Effect of N-Acetyl-L-Cysteine (NAC) on Inflammation After Intraperitoneal Mesh Placement in an Escherichia coli Septic Rat Model: A Randomized Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Groups and Interventions
- Group A (Control, clean field): Mesh placement followed by intraperitoneal ciprofloxacin and 0.9% saline. No deliberate contamination or bowel surgery was performed.
- Group B (Escherichia coli septic field): Mesh placement followed by intraperitoneal inoculation with E. coli (200 μL containing 106 CFU) and ciprofloxacin; animals received 0.9% saline as vehicle.
- Group C (Clean-contaminated bowel surgery): Mesh placement plus a standardized wedge colectomy with primary end-to-end anastomosis (6–8 extramucosal 5/0 Prolene® sutures), with ciprofloxacin and 0.9% saline.
- Group D (Septic field + NAC): Identical to Group B (E. coli inoculation and ciprofloxacin) with the addition of intraperitoneal N-acetyl-L-cysteine (NAC) at 150 mg/kg (0.2 Ml).
- Group E (Clean-contaminated bowel surgery + NAC): Identical to Group C (wedge colectomy and ciprofloxacin) with the addition of intraperitoneal NAC 150 mg/kg (0.2 mL).
2.3. Randomization and Masking
2.4. Operative and Perioperative Procedures
2.4.1. Anaesthesia and Preparation
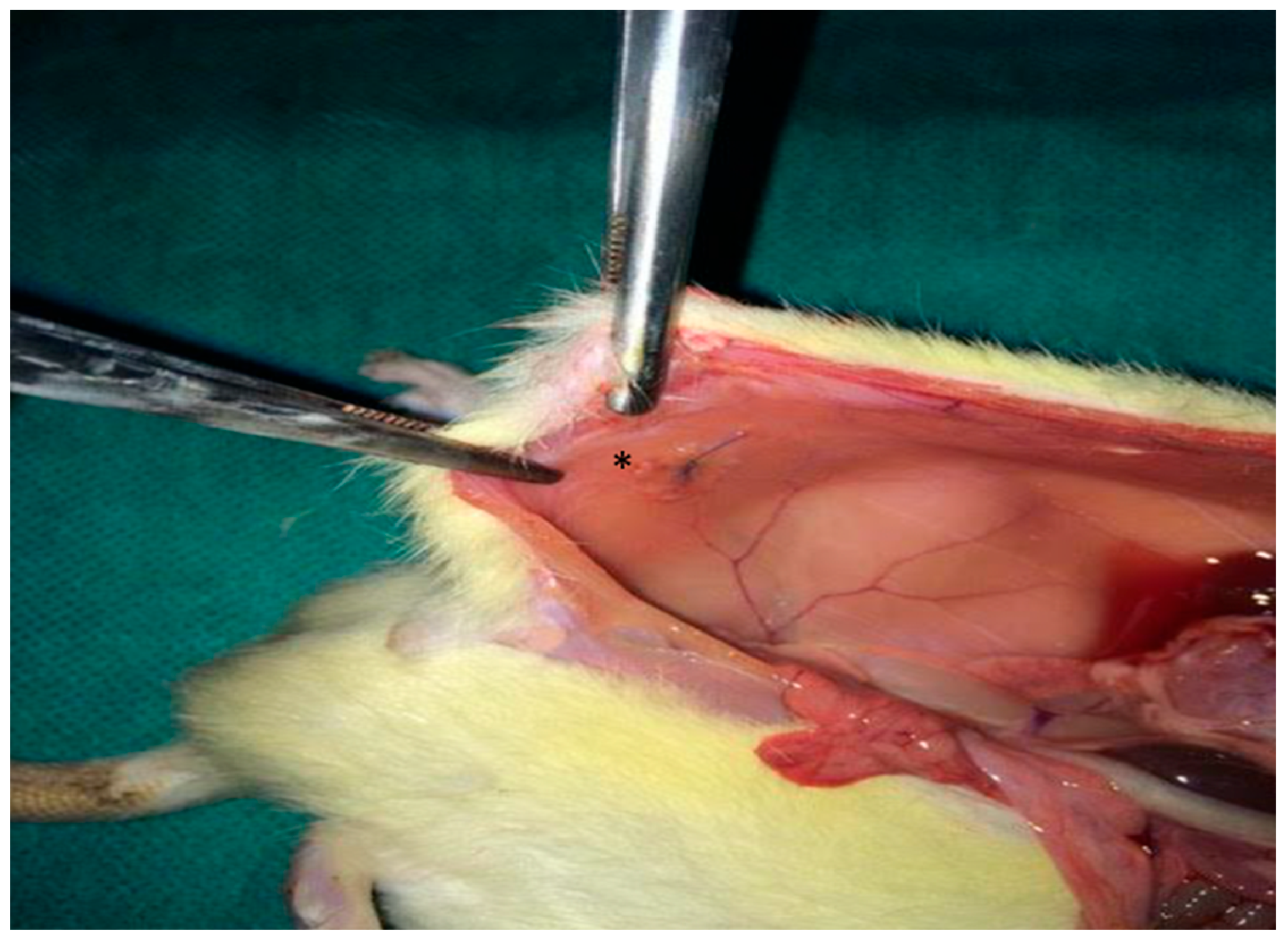
2.4.2. Mesh Implantation
2.4.3. Creation of Contamination Models
2.5. Pharmacologic Interventions
2.6. Postoperative Care
2.7. Euthanasia
2.8. Outcome Measures
2.8.1. Cytokine Assays
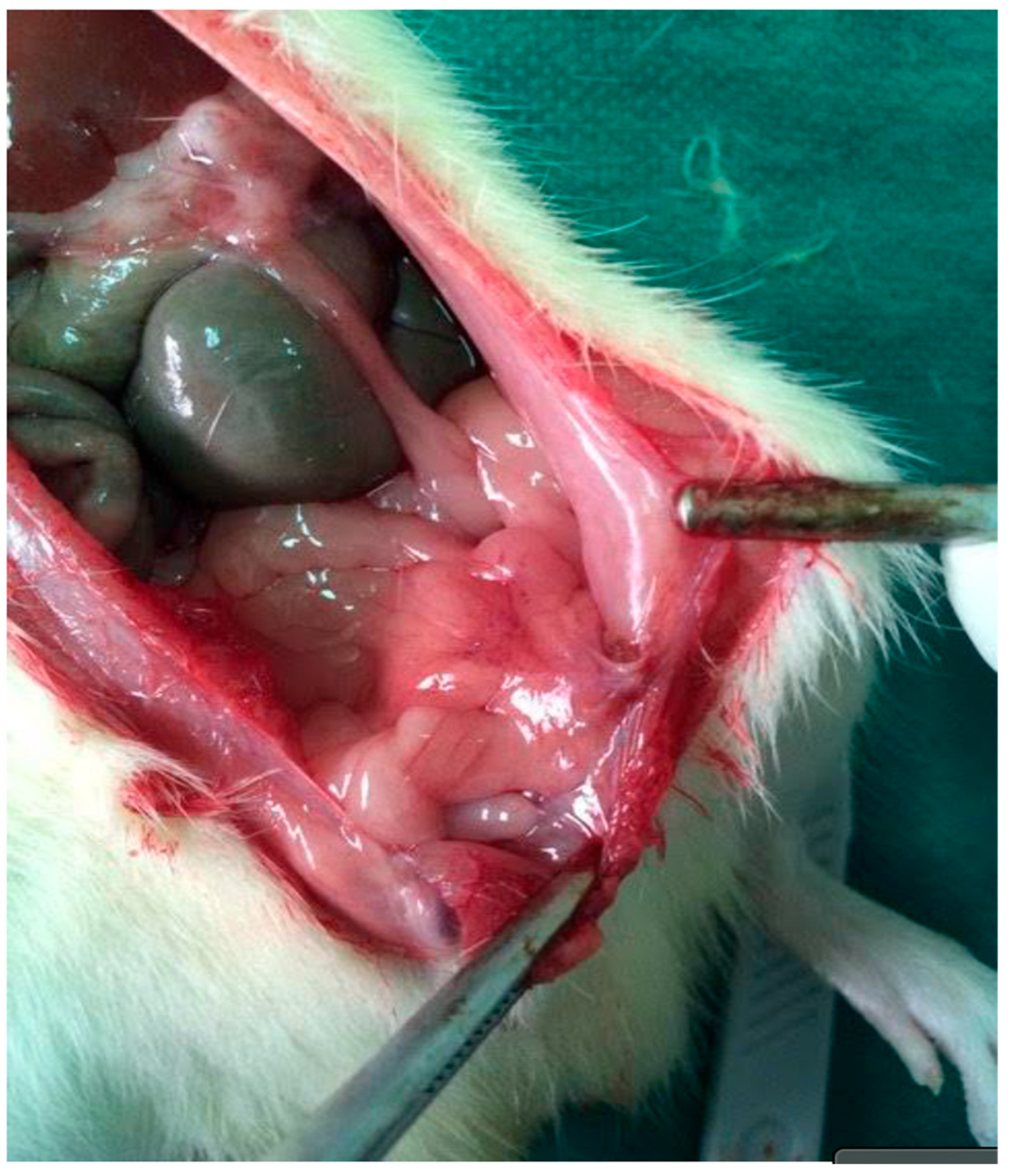
2.8.2. Macroscopic Adhesions
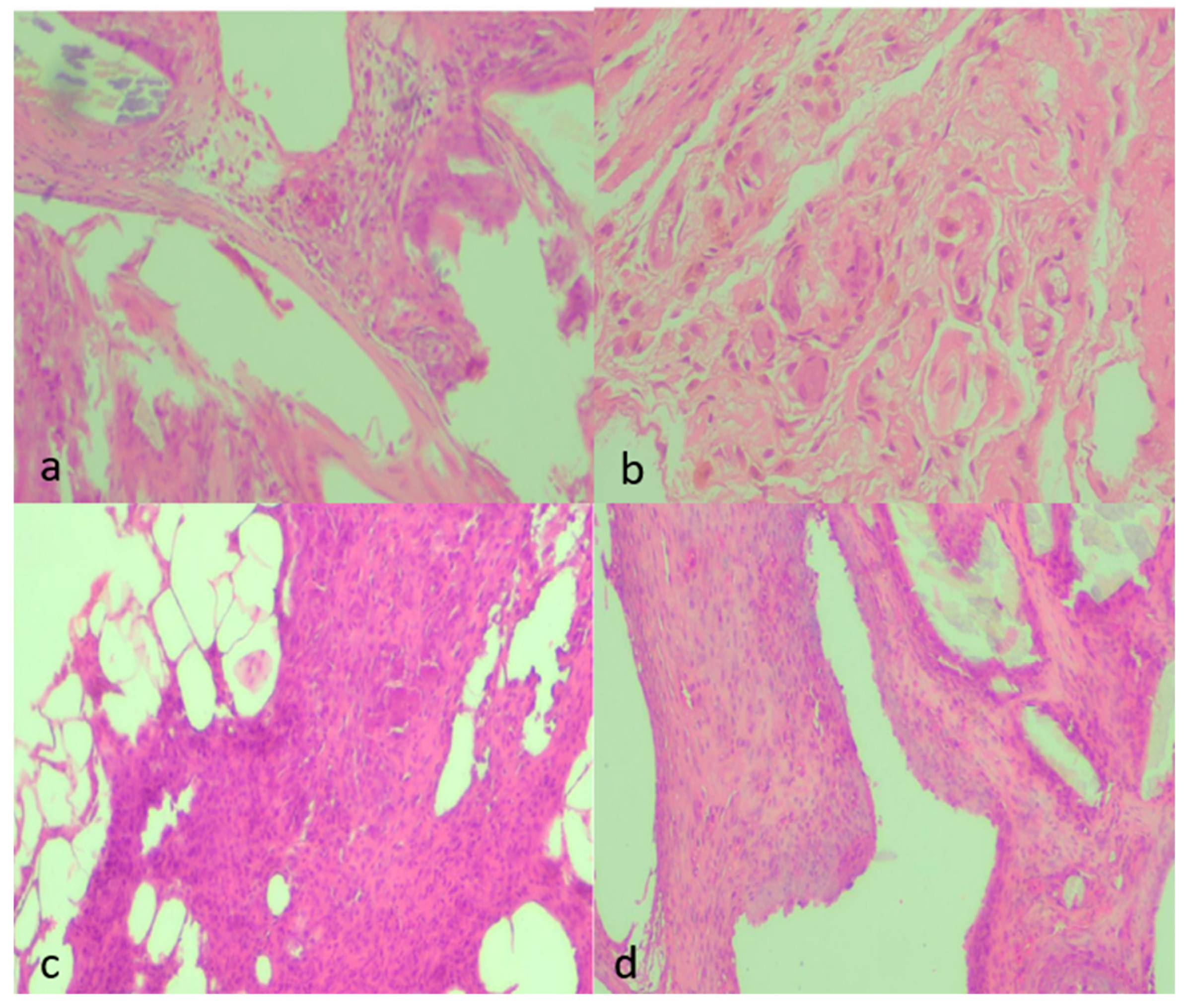
2.8.3. Histopathology
2.8.4. Microbiology
2.9. Sample Size and Statistical Analysis
2.10. Animal Housing and Welfare
2.11. Ethical Approval
3. Results
3.1. Cohort and Protocol Adherence
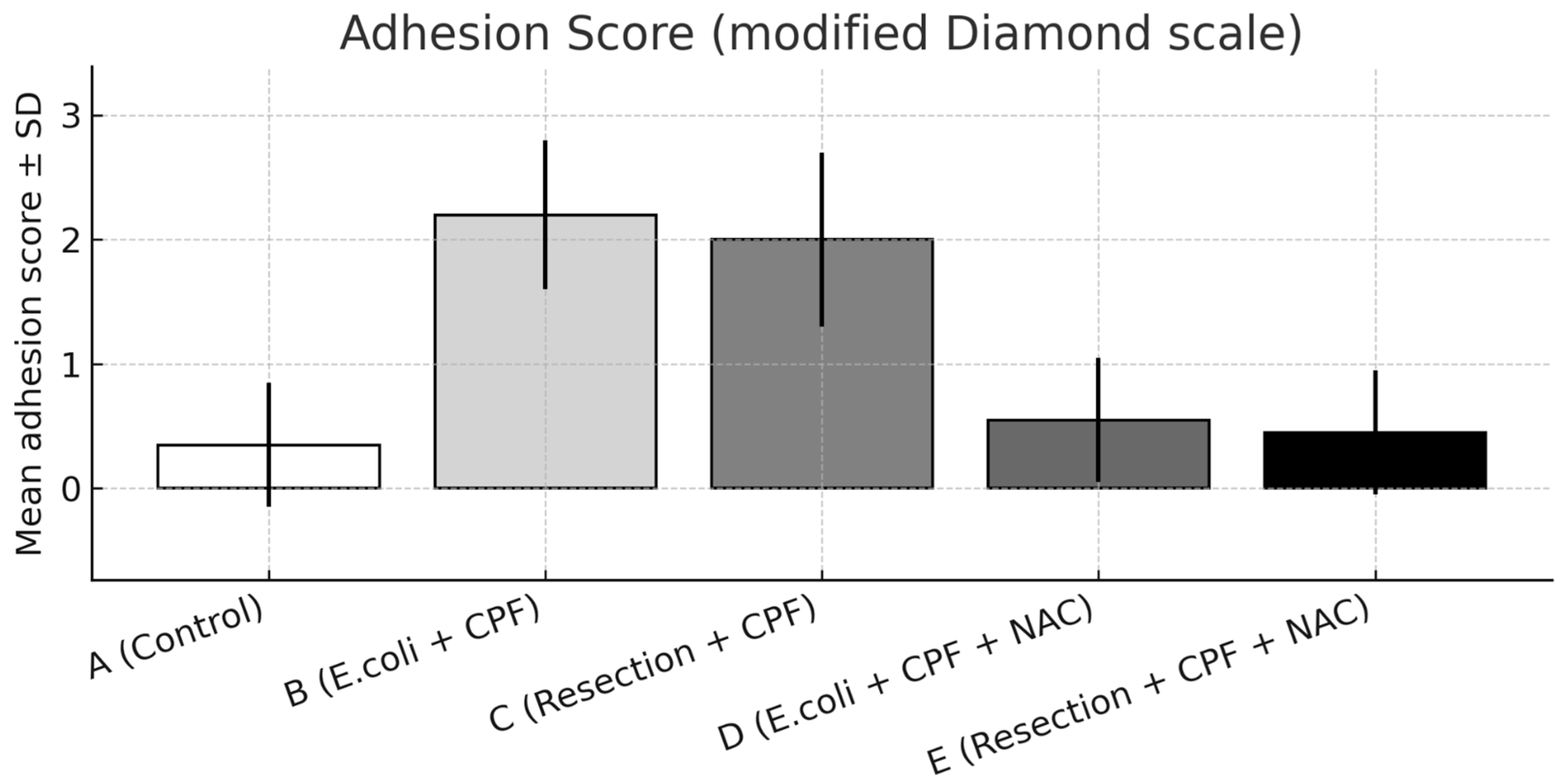
3.2. Adhesions
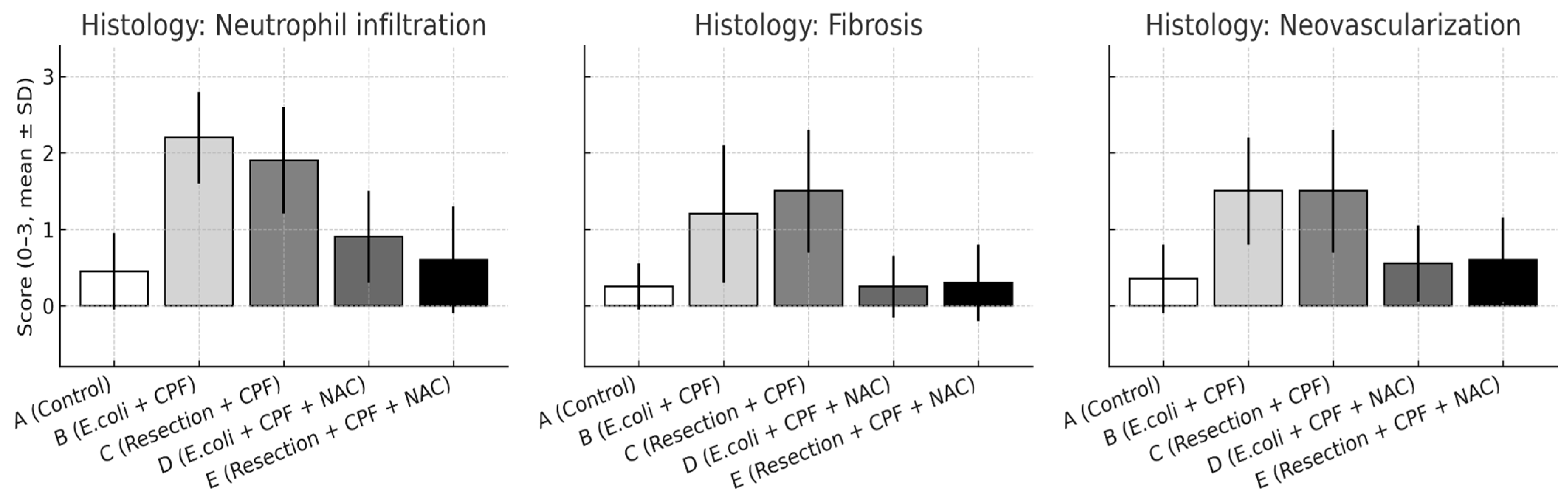
3.3. Histology
- Neutrophil infiltration: B > A (p < 0.001); D < B (p < 0.001); A vs. D non-significant. Additional contrasts: B > C (p < 0.05); D vs. E non-significant.
- Fibrosis: A vs. B (p = 0.002); B vs. D (p = 0.002); A vs. D non-significant; B vs. C (p < 0.05); D vs. E non-significant.
- Neovascularization: A vs. B (p < 0.001); B vs. D (p = 0.003); A vs. D non-significant; B vs. C (p < 0.05); D vs. E non-significant.
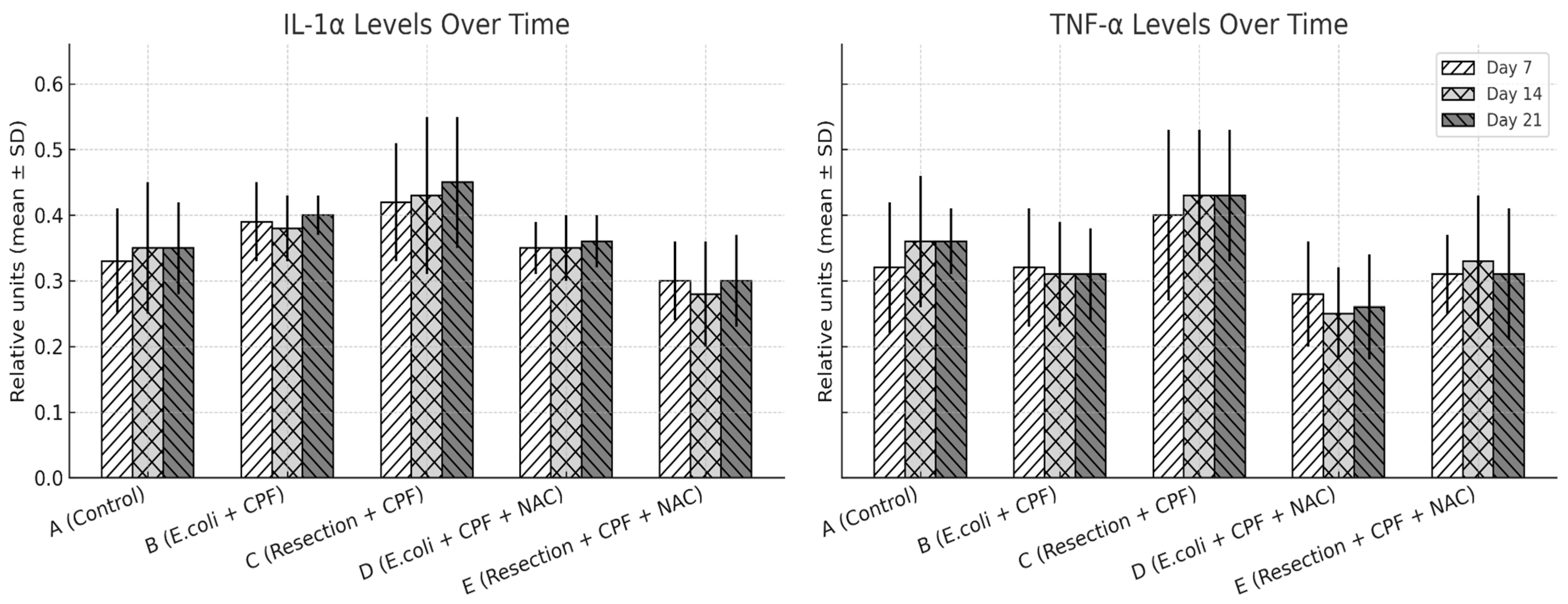
3.4. Serum Cytokines
- Neutrophil infiltration: B > A (p < 0.001); D < B (p < 0.001); A vs. D non-significant. Additional contrasts: B > C (p < 0.05); D vs. E non-significant.
- Fibrosis: A vs. B (p = 0.002); B vs. D (p = 0.002); A vs. D non-significant; B vs. C (p < 0.05); D vs. E non-significant.
- Neovascularization: A vs. B (p < 0.001); B vs. D (p = 0.003); A vs. D non-significant; B vs. C (p < 0.05); D vs. E non-significant.
3.5. Microbiology and Follow-Up
4. Discussion
5. Strengths, Limitations and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAC | N-acetyl-L-cysteine |
| E. coli | Escherichia coli |
| IL-1α | Interleukin-1 alpha |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| NF-κB | Nuclear factor kappa B |
| CFU | Colony-forming units |
| POD | Postoperative day |
| H&E | Hematoxylin and eosin |
| ELISA | Enzyme-linked immunosorbent assay |
| ANOVA | Analysis of variance |
| SPSS | Statistical Package for the Social Sciences |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| EU | European Union |
| PK | Pharmacokinetics |
| PD | Pharmacodynamics |
| CV | Coefficient of variation |
| R | R Statistical Software |
| WSES | World Society of Emergency Surgery |
References
- Birindelli, A.; Sartelli, M.; Di Saverio, S.; Coccolini, F.; Ansaloni, L.; van Ramshorst, G.H.; Campanelli, G.; Khokha, V.; Moore, E.E.; Peitzman, A.; et al. 2017 Update of the WSES Guidelines for Emergency Repair of Complicated Abdominal Wall Hernias. World J. Emerg. Surg. 2017, 12, 37. [Google Scholar] [CrossRef]
- Birolini, C.; Utiyama, E.M.; Rodrigues, A.J.J.; Birolini, D. Elective Colonic Operation and Prosthetic Repair of Incisional Hernia: Does Contamination Contraindicate Abdominal Wall Prosthesis Use? J. Am. Coll. Surg. 2000, 191, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellatif, M.E.; Negm, A.; Elmorsy, G.; Al-Katary, M.; Yousef, A.E.-A.M.; Ellaithy, R. Feasibility of Mesh Repair for Strangulated Abdominal Wall Hernias. Int. J. Surg. 2012, 10, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Haskins, I.N.; Amdur, R.L.; Lin, P.P.; Vaziri, K. The Use of Mesh in Emergent Ventral Hernia Repair: Effects on Early Patient Morbidity and Mortality. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2016, 20, 1899–1903. [Google Scholar] [CrossRef]
- Moris, D.; Chakedis, J.; Rahnemai-Azar, A.A.; Wilson, A.; Hennessy, M.M.; Athanasiou, A.; Beal, E.W.; Argyrou, C.; Felekouras, E.; Pawlik, T.M. Postoperative Abdominal Adhesions: Clinical Significance and Advances in Prevention and Management. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2017, 21, 1713–1722. [Google Scholar] [CrossRef]
- Maciver, A.H.; McCall, M.; James Shapiro, A.M. Intra-Abdominal Adhesions: Cellular Mechanisms and Strategies for Prevention. Int. J. Surg. 2011, 9, 589–594. [Google Scholar] [CrossRef]
- Yao, V.; Platell, C.; Hall, J.C. Peritoneal Mesothelial Cells Produce Inflammatory Related Cytokines. ANZ J. Surg. 2004, 74, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Bronzatto, E.; Riccetto, C.L.Z. Pro-Inflammatory Cytokines and Metalloproteinase Activation in Polypropylene Mesh Implant in Rat Subcutaneous Tissue. Int. Braz. J. Urol. 2018, 44, 819–825. [Google Scholar] [CrossRef]
- Olofsson, A.-C.; Hermansson, M.; Elwing, H. N-Acetyl-L-Cysteine Affects Growth, Extracellular Polysaccharide Production, and Bacterial Biofilm Formation on Solid Surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef]
- Shahzamani, S.; Jahandideh, A.R.; Abedi, G.; Akbarzadeh, A.; Hesaraki, S. Effect of N-Acetyl-Cysteine Nanoparticles on Intra-Abdominal Adhesion after Laparotomy in Rats. Pol. J. Vet. Sci. 2019, 22, 581–588. [Google Scholar] [CrossRef]
- Eroshenko, D.; Polyudova, T.; Korobov, V. N-Acetylcysteine Inhibits Growth, Adhesion and Biofilm Formation of Gram-Positive Skin Pathogens. Microb. Pathog. 2017, 105, 145–152. [Google Scholar] [CrossRef]
- Chu, D.I.; Lim, R.; Heydrick, S.; Gainsbury, M.L.; Abdou, R.; D’Addese, L.; Reed, K.L.; Stucchi, A.F.; Becker, J.M. N-acetyl-L-cysteine decreases intra-abdominal adhesion formation through the upregulation of peritoneal fibrinolytic activity and antioxidant defenses. Surgery 2011, 149, 801–812. [Google Scholar] [CrossRef]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef]
- Parpoudi, S.; Mantzoros, I.; Gkiouliava, A.; Kyziridis, D.; Makrantonakis, A.; Chatzakis, C.; Gekas, C.; Konstantaras, D.; Ioannidis, O.; Bitsianis, S.; et al. Effect of N-Acetyl-L-Cysteine on Inflammation after Intraperitoneal Mesh Placement in a Potentially Contaminated Environment: An Experimental Study in the Rat. Asian J. Surg. 2022, 45, 2191–2196. [Google Scholar] [CrossRef]
- Kalimeris, K.; Briassoulis, P.; Ntzouvani, A.; Nomikos, T.; Papaparaskeva, K.; Politi, A.; Batistaki, C.; Kostopanagiotou, G. N-acetylcysteine ameliorates liver injury in a rat model of intestinal ischemia–reperfusion. J. Surg. Res. 2016, 206, 263–272. [Google Scholar] [CrossRef]
- Matthews, B.D.; Pratt, B.L.; Pollinger, H.S.; Backus, C.L.; Kercher, K.W.; Sing, R.F.; Heniford, B.T. Assessment of Adhesion Formation to Intra-Abdominal Polypropylene Mesh and Polytetrafluoroethylene Mesh. J. Surg. Res. 2003, 114, 126–132. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- ten Raa, S.; van den Tol, M.P.; Sluiter, W.; Hofland, L.J.; van Eijck, C.H.J.; Jeekel, H. The Role of Neutrophils and Oxygen Free Radicals in Post-Operative Adhesions. J. Surg. Res. 2006, 136, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Margetts, P.J.; Kolb, M.; Yu, L.; Hoff, C.M.; Holmes, C.J.; Anthony, D.C.; Gauldie, J. Inflammatory Cytokines, Angiogenesis, and Fibrosis in the Rat Peritoneum. Am. J. Pathol. 2002, 160, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Treutner, K.H.; Haase, G.; Kinzel, S.; Tietze, L.; Schumpelick, V. Effect of Intraperitoneal Antiadhesive Fluids in a Rat Peritonitis Model. Arch. Surg. 2003, 138, 286–290. [Google Scholar] [CrossRef]
- Sudirman, T.; Hatta, M.; Prihantono, P.; Bukhari, A.; Tedjasaputra, T.R.; Lie, H. Vitamin E administration as preventive measures for peritoneal/intra-abdominal adhesions: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 80, 104225. [Google Scholar] [CrossRef] [PubMed]
- Flutur, I.M.; Păduraru, D.N.; Bolocan, A.; Palcău, A.C.; Ion, D.; Andronic, O. Postsurgical adhesions: Is there any prophylactic strategy really working? J. Clin. Med. 2023, 12, 3931. [Google Scholar] [CrossRef]
- Reed, K.L.; Fruin, A.B.; Gower, A.C.; Stucchi, A.F.; Leeman, S.E.; Becker, J.M. A Neurokinin 1 Receptor Antagonist Decreases Postoperative Peritoneal Adhesion Formation and Increases Peritoneal Fibrinolytic Activity. Proc. Natl. Acad. Sci. USA 2004, 101, 9115–9120. [Google Scholar] [CrossRef]
- Mahdy, T.; Mohamed, G.; Elhawary, A. Effect of Methylene Blue on Intra-Abdominal Adhesion Formation in Rats. Int. J. Surg. 2008, 6, 452–455. [Google Scholar] [CrossRef]
- Galili, Y.; Ben-Abraham, R.; Rabau, M.; Klausner, J.; Kluger, Y. Reduction of Surgery-Induced Peritoneal Adhesions by Methylene Blue. Am. J. Surg. 1998, 175, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Heydrick, S.J.; Reed, K.L.; Cohen, P.A.; Aarons, C.B.; Gower, A.C.; Becker, J.M.; Stucchi, A.F. Intraperitoneal Administration of Methylene Blue Attenuates Oxidative Stress, Increases Peritoneal Fibrinolysis, and Inhibits Intraabdominal Adhesion Formation. J. Surg. Res. 2007, 143, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Zaidi, M.; Qadir, I.; Memon, A.A. Emergency Incisional Hernia Repair: A Difficult Problem Waiting for a Solution. Ann. Surg. Innov. Res. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, L.; Eriksson, E.; al-Jabreen, M.; Risberg, B. Fibrinolysis in Human Peritoneum during Operation. Surgery 1996, 119, 701–705. [Google Scholar] [CrossRef]
- Cohn, S.M.; Lipsett, P.A.; Buchman, T.G.; Cheadle, W.G.; Milsom, J.W.; O’Marro, S.; Yellin, A.E.; Jungerwirth, S.; Rochefort, E.V.; Haverstock, D.C.; et al. Comparison of Intravenous/Oral Ciprofloxacin plus Metronidazole versus Piperacillin/Tazobactam in the Treatment of Complicated Intraabdominal Infections. Ann. Surg. 2000, 232, 254–262. [Google Scholar] [CrossRef]
- Maatouk, M.; Ben Safta, Y.; Mabrouk, A.; Kbir, G.H.; Ben Dhaou, A.; Daldoul, S.; Sayari, S.; Haouet, K.; Dziri, C.; Ben Moussa, M. Surgical Site Infection in Mesh Repair for Ventral Hernia in Contaminated Field: A Systematic Review and Meta-Analysis. Ann. Med. Surg. 2021, 63, 102173. [Google Scholar] [CrossRef]
- Mandalà, V.; Bilardo, G.; Darca, F.; Marco, F.; Luzza, A.; Lupo, M.; Mirabella, A. Some Considerations on the Use of Heterologous Prostheses in Incisional Hernias at Risk of Infection. Hernia 2000, 4, 268–271. [Google Scholar] [CrossRef]
- Nieuwenhuizen, J.; van Ramshorst, G.H.; ten Brinke, J.G.; de Wit, T.; van der Harst, E.; Hop, W.C.J.; Jeekel, J.; Lange, J.F. The Use of Mesh in Acute Hernia: Frequency and Outcome in 99 Cases. Hernia 2011, 15, 297–300. [Google Scholar] [CrossRef]
- Sido, B.; Teklote, J.-R.; Hartel, M.; Friess, H.; Büchler, M.W. Inflammatory Response after Abdominal Surgery. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M. Host Modulation Therapy with Anti-Inflammatory Agents. Periodontol. 2000 2018, 76, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Aslan, G.I.; Otgun, I.; Acer, T.; Tepeoglu, M.; Hicsonmez, A. The Effect of Intraperitoneal N-Acetylcysteine on Postoperative Adhesions in Rat Models. Ann. Ital. Chir. 2017, 88, 258–262. [Google Scholar] [PubMed]
- Shankaran, V.; Weber, D.J.; Reed, R.L., 2nd; Luchette, F.A. A Review of Available Prosthetics for Ventral Hernia Repair. Ann. Surg. 2011, 253, 16–26. [Google Scholar] [CrossRef]
- Morris-Stiff, G.J.; Hughes, L.E. The Outcomes of Nonabsorbable Mesh Placed within the Abdominal Cavity: Literature Review and Clinical Experience. J. Am. Coll. Surg. 1998, 186, 352–367. [Google Scholar] [CrossRef]
- Kaufmann, R.; Jairam, A.P.; Mulder, I.M.; Wu, Z.; Verhelst, J.; Vennix, S.; Giesen, L.J.X.; Clahsen-van Groningen, M.C.; Jeekel, J.; Lange, J.F. Characteristics of Different Mesh Types for Abdominal Wall Repair in an Experimental Model of Peritonitis. Br. J. Surg. 2017, 104, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Reijnen, M.M.; Meis, J.F.; Postma, V.A.; van Goor, H. Prevention of Intra-Abdominal Abscesses and Adhesions Using a Hyaluronic Acid Solution in a Rat Peritonitis Model. Arch. Surg. 1999, 134, 997–1001. [Google Scholar] [CrossRef]
- Mendes, J.B.; Campos, P.P.; Rocha, M.A.; Andrade, S.P. Cilostazol and Pentoxifylline Decrease Angiogenesis, Inflammation, and Fibrosis in Sponge-Induced Intraperitoneal Adhesion in Mice. Life Sci. 2009, 84, 537–543. [Google Scholar] [CrossRef]
- Lim, R.; Morrill, J.M.; Prushik, S.G.; Reed, K.L.; Gower, A.C.; Leeman, S.E.; Stucchi, A.F.; Becker, J.M. An FDA Approved Neurokinin-1 Receptor Antagonist Is Effective in Reducing Intraabdominal Adhesions When Administered Intraperitoneally, but Not Orally. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2008, 12, 1754–1761. [Google Scholar] [CrossRef]
- Carbonell, A.M.; Criss, C.N.; Cobb, W.S.; Novitsky, Y.W.; Rosen, M.J. Outcomes of Synthetic Mesh in Contaminated Ventral Hernia Repairs. J. Am. Coll. Surg. 2013, 217, 991–998. [Google Scholar] [CrossRef]
- Papi, A.; Di Stefano, A.F.D.; Radicioni, M. Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study. Adv. Ther. 2021, 38, 468–478. [Google Scholar] [CrossRef]
- Cristina, M.; Graciliano, N.G.; Andr, F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F.; Aldini, G.; Altomare, A.; Baron, G.; et al. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.R. Antioxidant Properties of N-acetylcysteine: Their Relevance in Relation to Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2024, 23, 629–636. [Google Scholar] [CrossRef]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Binda, M.M.; Molinas, C.R.; Koninckx, P.R. Reactive Oxygen Species and Adhesion Formation: Clinical Implications in Adhesion Prevention. Hum. Reprod. 2003, 18, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Akgun, E.; Caliskan, C.; Celik, H.A.; Ozutemiz, A.O.; Tuncyurek, M.; Aydin, H.H. Effects of N-Acetylcysteine Treatment on Oxidative Stress in Acetic Acid-Induced Experimental Colitis in Rats. J. Int. Med. Res. 2005, 33, 196–206. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parpoudi, S.; Mantzoros, I.; Ioannidis, O.; Zapsalis, K.; Gamali, T.; Kyziridis, D.; Gekas, C.; Anestiadou, E.; Symeonidis, S.; Bitsianis, S.; et al. Effect of N-Acetyl-L-Cysteine (NAC) on Inflammation After Intraperitoneal Mesh Placement in an Escherichia coli Septic Rat Model: A Randomized Experimental Study. Med. Sci. 2025, 13, 318. https://doi.org/10.3390/medsci13040318
Parpoudi S, Mantzoros I, Ioannidis O, Zapsalis K, Gamali T, Kyziridis D, Gekas C, Anestiadou E, Symeonidis S, Bitsianis S, et al. Effect of N-Acetyl-L-Cysteine (NAC) on Inflammation After Intraperitoneal Mesh Placement in an Escherichia coli Septic Rat Model: A Randomized Experimental Study. Medical Sciences. 2025; 13(4):318. https://doi.org/10.3390/medsci13040318
Chicago/Turabian StyleParpoudi, Styliani, Ioannis Mantzoros, Orestis Ioannidis, Konstantinos Zapsalis, Thomai Gamali, Dimitrios Kyziridis, Christos Gekas, Elissavet Anestiadou, Savvas Symeonidis, Stefanos Bitsianis, and et al. 2025. "Effect of N-Acetyl-L-Cysteine (NAC) on Inflammation After Intraperitoneal Mesh Placement in an Escherichia coli Septic Rat Model: A Randomized Experimental Study" Medical Sciences 13, no. 4: 318. https://doi.org/10.3390/medsci13040318
APA StyleParpoudi, S., Mantzoros, I., Ioannidis, O., Zapsalis, K., Gamali, T., Kyziridis, D., Gekas, C., Anestiadou, E., Symeonidis, S., Bitsianis, S., Kotidis, E., Pramateftakis, M.-G., Miliaras, D., Bikouli, A., Iosifidis, G., & Angelopoulos, S. (2025). Effect of N-Acetyl-L-Cysteine (NAC) on Inflammation After Intraperitoneal Mesh Placement in an Escherichia coli Septic Rat Model: A Randomized Experimental Study. Medical Sciences, 13(4), 318. https://doi.org/10.3390/medsci13040318





