Decentralized Clinical Trials: Governance, Ethics and Medico-Legal Issues for the New Paradigm of Research with a Focus on Cardiovascular Field
Abstract
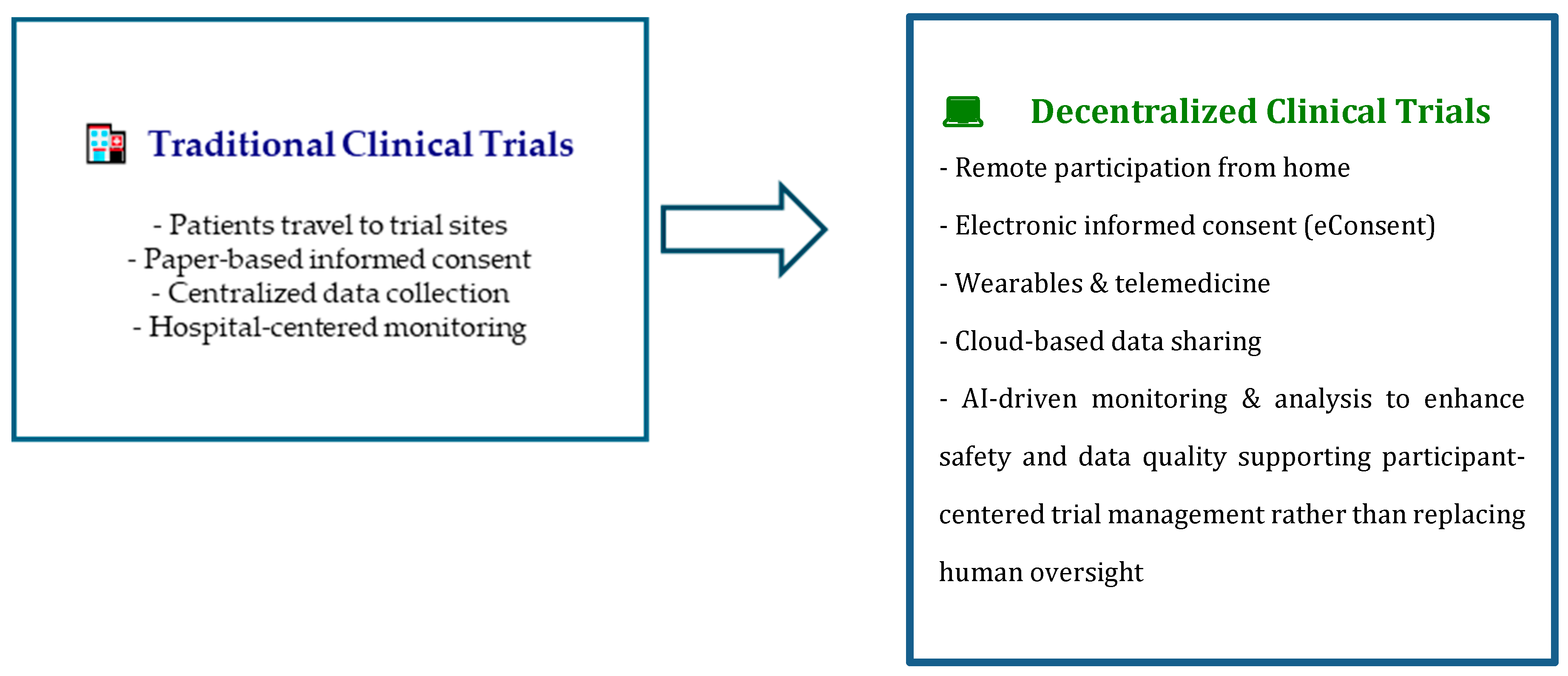
1. Introduction
- to increase the number of participants in each clinical trial and to collect useful data from participants in less time.
- to minimize the inconveniences and health risks for participants (and their caregivers) due to participation in the clinical trial by avoiding frequent travel and hospitalization.
- to improve participant adherence to trial procedures (compliance) and adverse events monitoring (safety).
- to easily share the data obtained from the clinical research studies using interactive platforms and analytics systems compliant by design and by default.
- recruitment, administration of informed consent both for privacy disclosure and health treatment consenting, and further participation in the study take place totally or partially (hybrid trial) remotely.
- the collection of data—clinical, biological, environmental, self-reported (outcomes, i.e., through participant self-assessment)—is implemented using telemonitoring, tele visits, tele assistance, and ePROs (electronic patient-reported outcome).
- the wide possibility of analysis of the data, as well as easily shareable in RWD (Real World Data) [4].
2. Discussion
2.1. Normative Principles and Ethical Requirements
2.2. European Recommendations for DCTs
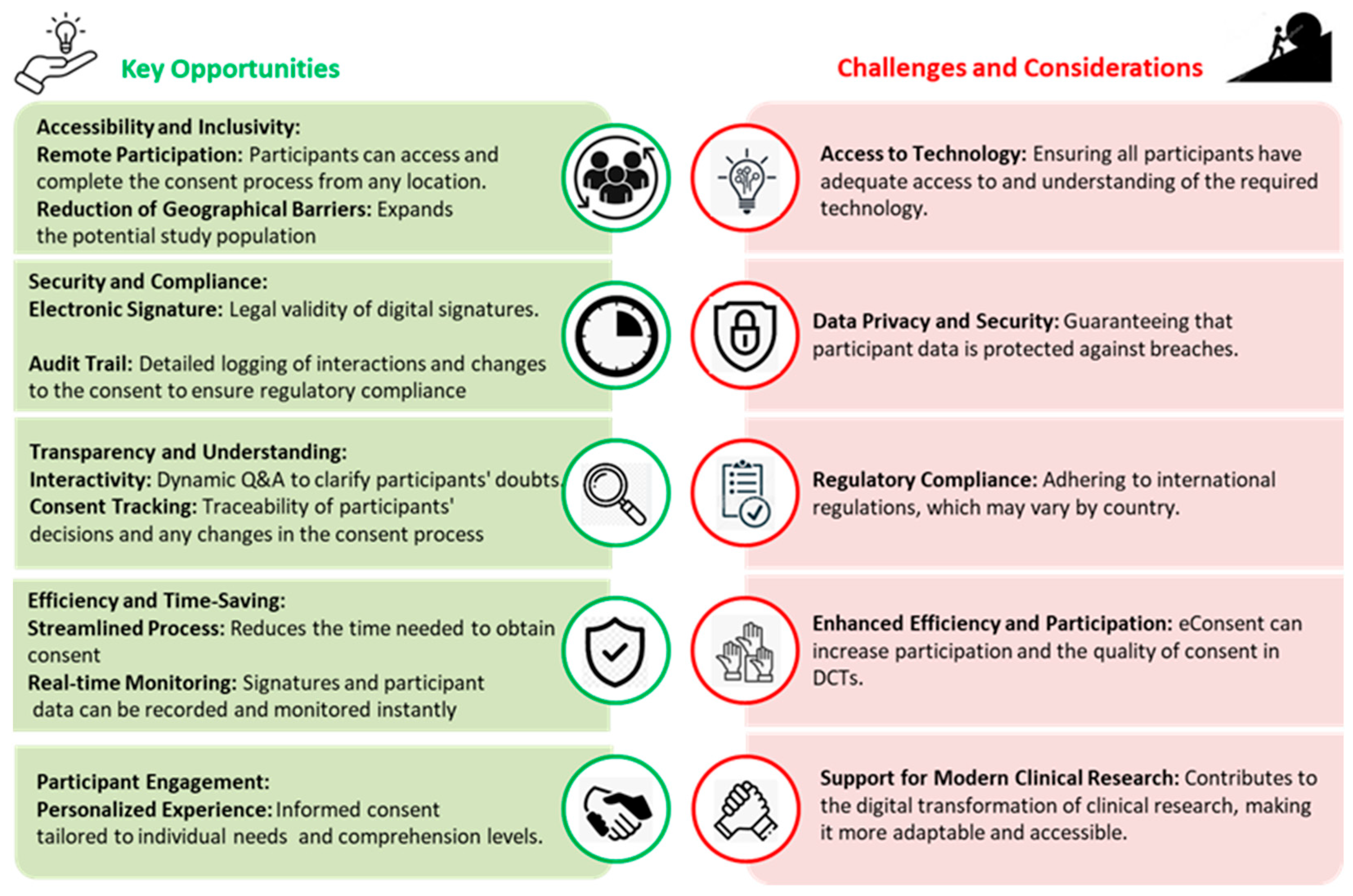
2.3. Participant Information Processing
2.4. Consenting for Health Research Participation
2.5. Health Literacy: A Matter of Ethics and Equity
| Aspect | Description/Challenge | Digital Solutions in DCTs | Impact on Cardiovascular Participants |
|---|---|---|---|
| Understanding information [25,27] | Participants with low health literacy struggle to understand study risks, benefits, and procedures | Interactive platforms with simplified text, explanatory videos, charts, and diagrams | Better trial adherence, reduced anxiety, and increased active participation |
| Digital literacy [29] | Difficulty using apps, wearables, and digital platforms | Guided tutorials, real-time support, multilingual assistance | Improved remote monitoring of blood pressure, heart rhythm, and other parameters |
| Autonomous engagement | Informed decision-making hindered by complex information | Interactive e-Consent with identity verification and consent traceability | Cardiovascular participants can clearly understand intervention or medication risks, increasing safety and trust |
| Accessibility [31] | Language, cognitive, or geographic barriers | Translations, subtitles, cultural adaptations, remote assistance | Inclusion of underrepresented groups, expanding diversity in cardiovascular trials |
| Continuous monitoring [32] | Limited understanding of self-management procedures | Wearables and telemonitoring with automatic alerts and educational feedback | Better control of critical parameters such as blood pressure, heart rhythm, and physical activity |
2.6. Medico-Legal Considerations
2.7. Specific Challenges to Cardiovascular Trials
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCT | Decentralized Clinical Trial |
| EMA | European Medicines Agency |
| EU | European Union |
| ePRO | electronic patient-reported outcome |
| GDPR | General Data Protection Regulation |
| IVDR | In Vitro Device Regulation |
| MDR | Medical Diagnostic Regulation |
| RWD | Real World Data |
| GMP | Good Manufacturing Practice |
| GDP | Good Distribution Practice |
References
- Petrini, C.; Mannelli, C.; Riva, L.; Gainotti, S.; Gussoni, G. Decentralized Clinical Trials (DCTs): A Few Ethical Considerations. Front. Public Health 2022, 10, 1081150. [Google Scholar] [CrossRef]
- Zagarrì, E.; Frasson, S.; Valerio, A.; Gussoni, G. Decentralized Clinical Trials in Italy: State of the Art and Future Perspectives. AboutOpen 2023, 10, 22–26. [Google Scholar] [CrossRef]
- Senbekov, M.; Saliev, T.; Bukeyeva, Z.; Almabayeva, A.; Zhanaliyeva, M.; Aitenova, N.; Toishibekov, Y.; Fakhradiyev, I. The Recent Progress and Applications of Digital Technologies in Healthcare: A Review. Int. J. Telemed. Appl. 2020, 2020, 8830200. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.J.; van Rijssel, T.I.; Zuidgeest, M.G.P.; van Thiel, G.J.M.W.; Askin, S.; Fons-Martínez, J.; De Smedt, T.; de Boer, A.; Santa-Ana-Tellez, Y.; Gardarsdottir, H.; et al. Opportunities and Challenges for Decentralized Clinical Trials: European Regulators’ Perspective. Clin. Pharmacol. Ther. 2022, 112, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, K.M.; van der Boon, T.A.B.; Devia-Rodriguez, R.; Schuurmann, R.C.L.; Sjollema, J.; van Huizen, L.; De Vries, J.P.P.M.; van Rijn, P. Recent Regulatory Developments in EU Medical Device Regulation and Their Impact on Biomaterials Translation. Bioeng. Transl. Med. 2024, 10, e10721. [Google Scholar] [CrossRef]
- Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 Amending, as Regards Pharmacovigilance, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use Text with EEA Relevance, 2010, EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2010/84/oj/eng (accessed on 27 August 2025).
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic Principles of Biobanking: From Biological Samples to Precision Medicine for Patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef]
- Park, J.; Huh, K.Y.; Chung, W.K.; Yu, K.S. The Landscape of Decentralized Clinical Trials (DCTs): Focusing on the FDA and EMA Guidance. Transl. Clin. Pharmacol. 2024, 32, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wilbanks, J. Design Issues in E-Consent. J. Law Med. Ethics 2018, 46, 110–118. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation), 2016, EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj/eng (accessed on 27 August 2025).
- Becker, R.; Chokoshvili, D.; Thorogood, A.; Dove, E.S.; Molnár-Gábor, F.; Ziaka, A.; Tzortzatou-Nanopoulou, O.; Comandè, G. Purpose Definition as a Crucial Step for Determining the Legal Basis under the GDPR: Implications for Scientific Research. J. Law Biosci. 2024, 11, lsae001. [Google Scholar] [CrossRef]
- Vos, I.M.L.; Schermer, M.H.N.; Bolt, I.L.L.E. Recent Insights into Decision-Making and Their Implications for Informed Consent. J. Med. Ethics 2018, 44, 734–738. [Google Scholar] [CrossRef]
- Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC Text with EEA Relevance, 2014, EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2014/536/oj/eng (accessed on 27 August 2025).
- Chen, J.; Di, J.; Daizadeh, N.; Lu, Y.; Wang, H.; Shen, Y.; Kirk, J.; Rockhold, F.W.; Pang, H.; Zhao, J.; et al. Decentralized Clinical Trials in the Era of Real-World Evidence: A Statistical Perspective. Clin. Transl. Sci. 2025, 18, e70117. [Google Scholar] [CrossRef]
- Kurtz-Rossi, S.; Okonkwo, I.A.; Chen, Y.; Dueñas, N.; Bilodeau, T.; Rushforth, A.; Klein, A. Development of a New Tool for Writing Research Key Information in Plain Language. Health Lit. Res. Pract. 2024, 8, e30–e37. [Google Scholar] [CrossRef]
- van Rijssel, T.I.; van Thiel, G.J.M.W.; van Delden, J.J.M. The Ethics of Decentralized Clinical Trials and Informed Consent: Taking Technologies’ Soft Impacts into Account. Health Care Anal. 2025, 33, 139–150. [Google Scholar] [CrossRef]
- Geier, C.; Adams, R.B.; Mitchell, K.M.; Holtz, B.E. Informed Consent for Online Research-Is Anybody Reading?: Assessing Comprehension and Individual Differences in Readings of Digital Consent Forms. J. Empir. Res. Hum. Res. Ethics 2021, 16, 154–164. [Google Scholar] [CrossRef]
- Bolcato, V.; Franzetti, C.; Fassina, G.; Basile, G.; Martinez, R.M.; Tronconi, L.P. Comparative Study on Informed Consent Regulation in Health Care among Italy, France, United Kingdom, Nordic Countries, Germany, and Spain. J. Forensic Leg. Med. 2024, 103, 102674. [Google Scholar] [CrossRef]
- Lepola, P.; Kindred, M.; Giannuzzi, V.; Glosli, H.; Dehlinger-Kremer, M.; Dalrymple, H.; Neubauer, D.; Boylan, G.B.; Conway, J.; Dewhurst, J.; et al. Informed Consent and Assent Guide for Paediatric Clinical Trials in Europe. Arch. Dis. Child. 2022, 107, 582–590. [Google Scholar] [CrossRef]
- Cousins, S.; Huttman, M.; Blencowe, N.; Tsang, C.; Elliott, D.; Blazeby, J.; Beard, D.J.; Campbell, M.K.; Gillies, K. Patient Information Leaflets for Placebo-Controlled Surgical Trials: A Review of Current Practice and Recommendations for Developers. Trials 2024, 25, 339. [Google Scholar] [CrossRef] [PubMed]
- Foe, G.; Larson, E.L. Reading Level and Comprehension of Research Consent Forms: An Integrative Review. J. Empir. Res. Hum. Res. Ethics 2016, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Aiyegbusi, O.L.; Davies, E.H.; Myles, P.; Williams, T.; Frost, C.; Haroon, S.; Hughes, S.E.; Wilson, R.; McMullan, C.; Subramanian, A.; et al. Digitally Enabled Decentralised Research: Opportunities to Improve the Efficiency of Clinical Trials and Observational Studies. BMJ Evid. Based Med. 2023, 28, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Mauffrey, C.; Prempeh, E.M.; John, J.; Vasario, G. The Influence of Written Information during the Consenting Process on Patients’ Recall of Operative Risks. A Prospective Randomised Study. Int. Orthop. 2008, 32, 425–429. [Google Scholar] [CrossRef]
- Urstad, K.H.; Andersen, M.H.; Larsen, M.H.; Borge, C.R.; Helseth, S.; Wahl, A.K. Definitions and Measurement of Health Literacy in Health and Medicine Research: A Systematic Review. BMJ Open 2022, 12, e056294. [Google Scholar] [CrossRef]
- Gazmararian, J.A.; Williams, M.V.; Peel, J.; Baker, D.W. Health Literacy and Knowledge of Chronic Disease. Patient Educ. Couns. 2003, 51, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.S.; Gazmararian, J.A.; Baker, D.W. Health Literacy and Functional Health Status among Older Adults. Arch. Intern. Med. 2005, 165, 1946–1952. [Google Scholar] [CrossRef]
- Rowlands, G.; Nutbeam, D. Health Literacy and the ‘Inverse Information Law’. Br. J. Gen. Pract. 2013, 63, 120. [Google Scholar] [CrossRef]
- Montalvo, W.; Larson, E. Participant Comprehension of Research for Which They Volunteer: A Systematic Review. J. Nurs. Scholarsh. 2014, 46, 423–431. [Google Scholar] [CrossRef]
- Kasahara, A.; Mitchell, J.; Yang, J.; Cuomo, R.E.; McMann, T.J.; Mackey, T.K. Digital Technologies Used in Clinical Trial Recruitment and Enrollment Including Application to Trial Diversity and Inclusion: A Systematic Review. Digit. Health 2024, 10, 20552076241242390. [Google Scholar] [CrossRef]
- Harmon, D.M.; Noseworthy, P.A.; Yao, X. The Digitization and Decentralization of Clinical Trials. Mayo Clin. Proc. 2023, 98, 1568–1578. [Google Scholar] [CrossRef]
- Bhaltadak, V.; Ghewade, B.; Yelne, S. A Comprehensive Review on Advancements in Wearable Technologies: Revolutionizing Cardiovascular Medicine. Cureus 2024, 16, e61312. [Google Scholar] [CrossRef] [PubMed]
- Betcheva, L.; Kim, J.Y.; Erhun, F.; Oraiopoulos, N.; Getz, K. Applying Systems Thinking to Inform Decentralized Clinical Trial Planning and Deployment. Ther. Innov. Regul. Sci. 2023, 57, 1081. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, C.; Contissa, G.; Basile, G. Healthcare AI, Explainability, and the Human-Machine Relationship: A (Not so) Novel Practical Challenge. Front. Med. 2025, 12, 1545409. [Google Scholar] [CrossRef]
- Raposo, V.L. Telemedicine: The Legal Framework (or the Lack of It) in Europe. GMS Health Technol Assess 2016, 12, Doc03. [Google Scholar] [CrossRef] [PubMed]
- Bolcato, V.; Basile, G.; Bianco Prevot, L.; Fassina, G.; Rapuano, S.; Brizioli, E.; Tronconi, L.P. Telemedicine in Italy: Healthcare Authorization Profiles in the Modern Medico-Legal Reading. Int. J. Risk Saf. Med. 2024, 35, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.R. Reflections about Blockchain in Health Data Sharing: Navigating a Disruptive Technology. Int. J. Environ. Res. Public Health 2024, 21, 230. [Google Scholar] [CrossRef]
- Hilgendorf, E.; Feldle, J. Digitization and the Law; Nomos Verlagsgesellschaft: Baden-Baden, Germany, 2018; Volume 15, Robotik und Recht; ISBN 978-3-8487-4700-9. [Google Scholar]
- Mastorci, F.; Lazzeri, M.F.L.; Ait-Ali, L.; Marcheschi, P.; Quadrelli, P.; Mariani, M.; Margaryan, R.; Pennè, W.; Savino, M.; Prencipe, G.; et al. Home-Based Intervention Tool for Cardiac Telerehabilitation: Protocol for a Controlled Trial. JMIR Res. Protoc. 2025, 14, e47951. [Google Scholar] [CrossRef]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef]
- Broadwin, C.; Azizi, Z.; Rodriguez, F. Clinical Trial Technologies for Improving Equity and Inclusion in Cardiovascular Clinical Research. Cardiol. Ther. 2023, 12, 215. [Google Scholar] [CrossRef]
| Domain | Challenge | Proposed Solution/Best Practice |
|---|---|---|
| Ethical and medico-legal | Adequate understanding of informed consent in digital settings | e-Consent with simplified language, videos/infographics, multilingual support, interactive Q&A |
| Legal | GDPR compliance and clear identification of data controller and data processor | Privacy by design, DPO appointment, access privilege definition, audit trail |
| Technological | Data security and traceability | Blockchain, advanced cryptography, immutable logs |
| Organizational | Transnational coordination and regulatory heterogeneity | Harmonization through EMA/ACT EU guidance, shared procedures |
| Clinical | Monitoring of fragile participants (e.g., complex cardiovascular cases) | Advanced telemedicine, wearable devices, remote healthcare teams |
| Equity | Inclusion of vulnerable populations with low health or digital literacy | User-friendly platforms, linguistic/cultural support, basic digital training |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenti, E.; Basile, G.; Giorgetti, C.; Sangiorgi, D.; Mikus, E.; Sebastiani, G.; Bolcato, V.; Tronconi, L.P.; Tremoli, E. Decentralized Clinical Trials: Governance, Ethics and Medico-Legal Issues for the New Paradigm of Research with a Focus on Cardiovascular Field. Med. Sci. 2025, 13, 222. https://doi.org/10.3390/medsci13040222
Tenti E, Basile G, Giorgetti C, Sangiorgi D, Mikus E, Sebastiani G, Bolcato V, Tronconi LP, Tremoli E. Decentralized Clinical Trials: Governance, Ethics and Medico-Legal Issues for the New Paradigm of Research with a Focus on Cardiovascular Field. Medical Sciences. 2025; 13(4):222. https://doi.org/10.3390/medsci13040222
Chicago/Turabian StyleTenti, Elena, Giuseppe Basile, Claudia Giorgetti, Diego Sangiorgi, Elisa Mikus, Gaia Sebastiani, Vittorio Bolcato, Livio Pietro Tronconi, and Elena Tremoli. 2025. "Decentralized Clinical Trials: Governance, Ethics and Medico-Legal Issues for the New Paradigm of Research with a Focus on Cardiovascular Field" Medical Sciences 13, no. 4: 222. https://doi.org/10.3390/medsci13040222
APA StyleTenti, E., Basile, G., Giorgetti, C., Sangiorgi, D., Mikus, E., Sebastiani, G., Bolcato, V., Tronconi, L. P., & Tremoli, E. (2025). Decentralized Clinical Trials: Governance, Ethics and Medico-Legal Issues for the New Paradigm of Research with a Focus on Cardiovascular Field. Medical Sciences, 13(4), 222. https://doi.org/10.3390/medsci13040222








