The Pathophysiology of Wharton’s Jelly and Its Impact on Fetal and Neonatal Outcomes: A Comprehensive Literature Review
Abstract
1. Introduction
2. Methods
2.1. The Spectrum of Wharton’s Jelly Pathologies
2.2. Pathophysiology
2.2.1. Reduction and Absence of Wharton’s Jelly (“Thin Cord Syndrome”)
Definition and Pathophysiology
Etiology and Pathogenesis
Complete and Segmental Absence
Association with Other Conditions
2.3. Abnormalities of Pathophysiology
2.3.1. Excessive Wharton’s Jelly and Umbilical Cord Edema (“Thick Cord Syndrome”)
Definition and Clinical Context
Histopathology and Hemodynamics
Associated Conditions
2.4. Structural Lesions and Degenerative Changes
2.4.1. Umbilical Cord Cysts (Pseudocysts)
Definition and Pathogenesis
Prevalence and Prognostic Significance
- Isolated, First-Trimester Cysts: When a single cyst is identified in the first trimester without any other sonographic abnormalities, the prognosis is generally excellent. These cysts are often transient, resolving spontaneously as the pregnancy progresses, and are typically considered a normal variant of development with a favorable outcome [25].
- Persistent, Second/Third-Trimester, and/or Multiple Cysts: The clinical implications become far more serious when cysts persist beyond the first trimester, are detected for the first time in the second or third trimester, or are multiple in number. In these scenarios, there is a strong association with underlying fetal pathology, with up to 50% of such cases having associated structural or chromosomal abnormalities [25]. The risk is further elevated if the cysts are located near the fetal or placental insertion sites of the cord [25]. These cysts are a prominent sonographic feature of severe aneuploidies, particularly Trisomy 18 and Trisomy 13 [25].
Association with Other Anomalies
Mucoid Degeneration
2.4.2. Impact on Fetus During Pregnancy and Neonatal Outcomes
2.4.3. Catastrophic Outcomes: Stillbirth, Fetal Demise, and Perinatal Mortality
2.4.4. Perinatal Morbidity and Compromised Development
Fetal Growth Restriction (FGR) and Placental Insufficiency
Intrapartum Complications and Neonatal Admission
2.4.5. Association with Congenital and Chromosomal Anomalies
2.4.6. Diagnosis
2.4.7. Prenatal Diagnosis: The Role and Limitations of Ultrasonography
Sonographic Assessment
Measuring Wharton’s Jelly Area
Diagnosing Cysts and Edema
The Diagnostic Challenge of Absent Wharton’s Jelly
2.5. Fetal Surveillance and Management
2.5.1. Management of Cord Cysts
- Detailed Anatomical Survey: The first step is a comprehensive, high-resolution ultrasound examination to meticulously search for any associated fetal structural anomalies [25].
- Karyotyping: Given the strong association with aneuploidy, invasive genetic testing via amniocentesis or chorionic villus sampling should be offered, particularly if the cysts are multiple, large, complex, persistent beyond the first trimester, or found in conjunction with any other sonographic marker of aneuploidy [43].
- Serial Surveillance: For pregnancies that continue, especially those with large or numerous cysts, serial ultrasound surveillance is recommended. These follow-up scans, typically performed every four weeks, are used to monitor fetal growth for any signs of FGR and to assess the cysts for any change in size or evidence of umbilical vessel compression [34].
- Delivery Planning: Advanced delivery planning is imperative. The potential for sudden vascular compromise and fetal demise means that decisions regarding the timing and mode of delivery must be carefully considered, often involving consultation with maternal-fetal medicine specialists. Continuous intrapartum electronic fetal monitoring is essential [34].
2.5.2. Management of Decreased/Absent Wharton’s Jelly
2.5.3. Management of Excessive Wharton’s Jelly/Edema
3. Conclusions and Future Directions
- Etiology: The fundamental molecular, genetic, and environmental causes of abnormal Wharton’s jelly development remain largely unknown. The pathways that lead to matrix degeneration, hypoplasia, or hyperplasia are poorly understood.
- Prenatal Diagnosis: A major deficiency exists in the ability of current ultrasound technology and screening protocols to reliably diagnose segmental absence of Wharton’s jelly prenatally. This “silent” pathology is responsible for some of the most catastrophic outcomes, yet it typically evades detection until after the adverse event has occurred.
- Standardized Protocols: There is a notable lack of consensus among international obstetric and ultrasound societies regarding the required depth and detail of a routine umbilical cord examination. Furthermore, for the majority of Wharton’s jelly pathologies, no standardized, evidence-based management protocols exist to guide clinicians in surveillance and delivery planning.
- Improved Diagnostics: Research efforts should be directed toward developing and validating novel diagnostic techniques that could improve the prenatal detection of subtle or segmental Wharton’s jelly abnormalities. This could include the application of high-frequency ultrasound transducers, advanced 3D/4D rendering techniques to better visualize the entire cord structure, or the investigation of potential biochemical markers in maternal serum or amniotic fluid that may reflect abnormal matrix turnover.
- Prospective Studies: There is a pressing need for large-scale, prospective, multicenter studies. Such studies are required to establish the true incidence of these pathologies, to identify risk factors, and to develop robust models for risk stratification. Specifically, studies that prospectively correlate sonographic measurements of Wharton’s jelly area with detailed postpartum placental pathology and long-term neonatal outcomes are needed to validate its potential use as a clinical marker of placental function.
- Basic Science Research: A renewed focus on the basic developmental biology of the umbilical cord is essential. Investigating the genetic and molecular pathways that govern the formation, maintenance, and degradation of the Wharton’s jelly matrix will provide the foundational knowledge needed to understand how and why these processes fail.
- Guideline Harmonization and Development: An international collaborative effort among professional societies is needed to harmonize clinical guidelines for the routine sonographic assessment of the umbilical cord. Consideration should be given to recommending a more detailed evaluation of Wharton’s jelly, particularly in pregnancies identified as high-risk for placental insufficiency or stillbirth. As more evidence becomes available, the development of standardized management algorithms will be crucial to ensure that pregnancies complicated by these pathologies receive optimal, evidence-based care.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brahmandam, G.; Lipsett, B.J. Anatomy, Abdomen and Pelvis: Umbilical Cord. In StatPearls; Updated 26 July 2025; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557389/ (accessed on 25 September 2025).
- Debebe, S.K.; Cahill, L.S.; Kingdom, J.C.; Whitehead, C.L.; Chandran, A.R.; Parks, W.T.; Serghides, L.; Baschat, A.; Macgowan, C.K.; Sled, J.G. Wharton’s jelly area and its association with placental morphometry and pathology. Placenta 2020, 94, 34–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Begum, F.; Mounika, P.; Pavane, M.; Ramya, C.H. Evaluation of role of umbilical cord anomalies in fetal death—An institutional experience. IP J. Diagn. Pathol. Oncol. 2022, 7, 178–182. [Google Scholar] [CrossRef]
- Santana, E.F.M.; Castello, R.G.; Rizzo, G.; Grisolia, G.; Júnior, E.A.; Werner, H.; Lituania, M.; Tonni, G. Placental and Umbilical Cord Anomalies Diagnosed by Two- and Three-Dimensional Ultrasound. Diagnostics 2022, 12, 2810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Troyer, D.L.; Weiss, M.L. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalluru, P.K.R.; Kalluru, H.R.; Allagadda, T.R.; Talur, M.; Gonepogu, M.C.; Gupta, S. Abnormal umbilical cord coiling and association with pregnancy factors. J. Turk. Gynecol. Assoc. 2024, 25, 44–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damasceno, E.B.; de Lima, P.P. Wharton’s jelly absence: A possible cause of stillbirth. Autops. Case Rep. 2013, 3, 43–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Liang, J.; Cui, H.; Wang, X.; Rong, H.; Shao, B.; Cui, H. Wharton’s jelly mesenchymal stem cells differentiate into retinal progenitor cells. Neural Regen. Res. 2013, 8, 1783–1792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drobiova, H.; Sindhu, S.; Ahmad, R.; Haddad, D.; Al-Mulla, F.; Al Madhoun, A. Wharton’s jelly mesenchymal stem cells: A concise review of their secretome and prospective clinical applications. Front. Cell Dev. Biol. 2023, 11, 1211217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, P.; Maurya, D.K. Wharton’s jelly mesenchymal stem cells: Future regenerative medicine for clinical applications in mitigation of radiation injury. World J. Stem Cells 2024, 16, 742–759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefańska, K.; Ożegowska, K.; Hutchings, G.; Popis, M.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Pieńkowski, W.; Gutaj, P.; Shibli, J.A.; et al. Human Wharton’s Jelly—Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J. Clin. Med. 2020, 9, 1102. [Google Scholar] [CrossRef]
- Thangappah, R.B.P.; Karnal, C.S.; Ravichandran, M.R.; Sowparnika, A.S.; Sundaravadivelu, P. Clinical significance of umbilical cord abnormalities: An observational study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2022, 11, 1727–1733. [Google Scholar] [CrossRef]
- Hammad, I.A.M.; Blue, N.R.; Allshouse, A.A.; Silver, R.M.; Gibbins, K.J.; Page, J.M.; Goldenberg, R.L.; Reddy, U.M.; Saade, G.R.; Dudley, D.J.; et al. NICHD Stillbirth Collaborative Research Network Group. Umbilical Cord Abnormalities and Stillbirth. Obstet. Gynecol. 2020, 135, 644–652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Redline, R.W. Placental Size, Shape, and Umbilical Cord Abnormalities (Chapter 16). In Placental and Gestational Pathology (Diagnostic Pediatric Pathology); Redline, R.W., Boyd, T.K., Roberts, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 157–180. [Google Scholar]
- Karaca, C.; Bostancıeri, N.; Ovayolu, A.; Kahraman, D.T. The effect of vascular complications of diabetes mellitus on human umbilical cord tissue and the number of Wharton Jelly's mesenchymal stem cells. Mol. Biol. Rep. 2020, 47, 9313–9323. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Gueye, M.; Mbodji, A.; Ndiaye, M.D. Absence of Wharton’s jelly around an umbilical artery. Int. J. Reprod. Contracept. Obstet. Gynecol. 2021, 11, 259–261. [Google Scholar] [CrossRef]
- Kulkarni, M.L.; Matadh, P.S.; Ashok, C.; Avinash, T.; Kulkarni, A.M. Absence of Wharton’s jelly around the umbilical arteries. Indian J. Pediatr. 2007, 74, 787–789. [Google Scholar] [CrossRef]
- Raio, L.; Ghezzi, F.; Di Naro, E.; Franchi, M.; Brühwiler, H.; Lüscher, K.P. Prenatal assessment of Wharton’s jelly in umbilical cords with single artery. Ultrasound Obstet. Gynecol. 1999, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Ennazhiyil, S.V.; Ramakrishnan, P.; Akshara, V.; Premlal, K.; Chitra, S.; Benjamin, W.; Nagalingam, S. Effects of gestational diabetes mellitus on umbilical cord morphology: A comparative study. J. Clin. Diagn. Res. 2019, 13, AC06–AC09. [Google Scholar] [CrossRef]
- Hemmati, F.; Barzegar, H.; Oboodi, R. Giant umbilical cord in a normal preterm infant: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundar Pavithira, P.; Raveenthiran, V. Giant Umbilical Cord due to Excessive Wharton’s Jelly. J. Pediatr. 2024, 274, 114195. [Google Scholar] [CrossRef]
- Dubetskyi, B.I.; Makarchuk, O.M.; Zhurakivska, O.Y.; Rymarchuk, M.I.; Andriets, O.A.; Lenchuk, T.L.; Delva, K.M.; Piron-Dumitrascu, M.; Bakun, O.V. Pregnancy and umbilical cord pathology: Structural and functional parameters of the umbilical cord. J. Med. Life 2023, 16, 1282–1291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coulter, J.B.; Scott, J.M.; Jordan, M.M. Oedema of the umbilical cord and respiratory distress in the newborn. Br. J. Obstet. Gynaecol. 1975, 82, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Martin, R. Wharton’s jelly umbilical cord cyst. Rev. Pediatr. Aten. Primaria 2016, 18, e121–e124. [Google Scholar]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Sepulveda, W. Beware of the umbilical cord ‘cyst’. Ultrasound Obstet. Gynecol. 2003, 21, 213–214. [Google Scholar] [CrossRef]
- Bergman, P.; Lundin, P.; Malmström, T. Mucoid Degeneration of Wharton’s Jelly. An Umbilical Cord Anomaly Threatening FŒTAL Life. Acta Obstet. Gynecol. Scand. 1961, 40, 372–378. [Google Scholar] [CrossRef]
- Kiran, H.; Kiran, G.; Kanber, Y. Pseudocyst of the umbilical cord with mucoid degeneration of Wharton’s jelly. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Tonni, G.; Lituania, M.; Cecchi, A.; Carboni, E.; Resta, S.; Bonasoni, M.P.; Ruano, R. Umbilical Cord Diseases Affecting Obstetric and Perinatal Outcomes. Healthcare 2023, 11, 2634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayes, D.J.L.; Warland, J.; Parast, M.M.; Bendon, R.W.; Hasegawa, J.; Banks, J.; Clapham, L.; Heazell, A.E.P. Umbilical cord characteristics and their association with adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0239630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Predanic, M.; Perni, S.C. Absence of a relationship between umbilical cord thickness and coiling patterns. J. Ultrasound Med. 2005, 24, 1491–1496. [Google Scholar] [CrossRef]
- Murphy, S.J.; Deegan, N.; O'LEary, B.D.; McParland, P. Absence of Wharton’s jelly. BMJ Case Rep. 2020, 13, e237222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lew, J.; Elmekkawi, A.; Kukreti, V. A giant cystic umbilical cord. Paediatr. Child Health 2019, 26, 12–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, C.K.Y.; Xean, K.Z.; Li, F.X.; Chandran, S. Umbilical cord anomalies: Antenatal ultrasound findings and postnatal correlation. BMJ Case Rep. 2018, 2018, bcr-2018-226651. [Google Scholar] [CrossRef]
- Bohîlțea, R.E.; Dima, V.; Ducu, I.; Iordache, A.M.; Mihai, B.M.; Munteanu, O.; Grigoriu, C.; Veduță, A.; Pelinescu-Onciul, D.; Vlădăreanu, R. Clinically Relevant Prenatal Ultrasound Diagnosis of Umbilical Cord Pathology. Diagnostics 2022, 12, 236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senapati, S.; Behera, S.S.; Chinara, P.K. Evaluation of Fetal Weight Sonographically Using Area of Wharton’s Jelly and Morphology of Umbilical Cord. Asian J. Pharm. Clin. Res. 2017, 10, 253–257. [Google Scholar] [CrossRef]
- Yuvaraj, H.; Aboda, A.; Pettigrew, I.; McCully, B. Angiomyxoma—A Rare Finding Associated with Congenital Cyst Malformation of the Umbilical Cord. Austin J. Clin. Case Rep. 2023, 10, 1283. [Google Scholar]
- Schiesser, M.; Lapaire, O.; Holzgreve, W.; Tercanli, S. Umbilical cord edema associated with patent urachus. Ultrasound Obstet. Gynecol. 2003, 22, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Sato, T.; Iwama, N.; Hamada, H.; Saito, M.; Takase, K. MR Imaging of Umbilical Cord Variations, Abnormalities, and Associated Placental Findings. Magn. Reson. Med. Sci. 2025, 24, 366–386. [Google Scholar] [CrossRef]
- Botezatu, R.; Raduteanu, S.; Ciobanu, A.M.; Gica, N.; Peltecu, G.; Panaitescu, A.M. Absence of Wharton’s Jelly at the Abdominal Site of the Umbilical Cord Insertion. Rare Case Report and Review of the Literature. Medicina 2021, 57, 1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masselli, G.; Gualdi, G. MR imaging of the placenta: What a radiologist should know. Abdom. Imaging 2012, 38, 573–587. [Google Scholar] [CrossRef]
- Available online: https://fetalmedicine.org/education/fetal-abnormalities/placenta-umbilical-cord/umbilical-cord-cyst (accessed on 20 August 2025).
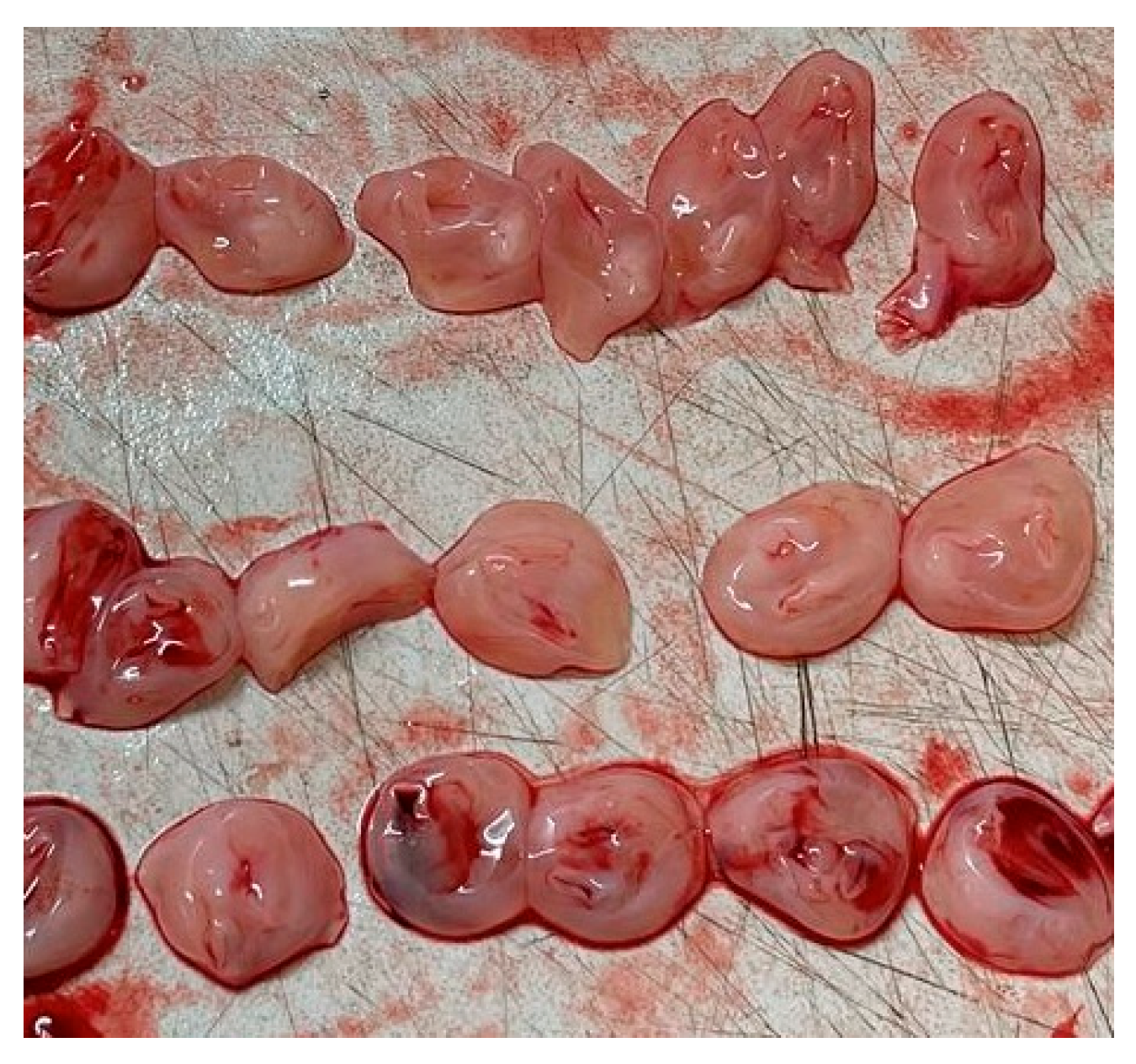
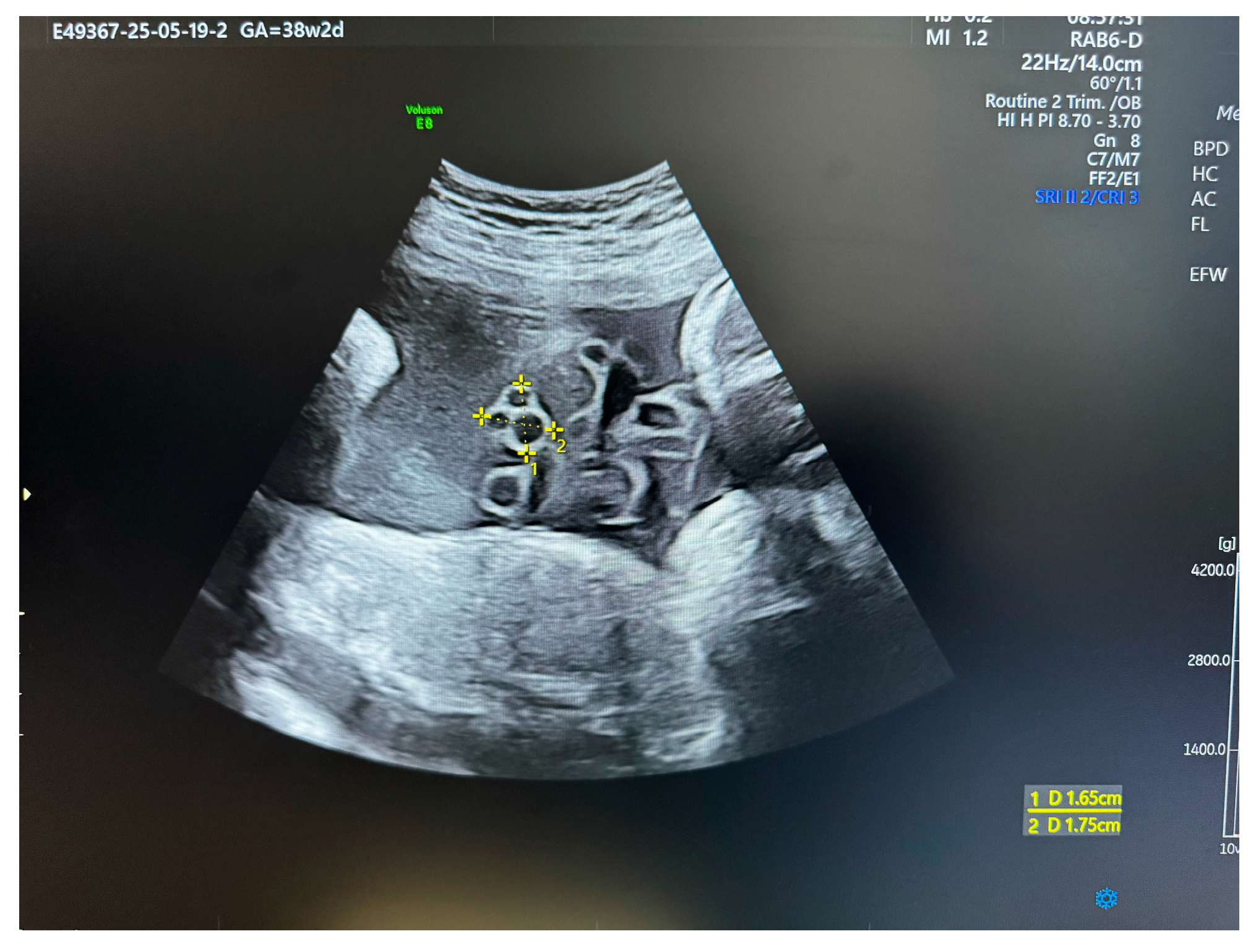
| Pathology | Key Sonographic and Histopathological Features | Associated Clinical Conditions (Maternal/Fetal) | Common Fetal/Neonatal Outcomes |
|---|---|---|---|
| Absence/Reduction (Thin Cord) | Cord diameter <10th percentile; decreased WJ area on ultrasound; constriction/stricture; exposed vessels; absent or attenuated matrix on histology [7]. | Single umbilical artery (SUA), early-onset preeclampsia, gestational diabetes, oligohydramnios [2] | Stillbirth, perinatal death, fetal growth restriction (FGR), fetal intolerance to labor, non-reassuring fetal heart rate, emergency cesarean section, low Apgar scores, NICU admission [2,14] |
| Edema/Excess (Thick Cord) | Cord diameter >90th percentile (>2 cm); giant cord (>5 cm); less dense, swollen WJ matrix; potential for cavity formation and hemorrhages [15] | Maternal diabetes, hydrops fetalis, Beckwith-Wiedemann syndrome, chromosomal abnormalities (rare) [15] | Often benign if isolated; outcomes are typically related to the underlying condition. Severe edema can cause vascular compression leading to fetal distress or demise [23] |
| Pseudocysts | Anechoic, avascular, non-epithelial lined space within WJ; can be single or multiple, variable in size and location [27] | Trisomy 18, Trisomy 13, omphalocele, patent urachus, hydrops, other structural anomalies [25] | Favorable if isolated and transient in the first trimester. High risk of miscarriage, intrauterine death (IUD), and neonatal complications if persistent, multiple, or associated with other anomalies [25] |
| Mucoid Degeneration | Histopathological finding of matrix liquefaction and breakdown; precursor to pseudocysts and potentially segmental absence of WJ [28] | Considered the underlying process for pseudocysts and some cases of absent WJ. | Threatens fetal life by compromising the structural integrity of the cord, leading to the risks associated with pseudocysts and absent WJ [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butureanu, T.-A. The Pathophysiology of Wharton’s Jelly and Its Impact on Fetal and Neonatal Outcomes: A Comprehensive Literature Review. Med. Sci. 2025, 13, 215. https://doi.org/10.3390/medsci13040215
Butureanu T-A. The Pathophysiology of Wharton’s Jelly and Its Impact on Fetal and Neonatal Outcomes: A Comprehensive Literature Review. Medical Sciences. 2025; 13(4):215. https://doi.org/10.3390/medsci13040215
Chicago/Turabian StyleButureanu, Tudor-Andrei. 2025. "The Pathophysiology of Wharton’s Jelly and Its Impact on Fetal and Neonatal Outcomes: A Comprehensive Literature Review" Medical Sciences 13, no. 4: 215. https://doi.org/10.3390/medsci13040215
APA StyleButureanu, T.-A. (2025). The Pathophysiology of Wharton’s Jelly and Its Impact on Fetal and Neonatal Outcomes: A Comprehensive Literature Review. Medical Sciences, 13(4), 215. https://doi.org/10.3390/medsci13040215






