Abstract
Background: The biosynthesis of Lewis (Le) antigens depends on the FUT3 gene, encoding an α(1,3/4)-fucosyltransferase. Individuals lacking functional FUT3 exhibit a Le(a–b–) phenotype, regardless of secretor status. Methods: This study determined the prevalence of FUT3 single nucleotide variants (SNVs) in Thai blood donors and characterised genotype and allele distributions. We also examined the association between FUT3 variants and the presence of Le antibodies to better understand variability in immune responses. A total of 112 blood donors were recruited, comprising 52 non-responders and 60 responders for Le antibody detection. The FUT3 coding sequence was amplified by polymerase chain reaction and directly sequenced to identify single nucleotide variants (SNVs) and haplotypes. Results: Associations between FUT3 SNVs, haplotypes, and Le antibody responsiveness were subsequently analysed. Thirteen FUT3 SNVs were identified, with c.59T>G (rs28362459) present in all Le(a–b–) cases. The FUT3*01N.17.03 (le59,1067) haplotype was most common (0.634) and showed the strongest association with Le antibody responsiveness (adjusted OR = 3.052, 95% CI: 1.683–5.534, p < 0.0001). Differences in antibody types, isotypes, and the FUT3*01N.17.03 genotype between groups were not statistically significant. Conclusions: This first study characterises FUT3 variations in Le(a–b–) Thai blood donors and identifies FUT3*01N.17.03 as associated with Le antibody responsiveness, highlighting its relevance for population-specific genetic diagnostics in transfusion medicine.
1. Introduction
The International Society of Blood Transfusion (ISBT) Working Party on Red Cell Immunogenetics and Blood Group Terminology designated the Lewis (Le) blood group system ISBT007, which is distinct from other blood group systems in that its glycan antigens are adsorbed onto the red cell membrane from various bodily fluids—plasma—rather than being synthesised intrinsically by erythrocytes [1]. Le antigens are carbohydrate moieties present on fucosylated glycosphingolipids and glycoproteins. They are synthesised by epithelial cells of the digestive, bronchial, mammary, seminal, urinary, and orbital tissues and are secreted either externally via exocrine pathways or into the interstitial space, from which they enter the plasma [2]. Le glycolipids are primarily derived from the epithelium of the digestive tract [3], whereas the glycoprotein-associated forms largely remain in plasma and other body fluids. The system currently comprises six antigens, including five polymorphic antigens (Lea, Leb, LebH, ALeb, and BLeb) and one high-prevalence antigen (Leab) [4].
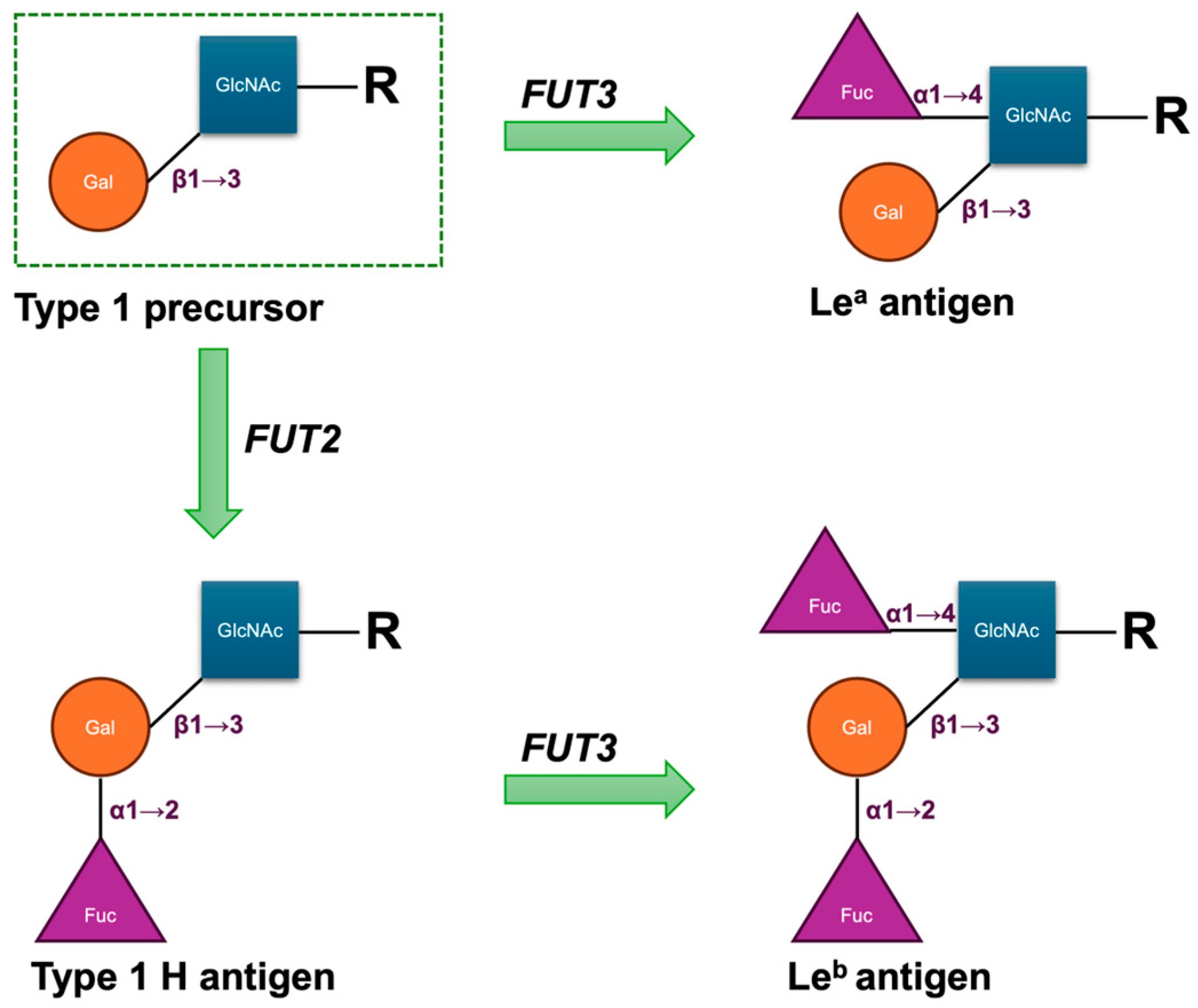
The biosynthesis of Le antigens depends on the LE or FUT3 gene, which encodes an α1,3/4-L-fucosyltransferase (FucT) [5]. The gene is located on chromosome 19q13.3 and comprises three exons spanning approximately 8 kbp of genomic DNA. Its primary product is an enzyme that transfers fucose in an α1→4 linkage to the penultimate N-acetylglucosamine of type 1 carbohydrate precursor chains, which are attached to proteins or lipids on the cell surface. Either the Lea or Leb antigen is formed depending on the sequential functionally activities of the FUT3 gene and the Secretor gene (FUT2), which encodes the α1,2-L-FucT. In secretors, the enzyme catalyses the addition of a L-fucose residue to the Type 1 H antigen to form the Leb antigen; in non-secretors, the enzyme catalyses the addition of the L-fucose residue to the Type 1 precursor to produce the Lea antigen (Figure 1). Consequently, as these structures are acquired from the plasma by the red cell membrane, red cells of most H secretors are Le(a–b+), and those of most H non-secretors are Le(a+b–). The Lewis transferase can also convert Type 1 A to ALeb and Type 1 B to BLeb; these are the result of the sequential actions of the FUT2, FUT3, and ABO genes [6]. The weak secretor allele (Sew), frequently observed in East Asian and Pacific populations, encodes a product with reduced competitive efficiency compared with the functional Se allele. This diminished activity favours enhanced Lea synthesis, such that individuals with either the Sew/Sew or Sew/se genotype secrete both Lea and Leb and generally exhibit the Le(a+b+) phenotype on red cells [6,7]. Fundamentally, the FUT3 locus comprises two principal alleles: Le, which encodes an active α1,4-L-FucT, and le, which lacks functional activity. Individuals homozygous for le fail to produce either Lea or Leb and consequently exhibit the Le(a–b–) red cell phenotype, irrespective of their ABH group or secretor status [6].
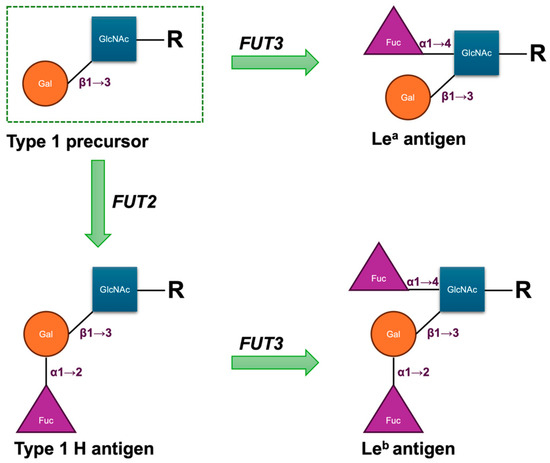
Figure 1.
Structural scheme of antigenic domains in Type 1 chain precursors and Lewis antigens. Fuc, fucose; Gal, galactose; GlcNAc, N-acetylglucosamine; R, side chain.
The Lea and Leb antigens are the most clinically relevant and are frequently encountered in practice [6]. Four principal Le phenotypes have been described. The reported frequencies of the Le(a+b–) and Le(a–b+) phenotypes are approximately 22% and 72% in Caucasians, 23% and 55% in Black populations, and 0.2% and 73% in Japanese populations, respectively [8]. The Le(a+b+) phenotype, while rare among European and African populations, occurs relatively commonly in East and Southeast Asia, the Pacific region, and Australasia, with a prevalence ranging from 10% to 40% [9,10,11]. In addition, studies in Black populations have demonstrated a comparatively higher frequency of the Le(a–b–) phenotype, with a prevalence of around 22% [6,12]. Three Thai cohorts of blood donors tested with anti-Lea and anti-Leb consistently showed Le(a–b+) as the predominant phenotype (51–66%), followed by Le(a+b–) (11–31%) and Le(a–b–) (18–24%), with little variation across decades. The Le(a+b+) phenotype, however, was not detected in these cohorts [13,14,15].
As with anti-Lea, anti-Leb antibodies occur primarily in individuals with the Le(a–b–) red cell phenotype [6]. Although Le antibodies are relatively common, they rarely cause haemolytic transfusion reactions (HTRs), with only a few reports for anti-Lea [16] and even fewer for anti-Leb [17]. This is largely because most are inactive at 37 °C, are neutralised by donor plasma, or are eluted from transfused Le(a–b–) red cells. As a result, they do not accelerate clearance of antigen-positive cells in vivo [18], allowing patients to receive indirect antiglobulin test (IAT)-compatible transfusions, and they rarely induce haemolytic disease of the foetus and newborn (HDFN), since Le antigens are present in foetal secretions but generally absent on foetal red cells [6]. Moreover, Le antibodies function as histocompatibility antibodies in renal transplantation, with Le(a–b–) recipients showing significantly reduced two-year graft survival compared with Le-positive recipients [19,20]. In Thailand, the relatively high prevalence of the Le(a–b–) phenotype contributes to the frequent detection of Le antibodies in both donors and patients [21]. To ensure transfusion safety, national practice mandates the use of Le(a–b–)-compatible blood units for patients who acquire these antibodies.
Allelic variation at the LE locus shows marked differences among populations and ethnic groups, and the genotype–phenotype relationship is often complicated [22,23]. The FUT3 gene spans 8562 bp and contains three exons, with the coding sequence (1086 bp) located entirely within the third exon [1]. Salomaa et al. reported that 90–95% of Le-negative individuals can be identified by screening for four single-nucleotide variants (SNVs)—c.59T>G (rs28362459), c.1067T>A (rs3894326), c.202T>C, and c.314C>T—which are relatively common in the general population [22]. The ISBT has catalogued several SNVs in both FUT3-active and -inactive individuals across diverse ethnic groups to date [1]. To our knowledge, no study has yet estimated the distribution of FUT3 gene SNVs in the Thai population.
Although anti-Lea is not uncommon, it is detected in only about 1 in 300 sera in Denmark and France [24]. Agglutinating alloanti-Lea is confined to individuals with Le(a–b–) red cells, predominantly ABH secretors, and occurs less frequently in group O than in other ABO types [24,25]; unlike anti-Lea, these individuals who possessed anti-Leb are generally ABH non-secretors [25]. No study has conclusively shown whether FUT3 genetic variation contributes to individual differences in anti-Le responsiveness. Certain SNVs may alter the α1,3/4-L-FucT binding region, reducing or abolishing enzyme activity and thereby affecting Le antigen expression. Yet, the wide allelic diversity of FUT3, population-specific distributions, and heterogeneous study designs have yielded inconsistent findings [1]. This underscores the need for systematic investigation of FUT3 polymorphisms and their role in Le antibody formation, particularly in under-studied populations. We aimed to determine the prevalence of FUT3 SNVs in a Thai blood donor population and to characterise the distribution of FUT3 genotypes and allele frequencies. In addition, we investigated the relationship between FUT3 SNVs, alleles, and the presence of Le antibodies in sera to provide further insight into the variability of immune responses to Le antigens.
2. Materials and Methods
2.1. Study Design, Population, and Data Collection
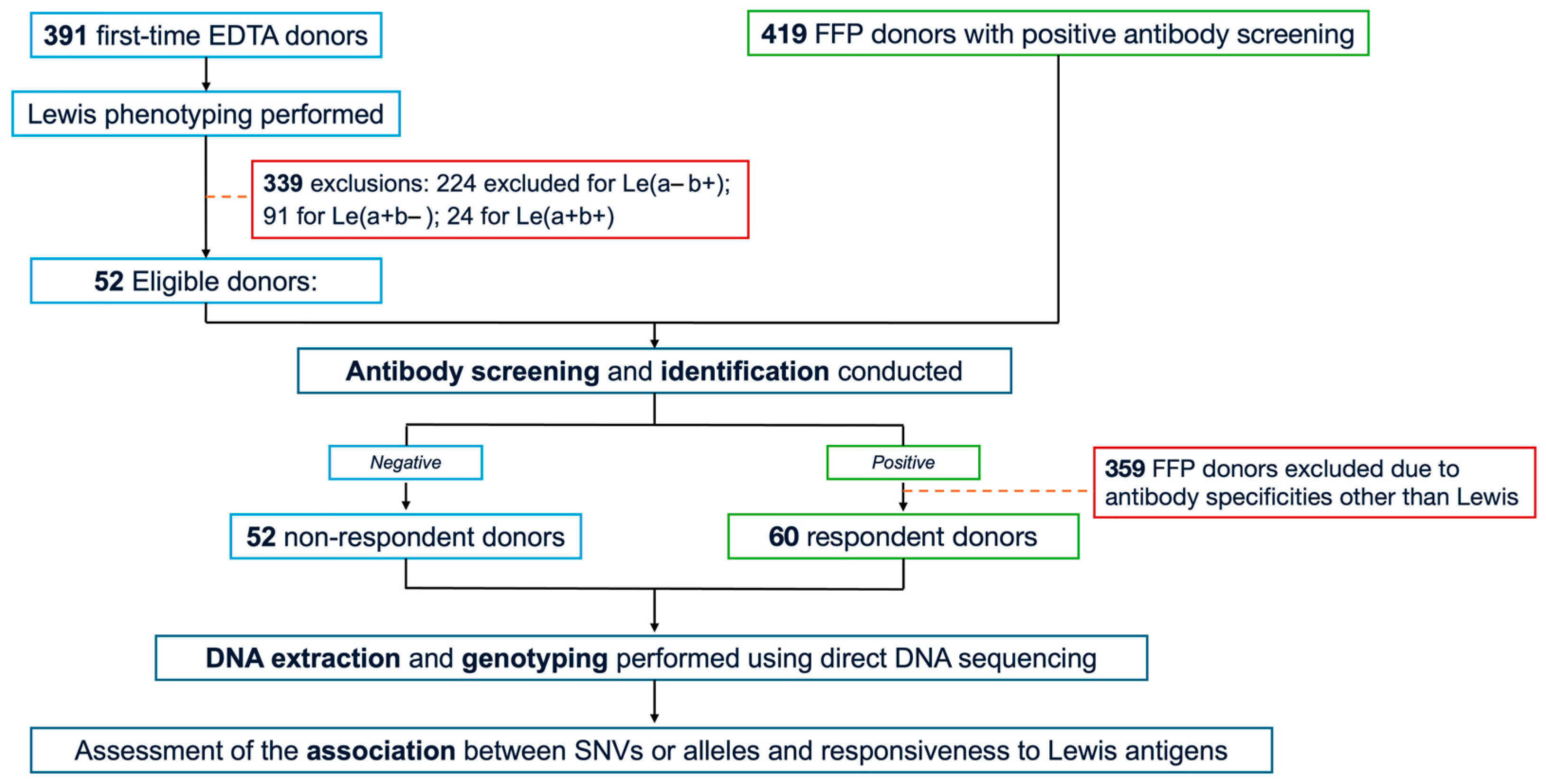
The study cohort comprised 112 randomly selected, unrelated Thai blood donors exhibiting the Le(a–b–) phenotype. From September to October 2024, 391 first-time donors were recruited from the Blood Bank Unit, Thammasat University Hospital (TUH), following informed consent. Whole peripheral blood samples anticoagulated with ethylenediaminetetraacetic acid (EDTA) were collected from all participants, and serological testing—including Le phenotyping and antibody screening—was subsequently performed. As a result, only 52 Le(a–b–) donors who were non-responders for Le antibodies were included in the study. In addition, 419 donors with positive antibody screens, collected from Fresh Frozen Plasma (FFP) donors between August 2022 and May 2025 at the National Blood Centre, Thai Red Cross Society, Bangkok, Thailand (NBC-TRC), underwent further antibody screening and identification. Of these, 60 FFP donors met the eligibility criteria for the study and were categorised as the responder group. Serum samples were immediately isolated and stored at −20 °C until testing. Demographic variables, including age, sex, and ABO blood group, were obtained from the TUH donor database. The complete study workflow is illustrated in Figure 2.
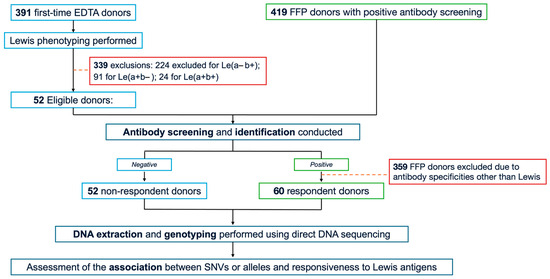
Figure 2.
Study flow chart. EDTA, ethylenediaminetetraacetic acid; FFP, fresh-frozen plasma; SNVs, single nucleotide variants.
2.2. Lewis Blood Group Phenotyping and Antibody Characterisation
A 5% suspension of red blood cells (RBCs) in Diluent-I (BIO-RAD, Cressier, Switzerland) was prepared and incubated at room temperature (18–25 °C) for 10 min. Microtubes of the ID card “DiaClon Anti-Lea” and “DiaClon Anti-Leb” (BIO-RAD, Cressier, Switzerland) were appropriately labelled. Ten microlitres of the RBC suspension were dispensed into each corresponding microtube, and the ID card was centrifuged in an ID centrifuge (BIO-RAD, Cressier, Switzerland) for 10 min. Results were interpreted and recorded according to the manufacturer’s instructions.
All donor samples were screened for antibodies using the column agglutination technique (CAT) with Low Ionic Strength Saline (LISS) Coombs and neutral gels on a fully automated ORTHO VISION MAX Analyser (Ortho Clinical Diagnostics, Raritan, NJ, USA) at the NBC-TRC. Samples yielding a positive screening result underwent antibody identification using 11 in-house panel cells (NBC-TRC, Bangkok, Thailand) alongside an auto-control. Antibody specificities were confirmed using both conventional tube testing (CTT).
Briefly, two drops of each plasma sample were mixed with one drop of in-house panel cells (NBC-TRC, Bangkok, Thailand), centrifuged, and examined immediately for agglutination or haemolysis. Reactions were read macroscopically, graded, and recorded. Tubes were then incubated at 37 °C for 30 min, centrifuged, and observed for agglutination. RBCs were washed three times with normal saline, decanted, and two drops of antihuman globulin reagent (CE-Immunodiagnostika GmbH, Neckargemünd, Germany) were added. Agglutination at the IAT phase was graded, following standard guidelines, recorded with weak or negative reactions confirmed microscopically (×10), and validated using IgG-coated RBCs. Reactions at room temperature typically indicate IgM antibodies, whereas those at 37 °C and/or during IAT indicate IgG antibodies.
2.3. DNA Extraction
Genomic DNA was isolated from donor whole blood and plasma using QIAamp DNA Blood Kits (Qiagen, Hilden, Germany) in strict accordance with the manufacturer’s protocol. The DNA samples were stored at −20 °C until further analysis.
2.4. Polymerase Chain Reaction (PCR) and Direct DNA Sequencing
FUT3 gene fragments (NG_007482.2 and NM_000149.4), encoding 361 amino acids, were used as references for comparison with reported data and were amplified and sequenced using the primers listed in Table 1. PCR was conducted to target amplify the coding sequence of exon 3 of the FUT3 gene, using newly designed forward and reverse primers (Table 1). PCR amplification was conducted in a total reaction volume of 40 µL, consisting of 3 µL of genomic DNA (50 ng/µL), 0.75 µM of each forward and reverse primer, 20 µL of 2× PCR Master Mix (i-StarMAXII, iNtRON Biotechnology, Seongnam-Si, Korea), and 11 µL of sterile distilled water. Reactions were performed using a T100 Thermal Cycler (Bio-Rad, Waltham, MA, USA). The target genomic regions were amplified from DNA templates using PCR under the following cycling conditions: an initial denaturation at 95 °C for 1 min; 10 cycles of denaturation at 95 °C for 30 s and annealing at 69 °C for 60 s; followed by 30 cycles of denaturation at 95 °C for 10 s, annealing at 62 °C for 50 s, and extension at 72 °C for 30 s; and a final extension at 72 °C for 5 min. This protocol yielded an 869 bp amplicon (EX3-A1) or a 1042 bp amplicon (EX3-A2).

Table 1.
Oligonucleotide primers for PCR and sequencing.
PCR products were separated on a 1.5% agarose gel containing SYBR™ Safe DNA Gel Stain (Invitrogen, Paisley, UK) and electrophoresed in 1× TBE buffer at 100 Volts. The DNA bands were visualised using a blue-light transilluminator. The desired amplicons were subsequently purified using the GeneJET Gel Extraction Kit (Thermo Scientific, Waltham, MA, USA), and the eluted DNA fragments were sequenced by U2Bio DNA Sequencing Services (Bangkok, Thailand) using the same PCR primers. Alleles for heterozygous mutations were resolved by TOPO TA cloning (Invitrogen, Carlsbad, CA, USA), and ten clones per PCR product were randomly selected for sequencing. Heterozygotes were identified using TOPO cloning of PCR products, followed by Sanger sequencing. Each chromatogram was manually inspected to confirm the presence of both alleles at each variant position.
Linkage disequilibrium (LD) between SNVs was evaluated using haplotype frequencies derived from genotyping data. Specifically, the two variants defining FUT3*01N.17.03, c.59T>G and c.1067T>A, were analysed for LD. Strong LD was observed between these sites, with a D′ value of 1. In addition, D, D′, and r2 metrics were calculated to quantify the correlation between alleles and to inform haplotype-based association analyses.
2.5. Statistical Analysis
All data cleaning and statistical analyses were performed using SPSS, Version 25 (SPSS Inc., Chicago, IL, USA). Phenotype prevalence was summarised using descriptive statistics and reported as percentages. Genotype and allele frequencies were derived by direct counting. Deviations of all SNV genotype frequencies from Hardy–Weinberg equilibrium (HWE) were assessed using a chi-squared goodness-of-fit test. Differences in genotype distributions between Le antibody responders and non-responders were evaluated using chi-square (χ2) test. Logistic regression models assessing the association between FUT3 SNVs and Le antibody responsiveness included age and sex as covariates in the final model. Sensitivity analyses incorporating ABO blood group confirmed that the associations remained significant. Associations between FUT3 SNV alleles and types of antibody responses were further assessed by Fisher’s exact test. Statistical significance was defined as p < 0.05 or a 95% confidence interval (CI) excluding unity. The primary endpoint was the association between FUT3 haplotypes and Le antibody responses. Analyses at the SNV level were considered exploratory. To account for multiple testing across both tiers, p-values were adjusted using the Bonferroni correction, yielding an adjusted significance threshold of p < 0.006 (0.05/9). For the current post hoc analyses, detectable effect sizes were assessed for the study sample at the Bonferroni-corrected significance threshold and 80% power. Detectable odds ratios were estimated for varying baseline prevalences of Le antibody responses and haplotype frequencies. These analyses were conducted to provide context for the interpretation of association results.
3. Results
3.1. Participant Characteristics and Responsiveness to Le Antibodies
In total, 112 participants were analysed, of whom 52 were categorised as non-respondent donors and 60 as respondent donors. Their principal characteristics are presented in Table 2. Of the 60 respondent donors, 46 (76.7%) were female and 14 (23.3%) male, with a median age of 28 years (IQR 22–35); 76.7% were aged 21–40 years. Both female representation and this age group were significantly higher than in non-respondents (p < 0.05), with a higher female-to-male ratio of 3.29:1, while ABO blood group distribution remained comparable between groups. The existing antibodies, as determined by screening and identification, are summarised in Table 2. In the respondent group, anti-Leb was most frequent (46.6%), followed by anti-Lea (41.7%) and anti-Leab (11.7%), with IgM antibodies predominating (66.7%), IgM + IgG detected in 25.0%, and IgG alone in 8.3% of these donors.

Table 2.
Principal demographic characteristics and distribution of Le antibody types among respondent and non-respondent donors.
3.2. FUT3 Genetic Variants and Their Association with Le Antigen Responsiveness
PCR amplification of the FUT3 gene produced the expected fragments of 869 and 1042 bp in all 112 donors. A total of thirteen SNVs were detected within the coding sequence of the FUT3 gene. Table 3 summarises the genetic and allelic frequencies of the SNVs in the coding sequence. All observed SNV genotype frequencies were consistent with HWE (p > 0.05), and call rates exceeded 95%. Notably, the c.59T>G mutation was the most prevalent, occurring in 100.00% of Le(a–b–) cases. The c.1067T>A and c.508G>A variants were observed at frequencies of 87.50% and 35.71%, respectively. By contrast, the variants c.146G>A, c.548C>T, c.612A>G, and c.645T>C were rare, each detected at a frequency of 0.89%. As shown in Table 3, significant differences were observed in the genotype and/or allele frequencies of c.59T>G, c.202T>C, c.314C>T, c.508G>A, and c.1067T>A between respondent and non-respondent groups (p < 0.05). However, after adjustment for age and sex using logistic regression models, no genotypes were found to be associated with the Le antibody respondent cohort (p > 0.05).

Table 3.
Genotype distribution of FUT3 SNVs in respondent and non-respondent cohorts.
3.3. Haplotypes or Alleles and Their Association with Le Antigen Responsiveness
As shown in Table 4, eight haplotypes (alleles) of the FUT3 gene have been assigned ISBT numbers, while other alleles remain unassigned [1]. Analysis of the FUT3 coding sequence revealed that the FUT3*01N.17.03 (le59,1067) haplotype was the most prevalent, representing 142 haplotypes (0.634 of the total). This was closely followed by FUT3*01N.17.05 (le59,508), with a frequency of 0.192. Rare haplotypes, including FUT3*01N.17.13 (le59,548,612,1067) and FUT3*01N.17.18 (le59,146,508), were each observed in only a single donor, corresponding to a frequency of 0.004. Moreover, a total of ten novel alleles were discovered in 26 (23.2%) donors and unofficially assigned by ISBT, as summarised in Table 5, arranged according to their occurrence in the respondent and non-respondent cohorts. Among all respondent donors, the le59,796, le59,508,1067, and le59,796,1067 haplotypes were the most prevalent, with five cases observed for each, accounting for 4.6% of the total respondent haplotypes. In addition, LD analysis between the two SNVs defining FUT3*01N.17.03, c.59T>G and c.1067T>A, showed moderate correlation, with D = 0.0245, D′ = 0.717, and r2 = 0.078, indicating that these variants are partially linked but not fully predictive of each other.

Table 4.
Haplotype and allele frequencies of the FUT3 coding sequence in respondent and non-respondent donors.

Table 5.
Supplementary haplotype frequencies of the FUT3 coding sequence in respondent and non-respondent donors.
Multivariable analysis in the final model, adjusted for age and sex, indicated that two alleles—FUT3*01N.17.03 and FUT3*01N.17.05—were significantly associated with Le antibody production (p < 0.05). Notably, the most influential allele, based on the highest adjusted odds ratio, was FUT3*01N.17.03 (aOR = 3.052, 95% CI: 1.683–5.534, p < 0.0001), which remained significant after Bonferroni correction (Table 4). Individuals with the Le(a–b–) phenotype carrying the FUT3*01N.17.03 allele had a 3.052-fold higher likelihood of developing Le-antibodies compared with carriers of other alleles, after controlling for other variables. Post hoc power analysis confirmed that, given the study sample (n = 112), a baseline prevalence of approximately 17%, and a haplotype frequency of approximately 63%, this study had 80% power to detect this effect, supporting the robustness of the observed association.
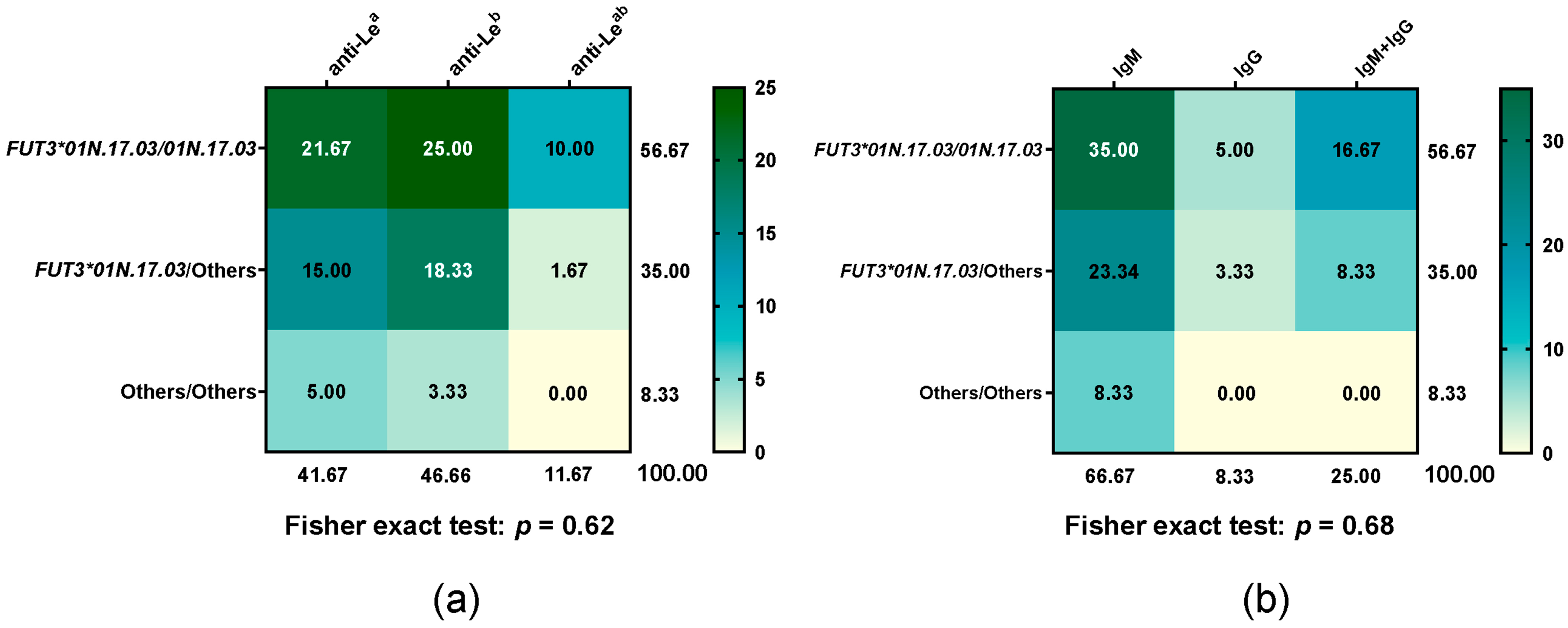
3.4. Distribution of Le Antibody Types in FUT3*01N.17.03 Diplotype
All participant donors underwent antibody screening and identification. All 52 non-respondent donors tested negative. Among the 60 respondent donors, the distribution of the FUT3*01N.17.03 diplotype and corresponding Le antibody types is summarised in Figure 3. Responders were classified according to their predominant type of Le antibody production for analysis. Differences in Le antibody types, their isotypes, and the FUT3*01N.17.03 genotype between groups were not statistically significant (Fisher exact test: p = 0.62 for antibody types, p = 0.68 for isotypes). Within respondents, neither anti-Lea antibodies (IgM or IgG) nor the FUT301N.17.03/01N.17.03 genotype showed a similar response compared with anti-Leb antibodies or carriers of FUT301N.17.03 combined with other alleles.
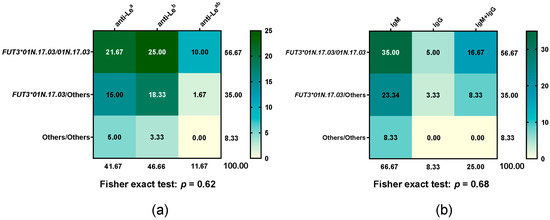
Figure 3.
Heatmap of Le antibody type frequencies across FUT3*01N.17.03 diplotypes, with each cell showing the percentage of that diplotype relative to the total. (a) Le antibody types and (b) antibody isotypes across FUT3*01N.17.03 diplotypes. Green indicates higher percentages (with darker shades representing stronger values), while yellow indicates lower percentages.
4. Discussion
Irregular erythrocyte antibodies are observed in the general population. Standardised guidelines for the screening of donor blood to detect isoimmune erythrocyte antibodies have been promulgated at the international level and adopted nationally in Thailand. Previous reports indicate that the predominant incidences of erythrocyte alloantibodies are approximately 86% in donor populations and 52% in patient populations when assessed by CTT [21]. These observations are consistent with the relatively high prevalence of the Le(a–b–) phenotype in the Thai population, estimated at 18–24% [13,14,15]. This concordance underscores the clinical relevance of antibody screening in Thailand, where national standards highlight the importance of ensuring the provision of the most compatible blood for transfusion. According to our demographic results, females were significantly over-represented among respondents, with a female-to-male ratio of 3.29:1. This finding is consistent with the observations of Ameen et al. [26], who reported a 0.49% prevalence of alloantibodies in the Kuwaiti population and 2.3% among blood donors, with females exhibiting a threefold higher frequency than males. Similarly, Zhu et al. reported a prevalence of 0.279% among blood donors in the Shaoguan area [27], again noting a higher frequency of irregular erythrocyte alloantibodies in females compared with males. These concordant findings suggest that sex-related immunological factors, particularly sensitisation during pregnancy, may contribute to the increased prevalence of alloantibodies in females. Furthermore, alloantibody frequency was highest among respondent donors aged 21–40 years, significantly exceeding that in non-respondents. This observation is consistent with reports by Makroo et al. [28] and Pahuja et al. [29], highlighting the potential importance of age-related factors in alloantibody formation and the need for targeted donor screening. Our results indicate that ABO/Rh blood type diversity does not significantly influence Le antibody production. Although both Le and ABO antigens are derived from oligosaccharide precursors, the Le system is regulated by fucosyltransferases (FUT3 and FUT2), whereas the ABO system relies on distinct glycosyltransferases. These findings support the view that ABO antigen expression does not impact FUT3 and therefore does not induce the Le(a–b–) phenotype or subsequent Lewis antibody formation [6].
To further understand the Le antibody response in Le(a–b–) individuals, we assessed all SNVs in the FUT3 for associations with Le antigen responsiveness. Variants of the FUT3 gene have been reported in diverse populations, showing ethnic specificity [1,30]. This study discusses the first analysis of FUT3 variants in Thai individuals with the Le(a–b–) phenotype. Through sequencing and analysis of the coding region, we identified 13 SNVs. All were in HWE with high call rates, indicating a representative population sample and reliable genotyping. Among these, the c.59T>G site was the most prevalent, with a mutation frequency of 100% in Le(a–b–) individuals. This aligns with studies in Northern and Eastern China, which reported frequencies of 43.13% and 39.87% among all Le phenotypes [30,31], suggesting that c.59T>G is a common variant influencing the Le(a–b–) phenotype across Asian populations. Afterwards, c.1067T>A and c.508G>A variants were observed at frequencies of 87.5% and 35.7%, respectively. Together with the other common variants, these findings indicate that these mutations are relatively prevalent and may contribute substantially to the Le(a–b–) phenotype in the population [22]. The rare mutations c.146G>A, c.548C>T, c.612A>G, and c.645T>C, each observed in a single Thai subject, may represent novel or population-specific variants relevant to the Le(a–b–) phenotype. Although the c.1067A and c.508G alleles were associated with Le antibody formation, no genotype differed significantly between responder and non-responder groups after adjusting for age and sex, suggesting additional factors may influence antibody response.
We identified eight ISBT-assigned and ten unassigned haplotypes among the Le(a–b–) Thai donors, with FUT301N.17.03 (le59,1067) and FUT301N.17.05 (le59,508) being the most frequent. This pattern is consistent with reports by Cooling et al. [32] and Pang et al. [33], who noted le59,1067 and le59,508 as the principal Lewis-negative alleles in Asian and African populations. The le59,508 allele is also common in Americans and Japanese but rare in Europeans—Swedish [32,33], indicating population-specific distributions of non-functional FUT3 variants. Such variation is clinically relevant, as different haplotypes may influence the likelihood and immunogenicity of Le antibody formation, underscoring the need to consider population background in transfusion practice. It is noteworthy that FUT3*01.10 (Le1067) and FUT3*01.17.01 (Le59) were detected only in six non-respondent donors. These functional FUT3 variants have been reported to reduce enzyme activity by approximately 90% [1,34,35]. The c.59T>G substitution predominantly decreases enzyme quantity rather than abolishing activity, as it lies within the transmembrane domain and results in a leucine-to-arginine change (p.Leu20Arg) [23]. Importantly, this alteration does not directly affect the catalytic region, which may explain the absence of antibody production in this group. Nevertheless, this observation illustrates that serological phenotyping does not always reflect the underlying genotype. Further genotyping showed that all six individuals carried a functional FUT2 allele and were therefore secretors. Based on these combined genetic results, their predicted phenotype would be Le(a–b+). As Le antigens are adsorbed onto RBCs, a 90% reduction in their production may render them undetectable by serology.
Our findings highlight that the FUT3*01N.17.03 haplotype, the most prevalent among Thai donors, is strongly associated with Le antibody responsiveness. Even after adjusting for age and sex in logistic regression models, the association remained significant (aOR = 3.05), indicating that this effect is robust and not confounded by these covariates. Although genotype-level analyses of individual SNVs showed no significant associations, the FUT3*01N.17.03 haplotype captured their combined effects. Notably, this signal reflects the cumulative impact of its defining SNVs rather than being driven solely by LD, supporting its biological relevance in modulating Le antibody formation. Importantly, this association was not accompanied by heterozygosity differences in either the type or isotype of Le antibodies, suggesting that the underlying genetic mechanism does not alter the qualitative nature of the immune response. Instead, the combined c.59T>G and c.1067T>A substitutions appear to inactivate the α1,3/4-L-FucT enzyme [23], thereby abolishing Le antigen expression on the red cell surface. This lack of antigenic presentation likely serves as the immunological trigger for Le antibody production in otherwise healthy donors. Moreover, the cumulative effect of haplotypes may contribute to the observed statistical significance, highlighting the complex genetic basis of Le antibody formation. These results enhance our understanding of population-level variation in immune responses to Le antigens and suggest practical applications for transfusion medicine. Specifically, the SNVs and haplotypes identified here could guide the refinement of FUT3 genotyping strategies, including improved SNV filtering and scoring, for the selection of Le-negative phenotypes. In particular, FUT3 variants with high prevalence and demonstrated correlation with Le antibody formation in the Thai population should be prioritised when designing genetic tests to improve diagnostic accuracy and specificity. Beyond Le phenotyping, FUT3 variations have been associated with ulcerative colitis [36], ankylosing spondylitis [37], and colonic polyps [38], suggesting that altered Le antigen expression may influence immunity, inflammation, and disease susceptibility. Together, these findings underscore the importance of investigating FUT3 variants both for understanding Le antibody formation and for their broader clinical relevance, including implications for not only genetic testing but also disease risk assessment.
However, our study has several limitations. First, the sample size is relatively modest, which may lead to wide confidence intervals and reduce the statistical power to detect true differences. Although adjustment for age and sex helped to mitigate potential confounding, it cannot fully eliminate biases arising from non-random sampling. Moreover, for outcomes with low incidence (<10%), OR estimates are inherently unstable and should therefore be interpreted with caution. Second, the Le antibodies identified in this study reflect only the humoral response directed against the LE system, without accounting for possible concomitant antibodies to other blood group antigens. As Le antigens share structural features with several other carbohydrate-based systems, particularly those with terminal oligosaccharide epitopes, cross-reactivity cannot be excluded. The processing of such polysaccharide antigens within endosomes is complex, and only a limited number of highly antigenic domains (e.g., Galili, Forssman, LeX/LeY, and P1PK system-antigens) are capable of eliciting strong immune responses, which may contribute to overlapping antibody patterns. Third, additional immune-related genetic factors and health conditions, such as polymorphisms in the HLA and FUT2 genes, which were beyond the scope of the present analysis, may also influence the antibody response to Le antigens.
5. Conclusions
To the best of our knowledge, this study provides the first characterisation of FUT3 gene variants in Thai blood donors with the Le(a–b–) phenotype and identifies FUT3*01N.17.03 as the key allele associated with Le antibody responsiveness. These findings highlight the population-specific distribution of FUT3 variants and their potential relevance for genetic diagnostics in transfusion practice. Further studies are warranted to clarify the underlying mechanisms by which FUT3 SNVs influence antibody formation and to assess their broader clinical implications.
Author Contributions
Conceptualization, O.N. and K.I.; methodology, K.I.; software, K.I.; validation, W.S. and K.I.; formal analysis, K.I.; investigation, W.S.; resources, P.K.; data curation, W.S. and K.I.; writing—original draft preparation, K.I.; writing—review and editing, O.N. and K.I.; visualization, O.N. and K.I.; supervision, K.I.; project administration, O.N. and K.I.; funding acquisition, K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by Thailand Science Research and Innovation (TSRI) Fundamental Fund, fiscal year 2025 (Grant No. TUFF 75/2568).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Committee on Human Rights Related to Research Involving Human Subjects, Thammasat University, Pathumtani, Thailand (protocol code 67AH191 and date of approval: 13 January 2024) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge and pay respects to all Thai blood donors in this study. The authors would like to thank Dollapak Apipongrat at the Hematology Laboratory, Department of Medicine, Phramongkutklao Hospital, Bangkok, Thailand, for their support in modifying the graphical heatmap needed for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAT | Column Agglutination Technique |
| CTT | Conventional Tube Testing |
| EDTA | Ethylenediaminetetraacetic Acid |
| FFP | Fresh Frozen Plasma |
| FucT | Fucosyltransferase |
| HDFN | Haemolytic Disease of the Foetus and Newborn |
| HTRs | Haemolytic Transfusion Reactions |
| IAT | Indirect Antiglobulin Test |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IQR | Interquartile Range |
| ISBT | International Society of Blood Transfusion |
| LD | Linkage Disequilibrium |
| Le | Lewis |
| LISS | Low Ionic Strength Saline |
| NBC-TRC | National Blood Centre, Thai Red Cross Society |
| PCR | Polymerase Chain Reaction |
| RBCs | Red Blood Cells |
| SNVs | Single-Nucleotide Variants |
| TUH | Thammasat University Hospital |
References
- Names for Lewis (ISBT 007) Blood Group Alleles. Available online: https://www.isbtweb.org/resource/007lealleles.html (accessed on 15 August 2025).
- Oriol, R.; Le Pendu, J.; Sparkes, R.S.; Sparkes, M.C.; Crist, M.; Gale, R.P.; Terasaki, P.I.; Bernoco, M. Insights into the expression of ABH and Lewis antigens through human bone marrow transplantation. Am. J. Hum. Genet. 1981, 33, 551–560. [Google Scholar] [PubMed]
- Hauser, R. Le(a) and Le(b) tissue glycosphingolipids. Transfusion 1995, 35, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Table of Blood Group Antigens within Systems. Available online: https://www.isbtweb.org/resource/tableofbloodgroupantigenswithinsystems.html (accessed on 15 August 2025).
- Chester, M.A.; Watkins, W.M. Alpha-L-fucosyltransferases in human submaxillary gland and stomach tissues associated with the H, Lea and Leb blood-group characters and ABH secretor status. Biochem. Biophys. Res. Commun. 1969, 34, 835–842. [Google Scholar] [CrossRef]
- Daniels, G. (Ed.) ABO, H, and Lewis Systems. In Human Blood Groups, 3rd ed.; Wiley-Blackwell Publishing: Oxford, UK, 2013; pp. 11–95. [Google Scholar]
- Henry, S.; Mollicone, R.; Fernandez, P.; Samuelsson, B.; Oriol, R.; Larson, G. Molecular basis for erythrocyte Le(a+ b+) and salivary ABH partial-secretor phenotypes: Expression of a FUT2 secretor allele with an A-->T mutation at nucleotide 385 correlates with reduced alpha(1,2) fucosyltransferase activity. Glycoconj. J. 1996, 13, 985–993. [Google Scholar] [CrossRef]
- Reid, M.E.; Lomas-Francis, C.; Olsson, M.L. Lewis blood group system. In The Blood Group Antigen Facts Book, 3rd ed.; Reid, M.E., Lomas-Francis, C., Olsson, M.L., Eds.; Elsevier Academic Press: Waltham, MA, USA, 2012; pp. 347–359. [Google Scholar]
- Broadberry, R.E.; Lin-Chu, M. The Lewis blood group system among Chinese in Taiwan. Hum. Hered. 1991, 41, 290–294. [Google Scholar] [CrossRef]
- Henry, S.M.; Simpson, L.A.; Woodfield, D.G. The Le(a+b+) phenotype in Polynesians. Hum. Hered. 1988, 38, 111–116. [Google Scholar] [CrossRef]
- Boettcher, B.; Kenny, R. A quantitative study of Lea, A and H antigens in salivas of Australian caucasians and aborigines. Hum. Hered. 1971, 21, 334–345. [Google Scholar] [CrossRef]
- Molthan, L. Lewis phenotypes of American Caucasians, American Negroes and their children. Vox Sang. 1980, 39, 327–330. [Google Scholar] [CrossRef]
- Chandanayingyong, D.; Sasaki, T.T.; Greenwalt, T.J. Blood groups of the Thais. Transfusion 1967, 7, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Nathalang, O.; Kuvanont, S.; Punyaprasiddhi, P.; Tasaniyanonda, C.; Sriphaisal, T. A preliminary study of the distribution of blood group systems in Thai blood donors determined by the gel test. Southeast Asian J. Trop. Med. Public Health 2001, 32, 204–207. [Google Scholar]
- Bejrachandra, S.; Nathalang, O.; Saipin, J.; Kuvanont, S.; Wichitchinda, K.; Vongpattranon, A. Distribution of blood group systems in Thai blood donors determined by gel test. Siriraj Hosp. Gaz. 2002, 54, 403–409. [Google Scholar]
- Roy, R.B.; Wesley, R.H.; Fitzgerald, J.D. Haemolytic transfusion reaction caused by anti-Le. Vox Sang. 1960, 5, 546–550. [Google Scholar] [CrossRef]
- Contreras, M.; Mollison, P.L. Delayed haemolytic transfusion reaction caused by anti-LebH antibody. Vox Sang. 1989, 56, 290. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Kennedy, M.S.; Gerhan, S.; Senhauser, D.A. Transfusion significance of Lewis system antibodies. Success in transfusion with crossmatch-compatible blood. Am. J. Clin. Pathol. 1981, 76, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Oriol, R.; Cartron, J.P.; Cartron, J.; Mulet, C. Biosynthesis of ABH and Lewis antigens in normal and transplanted kidneys. Transplantation 1980, 29, 184–188. [Google Scholar] [CrossRef]
- Oriol, R.; Cartron, J.; Yvart, J.; Bedrossian, J.; Duboust, A.; Bariety, J.; Gluckman, J.C.; Gagnadoux, M.F. The Lewis system: New histocompatibility antigens in renal transplantation. Lancet 1978, 1, 574–575. [Google Scholar] [CrossRef]
- Promwong, C.; Siammai, S.; Hassarin, S.; Buakaew, J.; Yeela, T.; Soisangwan, P.; Roxby, D. Frequencies and specificities of red cell alloantibodies in the Southern Thai population. Asian J. Transfus. Sci. 2013, 7, 16–20. [Google Scholar] [CrossRef]
- Salomaa, V.; Pankow, J.; Heiss, G.; Cakir, B.; Eckfeldt, J.H.; Ellison, R.C.; Myers, R.H.; Hiller, K.M.; Brantley, K.R.; Morris, T.L.; et al. Genetic background of Lewis negative blood group phenotype and its association with atherosclerotic disease in the NHLBI family heart study. J. Intern. Med. 2000, 247, 689–698. [Google Scholar] [CrossRef]
- Elmgren, A.; Börjeson, C.; Svensson, L.; Rydberg, L.; Larson, G. DNA sequencing and screening for point mutations in the human Lewis (FUT3) gene enables molecular genotyping of the human Lewis blood group system. Vox Sang. 1996, 70, 97–103. [Google Scholar] [CrossRef]
- Kissmeyer-Nielsen, F. Irregular blood group antibodies in 200,000 individuals. Scand. J. Haematol. 1965, 2, 331–342. [Google Scholar] [CrossRef]
- Miller, E.B.; Rosenfield, R.E.; Vogel, P.; Haber, G.; Gibbel, N. The Lewis blood factors in American Negroes. Am. J. Phys. Anthropol. 1954, 12, 427–443. [Google Scholar] [CrossRef]
- Ameen, R.; Al-Eyaadi, O.; Al-Shemmari, S.; Chowdhury, R.; Al-Bashir, A. Frequency of red blood cell alloantibody in Kuwaiti population. Med. Princ. Pract. 2005, 14, 230–234. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Lan, J.C.; Luo, H.Q. Screening analysis of irregular antibodies from random donor population in Shaoguan area. J. Exp. Haematol. 2007, 15, 630–631. [Google Scholar]
- Makroo, R.N.; Rajput, S.; Agarwal, S.; Chowdhry, M.; Prakash, B.; Karna, P. Prevalence of irregular red cell antibody in healthy blood donors attending a tertiary care hospital in North India. Asian J. Transfus. Sci. 2018, 12, 17–20. [Google Scholar] [CrossRef]
- Pahuja, S.; Kushwaha, S.; Sethi, N.; Pujani, M.; Jain, M. Screening of blood donors for erythrocyte alloantibodies. Hematology 2012, 17, 302–305. [Google Scholar] [CrossRef]
- Zhou, S.; Zheng, Z.; Wang, L.; Song, W.; Xia, Y.; Shao, L.; Liang, X. Genetic variation of FUT3 gene in the Han population from Northern China. Transfus. Med. 2024, 34, 437–444. [Google Scholar] [CrossRef]
- Guo, M.; Luo, G.; Lu, R.; Shi, W.; Cheng, H.; Lu, Y.; Jin, K.; Yang, C.; Wang, Z.; Long, J.; et al. Distribution of Lewis and Secretor polymorphisms and corresponding CA19-9 antigen expression in a Chinese population. FEBS Open Bio 2017, 7, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Cooling, L.; Gu, Y. Identification of two new single-nucleotide polymorphisms in FUT3 associated with the Lewis-null phenotype. Transfusion 2003, 43, 1760–1761. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Liu, Y.; Koda, Y.; Soejima, M.; Jia, J.; Schlaphoff, T.; Du Toit, E.D.; Kimura, H. Five novel missense mutations of the Lewis gene (FUT3) in African (Xhosa) and Caucasian populations in South Africa. Hum. Genet. 1998, 102, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, S.; Narimatsu, H.; Iwasaki, H.; Yazawa, S.; Akamatsu, S.; Ando, T.; Seno, T.; Narimatsu, I. Molecular genetic analysis of the human Lewis histo-blood group system. J. Biol. Chem. 1994, 269, 29271–29278. [Google Scholar] [CrossRef]
- Nishihara, S.; Yazawa, S.; Iwasaki, H.; Nakazato, M.; Kudo, T.; Ando, T.; Narimatsu, H. Alpha (1,3/1,4)fucosyltransferase (FucT-III) gene is inactivated by a single amino acid substitution in Lewis histo-blood type negative individuals. Biochem. Biophys. Res. Commun. 1993, 196, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhang, D.; Zheng, S.; Guo, M.; Lin, X.; Jiang, Y. Association of Ulcerative Colitis with FUT2 and FUT3 Polymorphisms in Patients from Southeast China. PLoS ONE 2016, 11, e0146557. [Google Scholar] [CrossRef]
- Jiang, G.; Han, R.; Chen, M.; Liu, R.; Wu, M.; Zhang, X.; Ma, Y.; Yuan, Y.; Wang, R.; Shuai, Z.; et al. Associations between fucosyltransferase 3 gene polymorphisms and ankylosing spondylitis: A case-control study of an east Chinese population. PLoS ONE 2020, 15, e0237219. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, F.; Ji, W.; Wang, Y. Correlations of FUT3 gene polymorphisms with colon polyps. Cell Cycle 2022, 21, 117–125. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).