Incidence and Survival of IDH-Wildtype Glioblastoma and IDH-Mutant Astrocytoma by Treatment and Sex: A Regional Study in Spain (2011–2021)
Abstract
1. Introduction
2. Methods
2.1. Ethical Considerations
2.2. Patient Selection
2.3. Clinical and Demographic Characteristics
2.4. Survival and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. IDH-Wildtype Glioblastoma Results
3.1.1. Clinical and Demographic Characteristics
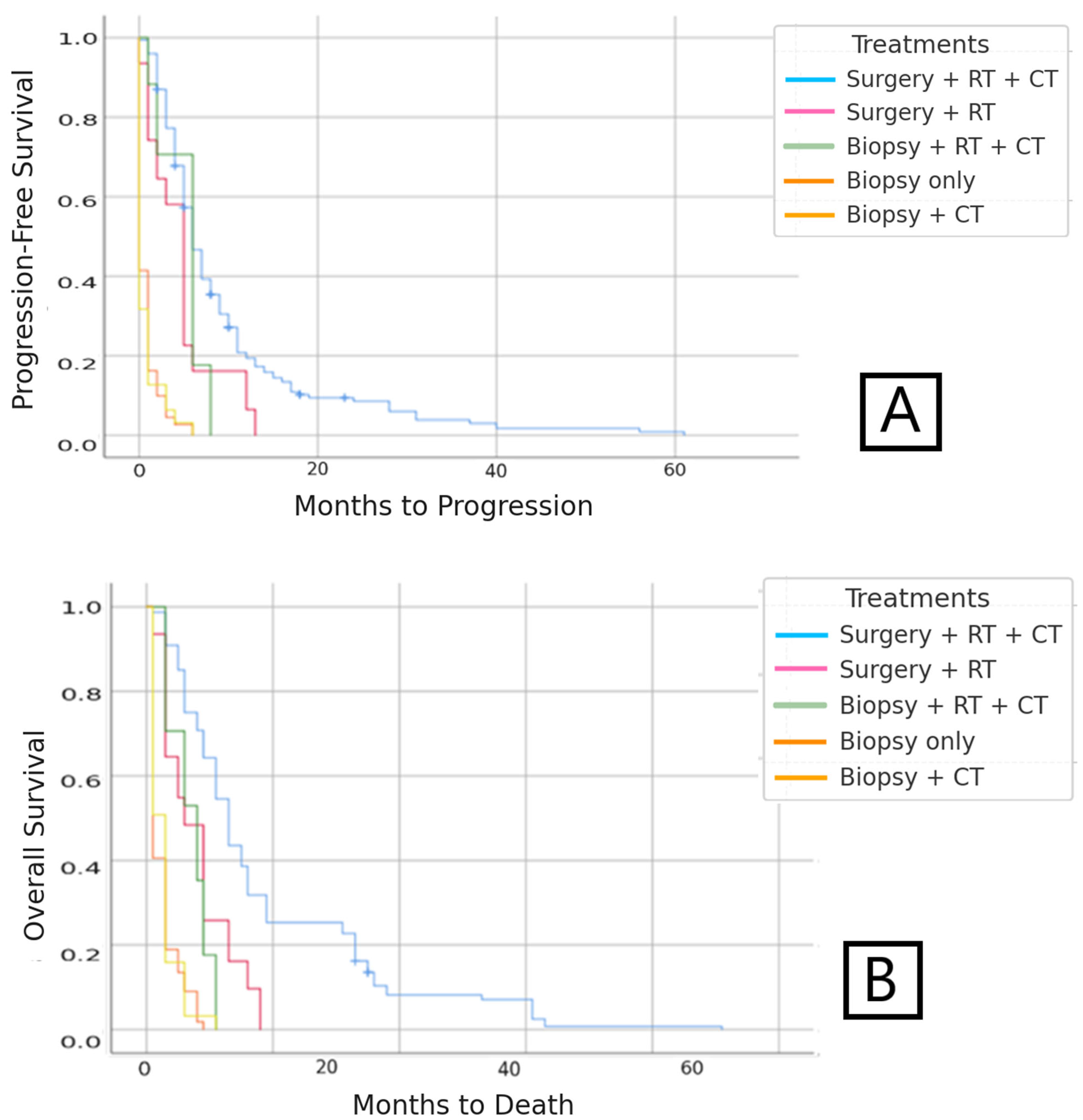
3.1.2. Progression-Free Survival (PFS)
3.1.3. Overall Survival (OS)
3.2. IDH-Mutant Astrocytoma Results
3.2.1. Clinical and Demographic Characteristics
3.2.2. Progression-Free Survival (PFS)
3.2.3. Overall Survival (OS)
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Institutional Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohler, B.A.; Ward, E.; McCarthy, B.J.; Schymura, M.J.; Ries, L.A.G.; Eheman, C.; Jemal, A.; Anderson, R.N.; Ajani, U.A.; Edwards, B.K. Annual Report to the Nation on the Status of Cancer, 1975–2007, Featuring Tumors of the Brain and Other Nervous System. JNCI J. Natl. Cancer Inst. 2011, 103, 714–736. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96, Erratum in: Neuro Oncol. 2022, 24, 1214. [Google Scholar] [CrossRef]
- Galceran, J.; Ameijide, A.; Carulla, M.; Mateos, A.; Quirós, J.R.; Rojas, D.; Aleman, A.; Torrella, A.; Chico, M.; Vicente, M.; et al. Cancer incidence in Spain, 2015. Clin. Transl. Oncol. 2017, 19, 799–825. [Google Scholar] [CrossRef] [PubMed]
- de Oncología Médica, S.S.E. Sociedad Española de Oncologia Médica. In Cifras del Cancer en España; Biblioteca Virtual em Saude: São Paulo, Brazil, 2020; 36p. [Google Scholar]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System tumours: A practical update on what neurosurgeons need to know-a minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wrensch, M.; Minn, Y.; Chew, T.; Bondy, M.; Berger, M.S. Epidemiology of primary brain tumors: Current concepts and review of theliterature. Neuro Oncol. 2002, 4, 278–299. [Google Scholar] [CrossRef]
- Davis, F.G.; Freels, S.; Grutsch, J.; Barlas, S.; Brem, S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J. Neurosurg. 1998, 88, 1–10. [Google Scholar] [CrossRef]
- Las Cifras del Cáncer en España. 2022. Available online: https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf (accessed on 15 January 2025).
- Sociedad Española de Oncología Médica (SEOM). Las Cifras del Cáncer en España 2017; Depósito legal: M-2172-2017;SEOM: Madrid, 2017. Available online: https://www.seom.org/seomcms/images/stories/recursos/Las_cifras_del_cancer_en_Esp_2017.pdf (accessed on 15 January 2025).
- Las Cifras del Cáncer en España. 2023. Available online: https://seom.org/images/Las_cifras_del_Cancer_en_Espana_2023.pdf (accessed on 15 January 2025).
- Radhakrishnan, K.; Mokri, B.; Parisi, J.E.; O’FAllon, W.M.; Sunku, J.; Kurland, L.T. The trends in incidence of primary brain tumors in the population of rochester, minnesota. Ann. Neurol. 1995, 37, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Polednak, A.P. Interpretation of Secular Increases in Incidence Rates for Primary Brain Cancer in Connecticut Adults, 1965–1988. Neuroepidemiology 1996, 15, 51–56. [Google Scholar] [CrossRef]
- Wen, P.Y.; Fine, H.A.; Black, P.M.; Shrieve, D.C.; Alexander, E.; Loeffer, J.S. High-grade astrocytomas. Neurol. Clin. 1995, 13, 875–900. [Google Scholar] [CrossRef]
- Gao, J.; Gu, D.; Yang, K.; Zhang, J.; Lin, Q.; Yuan, W.; Zhu, X.; Dixit, D.; Gimple, R.C.; You, H.; et al. Infiltrating plasma cells maintain glioblastoma stem cells through IgG-Tumor binding. Cancer Cell 2025, 43, 122–143.e8. [Google Scholar] [CrossRef] [PubMed]
- Epidemiología de Tumores Cerebrales. 2017. Available online: https://www.elsevier.es/es-revista-revista-medica-clinica-las-condes-202-articulo-epidemiologia-de-tumores-cerebrales-S0716864017300585 (accessed on 15 January 2025).
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma multiforme: Insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; A Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017-2021. Neuro Oncol. 2024, 26 (Suppl. S6), vi1–vi85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aibaidula, A.; Chan, A.K.-Y.; Shi, Z.; Li, Y.; Zhang, R.; Yang, R.; Li, K.K.-W.; Chung, N.Y.-F.; Yao, Y.; Zhou, L.; et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017, 19, 1327–1337. [Google Scholar] [CrossRef]
- Aoki, K.; Nakamura, H.; Suzuki, H.; Matsuo, K.; Kataoka, K.; Shimamura, T.; Motomura, K.; Ohka, F.; Shiina, S.; Yamamoto, T.; et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018, 20, 66–77. [Google Scholar] [CrossRef]
- Reuss, D.E.; Kratz, A.; Sahm, F.; Capper, D.; Schrimpf, D.; Koelsche, C.; Hovestadt, V.; Bewerunge-Hudler, M.; Jones, D.T.W.; Schittenhelm, J.; et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015, 130, 407–417. [Google Scholar] [CrossRef]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hänggi, D.; Wick, W.; et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Fresnedo, A.; Pullen, M.W.; Perez-Vega, C.; Domingo, R.A.; Akinduro, O.O.; Almeida, J.P.; Suarez-Meade, P.; Marenco-Hillembrand, L.; Jentoft, M.E.; Bendok, B.R.; et al. The survival outcomes of molecular glioblastoma IDH-wildtype: A multicenter study. J. Neurooncol. 2022, 157, 177–185. [Google Scholar] [CrossRef]
- Chen, L.; Guerrero-Cazares, H.; Ye, X.; Ford, E.; McNutt, T.; Kleinberg, L.; Lim, M.; Chaichana, K.; Quinones-Hinojosa, A.; Redmond, K. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int. J. Radiat. Oncol. 2013, 86, 616–622. [Google Scholar] [CrossRef]
- Kim, K.H.; Yoo, J.; Kim, N.; Moon, J.H.; Byun, H.K.; Kang, S.-G.; Chang, J.H.; Yoon, H.I.; Suh, C.-O. Efficacy of whole-ventricular radiotherapy in patients undergoing maximal tumor resection for glioblastomas involving the ventricle. Front. Oncol. 2021, 11, 736482. [Google Scholar] [CrossRef]
- Gandhi, M.J.; Strong, D.M. Donor derived malignancy following transplantation: A review. Cell Tissue Bank. 2007, 8, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne, P.; Soler, C.; Mosnier, J.-F. Diffuse vertebral body metastasis from a glioblastoma multiforme: A technetium-99m Sestamibi single-photon emisión computerized tomography study: Case report. J. Neurosurg. 2000, 93, 887–890. [Google Scholar] [CrossRef]
- Didelot, A.; Taillandier, L.; Grignon, Y.; Vespignani, H.; Beauchesne, P. Concomitant bone marrow metastasis of a glioblastoma multiforme revealed at the diagnosis. Acta Neurochir. 2006, 148, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012, 14 (Suppl. S5), v1–v49. [Google Scholar] [CrossRef]
- Deorah, S.; Lynch, C.F.; Sibenaller, Z.A.; Ryken, T.C. Trends in brain cancer incidence and survival in the United States: Surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg. Focus 2006, 20, E1. [Google Scholar] [CrossRef]
- Korja, M.; Raj, R.; Seppä, K.; Luostarinen, T.; Malila, N.; Seppälä, M.; Mäenpää, H.; Pitkäniemi, J. Glioblastoma survival is improving despite increasing incidence rates: A nationwide study between 2000 and 2013 in Finland. Neuro Oncol. 2019, 21, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.P.; Harrison, R.A.; Lapointe, S.; Lim-Fat, M.J.; MacNeil, M.V.; Mathieu, D.; Perry, J.R.; Pitz, M.W.; Roberge, D.; Tsang, D.S.; et al. Canadian Expert Consensus Recommendations for the Diagnosis and Management of Glioblastoma: Results of a Delphi Study. Curr. Oncol. 2025, 32, 207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Encarnación, J.A.; Hernández, A.L.; López, G.L.; Alonso-Romero, J.L.; De la Fuente Muñoz, M.I.; Cánovas, E.C.; Carreño, P.R.; Royo-Villanova, M.; Manso, C. Probable chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids syndrome: Management with corticosteroids and intravenous immunoglobulin—A case report. J. Med. Case Rep. 2025, 19, 351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myung, S.-K.; Ju, W.; McDonnell, D.D.; Lee, Y.J.; Kazinets, G.; Cheng, C.-T.; Moskowitz, J.M. Mobile phone use and risk of tumors: A meta-analysis. J. Clin. Oncol. 2009, 27, 5565–5572. [Google Scholar] [CrossRef]
- Philips, A.; Henshaw, D.L.; Lamburn, G.; O’cArroll, M.J. Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995–2015 Suggests an Adverse Environmental or Lifestyle Factor. J. Environ. Public Health 2018, 2018, 7910754. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, J.; He, Y.; Li, X.; Yin, S.; Chen, F.; Li, W. Association Between Dietary Nitrite intake and Glioma Risk: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Front. Oncol. 2022, 12, 910476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesa, J.A.E.; Güiza, L.V.F.; Romero, C.M.; Vela, J.S. Relationship between the incidence of glioblastoma and natural radiation. Neurocirugia, 2025; 500711, in press. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Prasad, R.N.; Cioffi, G.; Kruchtko, C.; Zaorsky, N.G.; Trifiletti, D.M.; Gondi, V.; Brown, P.D.; Perlow, H.K.; Mishra, M.V.; et al. Exposure to radon and heavy particulate pollution and incidence of brain tumors. Neuro Oncol. 2023, 25, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Encarnación Navarro, J.A. Donación de Órganos en Pacientes con Tumor Maligno Primario Cerebral: Posibles Donantes en la Región de Murcia. Análisis De los Registros Internacionales y Nacionales. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2022. [Google Scholar]
- Encarnación, J.A.; Coll, E.; Manso, C.; Llorente, S.; Morales, F.; Saura, I.; López-Cubillana, P.; Martínez-Valls, P.L.G.; Martínez, G.; De la Fuente, I.; et al. Outcomes of Kidney Transplantation from Deceased Donors with Severe Acute Kidney Injury (AKIN Stage 3): A Preliminary Single-Centre Analysis. Med. Sci. 2025, 13, 188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Percentage | IDH-Mutant Astrocytoma Age (Years) | Percentage |
|---|---|---|
| 18–30 | 18–30 | 6.5 |
| 30–40 | 30–40 | 14.7 |
| 40–60 | 40–60 | 34.7 |
| >60 | >60 | 44.1 |
| Number of Patients | Percentage | Progression-Free Survival (Months) | Overall Survival (Months) | |||
|---|---|---|---|---|---|---|
| Surgery + CT + RT | 309 | 58.30% | 9.493 | 18.645 | ||
| Surgery + RT | 28 | 5.28% | 4.742 | 7.871 | ||
| Biopsy only | 18 | 3.40% | 0.775 | p < 0.001 | 2.523 | p < 0.001 |
| Biopsy + RT + CT | 115 | 21.70% | 5.059 | 6.882 | ||
| Biopsy + CT | 60 | 11.32% | 0.698 | 2.651 |
| Total N | Percentage | Progression-Free Survival (Months) | Overall Survival (Months) | |||
|---|---|---|---|---|---|---|
| Surgery + RT + CT | 101 | 73.72 | 34.255 | 50.287 | ||
| Biopsy + RT + CT | 12 | 8.76 | 8.250 | p < 0.001 | 12.000 | p < 0.001 |
| Biopsy only | 24 | 17.52 | 0.556 | 1.875 | ||
| Overall | 137 | 100 | 25.068 | 38.453 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnación, J.A.; Manso, C.; Royo-Villanova, M.; Ruiz, P.; De la Fuente, M.I.; Cárdenas, E.; Ros, S.; Alonso-Romero, J.L. Incidence and Survival of IDH-Wildtype Glioblastoma and IDH-Mutant Astrocytoma by Treatment and Sex: A Regional Study in Spain (2011–2021). Med. Sci. 2025, 13, 233. https://doi.org/10.3390/medsci13040233
Encarnación JA, Manso C, Royo-Villanova M, Ruiz P, De la Fuente MI, Cárdenas E, Ros S, Alonso-Romero JL. Incidence and Survival of IDH-Wildtype Glioblastoma and IDH-Mutant Astrocytoma by Treatment and Sex: A Regional Study in Spain (2011–2021). Medical Sciences. 2025; 13(4):233. https://doi.org/10.3390/medsci13040233
Chicago/Turabian StyleEncarnación, J. A., C. Manso, M. Royo-Villanova, P. Ruiz, M. I. De la Fuente, E. Cárdenas, S. Ros, and J. L. Alonso-Romero. 2025. "Incidence and Survival of IDH-Wildtype Glioblastoma and IDH-Mutant Astrocytoma by Treatment and Sex: A Regional Study in Spain (2011–2021)" Medical Sciences 13, no. 4: 233. https://doi.org/10.3390/medsci13040233
APA StyleEncarnación, J. A., Manso, C., Royo-Villanova, M., Ruiz, P., De la Fuente, M. I., Cárdenas, E., Ros, S., & Alonso-Romero, J. L. (2025). Incidence and Survival of IDH-Wildtype Glioblastoma and IDH-Mutant Astrocytoma by Treatment and Sex: A Regional Study in Spain (2011–2021). Medical Sciences, 13(4), 233. https://doi.org/10.3390/medsci13040233






