The Role of Delayed Interval Debulking Surgery (DIDS) in the Surgical Treatment of Advanced Epithelial Ovarian Cancer: A Retrospective Cohort from an ESGO-Certified Center †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics
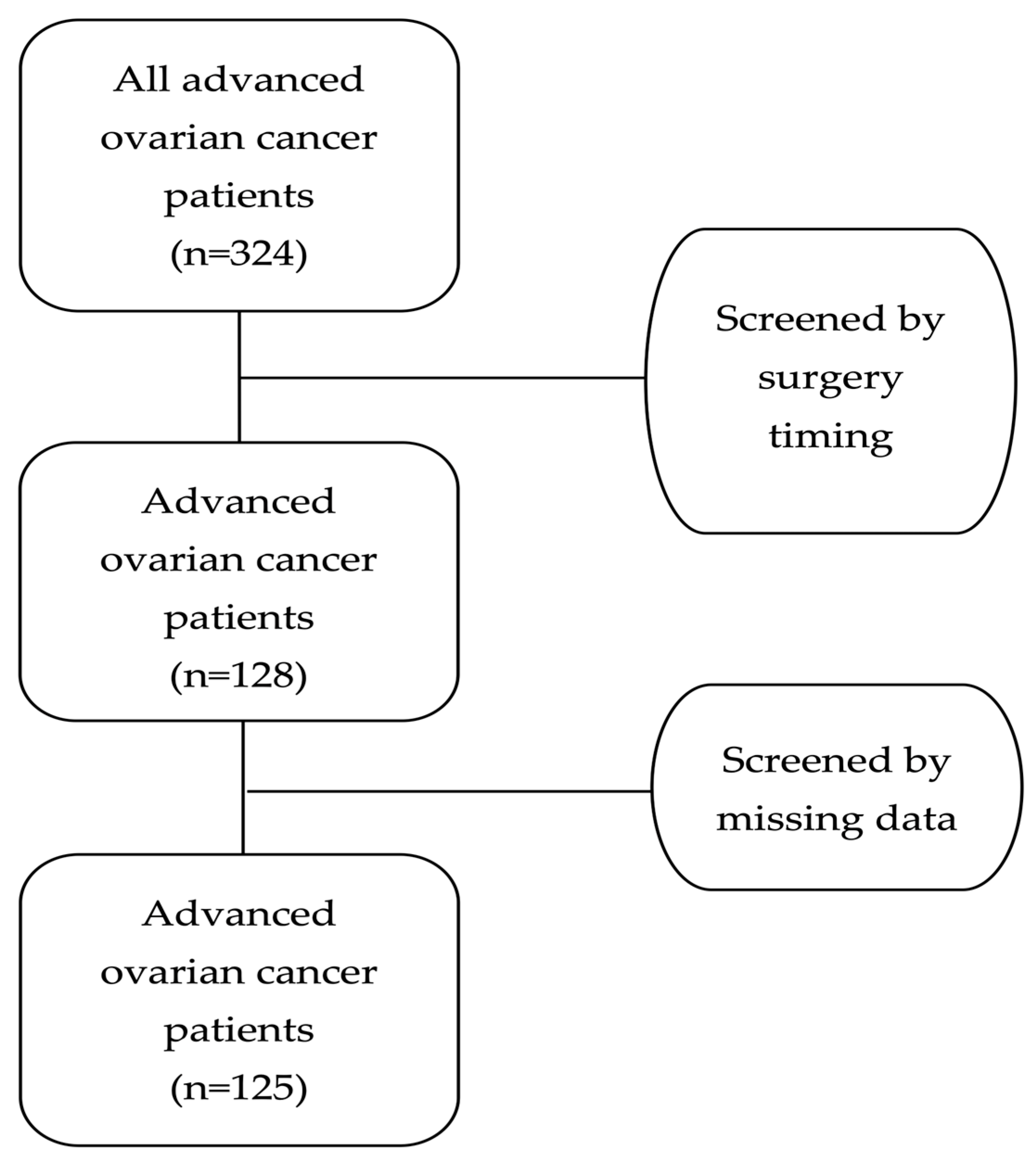
2.2. Patients
- Inclusion criteria:
- Age at diagnosis ≥ 18 years.
- Histologically confirmed epithelial ovarian carcinoma.
- Patients ultimately underwent surgery at out Gynecological–Oncology Unit.
- Exclusion criteria:
- Primary debulking surgery.
- Recurrent ovarian cancer.
- Incomplete or missing registry data.
2.3. Ethics
2.4. Data Collection
- Patient identification number;
- Age at diagnosis;
- Body Mass Index (BMI);
- Charlson Comorbidity Index (CCI);
- Number of NACT cycles;
- Histological types;
- Aletti score;
- CA-125 level;
- FIGO classification (2014 International Federation of Gynecology and Obstetrics staging system);
- Intraoperative blood loss;
- Duration of surgery;
- Intensive Care Unit (ICU) admission;
- Clavien–Dindo classification for postoperative complications;
- Hospital stay length;
- Residual disease (RD) after cytoreduction;
- Timeline data:
- ○
- Date of surgery;
- ○
- Date of recurrence;
- ○
- Date of last follow-up or death.
2.5. Statistical Analysis
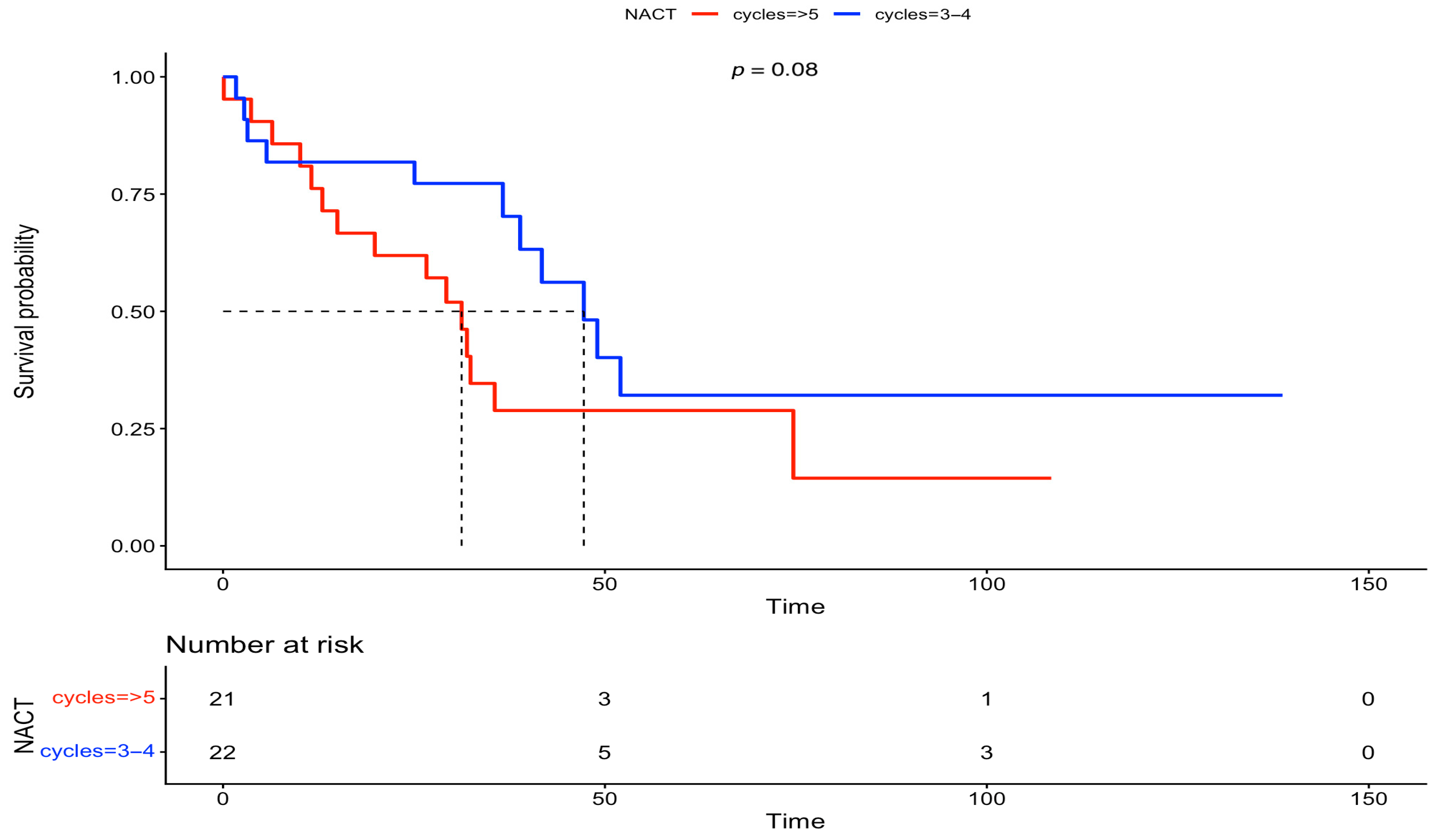
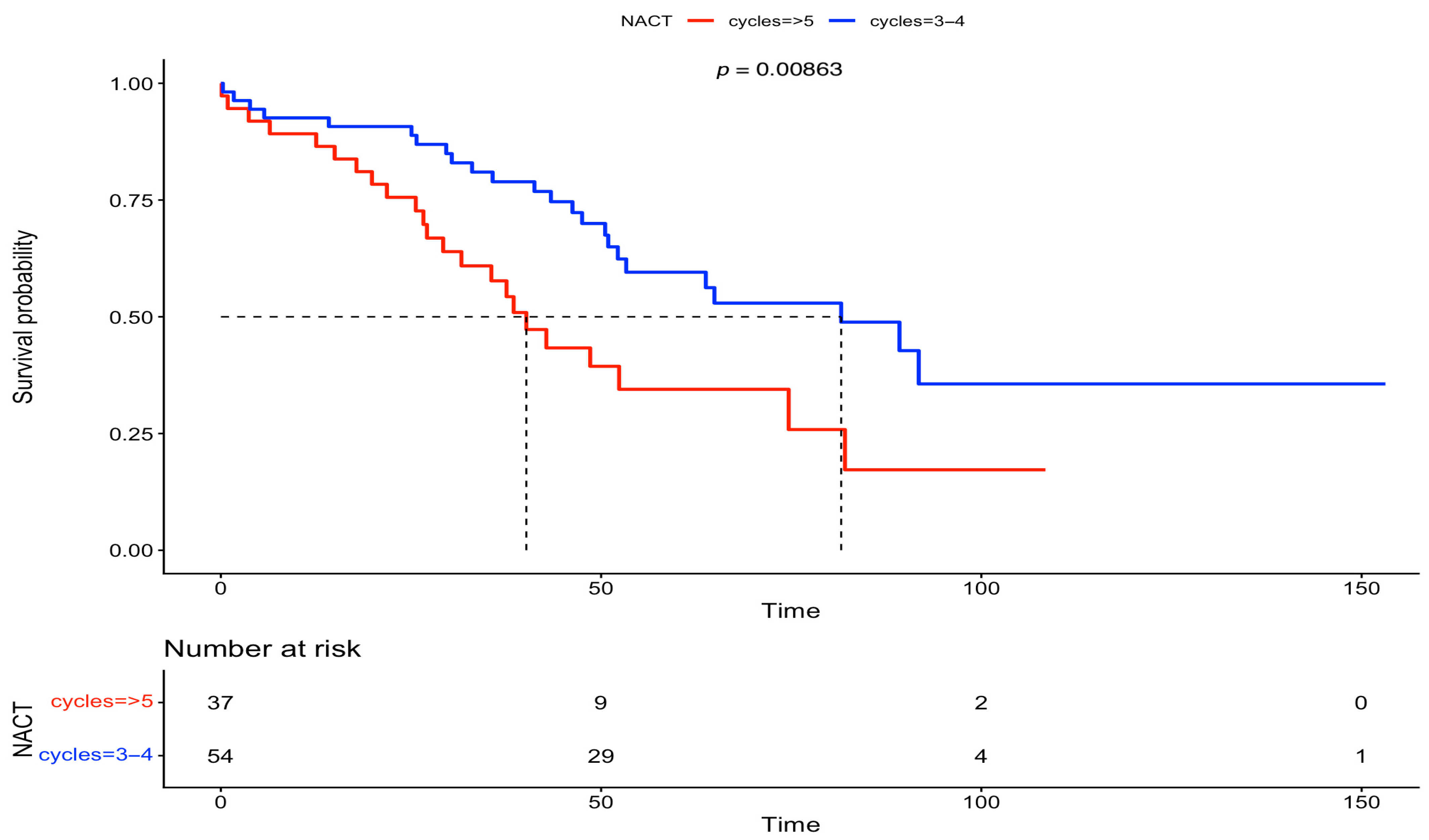
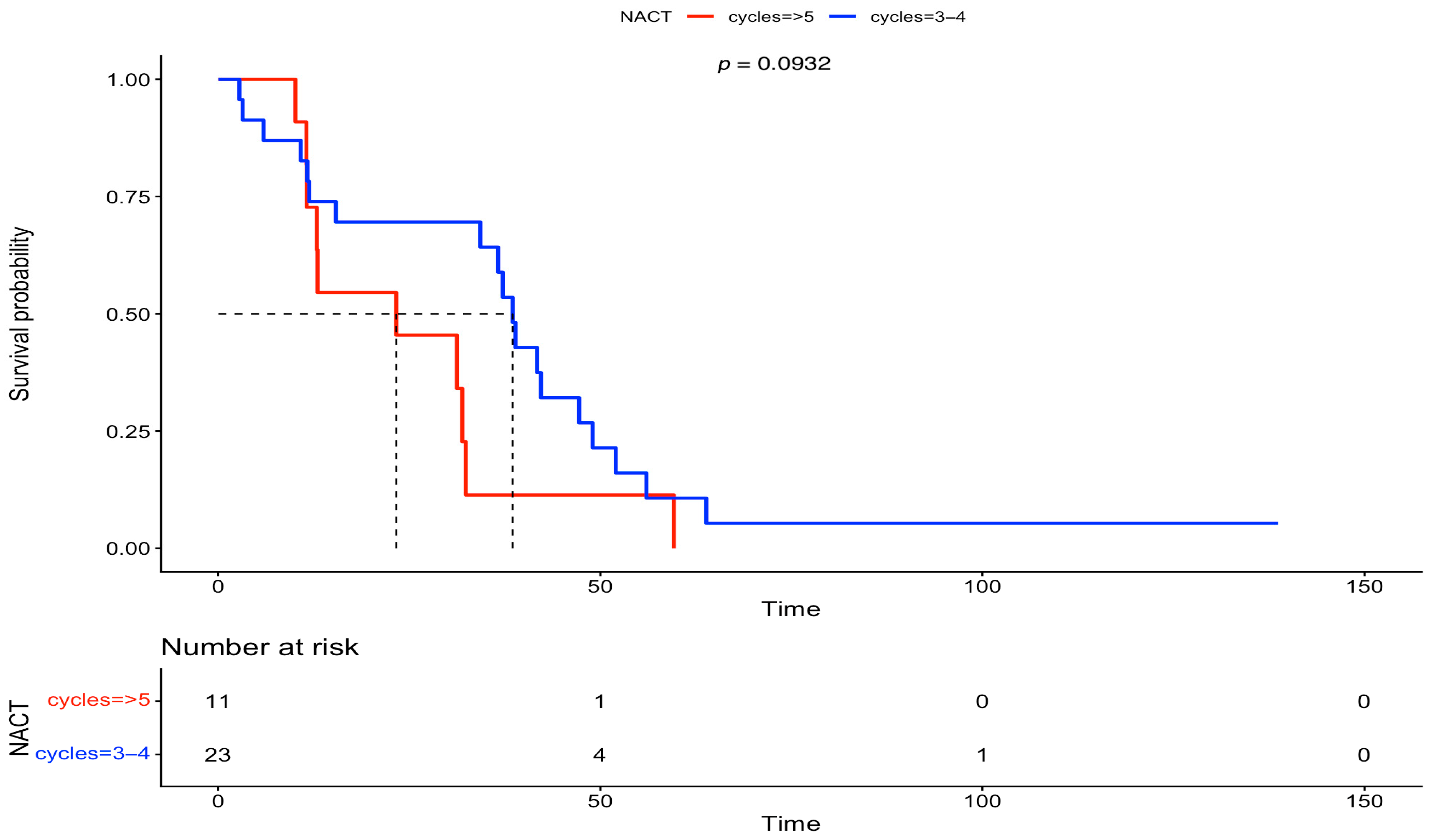
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DIDS | Delayed interval debulking surgery |
| IDS | Interval debulking surgery |
| NACT | Neoadjuvant chemotherapy |
| BMI | Body mass index |
| ICU | Intensive care unit |
| FIGO | International Federation of Gynecology and Obstetrics |
| PDS | Primary debulking surgery |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| KELIM | CA-125 elimination rate constant K |
| CCI | Charlson Comorbidity Index |
| RD | Residual disease |
| OS | Overall survival |
| PFS | Progression-free survival |
| COVID-19 | Coronavirus Disease 2019 |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.-M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol. Oncol. 2016, 143, 3–15. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Gueli Alletti, S.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Mahner, S.; Heitz, F.; Salehi, S.; Reuss, A.; Guyon, F.; Du Bois, A.; Harter, P.; Fotopoulou, C.; Querleu, D.; Mosgaard, B.J.; et al. TRUST: Trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). J. Clin. Oncol. 2025, 43, 17. [Google Scholar] [CrossRef]
- Classe, J.-M.; Ferron, G.; Ouldamer, L.; Gauthier, T.; Emambux, S.; Gladieff, L.; Dupre, P.-F.; Anota, A. CHRONO: Randomized trial of the CHROnology of surgery after Neoadjuvant chemotherapy for Ovarian cancer. Int. J. Gynecol. Cancer 2022, 32, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Faruqi, A.; Said, I.; Lockley, M.; Brockbank, E.; Jeyarajah, A.; Fitzpatrick, A.; Ennis, D.; Dowe, T.; Santos, J.L.; et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarian High-Grade Serous Carcinoma. J. Clin. Oncol. 2015, 33, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.J.; van de Vrie, R.; Bruining, A.; Spijkerboer, A.M.; Mol, B.W.; Kenter, G.G.; Buist, M.R. Predicting surgical outcome in patients with International Federation of Gynecology and Obstetrics stage III or IV ovarian cancer using computed tomography: A systematic review of prediction models. Int. J. Gynecol. Cancer 2015, 25, 407–415. [Google Scholar] [CrossRef] [PubMed]
- van de Vrie, R.; Rutten, M.J.; Asseler, J.D.; Leeflang, M.M.; Kenter, G.G.; Mol, B.W.J.; Buist, M. Laparoscopy for diagnosing resectability of disease in women with advanced ovarian cancer. Cochrane Database Syst. Rev. 2019, 3, CD009786. [Google Scholar] [CrossRef] [PubMed]
- Zouzoulas, D.; Tsolakidis, D.; Tzitzis, P.; Sofianou, I.; Chatzistamatiou, K.; Theodoulidis, V.; Topalidou, M.; Timotheadou, E.; Grimbizis, G. The Use of CA-125 KELIM to Identify Which Patients Can Achieve Complete Cytoreduction after Neoadjuvant Chemotherapy in High-Grade Serous Advanced Ovarian Cancer. Cancers 2024, 16, 1266. [Google Scholar] [CrossRef] [PubMed]
- Krankenberg, D.J.; Muallem, M.Z.; Pietzner, K.; Chekerov, R.; Armbrust, R.; Beteta, C.; Schöning, W.; Lee, M.; Klews, J.; Sehouli, J. Ovarian cancer management in an ESGO ovarian cancer center of excellence: A systematic case study of the interprofessional and interdisciplinary interaction. Arch. Gynecol. Obstet. 2024, 309, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, E.; Boubli, B.; Floquet, A.; Brouste, V.; Sire, M.; Croce, S.; Thomas, L.; Guyon, F. Optimal timing of interval debulking surgery in advanced ovarian cancer: Yet to be defined? Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Yoneoka, Y.; Ishikawa, M.; Uehara, T.; Shimizu, H.; Uno, M.; Murakami, T.; Kato, T. Treatment strategies for patients with advanced ovarian cancer undergoing neoadjuvant chemotherapy: Interval debulking surgery or additional chemotherapy? J. Gynecol. Oncol. 2019, 30, e81. [Google Scholar] [CrossRef] [PubMed]
- Plett, H.; Filippova, O.T.; Garbi, A.; Kommoss, S.; Rosendahl, M.; Langstraat, C.; Phadnis, S.; Muallem, M.Z.; Baert, T.; Chi, D.S.; et al. Role of delayed interval debulking for persistent residual disease after more than 5 cycles of chemotherapy for primary advanced ovarian cancer. An international multicenter study. Gynecol. Oncol. 2020, 159, 434–441. [Google Scholar] [CrossRef]
- Yao, S.-E.; Tripcony, L.; Sanday, K.; Robertson, J.; Perrin, L.; Chetty, N.; Land, R.; Garrett, A.; Obermair, A.; Nascimento, M.; et al. Survival outcomes after delayed cytoreduction surgery following neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2020, 30, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Arevalo, O.; Gysler, S.; Ko, E.M.; Cory, L.; Kim, S.H.; Giuntoli, R.L.; Latif, N.A. Impact of delayed interval cytoreductive surgery on the survival of patients with advanced stage high-grade epithelial ovarian carcinoma. Int. J. Gynecol. Cancer 2024, 34, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gaba, F.; Blyuss, O.; Ash, K. GO SOAR Collaborators Survival after interval and delayed cytoreduction surgery in advanced ovarian cancer: A Global Gynaecological Oncology Surgical Outcomes Collaborative-Led Study (GO SOAR2). Int. J. Gynecol. Cancer 2025, 35, 101650. [Google Scholar] [CrossRef] [PubMed]




| Number of Patients (N) | Percentage (%) | ||
|---|---|---|---|
| Age (years) | mean: 60.8 | SD: 12.4 | |
| BMI (kg/m2) | mean: 28.1 | SD: 5.6 | |
| CCI | median: 2 | IQR: 1–3 | |
| 0–2 | 73 | 58.4 | |
| 3–4 | 34 | 27.2 | |
| ≥5 | 18 | 14.4 | |
| NACT cycles | |||
| 3–4 | 77 | 61.6 | |
| ≥5 | 48 | 38.4 | |
| FIGO stage | |||
| III | 82 | 65.6 | |
| IV | 43 | 34.4 | |
| Aletti score | median: 4 | IQR: 3–6 | |
| ≤3 | 50 | 40 | |
| 4–7 | 59 | 47.2 | |
| ≥8 | 16 | 12.8 | |
| Histological types | |||
| Serous | 88 | 70.4 | |
| Endometrioid | 17 | 13.6 | |
| Clear-cell | 9 | 7.2 | |
| Undifferentiated | 7 | 5.6 | |
| Mixed | 4 | 3.2 | |
| CA-125 (U/mL) | median: 32 | IQR: 14.7–117 | |
| Intraoperative blood loss (mL) | median: 300 | IQR: 200–500 | |
| Surgery duration (min) | median: 230 | IQR: 180–300 | |
| Clavien–Dindo classification | median: 24.2 | IQR: 12.2–32 | |
| Grade ≥ III | 15 | 12 | |
| ICU admission | 28 | 22.4 | |
| Hospital stay (days) | median: 8 | IQR: 7–9 | |
| Residual disease (cm) | |||
| 0 | 91 | 72.8 | |
| <1 | 24 | 19.2 | |
| ≥1 | 10 | 8 | |
| Characteristics | Group A (IDS) 77 (61.6%) | Group B (DIDS) 48 (38.4%) | p-Value | |
| Age (years) mean (SD) | 59.7 (13) | 62.6 (11.3) | 0.2036 | |
| BMI (kg/m2) mean (SD) | 28.1 (5.6) | 28.2 (5.8) | 0.9302 | |
| CCI median (IQR) | 2 (1–3) | 2 (1–4) | 0.6319 | |
| FIGO stage | 0.08231 | |||
| III | 55 (71.4%) | 27 (56.3%) | ||
| IV | 22 (28.6%) | 21 (43.7%) | ||
| Aletti score median (IQR) | 4 (3–5.75) | 4 (2–6) | 0.8825 | |
| Histological types | 0.8831 | |||
| Serous | 55 (71.4%) | 33 (68.8%) | ||
| Other | 22 (28.6%) | 15 (31.2%) | ||
| CA-125 (U/mL) median (IQR) | 34.2 (12.2–30.2) | 24.9 (11.7–111) | 0.2841 | |
| Intraoperative blood loss (mL) median (IQR) | 300 (200–500) | 450 (300–525) | <0.05 | |
| Surgery duration (min) median (IQR) | 210 (150–300) | 240 (180–330) | 0.176 | |
| Clavien–Dindo classification median (IQR) | 22.6 (12.2–32) | 24.2 (15–32) | 0.4524 | |
| Clavien–Dindo classification Grade ≥ III | 9 (60%) | 6 (40%) | 0.754 | |
| ICU admission | 0.1519 | |||
| Yes | 14 (18.2%) | 14 (29.2%) | ||
| No | 63 (81.8%) | 34 (70.8%) | ||
| Hospital stay (days) median (IQR) | 8 (7–9) | 8 (6–10) | 0.867 | |
| Residual disease (cm) | 0.5791 | |||
| 0 | 54 (70.1%) | 37 (77.1%) | ||
| <1 | 17 (22.1%) | 7 (14.6%) | ||
| ≥1 | 6 (7.8%) | 4 (8.3%) | ||
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Age (years) | 1.01 | 0.99, 1.02 | 0.587 | ||||
| BMI (kg/m2) | 1.01 | 0.96, 1.07 | 0.688 | ||||
| CCI | 1.08 | 0.97, 1.29 | 0.196 | 1.09 | 0.98, 1.22 | 0.104 | |
| NACT cycles | |||||||
| 3–4 | 0.88 | 0.57, 1.36 | 0.56 | ||||
| ≥5 | 1 | 1 | 1 | ||||
| FIGO stage | |||||||
| III | 1 | 1 | 1 | 1 | 1 | 1 | |
| IV | 1.69 | 1.07, 2.68 | 0.0258 | 1.48 | 0.90, 2.42 | 0.12 | |
| Aletti score | 1.14 | 1.02, 1.27 | 0.0167 | ||||
| Histological types | |||||||
| Serous | 0.90 | 0.40, 2.02 | 0.796 | ||||
| Other | 1 | 1 | 1 | ||||
| CA-125 (U/mL) | 1 | 1, 1 | 0.051 | ||||
| Intraoperative blood loss (mL) | 1 | 0.99, 1.001 | 0.419 | ||||
| Surgery duration (min) | 1.004 | 1.001, 1.01 | <0.01 | ||||
| Clavien–Dindo classification | 1.02 | 1.007, 1.04 | <0.01 | 1.03 | 1.01, 1.05 | <0.01 | |
| ICU | |||||||
| Yes | 1.32 | 0.77, 2.25 | 0.317 | ||||
| No | 1 | 1 | 1 | ||||
| Hospital stay (days) | 1.03 | 0.98, 1.10 | 0.263 | ||||
| Residual disease (cm) | |||||||
| 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| <1 | 1.18 | 0.71, 1.95 | 0.532 | 0.97 | 0.57, 1.66 | 0.920 | |
| ≥1 | 3.51 | 1.68, 7.35 | <0.01 | 2.84 | 1.33, 6.06 | <0.01 | |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Age (years) | 1.02 | 0.99, 1.07 | 0.094 | 1.02 | 0.99, 1.04 | 0.058 | |
| BMI (kg/m2) | 0.99 | 0.94, 1.05 | 0.78 | ||||
| CCI | 0.97 | 0.87, 1.09 | 0.614 | 1.11 | 0.96, 1.28 | 0.160 | |
| NACT cycles | |||||||
| 3–4 | 1 | 1 | 1 | 1 | 1 | 1 | |
| ≥5 | 1.83 | 1.16, 2.9 | <0.01 | 1.84 | 1.15, 2.96 | 0.0116 | |
| FIGO stage | |||||||
| III | 1 | 1 | 1 | ||||
| IV | 1.45 | 0.90, 2.34 | 0.13 | ||||
| Aletti score | 1.04 | 0.93, 1.16 | 0.477 | ||||
| Histological types | |||||||
| Serous | 0.75 | 0.22, 2.49 | 0.633 | ||||
| Others | 1 | 1 | 1 | ||||
| CA-125 (U/mL) | 1 | 1, 1.001 | 0.018 | ||||
| Intraoperative blood loss (mL) | 1.001 | 1, 1.001 | <0.01 | ||||
| Surgery duration (min) | 0.99 | 0.99, 1.002 | 0.63 | ||||
| Clavien–Dindo classification | 1.01 | 0.99, 1.03 | 0.181 | 1.02 | 1.002, 1.04 | 0.0472 | |
| ICU | |||||||
| Yes | 1.13 | 0.64, 2.01 | 0.674 | ||||
| No | 1 | 1 | 1 | ||||
| Hospital stay (days) | 0.96 | 0.90, 1.03 | 0.247 | ||||
| Residual disease (cm) | |||||||
| 0 | 0.23 | 0.11, 0.47 | <0.01 | 0.18 | 0.08, 0.38 | <0.01 | |
| <1 | 0.53 | 0.24, 1.19 | 0.125 | 0.48 | 0.21, 1.11 | 0.085 | |
| ≥1 | 1 | 1 | 1 | 1 | 1 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouzoulas, D.; Sofianou, I.; Tzitzis, P.; Theodoulidis, V.; Chatzistamatiou, K.; Timotheadou, E.; Grimbizis, G.; Tsolakidis, D. The Role of Delayed Interval Debulking Surgery (DIDS) in the Surgical Treatment of Advanced Epithelial Ovarian Cancer: A Retrospective Cohort from an ESGO-Certified Center. Med. Sci. 2025, 13, 217. https://doi.org/10.3390/medsci13040217
Zouzoulas D, Sofianou I, Tzitzis P, Theodoulidis V, Chatzistamatiou K, Timotheadou E, Grimbizis G, Tsolakidis D. The Role of Delayed Interval Debulking Surgery (DIDS) in the Surgical Treatment of Advanced Epithelial Ovarian Cancer: A Retrospective Cohort from an ESGO-Certified Center. Medical Sciences. 2025; 13(4):217. https://doi.org/10.3390/medsci13040217
Chicago/Turabian StyleZouzoulas, Dimitrios, Iliana Sofianou, Panagiotis Tzitzis, Vasilis Theodoulidis, Kimon Chatzistamatiou, Eleni Timotheadou, Grigoris Grimbizis, and Dimitrios Tsolakidis. 2025. "The Role of Delayed Interval Debulking Surgery (DIDS) in the Surgical Treatment of Advanced Epithelial Ovarian Cancer: A Retrospective Cohort from an ESGO-Certified Center" Medical Sciences 13, no. 4: 217. https://doi.org/10.3390/medsci13040217
APA StyleZouzoulas, D., Sofianou, I., Tzitzis, P., Theodoulidis, V., Chatzistamatiou, K., Timotheadou, E., Grimbizis, G., & Tsolakidis, D. (2025). The Role of Delayed Interval Debulking Surgery (DIDS) in the Surgical Treatment of Advanced Epithelial Ovarian Cancer: A Retrospective Cohort from an ESGO-Certified Center. Medical Sciences, 13(4), 217. https://doi.org/10.3390/medsci13040217





