Evaluation of Platelet Indices and Reticulated Platelets Using the ADVIA 2120 Analyzer in Patients with Acute Infection or Acute Coronary Syndrome, at Onset
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Inclusion Criteria
2.3. Sample Collection
2.4. Analytical Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhou, P.; Zhang, H. From reticulated platelets to immature platelet fraction: Structure, function, and clinical applications. Platelets 2025, 36, 2467383. [Google Scholar] [CrossRef] [PubMed]
- Hille, L.; Lenz, M.; Vlachos, A.; Grüning, B.; Hein, L.; Neumann, F.-J.; Nührenberg, T.G.; Trenk, D. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J. Thromb. Haemost. 2020, 18, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Bodrova, S.V.V.; On, K.S.; Mazurov, A.V.; Mazurov, A.V. Platelet reticulated forms, size indexes and functional activity. Interactions in healthy volunteers. Platelets 2022, 33, 398–403. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagasaki, M.; Kunishima, S.; Sawaguchi, A.; Sakata, A.; Sakaguchi, H.; Ohmori, T.; Manabe, I.; Italiano, J.E., Jr.; Ryu, T.; et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J. Cell Biol. 2015, 209, 453–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Corpataux, N.; Franke, K.; Kille, A.; Valina, C.M.; Neumann, F.J.; Nührenberg, T.; Hochholzer, W. Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. J. Clin. Med. 2020, 9, 3737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Josefsson, E.C.; Vainchenker, W.; James, C. Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases. Int. J. Mol. Sci. 2020, 21, 7591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meintker, L.; Krause, S.W. Krause Reticulated platelets—Clinical application and future perspectives. J. Lab. Med. 2020, 44, 241–253. [Google Scholar] [CrossRef]
- Ittermann, T.; Feig, M.A.; Petersmann, A.; Radke, D.; Greinacher, A.; Völzke, H.; Thiele, T. Mean platelet volume is more important than age for defining reference intervals of platelet counts. PLoS ONE 2019, 14, e0213658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ault, K.A.; Rinder, H.M.; Mitchell, J.; Carmody, M.B.; Vary, C.P.; Hillman, R.S. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am. J. Clin. Pathol. 1992, 98, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Weiler, K.; Gibbs, G.; Prechtl, G.; Bauer, N.; Moritz, A. Evaluation of a novel moving threshold gating strategy for assessment of reticulated platelets in dogs using the ADVIA 2120 analyzer. Vet. Clin. Pathol. 2023, 52, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.J.; van den Broek, N.M.; Curvers, J. Reference intervals of reticulated platelets and other platelet parameters and their associations. Arch. Pathol. Lab. Med. 2013, 137, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Takagi, Y.; Kono, M.; Morikawa, T. Accuracy of a New Platelet Count System (PLT-F) Depends on the Staining Property of Its Reagents. PLoS ONE 2015, 10, e0141311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buttarello, M.; Mezzapelle, G.; Freguglia, F.; Plebani, M. Reticulated platelets and immature platelet fraction: Clinical applications and method limitations. Int. J. Lab. Hematol. 2020, 42, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bernlochner, I.; Klug, M.; Larasati, D.; Von Scheidt, M.; Santovito, D.; Hristov, M.; Weber, C.; Laugwitz, K.L.; Bongiovanni, D. Sorting and magnetic-based isolation of reticulated platelets from peripheral blood. Platelets 2021, 32, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Snyder, A.; Han, X.; Sokol, S.; Pettit, N.; Howell, M.D.; Edelson, D.P. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2017, 195, 906–911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367, Erratum in Eur. Heart J. 2021, 42, 1908. https://doi.org/10.1093/eurheartj/ehaa895; Erratum in Eur. Heart J. 2021, 42, 1925. https://doi.org/10.1093/eurheartj/ehab088; Erratum in Eur. Heart J. 2021, 42, 2298. https://doi.org/10.1093/eurheartj/ehab285; Erratum in Eur. Heart J. 2024, 45, 404–405. https://doi.org/10.1093/eurheartj/ehad879. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, A.; Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Ciavarella, D.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Serio, F.; et al. New Insights in Laboratory Testing for COVID-19 Patients: Looking for the Role and Predictive Value of Human epididymis secretory protein 4 (HE4) and the Innate Immunity of the Oral Cavity and Respiratory Tract. Microorganisms 2020, 8, 1718. [Google Scholar] [CrossRef]
- Türkmen, D.; Özsoylu, S.; Akyıldız, B.N. Comparison of the value of immature retyculocyte and immature platelet in the diagnosıs of sepsis. Pediatr. Int. 2022, 64, e14882. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, D.; Han, J.; Klug, M.; Kirmes, K.; Viggiani, G.; von Scheidt, M.; Schreiner, N.; Condorelli, G.; Laugwitz, K.L.; Bernlochner, I. Role of Reticulated Platelets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 527–539. [Google Scholar] [CrossRef] [PubMed]
- De Blasi, R.A.; Cardelli, P.; Costante, A.; Sandri, M.; Mercieri, M.; Arcioni, R. Immature platelet fraction in predicting sepsis in critically ill patients. Intensiv. Care Med. 2013, 39, 636–643. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From hemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, A.; Garzia, M.; Leone, F.; Arcangeli, A.; Pagano, L.; Zini, G. Immature platelet fraction (IPF) in hospitalized patients with neutrophilia and suspected bacterial infection. J. Infect. 2009, 59, 201–206. [Google Scholar] [CrossRef]
- Muronoi, T.; Koyama, K.; Nunomiya, S.; Lefor, A.K.; Wada, M.; Koinuma, T.; Shima, J.; Suzukawa, M. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb. Res. 2016, 144, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Katayama, S.; Muronoi, T.; Tonai, K.; Goto, Y.; Koinuma, T.; Shima, J.; Nunomiya, S. Time course of immature platelet count and its relation to thrombocytopenia and mortality in patients with sepsis. PLoS ONE 2018, 13, e0192064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Becker, M.; Bauer, N.; Moritz, A. Automated flow cytometric cell count and differentiation of canine cerebrospinal fluid cells using the ADVIA 2120. Vet. Clin. Pathol. 2008, 37, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Budak, Y.U.; Polat, M.; Huysal, K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: A systematic review. Biochem. Medica 2016, 26, 178–193. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Wang, L.; Shi, Q. Mean platelet volume (MPV) and platelet distribution width (PDW) predict clinical outcome of acute ischemic stroke: A systematic review and meta-analysis. J. Clin. Neurosci. 2022, 101, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, L.; Zhang, Q.; Zhou, L.; Liao, R.; Wu, A.; Wang, X.; Luo, J.; Huang, F.; Zou, W.; et al. Interleukins in Platelet Biology: Unraveling the Complex Regulatory Network. Pharmaceutical 2024, 17, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamad, M.A.; Schanze, N.; Schommer, N.; Nührenberg, T.; Duerschmied, D. Reticulated Platelets-Which Functions Have Been Established by In Vivo and In Vitro Data? Cells 2021, 10, 1172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magrini, L.; Gagliano, G.; Travaglino, F.; Vetrone, F.; Marino, R.; Cardelli, P.; Salerno, G.; Di Somma, S. Comparison between white blood cell count, procalcitonin and C reactive protein as diagnostic and prognostic biomarkers of infection or sepsis in patients presenting to emergency department. Clin. Chem. Lab. Med. 2014, 52, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Pogorzelska, K.; Krętowska, A.; Krawczuk-Rybak, M.; Sawicka-Żukowska, M. Characteristics of platelet indices and their prognostic significance in selected medical condition—A systematic review. Adv. Med. Sci. 2020, 65, 310–315. [Google Scholar] [CrossRef]
- Goel, G.; Semwal, S.; Khare, A.; Joshi, D.; Amerneni, C.K.; Pakhare, A.; Kapoor, N. Immature platelet fraction: Its clinical utility in thrombocytopenia patients. J. Lab. Physicians 2021, 13, 214–218. [Google Scholar] [CrossRef]
- Gargiulo, G.; Cirillo, P.; Sperandeo, L.; Castiello, D.S.; Manzi, L.; Forzano, I.; Florimonte, D.; Simonetti, F.; Canonico, M.E.; Avvedimento, M.; et al. Pharmacodynamic effects of cangrelor in patients with acute or chronic coronary syndrome undergoing percutaneous coronary intervention: The POMPEII Registry. EuroIntervention 2025, 21, 560–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beard, M.J.; Jeewa, Z.; Bashir, S.; Cardigan, R.; Thomas, S. Comparison of platelet activation in platelet concentrates measured by flow cytometry or ADVIA 2120. Vox Sang. 2011, 101, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Cesari, F.; Marcucci, R.; Gori, A.M.; Caporale, R.; Fanelli, A.; Casola, G.; Balzi, D.; Barchielli, A.; Valente, S.; Giglioli, C.; et al. Reticulated platelets predict cardiovascular death in acute coronary syndrome patients. Insights from the AMI-Florence 2 Study. Thromb. Haemost. 2013, 109, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, B.; Hannawi, Y.; Kleiman, N.S. Reticulated Platelets: Changing Focus from Basics to Outcomes. Thromb. Haemost. 2018, 118, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Eslick, R.; McLintock, C. Managing ITP and thrombocytopenia in pregnancy. Platelets 2020, 31, 300–306. [Google Scholar] [CrossRef]
- Everett, T.R.; Garner, S.F.; Lees, C.C.; Goodall, A.H. Immature platelet fraction analysis demonstrates a difference in thrombopoiesis between normotensive and preeclamptic pregnancies. Thromb. Haemost. 2014, 111, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.; Munhoz, T.P.; Pinheiro da Costa, B.E.; Hentschke, M.R.; Sontag, F.; Silveira Lucas, L.; Gadonski, G.; Antonello, I.C.; Poli-de-Figueiredo, C.E. Immature platelet fraction in hypertensive pregnancy. Platelets 2016, 27, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Surti, J.; Jain, I.; Shah, K.; Mishra, A.; Kandre, Y.; Garg, P.; Shah, J.; Shah, A.; Tripathi, P. Predictive efficacy of procalcitonin, platelets, and white blood cells for sepsis in pediatric patients undergoing cardiac surgeries who are admitted to intensive care units: Single-center experience. Ann. Pediatr. Cardiol. 2018, 11, 137–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolny, M.; Dittrich, M.; Knabbe, C.; Birschmann, I. Immature platelets in COVID-19. Platelets 2023, 34, 2184183. [Google Scholar] [CrossRef]
- Cohen, A.; Harari, E.; Yahud, E.; Cipok, M.; Bryk, G.; Lador, N.K.; Mann, T.; Mayo, A.; Lev, E.I. Immature platelets in patients with COVID-19: Association with disease severity. J. Thromb. Thrombolysis 2021, 52, 708–714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balmakov, Y.; Mark, T.; Barnett, I.; Cipok, M.; Lev, E.I.; Cohen, A.; Aviram, E.; Mayo, A. Immature Platelets and Platelet Reactivity in Patients with COVID-19. Clin. Appl. Thromb. Hemost. 2025, 31, 10760296251318320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thorup, C.V.; Christensen, S.; Hvas, A.M. Immature Platelets As a Predictor of Disease Severity and Mortality in Sepsis and Septic Shock: A Systematic Review. Semin. Thromb. Hemost. 2020, 46, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ha, S.O.; Cho, Y.U.; Park, C.J.; Jang, S.; Hong, S.B. Immature platelet fraction in septic patients: Clinical relevance of immature platelet fraction is limited to the sensitive and accurate discrimination of septic patients from non-septic patients, not to the discrimination of sepsis severity. Ann. Lab. Med. 2016, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dusse, L.M.; Freitas, L.G. Clinical applicability of reticulated platelets. Clin. Chim. Acta 2015, 439, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Song, M.Y.; Yang, B.X.; Xia, R.X. Clinical significance of measuring reticulated platelets in infectious diseases. Medicine 2017, 96, e9424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffin, M.M.; Penfield, C.A.; Hausvater, A.; Schaap, A.; Roman, A.S.; Xia, Y.; Gossett, D.R.; Quinn, G.P.; Berger, J.S. The relationship between platelet indices and hypertensive disorders of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 308, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.A.; Krauel, K.; Schanze, N.; Gauchel, N.; Stachon, P.; Nuehrenberg, T.; Zurek, M.; Duerschmied, D. Platelet Subtypes in Inflammatory Settings. Front. Cardiovasc. Med. 2022, 9, 823549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meintker, L.; Haimerl, M.; Ringwald, J.; Krause, S.W. Measurement of immature platelets with Abbott CD-Sapphire and Sysmex XE-5000 in haematology and oncology patients. Clin. Chem. Lab. Med. 2013, 51, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
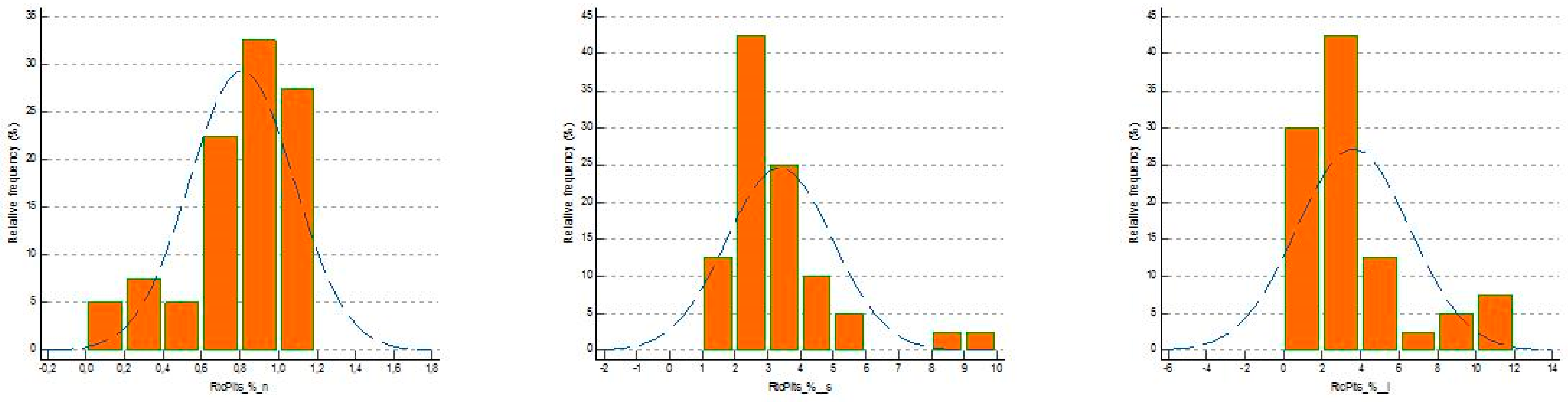
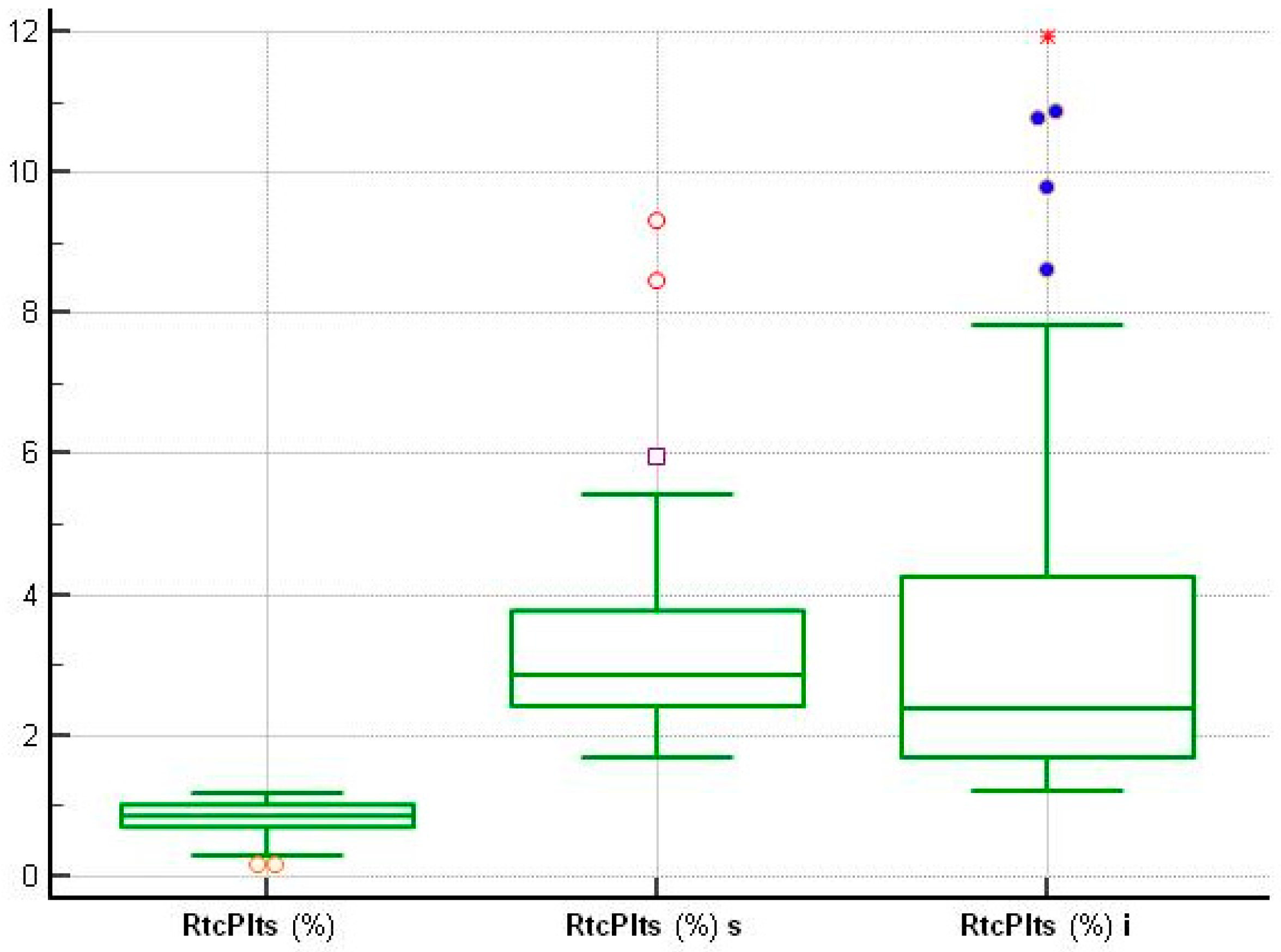

| PLT ×103/µL | RtcPlts % | MPC g/dL | MPM pg/dL | MPV fL | WBC ×103/µL | NG % | PSP pg/mL | Pct ng/mL | CRP mg/L | hs cTnI ng/L | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Minimum | 155.70 | 0.14 | 18.72 | 1.64 | 6.30 | 4.06 | 44.55 | 18.9 | 0.01 | 0.18 | 8.0 |
| Maximum | 342.0 | 1.16 | 28.10 | 2.46 | 10.10 | 8.55 | 71.30 | 48.00 | 0.03 | 1.40 | 25.0 |
| Mean | 231.37 | 0.81 | 25.06 | 2.00 | 8.22 | 6.46 | 57.51 | 33.82 | 0.01 | 0.77 | 14.60 |
| 95% CI | 216.38 to 246.34 | 0.72 to 0.89 | 24.44 to 25.66 | 1.93 to 2.06 | 7.93 to 8.49 | 6.04 to 6.88 | 55.9 to 59.63 | 31.36 to 36.28 | 0.011 to 0.02 | 0.67 to 0.86 | 13.23 to 15.96 |
| Median | 217.0 | 0.86 | 25.25 | 1.98 | 8.24 | 6.51 | 57.90 | 35.00 | 0.01 | 0.81 | 15.0 |
| 95% CI | 213.38 to 238.66 | 0.78 to 0.96 | 24.03 to 26.36 | 1.91 to 2.09 | 7.81 to 8.66 | 5.85 to 7.11 | 54.18 to 60.20 | 31.50 to 36.90 | 0.01 to 0.02 | 0.61 to 0.90 | 12.67 to 15.0 |
| Normal Distribution | 0.024 | 0.030 | 0.040 | 0.877 | 1.0 | 0.584 | 0.257 | 0.235 | <0.0001 | 0.202 | 0.009 |
| PLT-s ×103 µL | RtcPlts-s % | MPC-s g/dL | MPM-s pg | MPV-s fL | WBC-s ×103/µL | NG-s % | Pct-s ng/mL | PSP-s pg/mL | CRP-s mg/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Minimum | 36.0 | 1.68 | 21.10 | 1.72 | 8.30 | 1.40 | 23.3 | 0.48 | 407 | 12.60 |
| Maximum | 479.0 | 9.30 | 28.38 | 3.19 | 15.62 | 39.41 | 99.29 | 54.01 | 22,000 | 328.90 |
| Mean | 187.73 | 3.36 | 25.37 | 2.40 | 10.84 | 15.66 | 84.89 | 4.97 | 2921.15 | 97.49 |
| 95% CI | 150.75 to 224.69 | 2.84 to 3.88 | 24.76 to 25.981 | 2.28 to 2.51 | 10.23 to 11.45 | 13.50 to 17.83 | 79.65 to 90.12 | 1.41 to 8.54 | 1507.78 to 4334.51 | 76.19 to 118.79 |
| Median | 154.00 | 2.85 | 25.25 | 2.37 | 10.32 | 15.56 | 87.15 | 1.78 | 1522.50 | 83.80 |
| 95% CI | 111.01 to 236.25 | 2.53 to 3.45 | 24.71 to 26.21 | 2.25 to 2.54 | 9.93 to 11.07 | 14.46 to 16.63 | 85.02 to 92.16 | 1.29 to 2.34 | 1096.62 to 2297.06 | 71.10 to 114.44 |
| Normal Distribution | 0.0525 | 0.0028 | 0.2193 | 0.7917 | 0.0317 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | 0.0733 |
| Control Group | Patients with Suspected Sepsis | Two-Tailed Probability |
|---|---|---|
| RtcPlts | RtcPlts-s | p < 0.0001 |
| MPC | MPC-s | p = 0.3681 |
| MPM | MPM-s | p < 0.0001 |
| MPV | MPV-s | p < 0.0001 |
| PLT | PLT-s | p = 0.0130 |
| WBC | WBC-s | p < 0.0001 |
| NG | NG-s | p < 0.0001 |
| CRP | CRP-s | p < 0.0001 |
| Pct | Pct-s | p < 0.0001 |
| PSP | PSP-s | p < 0.0001 |
| Parameters | AUC | 95% CI |
|---|---|---|
| PLT-s | 0.66 | 0.52 to 0.79 |
| MPV-s | 0.93 | 0.88 to 0.98 |
| MPM-s | 0.84 | 0.74 to 0.92 |
| MPC-s | 0.56 | 0.42 to 0.68 |
| RtcPlts-s | 0.96 | 0.90 to 1.00 |
| Variable | Sensitivity | Specificity | Best Cutoff Values |
|---|---|---|---|
| MPV-s | 87.5 | 87.5 | 9.1 |
| MPV-i | 77.5 | 90.0 | 9.2 |
| MPC-s | 27.5 | 92.5 | 27.1 |
| MPC-i | 82.5 | 40.0 | 24.03 |
| MPM-s | 65.0 | 95.0 | 2.27 |
| MPM-i | 65.0 | 90.0 | 2.21 |
| RtcPlts-s | 95.0 | 100 | 1.15 |
| RtcPlts-i | 95.0 | 100 | 1.16 |
| PLT-i ×103 µL | RtcPlts-i % | MPC-i g/dL | MPM-i pg | MPV-i fL | hs cTnI -i ng/L | |
|---|---|---|---|---|---|---|
| Number | 40 | 40 | 40 | 40 | 40 | 40 |
| Minimum | 36.0 | 1.21 | 18.70 | 1.79 | 7.80 | 217 |
| Maximum | 547.0 | 11.95 | 29.59 | 3.38 | 18.37 | 104,883 |
| Mean | 183.58 | 3.62 | 25.40 | 2.38 | 10.83 | 16,596.53 |
| 95% CI | 147.01 to 220.13 | 2.68 to 4.56 | 24.60 to 26.19 | 2.26 to 2.49 | 10.04 to 11.61 | 6875.36 to 26,317.68 |
| Median | 177.50 | 2.38 | 25.55 | 2.29 | 10.23 | 3291.50 |
| 95% CI | 127.37 to 240.66 | 2.11 to 3.20 | 24.70 to 26.79 | 2.19 to 2.43 | 9.36 to 11.07 | 1130.84 to 7334.81 |
| Normal Distribution | 0.0958 | <0.0001 | 0.0446 | 0.1204 | 0.0024 | <0.0001 |
| Control Group | Patients with ACS | Two-Tailed Probability |
|---|---|---|
| RtcPlts | RtcPlts-i | p < 0.0001 |
| MPC | MPC-i | p = 0.1447 |
| MPM | MPM-i | p < 0.0001 |
| MPV | MPV-i | p < 0.0001 |
| PLT | PLT-i | p = 0.0189 |
| hs cTnI | hs cTnI-i | p < 0.0001 |
| Variable | AUC | 95% CI |
|---|---|---|
| PLT-i | 0.66 | 0.52 to 0.78 |
| MPV-i | 0.90 | 0.84 to 0.96 |
| MPC-i | 0.59 | 0.46 to 0.72 |
| MPM-i | 0.84 | 0.75 to 0.92 |
| RtcPlts-i | 0.95 | 0.89 to 1.00 |
| Patients with ACS | Patients with Suspected Sepsis | Two-Tailed Probability |
|---|---|---|
| RtcPlts-i | RtcPlts-s | p = 0.1321 |
| MPC-i | MPC-s | p = 0.7727 |
| MPM-i | MPM-s | p = 0.5252 |
| MPV-i | MPV-s | p = 0.5832 |
| PLT-i | PLT-s | p = 0.8398 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brescia, V.; Mileti, A.; Lovero, R.; Varraso, L.; Pignataro, F.; Di Serio, F.; Cazzolla, A.P.; Santacroce, L.; Bizzoca, M.E.; Crincoli, V.; et al. Evaluation of Platelet Indices and Reticulated Platelets Using the ADVIA 2120 Analyzer in Patients with Acute Infection or Acute Coronary Syndrome, at Onset. Med. Sci. 2025, 13, 232. https://doi.org/10.3390/medsci13040232
Brescia V, Mileti A, Lovero R, Varraso L, Pignataro F, Di Serio F, Cazzolla AP, Santacroce L, Bizzoca ME, Crincoli V, et al. Evaluation of Platelet Indices and Reticulated Platelets Using the ADVIA 2120 Analyzer in Patients with Acute Infection or Acute Coronary Syndrome, at Onset. Medical Sciences. 2025; 13(4):232. https://doi.org/10.3390/medsci13040232
Chicago/Turabian StyleBrescia, Vincenzo, Antonella Mileti, Roberto Lovero, Lucia Varraso, Francesco Pignataro, Francesca Di Serio, Angela Pia Cazzolla, Luigi Santacroce, Maria Eleonora Bizzoca, Vito Crincoli, and et al. 2025. "Evaluation of Platelet Indices and Reticulated Platelets Using the ADVIA 2120 Analyzer in Patients with Acute Infection or Acute Coronary Syndrome, at Onset" Medical Sciences 13, no. 4: 232. https://doi.org/10.3390/medsci13040232
APA StyleBrescia, V., Mileti, A., Lovero, R., Varraso, L., Pignataro, F., Di Serio, F., Cazzolla, A. P., Santacroce, L., Bizzoca, M. E., Crincoli, V., & Di Comite, M. S. (2025). Evaluation of Platelet Indices and Reticulated Platelets Using the ADVIA 2120 Analyzer in Patients with Acute Infection or Acute Coronary Syndrome, at Onset. Medical Sciences, 13(4), 232. https://doi.org/10.3390/medsci13040232








