Sex-Specific Safety Signals of Trelegy Ellipta: A FAERS Pharmacovigilance Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Inclusion and Exclusion Criteria
2.3. Data Cleaning and Coding
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.K.; Raja, A.; Brown, B.D. Chronic Obstructive Pulmonary Disease; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559281/ (accessed on 28 August 2025).
- Oba, Y.; Keeney, E.; Ghatehorde, N.; Dias, S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2018, 12, CD012620. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K. Fluticasone furoate/vilanterol: A review of its use in chronic obstructive pulmonary disease. Drugs 2014, 74, 1509–1522. [Google Scholar] [CrossRef]
- Mashaal, H.; Fogel, J.; Sayedy, N.; Sahota, R.J.; Akella, J. Chronic obstructive pulmonary disease patients’ experience using Trelegy as compared with other inhalers. Can. J. Respir. Ther. 2022, 58, 44–48. [Google Scholar] [CrossRef]
- Ruan, Z.; Wang, C.; Yuan, S.; Fan, Y.; Xu, B.; Cong, X.; Li, D.; Miao, Q. High-risk adverse events in two types of single inhaler triple-therapy: A pharmacovigilance study based on the FAERS database. Front. Pharmacol. 2025, 15, 1460407. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Pattock, A.M.; Locke, E.R.; Hebert, P.L.; Simpson, T.; Battaglia, C.; Trivedi, R.B.; Swenson, E.R.; Edelman, J.; Fan, V.S. Predictors of patient-reported and pharmacy refill measures of maintenance inhaler adherence in veterans with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2024, 21, 384–392. [Google Scholar] [CrossRef]
- Malerba, M.; Nardin, M.; Santini, G.; Mores, N.; Radaeli, A.; Montuschi, P. Single-inhaler triple therapy utilizing the once-daily combination of fluticasone furoate, umeclidinium and vilanterol in the management of COPD: The current evidence base and future prospects. Ther. Adv. Respir. Dis. 2018, 12, 1753466618760779. [Google Scholar] [CrossRef]
- Langham, S.; Lewis, J.; Pooley, N.; Embleton, N.; Langham, J.; Han, M.K.; Chalmers, J.D. Single-inhaler triple therapy in patients with chronic obstructive pulmonary disease: A systematic review. Respir. Res. 2019, 20, 242. [Google Scholar] [CrossRef]
- Sportiello, L.; Capuano, A. Sex and gender differences and pharmacovigilance: A knot still to be untied. Front. Pharmacol. 2024, 15, 1397291. [Google Scholar] [CrossRef]
- Trudzinski, F.C.; Kellerer, C.; Jörres, R.A.; Alter, P.; Lutter, J.I.; Trinkmann, F.; Herth, F.J.F.; Frankenberger, M.; Watz, H.; Vogelmeier, C.F.; et al. Gender-specific differences in COPD symptoms and their impact for the diagnosis of cardiac comorbidities. Clin. Res. Cardiol. 2023, 112, 177–186. [Google Scholar] [CrossRef]
- Price, D.; Keininger, D.L.; Viswanad, B.; Gasser, M.; Walda, S.; Gutzwiller, F.S. Factors associated with appropriate inhaler use in patients with COPD—Lessons from the REAL survey. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 695–702. [Google Scholar] [CrossRef]
- López-Pintor, E.; Grau, J.; González, I.; Bernal-Soriano, M.C.; Quesada, J.A.; Lumbreras, B. Impact of patients’ perception of COPD and treatment on adherence and health-related quality of life in real-world: Study in 53 community pharmacies. Respir. Med. 2021, 176, 106280. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Hirano, T.; Oishi, K.; Fukatsu-Chikumoto, A.; Ohteru, Y.; Hamada, K.; Ohata, S.; Murata, Y.; Yamaji, Y.; Asami-Noyama, M.; et al. Gender Difference in the Relationship between Extrapulmonary Factors and Reduced Lung Function in Early Adulthood. J. Clin. Med. 2024, 13, 1769. [Google Scholar] [CrossRef]
- Darbà, J.; Ramírez, G.; Sicras, A.; Francoli, P.; Torvinen, S.; Sánchez-de la Rosa, R. The importance of inhaler devices: The choice of inhaler device may lead to suboptimal adherence in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2335–2345. [Google Scholar] [CrossRef]
- Chee, C.; Sellahewa, L.; Pappachan, J.M. Inhaled corticosteroids and bone health. Open Respir. Med. J. 2014, 8, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Maurier, A.; Largeau, B.; Destere, A.; Thillard, E.M.; Drici, M.; Micallef, J.; Jonville-Bera, A.P. Sex differences in adverse drug reactions: Are women more impacted? Therapies 2023, 78, 175–188. [Google Scholar] [CrossRef]
- de Vries, S.T.; Denig, P.; Ekhart, C.; Burgers, J.S.; Kleefstra, N.; Mol, P.G.M.; van Puijenbroek, E.P. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: An explorative observational study. Br. J. Clin. Pharmacol. 2019, 85, 1507–1515. [Google Scholar] [CrossRef]
- Moon, J.Y.; Sin, D.D. Inhaled corticosteroids and fractures in chronic obstructive pulmonary disease: Current understanding and recommendations. Curr. Opin. Pulm. Med. 2019, 25, 165–172. [Google Scholar] [CrossRef]
- van den Berge, M.; Heijink, H.I.; van Oosterhout, A.J.; Postma, D.S. The role of female sex hormones in the development and severity of allergic and non-allergic asthma. Clin. Exp. Allergy 2009, 39, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, R.; DuMouchel, W.; Shah, N.H.; Madigan, D.; Ryan, P.; Friedman, C. Novel data-mining methodologies for adverse drug event discovery and analysis. Clin. Pharmacol. Ther. 2012, 91, 1010–1021. [Google Scholar] [CrossRef]
- Alshammari, T.M.; Alshakka, M.; Aljadhey, H. Pharmacovigilance system in Saudi Arabia. Saudi Pharm. J. 2017, 25, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Cutroneo, P.; Trifirò, G. Clinical and economic burden of adverse drug reactions. J. Pharmacol. Pharmacother. 2013, 4 (Suppl. S1), S73–S77. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Montvida, O.; Shaw, J.; Atherton, J.J.; Stringer, F.; Paul, S.K. Long-term trends in antidiabetes drug usage in the U.S.: Real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 2018, 41, 69–78. [Google Scholar] [CrossRef]
- Brabete, A.C.; Greaves, L.; Maximos, M.; Huber, E.; Li, A.; Lê, M.L. A sex- and gender-based analysis of adverse drug reactions: A scoping review of pharmacovigilance databases. Pharmaceuticals 2022, 15, 298. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA’s Action Plan to Enhance the Collection and Availability of Demographic Subgroup Data. 2020. Available online: https://www.fda.gov/media/109618/download (accessed on 28 August 2025).
- Hendriksen, L.C.; van der Linden, P.D.; Lagro-Janssen, A.L.M.; van den Bemt, P.M.L.A.; Siiskonen, S.J.; Teichert, M.; Kuiper, J.G.; Herings, R.M.C.; Stricker, B.H.; Visser, L.E. Sex differences associated with adverse drug reactions resulting in hospital admissions. Biol. Sex Differ. 2021, 12, 34. [Google Scholar] [CrossRef]
- Scott, P.E.; Unger, E.F.; Jenkins, M.R.; Southworth, M.R.; McDowell, T.Y.; Geller, R.J.; Elahi, M.; Temple, R.J.; Woodcock, J. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J. Am. Coll. Cardiol. 2018, 71, 1960–1969. [Google Scholar] [CrossRef]
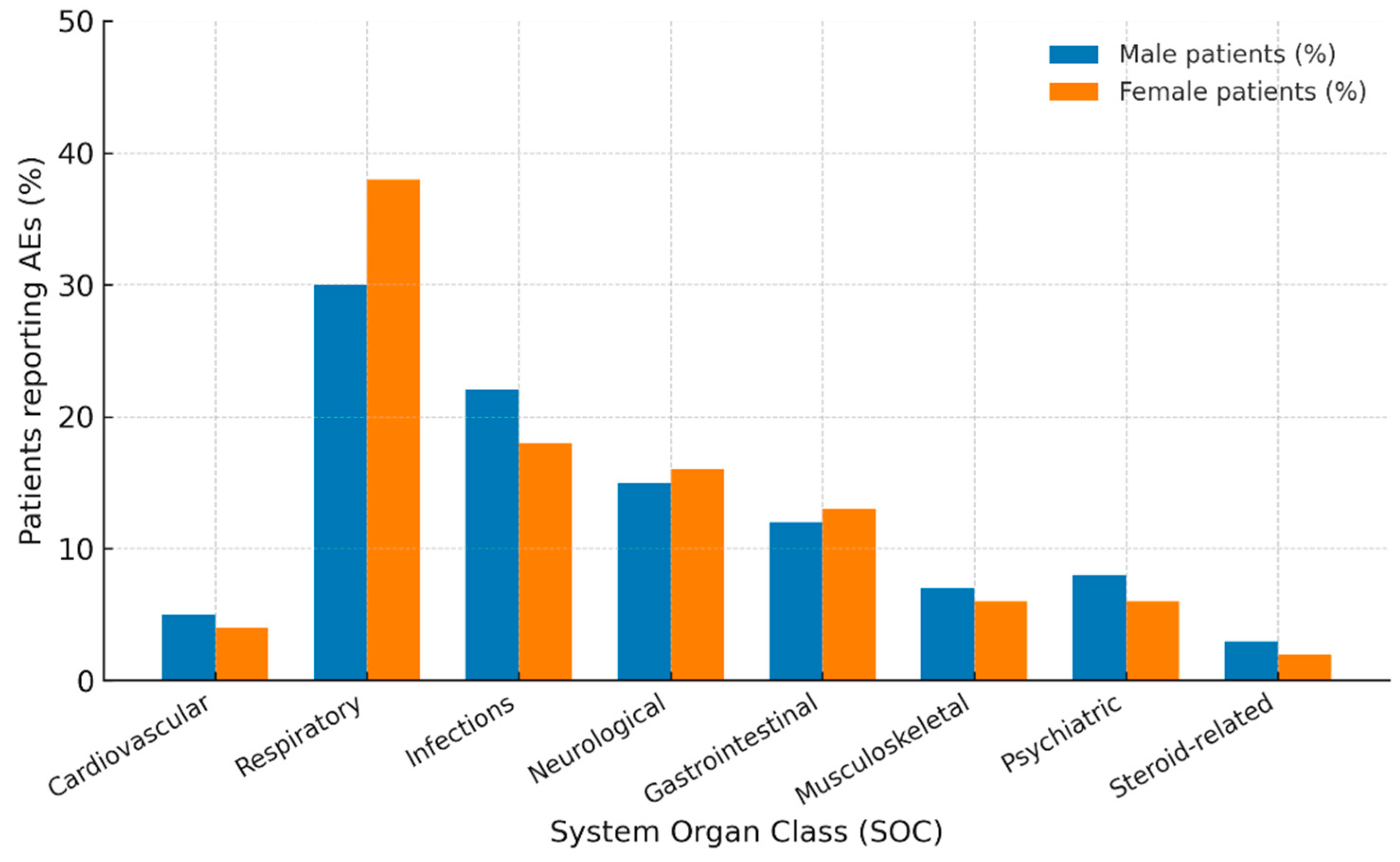

| Adverse Events (SOC/PT) | Male n (%) | Female n (%) | ROR (95% CI) | p Value |
|---|---|---|---|---|
| Cardiovascular | 93 (5.7) | 164 (5.6) | 1.03 (0.79–1.34) | 0.84 |
| – Tachycardia | 6 (6.5) | 22 (13.4) | – | 0.13 |
| – Palpitations | 28 (30.1) | 65 (39.6) | – | 0.16 |
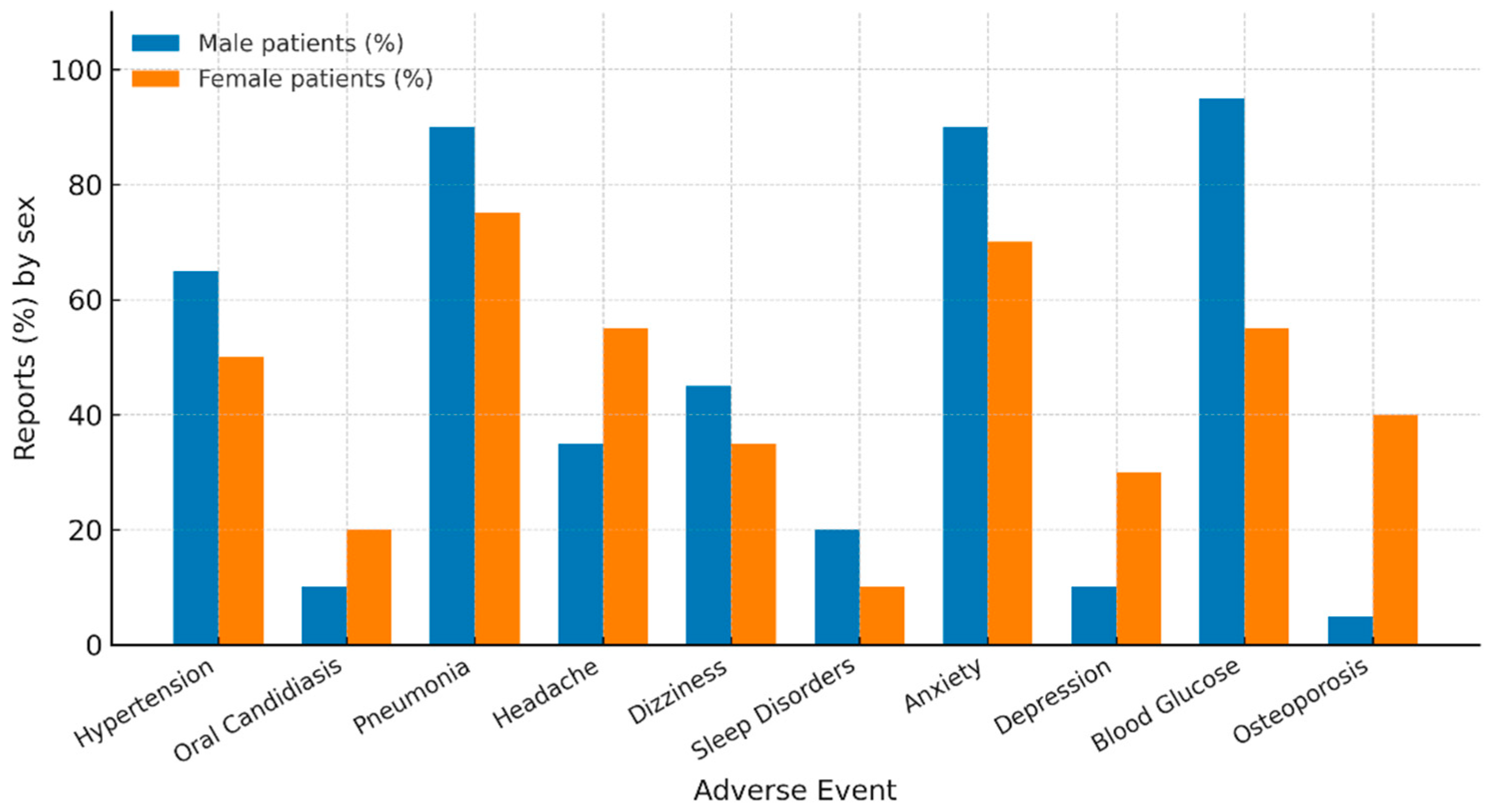
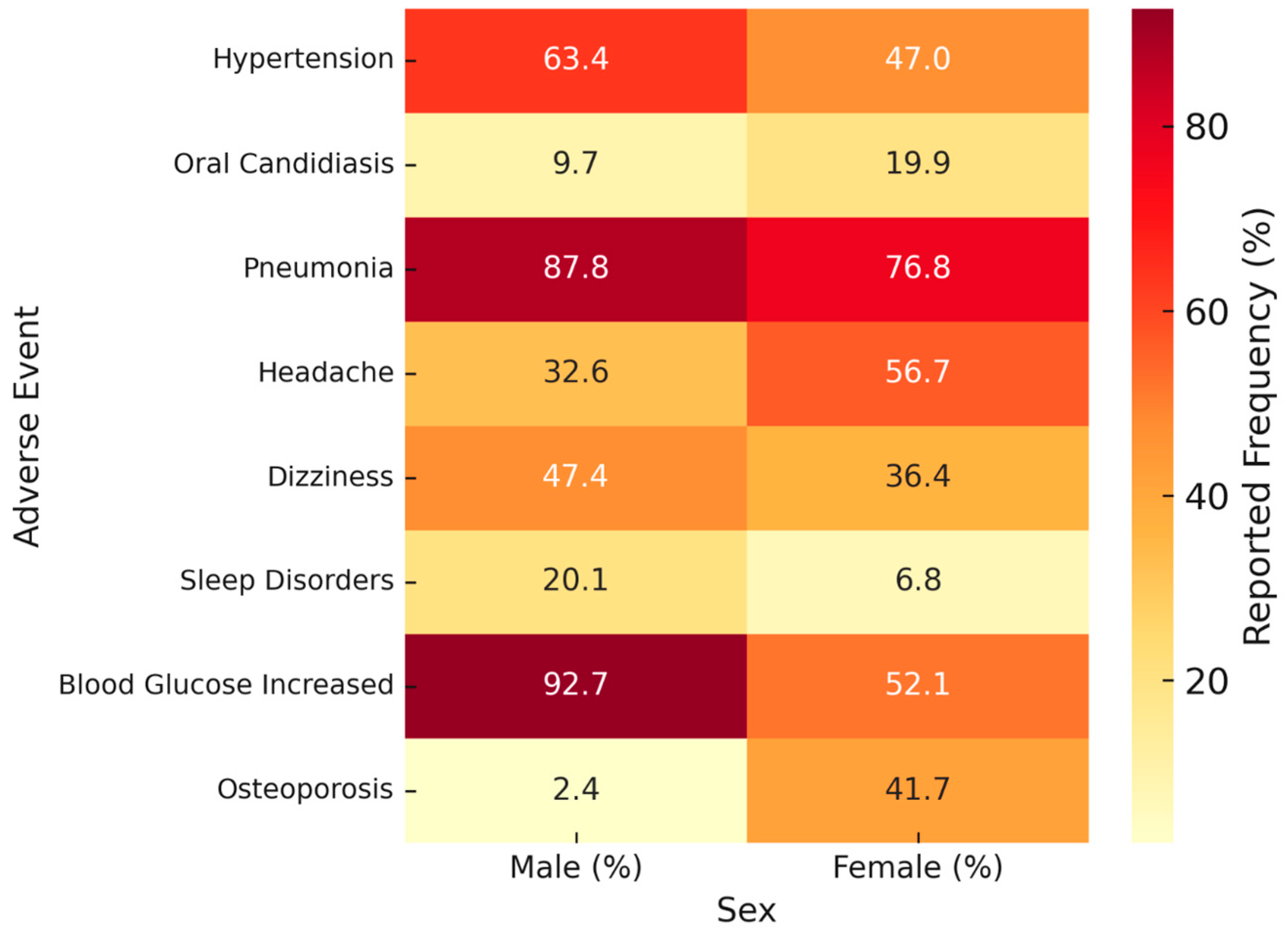
| – Hypertension | 59 (63.4) | 77 (47.0) | – | 0.016 |
| Respiratory | 535 (33.0) | 1275 (43.5) | 0.64 (0.56–0.73) | <0.001 |
| – Dysphonia | 198 (37.0) | 531 (41.6) | – | 0.08 |
| – Cough | 335 (62.6) | 741 (58.1) | – | 0.08 |
| – Paradoxical bronchospasm | 2 (0.4) | 3 (0.2) | – | 1.00 |
| Infections | 361 (22.7) | 483 (16.5) | 1.45 (1.25–1.63) | <0.001 |
| – Oral candidiasis | 35 (9.7) | 96 (19.9) | – | <0.001 |
| – URTI | 9 (2.5) | 16 (3.3) | – | 0.62 |
| – Pneumonia | 317 (87.8) | 371 (76.8) | – | <0.001 |
| Neurological | 209 (12.9) | 453 (15.4) | 0.81 (0.68–0.97) | 0.02 |
| – Headache | 68 (32.6) | 257 (56.7) | – | <0.001 |
| – Dizziness | 99 (47.4) | 165 (36.4) | – | 0.01 |
| – Sleep disorders | 42 (20.1) | 31 (6.8) | – | <0.001 |
| Gastrointestinal | 205 (12.6) | 237 (8.1) | 1.65 (1.35–2.01) | <0.001 |
| – Nausea | 99 (48.3) | 107 (45.1) | – | 0.57 |
| – Dry mouth | 83 (40.5) | 89 (37.6) | – | 0.60 |
| – Taste disorders | 23 (11.2) | 41 (17.3) | – | 0.09 |
| Musculoskeletal | 77 (4.8) | 147 (5.0) | 0.95 (0.71–1.25) | 0.70 |
| – Muscle disorders | 1 (1.3) | 0 | – | 0.74 |
| – Back pain | 76 (98.7) | 147 (100) | – | 0.74 |
| Psychiatric | 100 (6.2) | 127 (4.3) | 1.45 (1.11–1.90) | 0.006 |
| – Anxiety | 91 (91.0) | 85 (66.9) | – | <0.001 |
| – Depression | 9 (9.0) | 42 (33.1) | – | <0.001 |
| Steroid-related | 41 (2.5) | 48 (1.6) | 1.56 (1.02–2.38) | 0.037 |
| – Skin disorders | 2 (4.9) | 3 (6.3) | – | 0.86 |
| – Blood glucose increased | 38 (92.7) | 25 (52.1) | – | <0.001 |
| – Osteoporosis | 1 (2.4) | 20 (41.7) | – | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yayan, J.; Biancosino, C.; Krüger, M.; Rasche, K. Sex-Specific Safety Signals of Trelegy Ellipta: A FAERS Pharmacovigilance Analysis. Med. Sci. 2025, 13, 221. https://doi.org/10.3390/medsci13040221
Yayan J, Biancosino C, Krüger M, Rasche K. Sex-Specific Safety Signals of Trelegy Ellipta: A FAERS Pharmacovigilance Analysis. Medical Sciences. 2025; 13(4):221. https://doi.org/10.3390/medsci13040221
Chicago/Turabian StyleYayan, Josef, Christian Biancosino, Marcus Krüger, and Kurt Rasche. 2025. "Sex-Specific Safety Signals of Trelegy Ellipta: A FAERS Pharmacovigilance Analysis" Medical Sciences 13, no. 4: 221. https://doi.org/10.3390/medsci13040221
APA StyleYayan, J., Biancosino, C., Krüger, M., & Rasche, K. (2025). Sex-Specific Safety Signals of Trelegy Ellipta: A FAERS Pharmacovigilance Analysis. Medical Sciences, 13(4), 221. https://doi.org/10.3390/medsci13040221






