Abstract
Background: Graves–Basedow disease (GBD) is an autoimmune thyroid disorder characterized by loss of tolerance to the thyrotropin receptor, with clinical manifestations such as a hyperadrenergic state, goiter, orbitopathy, and myxedema, inter alia. Selenium is a micronutrient, essential for the synthesis of selenoproteins. Selenium deficiency has been linked to an increased risk and exacerbation of GBD and GBD orbitopathy; therefore, it has been suggested that supplementation with this micronutrient could modify some outcomes associated with both conditions. Objectives: The objective of this scoping review was to synthesize and analyze the clinical trials that have evaluated the effectiveness of selenium on different outcomes in patients with GBD or GBD orbitopathy. Methods: The following databases were consulted: PubMed/Medline, Scopus, Biosis, ProQuest, Web of Science, and Google Scholar; and the search terms ‘Graves-Basedow disease’ or ‘Graves’ disease’ or ‘hyperthyroidism’ or ‘Graves’ hyperthyroidism’ or ‘selenium or selenium supplementation’ and ‘effectiveness’ were used. The search was limited to articles published in English between January 2000 and March 2025. To reduce selection bias, each article was reviewed independently by three authors using the Rayyan web tool and the JBI Critical Appraisal Checklist. Results: A total of 15 studies were identified (11 on patients with GBD and 4 on patients with GBD orbitopathy). In GBD, selenium supplementation was associated with significant improvements in TSH, FT4, FT3, TPOAb, TgAb, and TRAb levels; while in GBD orbitopathy, a positive effect of selenium supplementation was found on multiple clinical outcomes. Conclusions: Selenium supplementation in patients with GBD or GBD orbitopathy is associated with favorable biochemical and clinical outcomes.
1. Introduction
Autoimmune diseases (AIDs) are a broad group of more than 100 heterogeneous diseases in which the common denominator is the loss of immune tolerance against one or multiple autoantigens. It is estimated that approximately 5% of the population has been diagnosed with an AID, and of these, 34% have been diagnosed with more than one AID. Furthermore, women are more frequently affected than men (almost 80% of all confirmed AIDs diagnoses are made in women) [1,2].
AIDs are generally classified as those that can affect multiple organs or systems [non-organ-specific (NOS)] or those that affect a single organ [organ-specific (OE)]. Among the OE AIDs, the most common is autoimmune thyroid disease (AITD) [3,4].
AITD includes Graves–Basedow disease (GBD) and Hashimoto’s disease (HT), which can exhibit two extremes of clinical presentation, hyperthyroidism (in GBD) and hypothyroidism (in HT) [5].
GBD is characterized by an infiltration of T lymphocytes (TLs) into thyroid tissue, as well as an increase in B lymphocytes (BL) activation and the synthesis and secretion of antibodies (Abs) directed against the thyrotropin (TSH) receptor (TSHR) TRAbs. This results in an autoimmune response that can clinically manifest as a goiter and as hyperthyroidism, ophthalmopathy, and dermopathy, inter alia [6].
GBD has a prevalence of 0.5–2.0%, while that of HT is 5–10%. Multiple associated factors (genetic, epigenetic, environmental, socioeconomic, and nutritional, inter alia) not only influence the pathogenesis, but also the overall frequency of AITD [7,8].
Among the nutritional factors at play, the status of some micronutrients in a given population is considered to affect susceptibility to AITD. Studies in this area have focused on determining the concentrations of micronutrients such as selenium, iodine, iron, zinc, and vitamin D, inter alia, in the blood (whole blood, serum, or plasma), urine (in random or 24 h urine samples), or various tissues [9,10].
In this sense, selenium is an essential micronutrient for the biosynthesis of selenoproteins containing selenocysteine. The thyroid contains the highest amount of selenium per gram of tissue, and most selenoproteins are expressed in the thyroid and participate in the metabolism of thyroid hormones [11,12,13].
Selenium deficiency has been associated with an increased risk of AITD, an effect that could be explained by several mechanisms, such as decreased synthesis and secretion of interferon gamma and other cytokines, accompanied by an alteration in the cellular immune response, along with increased activity of autoreactive TLs and low activity of regulatory TLs (Treg) [14,15,16,17].
The importance of selenium for health lies in its role as a component of selenocysteine (SeCys), which is present in various selenoproteins. SeCys is located in several active enzyme sites that play fundamental roles in the regulation of reactive oxygen species (ROS), energy metabolism, redox status, and the various cellular processes responsible for the innate and adaptive immune responses [16,17,18].
Moreover, among the well-characterized selenoproteins are iodothyronine deiodinases (DIOs), glutathione peroxidases (GPXs), and thioredoxin reductases (TXNRDs), all of which are enzymes involved in thyroid hormone metabolism, the regulation of redox states, and protection from oxidative damage [19,20].
Therefore, selenium deficiency can result in a reduction in the expression and activity of these enzymes, resulting in increased T4 levels and decreased T3 levels [20,21].
Additionally, selenium deficiency (corresponding to a serum concentration < 70 µg/L) has also been documented as being associated with AITD; in fact, multiple studies have shown that selenium supplementation in areas deficient in this micronutrient decreases thyroid Ab concentrations, suggesting that it could modify the natural course of AITD [22,23].
Several studies have documented that serum selenium concentrations are significantly lower in individuals with GBD (compared to healthy individuals), resulting in selenium deficiency being considered a risk factor for the development of GBD [22,23,24,25].
This has, in turn, led to the suggestion that selenium supplementation could have a favorable effect not only in terms of the prevention of AITD (and, in this sense, GBD), but also in terms of different biochemical outcomes (e.g., TSH, FT4, FT4 concentrations, and thyroid Ab titers) or clinical outcomes associated with the disease (e.g., signs and symptoms of hyperthyroidism or ocular findings—GBD orbitopathy) [23,24,25].
These concepts then led to the design and delivery of clinical studies that evaluated the effectiveness of selenium supplementation in patients with GBD, with or without ophthalmopathy. However, despite the evidence from these clinical studies, there is still no universally accepted criterion for the use of selenium in such patients.
The objective of this scoping review is to evaluate the effectiveness of the use of selenium in patients with GBD (or with GBD orbitopathy) who have undergone the usual treatment for the disease (or who have previously been treated) in relation to a range of clinical and/or biochemical outcomes.
2. Materials and Methods
2.1. Literature Search and Selection Criteria
Using a modified version of the Population, Interventions, Comparators, and Outcomes (PICO) framework, we formulated the research question and defined the eligibility criteria for the scoping review (Table 1).

Table 1.
Inclusion criteria adopted in the scoping review.
Subsequently, a structured literature search was carried out in PubMed/Medline, Scopus, Biosis, ProQuest, Web of Science, and Google Scholar for articles published from January 2000 to March 2025 (human trials, clinical trials, meta-analyses, reviews, scoping reviews, and systematic reviews).
The following search terms were used: ‘Graves-Basedow disease’ or ‘Graves’ disease’ or ‘hyperthyroidism’ or ‘Graves’ hyperthyroidism’ or ‘selenium or selenium supplementation’ and ‘effectiveness.’
The search strategy was as follows: Graves-Basedow disease [Title/Abstract] OR Graves’ disease [Title/Abstract] OR hyperthyroidism [Title/Abstract] OR Graves’ hyperthyroidism [Title/Abstract] OR Selenium intake [Title/Abstract] OR Selenium supplementation AND Effectiveness [Title/Abstract] OR Outcomes [Title/Abstract].
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a total of 15 studies were included: 11 on individuals with GBD and 4 on patients with GBD orbitopathy (Figure 1).
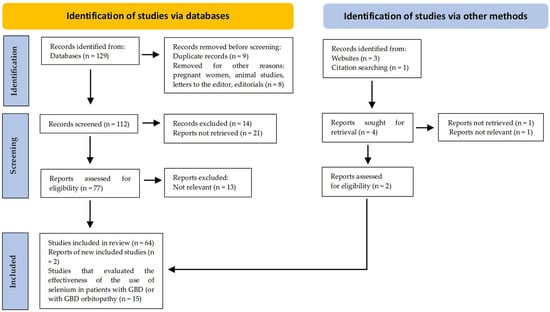
Figure 1.
PRISMA flow diagram. Method for the selection of articles.
2.2. Data Extraction
The titles and abstracts of all the studies were independently reviewed by three investigators (H.V.-U., A.C.-P., and H.V.-S) using the Rayyan web tool (this further helped reduce selection bias). Full texts of the studies that met the initial inclusion criteria were obtained and reviewed, and the data were extracted through a standardized template, using a predefined data form created in Excel.
In the presence of discrepancies in data extraction, the investigators collaboratively conducted a second round of analysis and extraction to validate the information obtained. Each article was scrutinized according to the JBI Critical Appraisal Checklist; only articles written in English were considered.
The eligible studies and the inclusion and exclusion criteria (according to the defined categories) are described in Table 2.

Table 2.
Categories, inclusion, and exclusion criteria of eligible studies.
2.3. Data Analysis
The following data were collected in the review: design type, country, inclusion criteria, interventions, selenium dose, number of participants, follow-up time, and clinical and/or biochemical outcomes.
The heterogeneity found in the 15 studies, in relation to aspects such as the definition of the disease, previous or current treatment of hyperthyroidism, clinical and/or biochemical outcomes, and follow-up time, did not allow for a statistical analysis or meta-analysis. Consequently, we conducted a descriptive analysis, using a narrative approach, to summarize and synthesize the most representative findings of the selected studies.
This scoping review was registered on the “International Platform of Registered Systematic Review and Meta–Analysis Protocols INPLASY” (Registration number: INPLASY202580095; DOI: 10.37766/inplasy2025.8.0095) and conducted according to the “Reporting Checklist for Systematic Reviews Based on the PRISMA guidelines” (Table S1).
3. Results
3.1. General Characteristics of the Studies and Participants
A total of 15 clinical trials were identified, 11 of which were developed with individuals with GBD [26,27,28,29,30,31,32,33,34,35,36] and 4 with patients with GBD orbitopathy [37,38,39,40]. Table 3 and Table 4 summarize the design of the clinical trials, the baseline statuses and selenium doses used, and the follow-up times and outcomes for participants diagnosed with GBD (or GBD orbitopathy) and assigned to receive selenium management.

Table 3.
Clinical trial design and general characteristics, follow-up time, and outcomes of participants diagnosed with GBD, assigned to receive selenium treatment.

Table 4.
Clinical trial design and general characteristics, follow-up time, and outcomes of participants diagnosed with GBD orbitopathy, assigned to receive selenium treatment.
These studies are generally notable for their small samples, with follow-ups ranging from the very short (e.g., 4 weeks) up to the very long (e.g., 5 years). The majority of the population evaluated consisted of women, with mean ages of 39.1 years in the intervention group (with selenium) and 39.7 years in the control group (in the 11 studies of participants with GBD), and 42.2 years in the intervention group and 44.6 years in the control group (in the 4 studies of participants with GBD orbitopathy).
3.2. Basal Selenium Concentrations and Doses Used in the Studies
Baseline selenium concentrations were assessed in 4 of the 11 studies of participants with GBD (normal concentrations were observed in 3 studies, and a low concentration was observed in 1); of the 4 studies of individuals with GBD orbitopathy, selenium concentrations were assessed in 2 (normal concentrations were observed in both studies, but in 1 study, concentrations were measured only in the intervention group).
The selenium doses used ranged from 60 to 300 µg/day; various types of selenium (selenite, selenium yeast, selenium glycinate, selenomethionine, selenomethionine + selenium yeast, L-selenomethionine, capsules of antioxidants, selenious yeast, and selenium glycinate) were used.
3.3. Concomitant or Previously Used Management for GBD
In 10 of the 11 studies on participants with GBD, methimazole was used as a baseline treatment. In 1 of these, baseline management was carried out with antioxidants. In contrast, in the studies concerning GBD orbitopathy, patients were previously managed with methimazole, radioactive iodine, or thyroidectomy (and remained euthyroid throughout the studies).
3.4. Severity of GBD Orbitopathy
In the studies on patients with GBD orbitopathy, participants were classified as follows: mild orbitopathy and euthyroidism (one study); mild and active orbitopathy (one study); moderate-to-severe inactive orbitopathy (one study); and mild-to-moderate orbitopathy (one study).
3.5. Clinical and Biochemical Outcomes (Before and After Selenium Intervention) for Participants with GBD or GBD Orbitopathy
The studies concerning the participants with GBD primarily assessed (before and after selenium intervention) outcomes such as TSH, FT4, FT3, TPOAb, TgAb, and TRAb concentrations; remission and recurrence rates of hyperthyroidism; and clinical and/or biochemical control of hyperthyroidism.
Meanwhile, in the studies of individuals with GBD orbitopathy, the effects of selenium treatment on specific outcomes—such as Clinical Activity Score (CAS), improvement in total GBD orbitopathy-related Quality of Life (GO–QOL), visual functioning score (GO–QOL change), psychological functioning score (GO–QOL), palpebral (eyelid) aperture change, improvement in palpebral (eyelid) aperture, exophthalmos change, and improvement in exophthalmos—were measured.
In 9 of the 11 studies concerning individuals with GBD, and in all 4 studies on individuals with GBD orbitopathy, at least one significant and favorable outcome was found in the group of participants who received selenium (Table 5).

Table 5.
Number of studies showing favorable results with selenium intervention, according to biochemical and/or clinical findings.
3.6. Favorable Biochemical Outcomes After Selenium Intervention for Participants with GBD
In summary, 9 of the 11 studies (on patients with GBD) in which at least one significant and favorable result (biochemical outcomes) was achieved with the use of selenium found the following:
One study demonstrated that a euthyroid state had been significantly achieved [26]; one demonstrated a significant reduction in FT4 levels [27]; one demonstrated a significant increase in TSH levels, with a decrease in FT4 levels [28]; one demonstrated a significant increase in TSH levels, with a decrease in FT3, FT4, TgAb, and TPOAb and TRAb titers [29]; one demonstrated an increase in the effect of antithyroid drugs in patients with recurrent GBD [30]; one demonstrated a reduction in TPOAb and TRAb titers and a reduction in the incidence of hypothyroidism [33]; one demonstrated a significant decrease in TRAb levels [34]; one demonstrated a decrease in FT3 and FT4 concentrations as well as TRAb, TPOAb, and TgAb titers [35]; and, one study demonstrated that the use of selenium enhances the effect of antithyroid drugs (when selenium and vitamin D levels are suboptimal) [36].
3.7. Favorable Clinical Outcomes After Selenium Intervention for Participants with GBD Orbitopathy
Furthermore, all four studies on patients with GBD orbitopathy showed at least two clinical outcomes in favor of the use of selenium [37,38,39,40]. The outcomes can be summarized as follows: CAS change at 6 months (three of four studies were in favor) and 12 months (two of two studies were in favor); improvement in total GO–QOL at 6 months (one of two studies in favor) and 12 months (one of two studies in favor); visual functioning score GO–QOL change at 6 months (one of three studies in favor) and/or 12 months (one of two studies was in favor); psychological functioning score GO–QOL change at 6 months (one of three studies in favor), and 12 months (one of two studies in favor); palpebral (eyelid) aperture change at 6 months (zero of two studies in favor); improvement in palpebral (eyelid) aperture at 6 months (one of two studies in favor); exophthalmos change at 6 months (zero of two studies in favor); and improvement in exophthalmos at 6 months (zero of two studies in favor).
4. Discussion
In this scoping review, we found that 9 of the 11 studies on patients with GBD and all 4 studies on patients with GBD orbitopathy demonstrated selenium use had a significant benefit.
Among patients with GBD, the benefits were achieved in six clinical scenarios (hyperthyroidism due to GBD; GBD treated with methimazole; newly diagnosed GBD; untreated hyperthyroid patients with GBD; recurring GBD; and patients with GBD after radioactive iodine treatment), with favorable outcomes in relation to increased TSH levels and decreased FT4, FT3, TPOAb, TgAb, and TRAb levels.
Meanwhile, in patients with GBD orbitopathy, benefits were achieved in four major clinical scenarios (mild GBD orbitopathy with euthyroidism; mild and active GBD orbitopathy (CAS > 3); inactive moderate-to-severe GBD orbitopathy; and mild-to-moderate GBD orbitopathy), with favorable outcomes in relation to quality of life, reduced ocular involvement, and slowed progression of the disease (in mild Graves’ orbitopathy); differences in palpebral fissure and CAS and eyelid aperture (even in inactive moderate-to-severe GBD orbitopathy); and the early course of mild-to-moderate GBD orbitopathy.
Trying to explain the beneficial effect of selenium on GBD and GBD orbitopathy can be difficult, given the biological and molecular complexity of AITD. GBD originates from the loss of host tolerance toward the TSHR, and TL activation induces the synthesis and secretion of inflammatory cytokines and the release of chemokines by thyroid follicular cells, generating an amplified inflammatory response, with an increase in the synthesis and secretion of TRAb [3,5,41].
Consequently, direct stimulation of TRAbs on the TSHR induces inappropriately high secretion of thyroid hormones, goiter, and extrathyroidal manifestations (especially orbitopathy and/or myxedema) [42].
The close interaction between genetic, non-genetic, epigenetic, and environmental factors influences the risk of developing GBD. In this context, among environmental factors, selenium is one of the most studied in relation to the association between deficiency and an increased risk of AITD (particularly with GBD and GBD orbitopathy) [16,42].
Therefore, the prevalence of selenium deficiency varies (depending on the geographic area studied), and some studies have suggested that the differences found in the effectiveness of selenium supplementation in patients with GBD and/or GBD orbitopathy could only be reflected in individuals with a deficiency in this micronutrient [16,43,44].
Furthermore, it should be noted that selenium intake from organic sources (e.g., selenomethionine and selenocysteine) is associated with greater absorption and bioavailability than that from inorganic sources (e.g., selenite and selenate), and that inorganic forms are retained in the body to a lesser extent than selenomethionine and selenocysteine [12,15,18,21]. This aspect related to absorption, bioavailability, and the source of selenium could also explain (at least in part) the findings of this review (and the variability of these results).
However, this scoping review describes the results in favor of selenium supplementation for people with GBD or GBD orbitopathy (in 13 of the 15 selected studies), taking into account that only 6 of the studies determined the baseline selenium status. Thus, the results suggest that, regardless of baseline selenium status, selenium supplementation (associated with baseline management with methimazole) has a positive effect on various biochemical outcomes (in GBD) and on multiple clinical and biochemical outcomes (in GBD orbitopathy).
Intriguingly, in one of the studies of mild-to-moderate GBD orbitopathy, a benefit of selenium use was found in study participants in the short term, but not in the long-term outcomes (which may be an effect of the natural evolution of GBD orbitopathy, where a decrease in its activity over time is observed) [22,23,40].
The above can be explained by the fact that selenium supplementation can induce an immunomodulatory effect in patients with AITD; for example, in a mouse model of autoimmune thyroiditis, selenium was observed to decrease lymphocytic infiltration in the thyroid, with the upregulation of Treg and the expression of GPX and TXNRD [45].
Other studies have found that selenoprotein deficiency can decrease calcium influx during the activation of various cells involved in the immune response, affecting the ability to respond to host and foreign antigens [46]. It has also been proposed that selenium may inhibit cell proliferation and the secretion of several proinflammatory cytokines (e.g., IFN-γ and TNF-α) [14,15,42,43].
However, most of the evidence for selenium’s potential immunomodulatory effect on GBD and GBD orbitopathy comes from animal models. Therefore, information from RCTs is quite scarce and limited, with studies varying considerably in terms of study design, methods for assessing immune function and response, selenium dose used, and follow-up time, among other factors.
Several studies suggest that the effect of selenium supplementation on humoral immunity is of low impact and magnitude [9,43,44]. However, this review found that supplementation has a beneficial effect on the levels of different thyroid Abs, while the effect on the cellular immune response is less clear (and among the RCTs selected in this review, none directly evaluated this aspect) Figure 2.
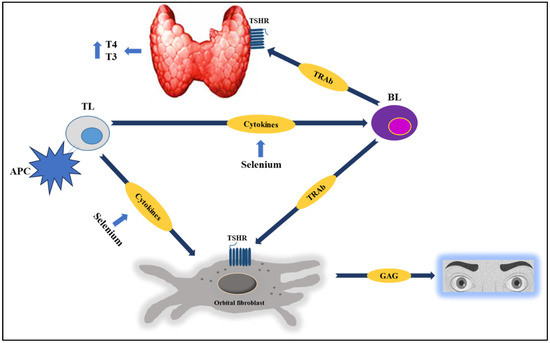
Figure 2.
In AITD, selenium has a potential immunomodulatory effect, as it is capable of inhibiting the synthesis of inflammatory cytokines, attenuating TL infiltration into the thyroid, and reducing thyroid Abs (TRAb) levels, among other effects. Studies suggest that selenium supplementation (along with basic management, especially with methimazole) favorably modifies multiple clinical and/or biochemical outcomes in patients with GBD or GBD orbitopathy. See the text for further details. Abbreviations: TSHR: Thyrotropin receptor; BL: B lymphocyte; TL: T lymphocyte; APC: antigen-presenting cell; GAG: glycosaminoglycans.
On the other hand, when selenium intake is adequate, the intracellular glutathione peroxidase and thioredoxin peroxidase systems protect thyrocytes from these peroxides. Additionally, selenium deficiency produces a significant reduction in the activity of the selenoprotein glutathione peroxidase, which removes H2O2 (promoting lipid peroxidation). Selenoproteins also prevent the excessive formation of ROS, which promote chronic inflammation and autoimmunity, as they are capable of regulating the effector function of TLs [10,11,42,43].
Consequently, adequate selenium intake could promote an effective immune response toward Th1 lymphocytes, thus avoiding a “switch” to a Th2 lymphocyte-mediated response (one of the hallmarks of GBD). In fact, some studies suggest that selenium supplementation may attenuate the Th2-mediated immune response, inducing a predominantly Th1-mediated response [6,10,42].
5. Strengths and Weaknesses
This scoping review has several limitations, such as the number of RCTs with small sample sizes, the widely varying follow-ups of participants, the heterogeneity of the inclusion criteria, and the lack of clear descriptions of the methods of diagnosis, disease severity, and baseline selenium statuses. Furthermore, the outcomes assessed were not standardized.
The use of other ATDs (propylthiouracil or carbimazole) and concomitant therapies (such as cholestyramine) was not taken into account either. It should also be noted that none of the studies assessed the basal statuses of other micronutrients, which may influence selenium metabolism and basal status (e.g., iodine, vitamin D, zinc, iron, etc.). Additionally, some studies did not provide sufficient information on and/or the clinical and biochemical characteristics of participants.
Therefore, conclusions based on our findings should only be extrapolated to patients with conditions similar to those of the participants in the selected studies.
However, this review has some strengths—for example, the rigorous process carried out in the search for information and current evidence on the subject, and the collection and selection of studies from the described databases (PubMed/Medline, Scopus, Biosis, ProQuest, Web of Science, and Google Scholar)—which allowed us to establish in a broad and precise manner the current evidence of the effectiveness of selenium in terms of its various clinical and/or biochemical outcomes for patients with GBD and GBD orbitopathy.
6. Future Implications
Finally, there is an urgent need to develop RCTs with more robust designs and that involve humans, evaluating the mechanisms by which selenium supplementation could affect the different clinical, biochemical, metabolic, and/or imaging outcomes in patients with GBD and with GBD orbitopathy, at different stages of severity and activity of the disease, as well as the doses to be administered, the duration of treatment, and the effect on the risk of relapse and clinical progression.
It should also be evaluated and clarified in this type of study (RCT) whether the basal selenium status determines the success of selenium supplementation in patients with GBD and/or GBD orbitopathy, especially given the fact that the only two studies that did not show a benefit from such supplementation were those that evaluated the basal selenium status prior to the intervention (and in which its serum concentration was normal). This concept can also be extrapolated to the basal status of other micronutrients involved in a greater susceptibility to the presence of AITD.
Furthermore, because few studies measured selenium levels at baseline or follow-up, it is unknown whether participants in the studies assessed in this review may have had elevated serum selenium concentrations (and whether the doses used were sufficient or supraphysiological); therefore, future studies should focus on maintaining normal serum selenium concentrations (63–159 μg/L) during follow-up, since supraphysiological doses have been associated with outcomes such as hair loss, nausea, vomiting, dermatitis, different types of cancer, and increased mortality [24,26,47].
Additionally, the concomitant use of selenium should also be considered in patients with GBD orbitopathy who are receiving other non-surgical treatments (glucocorticoids, azathioprine, rituximab, mycophenolate mofetil, teprotumumab, tocilizumab, and orbital radiotherapy, inter alia), specifically to determine whether the use of selenium results in a synergistic effect or decreases the need for more aggressive treatments (orbital radiotherapy or surgical management) [48,49].
7. Conclusions
In patients with GBD, selenium supplementation combined with methimazole therapy is significantly associated with an increase in TSH levels and a reduction in FT4, FT3, TPOAb, TgAb, and TRAb levels. These findings were consistent across six clinical scenarios (hyperthyroidism due to GBD, GBD treated with methimazole, newly diagnosed GBD, untreated hyperthyroid patients with GBD, recurring GBD, and patients with GBD after radioactive iodine treatment).
For patients with GBD orbitopathy, selenium supplementation is significantly associated with multiple clinical outcomes (improved quality of life, reduced ocular involvement, and slowed progression of the disease; differences in palpebral fissure, CAS, and eyelid aperture; and an improved early course of GBD orbitopathy), specifically in four clinically possible scenarios (mild GBD orbitopathy with euthyroidism; mild and active GBD orbitopathy; inactive moderate-to-severe GBD orbitopathy; and mild-to-moderate GBD orbitopathy).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medsci13040241/s1, Table S1: PRISMA 2020 Main Checklist.
Author Contributions
Conceptualization, H.V.-U., A.C.-P., I.A.M.-C., M.V.P.-F., K.U.-N. and H.V.-S.; methodology, H.V.-U., A.C.-P., M.V.P.-F. and I.A.M.-C.; investigation, H.V.-U., I.A.M.-C., A.C.-P. and M.V.P.-F.; resources, H.V.-U., I.A.M.-C., K.U.-N. and M.V.P.-F.; data curation, H.V.-U. and I.A.M.-C., K.U.-N., H.V.-S., A.C.-P. and M.V.P.-F.; writing—original draft preparation, H.V.-U., I.A.M.-C., K.U.-N., A.C.-P., H.V.-S. and M.V.P.-F.; writing—review and editing, H.V.-U., A.C.-P., I.A.M.-C. and H.V.-S.; visualization, M.V.P.-F., K.U.-N., I.A.M.-C. and H.V.-S.; supervision, H.V.-U.; project administration, H.V.-U. and M.V.P.-F.; funding acquisition, H.V.-U., A.C.-P. and M.V.P.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from the Colombian Association of Endocrinology, Diabetes, and Metabolism (008-232025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Abend, A.H.; He, I.; Bahroos, N.; Christianakis, S.; Crew, A.B.; Wise, L.M.; Lipori, G.P.; He, X.; Murphy, S.N.; Herrick, C.D.; et al. Estimation of prevalence of autoimmune diseases in the United States using electronic health record data. J. Clin. Investig. 2024, 135, e178722. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee for the Assessment of NIH Research on Autoimmune Diseases. Chapter 2, Background on Autoimmune Diseases. In Enhancing NIH Research on Autoimmune Disease; National Academies Press (USA): Washington, DC, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK605884/ (accessed on 2 May 2025).
- Petranović Ovčariček, P.; Görges, R.; Giovanella, L. Autoimmune Thyroid Diseases. Semin. Nucl. Med. 2024, 54, 219–236. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Gallo, D.; Piantanida, E.; Bartalena, L.; Lai, A.; Zerbinati, N.; Tanda, M.L.; Mortara, L. Regulatory T Cells in the Pathogenesis of Graves’ Disease. Int. J. Mol. Sci. 2023, 24, 16432. [Google Scholar] [CrossRef]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Castellanos-Pinedo, A.; Urrego-Noguera, K.; Pinzón-Fernández, M.V.; Meza-Cabrera, I.A.; Vargas-Sierra, H. A Scoping Review on the Prevalence of Hashimoto’s Thyroiditis and the Possible Associated Factors. Med. Sci. 2025, 13, 43. [Google Scholar]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Castellanos-Pinedo, A.; Meza-Cabrera, I.A.; Pinzón-Fernández, M.V.; Urrego-Noguera, K.; Vargas-Sierra, H. Iodine Intake from Universal Salt Iodization Programs and Hashimoto’s Thyroiditis: A Systematic Review. Diseases 2025, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87 (Suppl. S1), S168–S177. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, C.B.; Muscurel, C.; Duță, C.; Stoian, I. “Alphabet” Selenoproteins: Their Characteristics and Physiological Roles. Int. J. Mol. Sci. 2023, 24, 15992. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J.; Jakob, F.; Contempre, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedus, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Bryliński, Ł.; Kostelecka, K.; Woliński, F.; Komar, O.; Miłosz, A.; Michalczyk, J.; Biłogras, J.; Machrowska, A.; Karpiński, R.; Maciejewski, M.; et al. Effects of Trace Elements on Endocrine Function and Pathogenesis of Thyroid Diseases–A Literature Review. Nutrients 2025, 17, 398. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Chen, P.; Wei, J.; Lv, H.; Wang, S.; Wu, Y.; Zhao, X.; Peng, X.; Rijntjes, E.; et al. Increased Incidence of Hashimoto Thyroiditis in Selenium Deficiency: A Prospective 6–Year Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e3603–e3611. [Google Scholar] [CrossRef]
- Shahidin; Wang, Y.; Wu, Y.; Chen, T.; Wu, X.; Yuan, W.; Zhu, Q.; Wang, X.; Zi, C. Selenium and Selenoproteins: Mechanisms, Health Functions, and Emerging Applications. Molecules 2025, 30, 437. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- Negro, R.; Hegedüs, L.; Attanasio, R.; Papini, E.; Winther, K.H. A 2018 European Thyroid Association Survey on the Use of Selenium Supplementation in Graves’ Hyperthyroidism and Graves’ Orbitopathy. Eur. Thyroid J. 2019, 8, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.H.; Papini, E.; Attanasio, R.; Negro, R.; Hegedüs, L. A 2018 European Thyroid Association Survey on the Use of Selenium Supplementation in Hashimoto’s Thyroiditis. Eur. Thyroid J. 2020, 9, 99–105. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef]
- Fatourechi, V.; Heshmati, H.M. Selenium and Graves’ Disease. Acta Med. Iran. 2024, 62, 6–14. [Google Scholar] [CrossRef]
- Bacić Vrca, V.; Skreb, F.; Cepelak, I.; Mayer, L. Supplementation with antioxidants in the treatment of Graves’ disease: The effect on the extracellular antioxidative parameters. Acta Pharm. 2004, 54, 79–89. [Google Scholar]
- Lai, J. Efficacy of selenious yeast tablets combined with methimidazole in the treatment of graves’ disease. Chin. J. Pharmacoepidemiol. 2014, 23, 472–474. [Google Scholar]
- Calissendorff, J.; Mikulski, E.; Larsen, E.H.; Möller, M. A Prospective Investigation of Graves’ Disease and Selenium: Thyroid Hormones, Auto-Antibodies and Self-Rated Symptoms. Eur. Thyroid J. 2015, 4, 93–98. [Google Scholar] [CrossRef]
- Gong, M.; Wang, A. Clinical study on effect of selenium combining with methimazole in graves’ disease patients with hyperthyroidism. Chin. J. Tradit. Med. Sci. Technol. 2015, 22, 130–132. [Google Scholar]
- Wang, L.; Wang, B.; Chen, S.R.; Hou, X.; Wang, X.F.; Zhao, S.H.; Song, J.Q.; Wang, Y.G. Effect of Selenium Supplementation on Recurrent Hyperthyroidism Caused by Graves’ Disease: A Prospective Pilot Study. Horm. Metab. Res. 2016, 48, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Riedl, M.; König, J.; Diana, T.; Schomburg, L. Double-Blind, Placebo-Controlled, Randomized Trial of Selenium in Graves Hyperthyroidism. J. Clin. Endocrinol. Metab. 2017, 102, 4333–4341. [Google Scholar] [CrossRef]
- Leo, M.; Bartalena, L.; Rotondo Dottore, G.; Piantanida, E.; Premoli, P.; Ionni, I.; Di Cera, M.; Masiello, E.; Sassi, L.; Tanda, M.L.; et al. Effects of selenium on short-term control of hyperthyroidism due to Graves’ disease treated with methimazole: Results of a randomized clinical trial. J. Endocrinol. Investig. 2017, 40, 281–287. [Google Scholar] [CrossRef]
- Tang, H.; Su, H.; Su, L. Observation of TPO-Ab and TRAb in Blood of the Patients with Graves Disease after Iodine 131 Treatment and Selenium Yeast. J. Med. Theory Pract. 2017, 4, 476–478. [Google Scholar]
- Fu, H.; Wang, L. The clinical research of selenium yeast joint methimazole in the treatment for graves disease with hyperthyroidism. Chin. Foreign Med. Res. 2017, 15, 26–27. [Google Scholar]
- Xu, B.; Wu, D.; Ying, H.; Zhang, Y. A pilot study on the beneficial effects of additional selenium supplementation to methimazole for treating patients with Graves’ disease. Turk. J. Med. Sci. 2019, 49, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Mortara, L.; Veronesi, G.; Cattaneo, S.A.; Genoni, A.; Gallazzi, M.; Peruzzo, C.; Lasalvia, P.; Moretto, P.; Bruno, A.; et al. Add-On Effect of Selenium and Vitamin D Combined Supplementation in Early Control of Graves’ Disease Hyperthyroidism During Methimazole Treatment. Front. Endocrinol. 2022, 13, 886451. [Google Scholar] [CrossRef]
- Marcocci, C.; Kahaly, G.J.; Krassas, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. Selenium and the course of mild Graves’ orbitopathy. N. Engl. J. Med. 2011, 364, 1920–1931. [Google Scholar] [CrossRef]
- Almanza-Monterrubio, M.; Garnica-Hayashi, L.; Dávila-Camargo, A.; Nava-Castañeda, Á. Oral selenium improved the disease activity in patients with mild graves’ orbitopathy. J. Fr. Ophtalmol. 2021, 44, 643–651. [Google Scholar] [CrossRef]
- Potita, P.; Pruksakorn, V.; Srichomkwun, P.; Kingpetch, K.; Saonanon, P. Selenium supplementation in inactive moderate to severe Graves’ orbitopathy patients: A randomized controlled trial. Orbit 2024, 43, 329–336. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, J.; Liu, S.; Piao, S.; Zhou, Y.; Hu, Y.; Wan, C.; Sun, Y.; Ning, H.; Chen, L.; et al. Selenium in the treatment of mild-to-moderate Graves’ orbitopathy: A 5-year prospective controlled cohort study. Endocrine 2024, 84, 1072–1080. [Google Scholar] [CrossRef]
- Viola, N.; Colleo, A.; Casula, M.; Mura, C.; Boi, F.; Lanzolla, G. Graves’ Disease: Is It Time for Targeted Therapy? A Narrative Review. Medicina 2025, 61, 500. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Li, S.; Cui, L.; Zhao, J.; Liao, L. Selenium and thyroid diseases. Front. Endocrinol. 2023, 14, 1133000. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. Selenium intake and multiple health-related outcomes: An umbrella review of meta-analyses. Front. Nutr. 2023, 10, 1263853. [Google Scholar] [CrossRef]
- Xue, H.; Wang, W.; Li, Y.; Shan, Z.; Li, Y.; Teng, X.; Gao, Y.; Fan, C.; Teng, W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr. J. 2010, 57, 595–601. [Google Scholar] [CrossRef]
- Verma, S.; Hoffmann, F.W.; Kumar, M.; Huang, Z.; Roe, K.; Nguyen-Wu, E.; Hashimoto, A.S.; Hoffmann, P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J. Immunol. 2011, 186, 2127–2137. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Bastidas, B.; Pinzón, M.V. Population status of selenium in Colombia and associated factors: A cross-sectional study. Horm. Mol. Biol. Clin. Investig. 2022, 44, 153–158. [Google Scholar] [CrossRef]
- Lee, A.C.H.; Kahaly, G.J. Unravelling the pathogenic mechanisms in Graves’ orbitopathy. Eur. Thyroid J. 2025, 14, e250200. [Google Scholar] [CrossRef]
- La Rocca, M.; Leonardi, B.F.; Lo Greco, M.C.; Marano, G.; Milazzotto, R.; Liardo, R.L.E.; Acquaviva, G.; La Monaca, V.A.; Salamone, V.; Basile, A.; et al. Orbital Radiotherapy for Graves’ Ophthalmopathy: Single Institutional Experience of Efficacy and Safety. Diseases 2025, 13, 61. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).