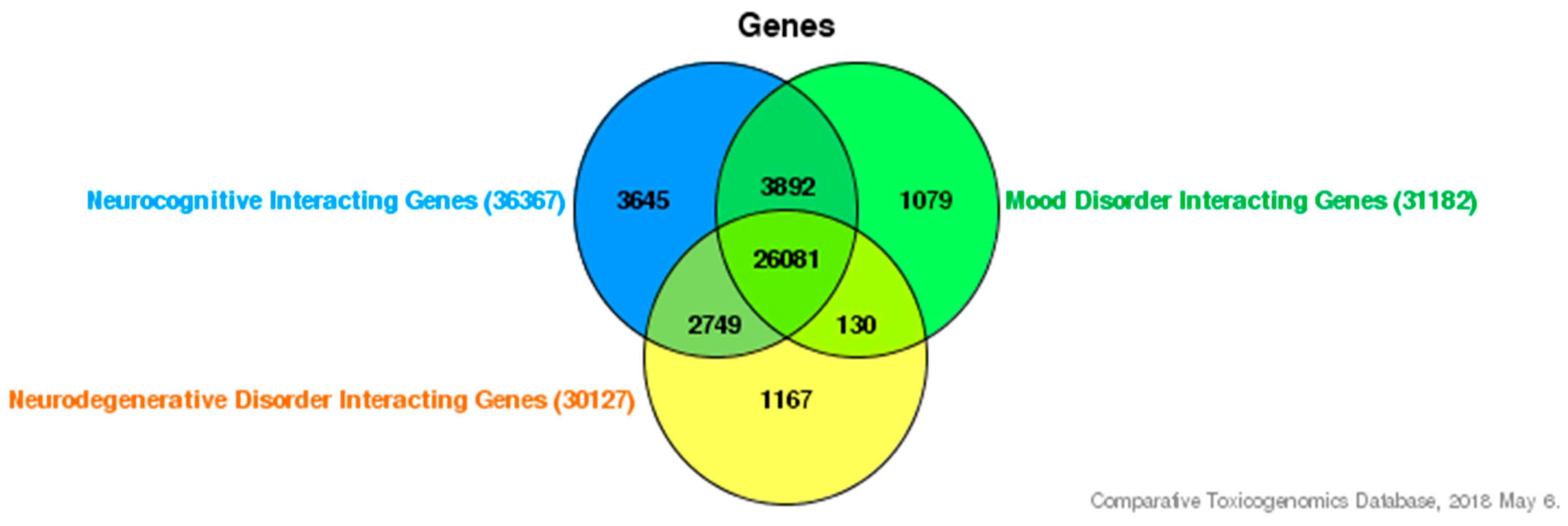
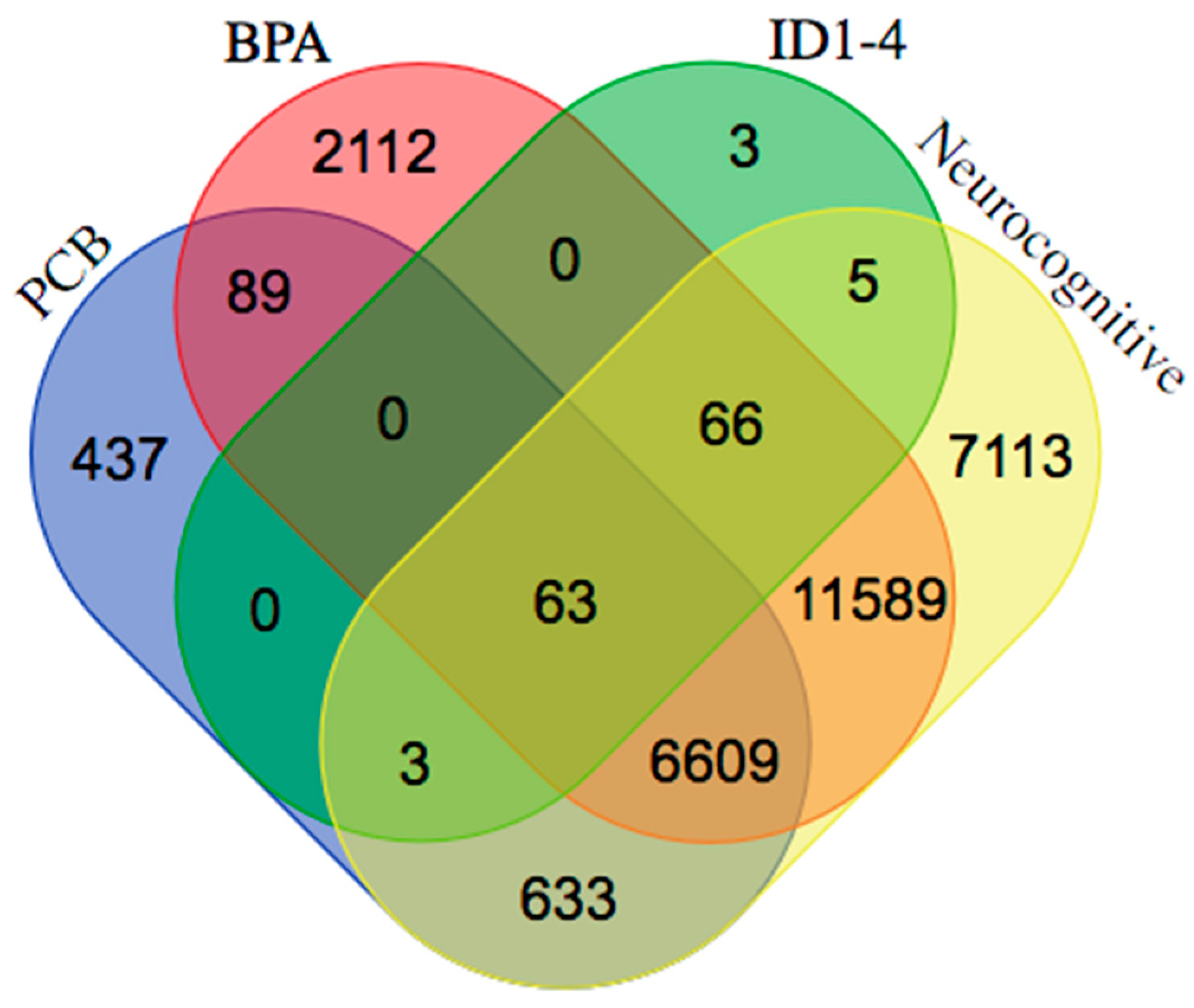
4. Interaction of Inhibitor of Differentiation Proteins and Estrogenic Endocrine Disruptors in Neurocognitive Disorders
5. Conclusions
Conflicts of Interest
References
- Grohol, J. Symptoms of Major Neurocognitive Disorder. Psych Central, 2017. Available online: https://psychcentral.com/disorders/symptoms-of-major-neurocognitive-disorder/ (accessed on 9 May 2018).
- Nall, R. What is dementia (neurocognitive disorder). Medical News Today. 2017. Available online: https://www.medicalnewstoday.com/articles/314850.php (accessed on 6 February 2018).
- Zhang, M.R.; Qu, C.; Sun, J.; Wang, C.; Li, H.Y.; Zhang, Y.J.; Zhang, B.Q.; Zou, W. Different subtypes of estrogen receptor α and related signal molecules in the hippocampus are associated with spatial cognitive impairment of diabetic mice. Sheng Li Xue Bao 2017, 69, 252–260. [Google Scholar] [PubMed]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 2017, 61, 143–187. [Google Scholar] [CrossRef] [PubMed]
- Shafi, O. Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: A systematic review. BMC Neurol. 2016, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, V.L.; Must, A.; Horvath, S.; Király, A.; Kincses, Z.T.; Vécsei, L. Gender-specific degeneration of dementia-related subcortical structures throughout the lifespan. J. Alzheimers Dis. 2017, 55, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.C.; Fraga, V.G.; Guimarães, H.C.; Teixeira, A.L.; Barbosa, M.T.; Carvalho, M.D.; Mota, A.P.; Silva, I.F.; Caramelli, P.; Gomes, K.B.; et al. Estrogen receptor-alpha gene XbaI A > G polymorphism influences short-term cognitive decline in healthy oldest-old individuals. Arq. Neuropsiquiatr. 2017, 75, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Liu, L.; Hu, X.T.; He, L.; Chen, G.J. Estrogen Modulates ubc9 Expression and Synaptic Redistribution in the Brain of APP/PS1 Mice and Cortical Neurons. J. Mol. Neurosci. 2017, 61, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Ropero, A.B.; Laribi, O.; Maillet, M.; Fuentes, E.; Soria, B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc. Natl. Acad. Sci. USA 2000, 97, 11603–11608. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.S.; Bulayeva, N.N.; Wozniak, A.L.; Finnerty, C.C. Signaling from the membrane via membrane estrogen receptor-α: Estrogens, xenoestrogens, and phytoestrogens. Steroids 2005, 70, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Dong, J. Binding and activation of the seven transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Korach, K.S.; Sarver, P.; Chae, K.; McLachlan, J.A.; McKinney, J.D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: Conformationally restricted structural probes. Mol. Pharmacol. 1988, 33, 120–126. [Google Scholar] [PubMed]
- Bonefeld-Jorgensen, E.C.; Andersen, H.R.; Rasmussen, T.H.; Vinggaard, A.M. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 2001, 158, 141–153. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, L.; Wang, F.; Ge, H.; Wu, P.C.; Li, X.W.; Chen, G.H. Accelerated reduction of serum thyroxine and hippocampal histone acetylation links to exacerbation of spatial memory impairment in aged CD-1 mice pubertally exposed to bisphenol-a. Age 2016, 38, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Masuo, Y.; Ishido, M. Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Avecilla, V.; Doke, M.; Felty, Q. Contribution of Inhibitor of DNA Binding/Differentiation-3 and Endocrine Disrupting Chemicals to Pathophysiological Aspects of Chronic Disease. Biomed. Res. Int. 2017, 2017, 6307109. [Google Scholar] [CrossRef] [PubMed]
- Lyden, D.; Young, A.Z.; Zagzag, D.; Yan, W.; Gerald, W.; O’Reilly, R.; Bader, B.L.; Hynes, R.O.; Zhuang, Y.; Manova, K.; et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999, 401, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Morrell, N.W. Id proteins in the vasculature: From molecular biology to cardiopulmonary medicine. Cardiovasc. Res. 2014, 104, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Benezra, R.; Davis, R.L.; Lockshon, D.; Turner, D.L.; Weintraub, H. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell 1990, 61, 49–59. [Google Scholar] [CrossRef]
- Doke, M.; Avecilla, V.; Felty, Q. Inhibitor of Differentiation-3 and Estrogenic Endocrine Disruptors: Implications for Susceptibility to Obesity and Metabolic Disorders. BioMed Res. Int. 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.D. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 2000, 113, 3897–3905. [Google Scholar] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Ruzinova, M.B.; Benezra, R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003, 13, 410–418. [Google Scholar] [CrossRef]
- Ellmeier, W.; Weith, A. Expression of the helix-loop-helix gene Id3 during murine embryonic development. Dev. Dyn. 1995, 203, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jen, Y.; Manova, K.; Benezra, R. Each member of the Id gene family exhibits a unique expression pattern in mouse gastrulation and neurogenesis. Dev. Dyn. 1997, 208, 92–106. [Google Scholar] [CrossRef]
- Neuman, T.; Keen, A.; Zuber, M.X.; Kristjansson, G.I.; Gruss, P.; Nornes, H.O. Neuronal Expression of Regulatory Helix-Loop-Helix Factor Id2 Gene in Mouse. Dev. Biol. 1993, 160, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Roschger, C.; Cabrele, C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun. Signal. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.F.; de Vellis, J. Id1, Id2, and Id3 gene expression in neural cells during development. Glia 1998, 24, 372–381. [Google Scholar] [CrossRef]
- Gupta, G.P.; Perk, J.; Acharyya, S.; de Candia, P.; Mittal, V.; Todorova-Manova, K.; Gerald, W.L.; Brogi, E.; Benezra, R.; Massague, J. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc. Natl. Acad. Sci. USA 2007, 104, 19506–19511. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Patel, D.; Chaudhary, J. Id1 and Id3 expression is associated with increasing grade of prostate cancer: Id3 preferentially regulates CDKN1B. Cancer Med. 2012, 1, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Akeel, S.; El-awady, A.; Hussein, K.; El-Refaey, M.; Elsalanty, M.; Sharawy, M.; Al-Shabrawey, M. Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: Role of reactive oxygen species. Arch. Oral Biol. 2012, 57, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Baudler, S.; Welzel, H.; Böhm, M.; Nickenig, G. Identification of a novel redox-sensitive gene, Id3, which mediates angiotensin II-induced cell growth. Circulation 2002, 105, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Baudler, S.; Müller, C.; Werner, C.; Werner, N.; Welzel, H.; Strehlow, K.; Böhm, M. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. FASEB J. 2002, 16, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Felty, Q. PCB153-induced overexpression of ID3 contributes to the development of microvascular lesions. PLoS ONE 2014, 9, e104159. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Felty, Q. Microvascular Lesions by Estrogen-Induced ID3: Its Implications in Cerebral and Cardiorenal Vascular Disease. J. Mol. Neurosci. 2014, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Felty, Q. Proteomic 2D DIGE profiling of human vascular endothelial cells exposed to environmentally relevant concentration of endocrine disruptor PCB153 and physiological concentration of 17β-estradiol. Cell Biol. Toxicol. 2011, 27, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Datla, S.R.; Griendling, K.K. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Shohami, E.; Beit-Yannai, E.; Horowitz, M.; Kohen, R. Oxidative Stress in Closed-Head Injury: Brain Antioxidant Capacity as an Indicator of Functional Outcome. J. Cereb. Blood Flow Metab. 1997, 17, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Chang, S.H.; Huang, C.T.; Yin, J.H.; Hwang, C.S.; Yang, L.Y.; Yang, D.I. Inhibitor of Differentiation-1 and Hypoxia-Inducible Factor-1 Mediate Sonic Hedgehog Induction by Amyloid Beta-Peptide in Rat Cortical Neurons. Mol. Neurobiol. 2016, 53, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Takahashi, R.; Yokota, Y. Localization of Id2 mRNA in the adult mouse brain. Brain Res. 2006, 1073–1074, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Enoki, Y.; Sakamoto, Y.; Yokota, K.; Okubo, M.; Matsumoto, M.; Hayashi, N.; Usui, M.; Kokabu, S.; Mimura, T.; et al. Donepezil prevents RANK-induced bone loss via inhibition of osteoclast differentiation by downregulating acetylcholinesterase. Heliyon 2015, 1, e00013. [Google Scholar] [CrossRef] [PubMed]
- Savalli, G.; Diao, W.; Schulz, S.; Todtova, K.; Pollak, D.D. Diurnal oscillation of amygdala clock gene expression and loss of synchrony in a mouse model of depression. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Ortiz, J.L.; Bordner, K.A.; Carlyle, B.C.; Gelernter, J.; Simen, A.A.; Kaufman, J. The role of genes involved in stress, neural plasticity, and brain circuitry in depressive phenotypes: Convergent findings in a mouse model of neglect. Behav. Brain Res. 2016, 315, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; Perepletchikova, F.; et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Guadalupe, T.; Franke, B.; Hibar, D.P.; Renteria, M.E.; Stein, J.L.; Thompson, P.M.; Francks, C.; Vernes, S.C.; Fisher, S.E. Early developmental gene enhancers affect subcortical volumes in the adult human brain. Hum. Brain Mapp. 2016, 37, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Kepa, A.; Martinez Medina, L.; Erk, S.; Srivastava, D.P.; Fernandes, A.; Toro, R.; Lévi, S.; Ruggeri, B.; Fernandes, C.; Degenhardt, F.; et al. Associations of the Intellectual Disability Gene MYT1L with Helix-Loop-Helix Gene Expression, Hippocampus Volume and Hippocampus Activation During Memory Retrieval. Neuropsychopharmacology 2017, 42, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Ribasés, M.; Bosch, R.; Hervás, A.; Ramos-Quiroga, J.A.; Sánchez-Mora, C.; Bielsa, A.; Gastaminza, X.; Guijarro-Domingo, S.; Nogueira, M.; Gómez-Barros, N.; et al. Case-control study of six genes asymmetrically expressed in the two cerebral hemispheres: Association of BAIAP2 with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2009, 66, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.W.; Rohac, D.J.; Bottom, R.T.; Patadia, S.; Huffman, K.J. Prenatal Ethanol Exposure and Neocortical Development: A Transgenerational Model of FASD. Cereb. Cortex 2018, 28, 2908–2921. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tian, H.; Wang, W.; Zhang, X.; Liu, J.; Ru, S. Disruption of the thyroid system by the thyroid-disrupting compound Aroclor 1254 in juvenile Japanese flounder (Paralichthys olivaceus). PLoS ONE 2014, 9, e104196. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, J.; Boyajian, A.; Brown, C.; Smith, S.D.; Hixon, M. Effects of in utero exposure to Bisphenol A or diethylstilbestrol on the adult male reproductive system. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.R.; Thompson, L.M.; Rodriguez, K.; Gore, A.C. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm. Behav. 2016, 78, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Elnar, A.A.; Allouche, A.A.; Desor, F.; Yen, F.T.; Soulimani, R.; Oster, T. Lactational exposure of mice to low levels of non-dioxin-like polychlorinated biphenyls increases susceptibility to neuronal stress at a mature age. Neurotoxicology 2016, 53, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Gillette, R.; Reilly, M.P.; Topper, V.Y.; Thompson, L.M.; Crews, D.; Gore, A.C. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm. Behav. 2017, 87, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.T.; Remick, D.; Creton, R.; Colwill, R.M. Effects of embryonic exposure to polychlorinated biphenyls (PCBs) on anxiety-related behavior in larval zebrafish. Neurotoxicology 2016, 53, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Elnar, A.A.; Desor, F.; Marin, F.; Soulimani, R.; Nemos, C. Lactational exposure to low levels of the six indicator non-dioxin-like polychlorinated biphenyls induces DNA damage and repression of neuronal activity in juvenile male mice. Toxicology 2015, 328, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Guam, P.M.; Esser, A.; Schettgen, T.; Gube, M.; Kraus, T.; Lang, J. Prevalence and iincidence rates of mental syndromes after occupational exposure to polychlorinated biphenyls. Int. J. Hyg. Environ. Health 2014, 217, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Guida, N.; Cocco, S.; Secondo, A.; Sirabella, R.; Ulianich, L.; Paturzo, F.; Di Renzo, G.; Canzoniero, L.M. The repressor element 1-silencing transcription factor is a novel molecular target for the neurotoxic effect of the polychlorinated biphenyl mixture aroclor 1254 in neuroblastoma SH-SY5Y cells. J. Pharmacol. Exp. Ther. 2011, 338, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Royland, J.E.; Wu, J.; Zawia, N.H.; Kodavanti, P.R. Gene expression profiles in the cerebellum and hippocampus following exposure to a neurotoxicant, Aroclor 1254: Developmental effects. Toxicol. Appl. Pharmacol. 2008, 231, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Guo, N.W.; Tsai, P.C.; Yang, C.Y.; Guo, Y. Neurocognitive changes among elderly exposed to PCBs/PCDFs in Taiwan. Environ. Health Perspect. 2008, 116, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, J.; Chan, S.; Hauser, R.; Hu, H. Longitudinal assessment of PCBs and chlorinated pesticides in pregnant women from Western Canada. Environ. Health. 2005, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.; Julvez, J.; Sunyer, J.; Vrijheid, M. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ. Res. 2015, 142, 671–679. [Google Scholar]
- Evans, S.F.; Kobrosly, R.W.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Weiss, B.; Stahlhut, R.; Yolton, K.; Swan, S.H. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology 2014, 45, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gioiosa, L.; Parmigiani, S.; vom Saal, F.S.; Palanza, P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm. Behav. 2013, 63, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.P.; Allardice, H.A.; Schenk, A.K.; Rissman, E.F. Effects of maternal or paternal bisphenol A exposure on offspring behavior. Horm. Behav. 2018, 101, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; King, B.L.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2017. Nucleic Acids Res. 2017, 45, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ene, L.; Marcotte, T.D.; Umlauf, A.; Grancea, C.; Temereanca, A.; Bharti, A.; Achim, C.L.; Letendre, S.; Ruta, S.M. Latent toxoplasmosis is associated with neurocognitive impairment in young adults with and without chronic HIV infection. J. Neuroimmunol. 2016, 299, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chen, Y.; Li, L.; Jiang, J.; Wu, G.; Zuo, Y.; Zhang, J.H.; Feng, H.; Yan, X.; Liu, F. Decorin alleviated chronic hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after subarachnoid hemorrhage in rats. Brain Res. 2016, 1630, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Hulsen, T.; de Vlieg, J.; Alkema, W. Biovenn—A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Sampey, G.C.; Saifuddin, M.; Schwab, A.; Barclay, R.; Punya, S.; Chung, M.C.; Hakami, R.M.; Zadeh, M.A.; Lepene, B.; Klase, Z.A.; et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J. Biol. Chem. 2016, 291, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Cheng, F.; Wang, X.; Zhai, C.; Yue, W.; Lian, Y.; Wang, Q. Erythropoietin Pathway: A Potential Target for the Treatment of Depression. Int. J. Mol. Sci. 2016, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, J.; Pucilowska, J.; Goodnough, L.H.; Atit, R.P.; Landreth, G.E. Dentate Gyrus Development Requires ERK Activity to Maintain Progenitor Population and MAPK Pathway Feedback Regulation. J. Neurosci. 2015, 35, 6836–6848. [Google Scholar] [CrossRef] [PubMed]
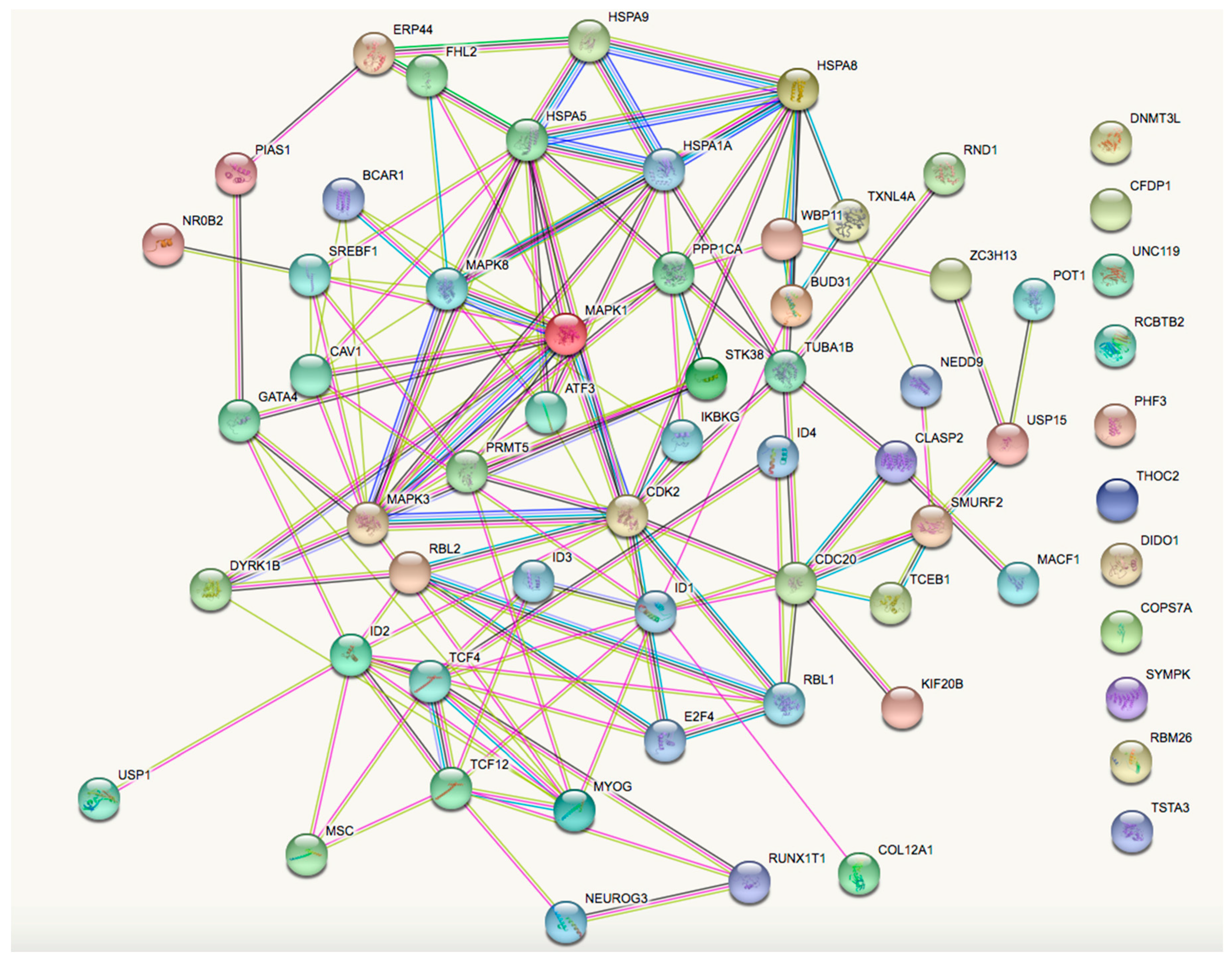
| Gene Symbol | Gene Name |
|---|---|
| ATF3 | Activating transcription factor 3 (ATF3) |
| BCAR1 | Breast cancer anti-estrogen resistance protein 1, Cas family scaffolding protein (BCAR1) |
| BUD31 | BUD31 homolog (BUD31) |
| CAV1 | Caveolin 1 (CAV1) |
| CDC20 | Cell division cycle 20 (CDC20) |
| CDK2 | Cyclin-dependent kinase 2 (CDK2) |
| CFDP1 | Craniofacial development protein 1 (CFDP1) |
| CLASP2 | Cytoplasmic linker associated protein 2 (CLASP2) |
| COL12A1 | Collagen type XII α 1 chain (COL12A1) |
| COPS7A | Constitutive photomorphogenesis 9 signalosome subunit 7A (COPS7A) |
| DIDO1 | Death inducer-obliterator 1 (DIDO1) |
| DNMT3L | DNA methyltransferase 3 like (DNMT3L) |
| DYRK1B | Dual-specificity tyrosine phosphorylation regulated kinase 1B (DYRK1B) |
| E2F4 | E2F transcription factor 4 (E2F4) |
| ELOC | Elongin c (ELOC) |
| ERP44 | Endoplasmic reticulum protein 44 (ERP44) |
| FHL2 | Four and a half LIM domains 2 (FHL2) |
| GATA4 | Global transcription factor binding protein 4 (GATA4) |
| HSPA1A | Heat shock protein family A member 1A (HSPA1A) |
| HSPA5 | Heat shock protein family A member 5 (HSPA5) |
| HSPA8 | Heat shock protein family A member 8 (HSPA8) |
| HSPA9 | Heat shock protein family A member 9 (HSPA9) |
| ID1 | Inhibitor of DNA binding 1, helix-loop-helix (HLH) protein (ID1) |
| ID2 | Inhibitor of DNA binding 2, HLH protein (ID2) |
| ID3 | Inhibitor of DNA binding 3, HLH protein (ID3) |
| ID4 | Inhibitor of DNA binding 4, HLH protein (ID4) |
| IKBKG | Inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma (IKBKG) |
| KIF20B | Kinesin family member 20B (KIF20B) |
| MACF1 | Microtubule-actin crosslinking factor 1 (MACF1) |
| MAPK1 | Mitogen-activated protein kinase 1 (MAPK1) |
| MAPK3 | Mitogen-activated protein kinase 3 (MAPK3) |
| MAPK8 | Mitogen-activated protein kinase 8 (MAPK8) |
| MSC | Musculin (MSC) |
| MYOG | Myogenin (MYOG) |
| NEDD9 | Neural precursor cell expressed, developmentally down-regulated 9 (NEDD9) |
| NEUROG3 | Neurogenin 3 (NEUROG3) |
| NR0B2 | Nuclear receptor subfamily 0 group B member 2 (NR0B2) |
| PHF3 | PHD finger protein 3 (PHF3) |
| PIAS1 | Protein inhibitor of activated STAT (signal transducer and activator of transcription) 1 (PIAS1) |
| POT1 | Protection of telomeres 1 (POT1) |
| PPP1CA | Protein phosphatase 1 catalytic subunit α (PPP1CA) |
| PRMT5 | Protein arginine methyltransferase 5 (PRMT5) |
| RBL1 | RB (retinoblastoma protein) transcriptional corepressor like 1 (RBL1) |
| RBL2 | RB transcriptional corepressor like 2 (RBL2) |
| RBM26 | RNA binding motif protein 26 (RBM26) |
| RCBTB2 | RCC1 (regulator of chromosome condensation 1) and BTB domain containing protein 2 (RCBTB2) |
| RND1 | RNA binding motif protein 26 family GTPase 1 (RND1) |
| RUNX1T1 | Runt related transcription factor 1 (RUNX1T1) |
| SMURF2 | SMAD specific E3 ubiquitin protein ligase 2 (SMURF2) |
| SREBF1 | Sterol regulatory element binding transcription factor 1 (SREBF1) |
| STK38 | Serine/threonine kinase 38 (STK38) |
| SYMPK | Symplekin (SYMPK) |
| TCF12 | Transcription factor 12 (TCF12) |
| TCF4 | Transcription factor 4 (TCF4) |
| THOC2 | THO complex 2(THOC2) |
| TSTA3 | Tissue-specific transplantation antigen P35B (TSTA3) |
| TUBA1B | Tubulin α 1b (TUBA1B) |
| TXNL4A | Thioredoxin like 4A (TXNL4A) |
| UNC119 | unc-119 Lipid Bind Chaperone (UNC119) |
| USP1 | Ubiquitin specific peptidase 1 (USP1) |
| USP15 | Ubiquitin specific peptidase 15 (USP15) |
| WBP11 | WW domain binding protein 11 (WBP11) |
| ZC3H13 | Zinc finger CCCH-type containing 13 (ZC3H13) |
| Pathway Name | Gene Count | Matching Genes in Network (Nodes) |
|---|---|---|
| TGF-β signaling pathway | 9 | E2F4, ID1, ID2, ID3, ID4, MAPK1, MAPK3, RBL1, SMURF2 |
| Toxoplasmosis | 6 | HSPA1A, HSPA8, IKBKG, MAPK1, MAPK3, MAPK8 |
| Focal adhesion | 6 | HSPA1A, HSPA5, MAPK1, MAPK3 |
| Viral carcinogenesis | 6 | FHL2, IKBKG, MAPK1, MAPK3, MAPK8 |
| MAPK (Mitogen-activated protein kinase) signaling pathway | 6 | BCAR1, CAV1, MAPK1, MAPK3, MAPK8, PPP1CA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avecilla, A.; Doke, M.; Jovellanos, J.; Avecilla, V. Contribution of Inhibitor of Differentiation and Estrogenic Endocrine Disruptors to Neurocognitive Disorders. Med. Sci. 2018, 6, 61. https://doi.org/10.3390/medsci6030061
Avecilla A, Doke M, Jovellanos J, Avecilla V. Contribution of Inhibitor of Differentiation and Estrogenic Endocrine Disruptors to Neurocognitive Disorders. Medical Sciences. 2018; 6(3):61. https://doi.org/10.3390/medsci6030061
Chicago/Turabian StyleAvecilla, Andrea, Mayur Doke, Jeremy Jovellanos, and Vincent Avecilla. 2018. "Contribution of Inhibitor of Differentiation and Estrogenic Endocrine Disruptors to Neurocognitive Disorders" Medical Sciences 6, no. 3: 61. https://doi.org/10.3390/medsci6030061
APA StyleAvecilla, A., Doke, M., Jovellanos, J., & Avecilla, V. (2018). Contribution of Inhibitor of Differentiation and Estrogenic Endocrine Disruptors to Neurocognitive Disorders. Medical Sciences, 6(3), 61. https://doi.org/10.3390/medsci6030061





