Right Heart Failure in Critical and Chronic Care: Current Concepts, Challenges and Mechanical Support Strategies
Abstract
1. Introduction
2. Relevant Sections
2.1. Right Ventricular Anatomy, Function and Interplay with the Pulmonary Circulation
2.2. Pathophysiology of Right Heart Failure: Acute vs. Chronic
2.2.1. Acute Right Heart Failure (ARHF)
- Pressure overload
- Contractile dysfunction
- Acute volume overload
- Additional precipitants
- Molecular mechanism in ARHF
2.2.2. Chronic Right Heart Failure (CRHF)
- Chronic RV volume overload
- Chronic RV pressure overload
2.2.3. RV Function in Pressure and Volume Overload
2.2.4. From Chronic to Acute: The Continuum of Right Ventricular Failure
2.3. Clinical Manifestations of Right Heart Failure
2.4. Specific Etiologies and Management Strategies in RHF
2.4.1. RHF Secondary to Pulmonary Hypertension: Pre- vs. Post-Capillary Mechanism
2.4.2. Congenital Heart Disease-Associated RHF
2.4.3. RHF Secondary to Left Heart Disease
- In HFrEF, evidence-based LV therapies (RAAS blockade, beta-blockade, mineralocorticoids receptor antagonists, sacubril/valsartan, resynchronization therapy) are central, though their direct effects on RV performance remain incompletely defined. Preservation of euvolemia and reduction of pulmonary venous congestion are critical for RV protection [45,90].
- In HFpEF, RV dysfunction is highly prevalent and strongly prognostic [91]. Strategies aimed at reducing pulmonary vascular load have yielded mixed results: sildenafil trials were largely negative, whereas vericiguat showed promise in improving quality of life [92,93]. At present, no guideline-directed therapy is specifically recommended for RHF in the setting of HFpEF. In this patient population, meticulous volume management remains the cornerstone of treatment [87].
2.4.4. RHF in Pulmonary and Thromboembolic Disease
2.4.5. RHF in the Setting of LVAD Support
2.4.6. Acute Right Ventricular Ischemia
2.4.7. Primary and Infiltrative Cardiomyopathies
2.4.8. Right Sided Valvular Heart Disease
2.4.9. Pericardial Disease
2.4.10. Iatrogenic Causes
2.5. Assessment of Right Ventricular Function: Current Concepts and Advanced Imaging Approaches
2.5.1. Echocardiography
- Systolic function indices: Fractional area change (FAC), derived from RV end-diastolic and end-systolic areas, serves as a validated surrogate for global RV systolic function (reduced FAC correlates independently with adverse outcomes, including mortality and major cardiovascular events) [113]; tricuspid annular plane systolic excursion (TAPSE), measured by M-mode, reflects longitudinal RV contractility (lower TAPSE values are predictive of increased mortality and urgent need for heart transplantation in chronic HFrEF patients) [114].
- Doppler-derived functional indices: RV myocardial performance index (RVMPI or Tei index) integrates systolic and diastolic performance [115]; dP/dT of the tricuspid valve and tissue Doppler imaging (TDI) of tricuspid annular systolic velocity (S’) provide additional prognostic insight, particularly in the setting of PH, RV infarction or post LVAD implantation [116].
- Right atrial function: although often overlooked, represents a crucial determinant of right sided hemodynamic and prognosis. The RA acts not merely as a passive reservoir but fulfills three complementary roles (reservoir during ventricular systole, conduit during early diastole and booster pump during atrial contraction). Impairment of any of these phases contributes to elevated right atrial pressure, systemic congestion and diminished RV filling. Advanced echocardiographic techniques, including speckle tracking-derived RA strain, have emerged as a sensitive indices of RA remodeling. Reduced RA reservoir and conduit strain are independently associated with adverse outcomes in PH, chronic heart failure and congenital heart diseas. Furthermore, atrial dysfunction frequently precedes overt RV failure and correlates with the severity of functional tricuspid regurgitation [117,118,119,120].
- Tricuspid regurgitation (TR) assessment: is a critical component of RV evaluation. Moderate-to-severe TR not only reflects annular dilatation and leaflet tethering secondary to RV enlargement, but also exacerbates systemic venous congestion and alters load dependent indices of RV performance. Severe TR can lead to underestimation of RV systolic pressure and confound the interpretation of echocardiographic parameters such as TAPSE or fractional area change. Moreover, TR severity has consistently been associated with worse prognosis, underscoring the importance of its systematic evaluation during RV functional assessment [121,122,123].
- Strain imaging: Speckle tracking echocardiography allows quantification of RV longitudinal strain which correlates with RV contractile reserve and has emerged as a sensitive predictor of adverse outcomes across multiple pathologies, including PH, chronic heart failure and congenital heart disease [124,125].
2.5.2. Advanced Imaging Modalities
- Cardiac magnetic resonance (CMR) is the gold standard for RV volumetric quantification and ejection fraction assessment [109]. CMR allows accurate delineation of RV end-diastolic and end-systolic volumes, mass and RVEF, while also enabling myocardial tissue characterization for infiltrative or fibrotic disorders such as arrhythmogenic right ventricular cardiomyopathy (ARVC), cardiac sarcoidosis or amyloidosis. Late gadolinium enhancement (LGE) and T1/T2 mapping can provide further prognostic information regarding fibrotic burden and arrhythmogenic risk [127,128].
- 4D Flow MRI: Four-dimensional flow cardiac magnetic resonance has recently emerged as a powerful tool for the comprehensive assessment of right-sided hemodynamics. Beyond static volumetric data, it enables visualization and quantification of complex intracardiac flow patterns, vorticity and kinetic energy dissipation across the RV and pulmonary arteries. Although still primarily a research modality, 4D flow MRI offers unique mechanistic insights and holds potential for refining prognostication and therapeutic monitoring in RHF [129,130].
- Cardiac computed tomography (CT), particularly with contrast-enhanced angiography, is valuable in evaluating RV morphology in the context of pulmonary vascular pathology, such as acute PE or chronic thromboembolic pulmonary hypertension and in pre-surgical planning [131].
2.5.3. Hemodynamic Assessment
- Right atrial pressure (RAP) to pulmonary capillary wedge pressure (PCWP) ratio is commonly used to characterize RV-pulmonary coupling, particularly after LVAD implantation or in acute myocardial infarction. The ratio is considered preserved when <0.6, whereas values ≥ 0.63 identify patients at increased risk of right ventricular disfunction in the setting of left ventricular assist device (LVAD) implantation [133,134].
- Pulmonary artery pulsatility index (PAPi), defined as (pulmonary artery systolic pressure (PASP)- pulmonary artery diastolic pressure (PADP))/RAP, is predictive of post-LVAD RV failure and overall prognosis in advanced heart failure. A PAPi < 1.5–2.0 is strongly associated with high RHF [134].
- RV stroke work index (RVSWI) quantifies RV contractile performance relative to afterload and reduced RVSWI is associated with increased risk of RV decompensation post LVAD or after acute RV infarction. Normal values typically range between 5–10 g×m/m2, whereas an RVSWI < 5 g×m/m2 indicates impaired RV contractility [135].
- Pulmonary arterial compliance (PAC), calculated as stroke volume divided by pulmonary pulse pressure (PAPP = pulmonary artery systolic pressure (PASP)-pulmonary artery diastolic pressure (PADP). PAC serves as a sensitive predictor of RV failure and adverse outcomes in chronic heart failure and PH, reflecting the dynamic interaction between the RV and pulmonary circulation. A PAC < 2 mL/mmHg indicates reduced RV-pulmonary coupling and portends a higher risk of progressive RHF [136,137].
- Transpulmonary gradient (TPG) serves as critical hemodynamic parameter for elucidating the etiology of right heart failure in the context of concomitant pulmonary hypertension. Hemodynamic profiling distinguishes between predominant pathophysiological mechanism: an elevated TPG (>12 mmHg) in conjunction with a normal PCWP (<15 mmHg) is indicative of a pre-capillary etiology, implicating pulmonary vascular disease. Conversely, a low TPG (<12 mmHg) with an elevated PCWP (>15 mmHg) signifies a post-capillary origin, typically due to left heart pathology. A scenario of both a high TPG and a high PCWP characterizes combined pre- and post-capillary pulmonary hypertension, reflecting the presence of overlapping vascular and cardiac dysfunction [138].
2.5.4. Risk Stratification and Prognostic Score in RHF
2.5.5. Emerging Biomarkers
2.6. Therapeutic Strategies in Acute Right Heart Failure
2.6.1. Medical Therapy
2.6.2. Targeting the Etiology
2.6.3. Preload Optimization
2.6.4. Afterload Reduction
2.6.5. Augmenting Contractility
2.7. Management of Chronic Right Ventricular Failure
- Volume regulation: diuretics are central to reducing congestion and preventing RV volume overload, with close renal surveillance to avoid prerenal injury [156].
- Iron deficiency therapy: Iron deficiency is highly prevalent in chronic RHF, contributing to reduced exercise capacity, impaired skeletal muscle function and systemic inflammation. Intravenous iron repletion, particularly with ferric carboxymaltose, has been shown to improve functional capacity and quality of life in chronic left-sided HF, with emerging evidence suggesting parallel benefits in patients with right-sided dysfunction. Correction of iron deficiency not only alleviates anemia but also improves mitochondrial function and skeletal muscle energetics, potentially mitigating fatigue and exercise intolerance in RHF. Routine screening and treatment of iron deficiency should therefore be considered an integral component of long-term management [157].
- Afterload management: therapy depends on the underlying mechanism. In left-sided systolic dysfunction, evidence-based heart failure therapies (beta-blockers, ACE inhibitors, mineralocorticoid antagonists, ARNi and SGLT-2 inhibitors) are indicated. SGLT-2 (sodium-glucose cotransporter-2) inhibitors have transformed the management of left-sided heart failure, with consistent reduction in hospitalization and mortality across both HFrEF and HFpEF phenotypes. Although their direct effects on RV function remains less well studied, emerging data suggest potential benefits in reducing pulmonary pressures, improving systemic congestion and enhancing renal-cardiac interaction [158,159,160]. In pulmonary hypertension, treatment is disease-specific: group I PH require advanced vasodilator therapy (PDE5 inhibitors, prostacyclin analogues, endothelin receptor antagonist), whereas group IV disease necessitates lifelong anticoagulation with potential surgical endarterectomy due to chronic thromboembolic disease. Management of RHF in congenital heart disease requires specialist involvement due to complexity [4].
2.8. Mechanical Circulatory Support
2.8.1. Short Term Support
- Peripheral V-A ECMO is a RA to Aorta device: remains a widely used form of temporary biventricular support. Venous drainage from the RA is passed through a centrifugal pump and oxygenator, with return into the arterial system, typically via femoral circulation. Variants such as veno-arterialvenous (VAV) ECMO or veno-venous-arterial (VVA) ECMO have been developed to enhance oxygenation or RV unloading, respectively. Hemodynamically, V-A ECMO reduces RAP but has variable effects on PA pressures, depending on LV function and pulmonary vascular tone. In the presence of LV dysfunction, concomitant LV unloading via IABP or Impella (ECPELLA configuration) is often required. The left atrial veno-arterial (LAVA)-ECMO configuration, using trans-septal left atrial cannulation, allows simultaneous unloading of both ventricles and has the advantage of avoiding additional arterial access. However, this approach often provides greater circulatory support than required for isolated right heart failure. An alternative strategy is the use of a rotaflow centrifugal pump in a dedicated RVAD configuration, with venous outflow cannulated from the femoral vein or RA and return directed into the PA via a surgically implanted graft. The principal advantage of this system lies in its relative simplicity and low cost [165,166,167].
- ProtekDuo (LivaNova) is a single dual-lumen cannula inserted percutaneously via the internal jugular vein. One lumen drains blood from the right atrium, while the other lumen returns blood directly into the pulmonary artery. The blood drained from the RA flows through an external centrifugal pump that actively propels the blood into the PA, bypassing the failing RV. The pulmonary circulation is fully perfused ensuring oxygenation and reducing RV strain. The device decreases RAP and systemic venous congestion, increases CO and improves end organ perfusion [168].
- TandemHeart-RVAD (LivaNova) temporarily replaces RV function by actively moving blood from the right atrium to the pulmonary artery using a percutaneous centrifugal pump. The drainage right atrial cannula is usually inserted by femoral or jugular vein. The centrifugal pump provides continuous flow and bypasses the failing right ventricle, reducing RV workload. The pumped blood is returned to the pulmonary artery, usually via another percutaneous cannula, ensuring oxygenation of blood through the lungs while supporting the failing RV. The device reduces central venous pressure, improves CO and end-organ perfusion and provides a bridge to recovery, to decision or to longer term support [169].
- Impella RP (Abiomed, Danvers, MA) is a single access, percutaneous micro-axial pump. Positioned via the femoral vein and advanced across the tricuspid and pulmonic valves into the PA, it withdraws blood from the RA and delivers up to 4 L/min into the pulmonary circulation. Unlike other systems, it cannot be combined with an oxygenator. Clinical data from RECOVER RIGHT trial demonstrated significant hemodynamic improvement, with reductions in right atrial pressure (RAP) and increases in cardiac index, with survival to discharge in approximately 73% of patients. The device has been successfully used in RHF complicating myocardial infarction, pulmonary embolism, LVAD implantation, post-cardiotomy shock and primary graft dysfunction after heart transplantation. Major limitations include femoral access, predisposing to bleeding, hemolysis and restricted patient mobility [171,172]. A new internal jugular configuration is under development to enable deambulation (Impella RP Flex, Abiomed) [173].
- Levitronix CentriMag RVAD (Abbott) is a surgically implanted extracorporeal centrifugal pump capable of flows up to 10 L/min. Unlike conventional pumps that rely on mechanical bearings, the CentriMag uses a fully magnetically levitated rotor, eliminating friction and reducing hemolysis and thrombosis. Cannulation typically involves sternotomy or thoracotomy with direct access to the RA/RV and PA. The pump withdraws blood from RA or RV through a venous cannula and propels it into the PA, thereby bypassing the failing right ventricle and ensuring adequate pulmonary circulation. While invasive, the device allows a rapid implantation and provides robust circulatory support. For these reasons it is frequently applied in post-cardiotomy shock, RHF following LVAD implantation and primary graft dysfunction after transplantation [174,175].
- PERkutane KATheroumptechnologie RV (PERKAT RV, NovaPump, Jena, Germany) is a percutaneous, pulsatile device designed to replicate physiologic RV ejection. Delivered via an 18F femoral catheter, it incorporates a nitinol stent-mounted balloon pump, electrocardiographically triggered to displace blood into the PA during diastolic inflation. Capable of generating flows up to 4 L/min, it has shown efficacy in preclinical models of RV failure, particularly in acute pulmonary embolism. Its key advantage is smaller bore access and pulsatile support, potentially mitigating microvascular dysfunction attributed to continuous flow devices [176,177].
- Spectrum medical dual lumen RV-PA cannula (Cheltenham, England) is a novel cannula placed via internal jugular vein, with inflow from the RA and RV and outflow into the PA. The outer lumen (which has multiple inflow openings) drains deoxygenated blood, while the inner lumen returns oxygenated blood back into the patient. Its dual-stage design addresses a key limitation of single-port RA drainage system (e.g., ProtekDuo), by capturing blood from both the RA and RV, thereby ensuring more complete RV unloading. Available in multiple sizes, it can provide 3–5 L/min of flow and is compatible with any extracorporeal centrifugal circuit with an oxygenator and allows preserved patient mobility [178].
2.8.2. Long Term Support
- Durable RVAD (LVADs in RV position): dedicated durable RVADs are not commercially available. In cases requiring chronic RV support, LVADs may be repositioned to draw from RA or RV and eject into the PA. This off label strategy poses unique challenges: thinner RV/RA walls predispose to suction events, while low systemic pressures and high venous pressures complicate hemodynamics. Modifications, such as shortening the inflow cannula or restricting the outflow graft are often necessary. Debate continues regarding whether inflow is best positioned in the RA (less suction risk) or RV (more effective unloading) [161]. HeartMate 3 (HM3, Abbott): its use as a RVAD or as a part of biventricular support (BiVAD) is off label, though it has been done by implanting two HM3 pumps, one to support RV [179]. Berlin Heart (EXCOR) is a paracorporeal, pulsatile-flow VAD, configurable for support of LV, RV or both (BiVAD) and offers a long-term RV support, especially in pediatric cases, as part of a BiVAD strategy [180].
- Total Artificial heart (TAH) provide biventricular support (the blood is drained from the right and the left atria and is pumped by the TAH into the PA and aorta). The SynCardia TAH is pneumatically driven and generates pulsatile flow. In contrast, the Carmat Aeson bioprosthetic heart (another TAH) is electromechanically driven and incorporates biological valves and surfaces. Both systems can provide flows up to 9–10 L/min, ensuring full systemic and pulmonary perfusion [181,182,183].
2.8.3. Special Consideration in Device Selection
- Tricuspid regurgitation (TR): TR is usually secondary to RV dysfunction with annular dilatation. While devices crossing the tricuspid valve may worsen or induce regurgitation, this should not preclude their use: TR may facilitate RV unloading, stabilize rotary-flow pump performance and, occasionally, improve with RV offloading [161,184].
- Concomitant LV dysfunction: In the presence of left ventricular dysfunction, the hemodynamic impact of right-sided devices must be carefully considered. Right- sided bypass systems (RA-PA or RV-PA) increase LV preload and may precipitate pulmonary edema in LV dysfunction, whereas RA-Ao configurations such as V-A ECMO elevate LV afterload, risking ventricular distension and pulmonary congestion. Under these circumstances, biventricular support provides a more balanced approach [161] (Table 5). Strategies such as BiPella (Impella RP combined with Impella CP/5.0/5.5), Propella (ProtekDuo and centrifugal pump combined with an Impella 5.0/5.5) or V-A ECMO with LV venting is preferred to achieve simultaneous unloading of both ventricles. Among these, BiPella offers the advantage of single arterial access, stepwise explantation and direct physiological decompression of the right and left ventricles, making it particularly attractive in selected patients with biventricular cardiogenic shock [185,186,187,188,189].
2.9. Heart Transplantation
2.10. Novel Strategies for RHF
2.10.1. Energy Metabolism Dysregulation
2.10.2. Mitochondrial Dysfunction, ROS and Antioxidant Capacity
2.10.3. Impaired Angiogenesis
3. Discussion
Limitations and Strengths
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RHF | Right heart failure |
| RV | Right ventricular |
| ARHF | Acute right heart failure |
| CRHF | Chronic right heart failure |
| RVAD | Right ventricular assist devices |
| LVAD | Left ventricular assist device |
| BiVAD | Biventricular assist device |
| V-A ECMO | Veno-arterial extracorporeal membrane oxygenation |
| CHD | Congenital heart disease |
| TAH | Total artificial heart |
| BiPella | Left and right Impella support |
| Propella | ProtekDuo with centrifugal combined with Impella 5.0/5.5 |
References
- Mehra, M.R.; Park, M.H.; Landzberg, M.J.; Lala, A.; Waxman, A.B.; International Right Heart Failure Foundation Scientific Working Group. Right heart failure: Toward a common language. J. Heart Lung Transplant. 2014, 33, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Vlahakes, G.J.; Turley, K.; Hoffman, J.I. The pathophysiology of failure in acute right ventricular hypertension: Hemodynamic and biochemical correlations. Circulation 1981, 63, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zochios, V.; Jones, N. Acute right heart syndrome in the critically ill patient. Heart Lung Vessel. 2014, 6, 157–170. [Google Scholar] [PubMed] [PubMed Central]
- Monteagudo-Vela, M.; Tindale, A.; Monguió-Santín, E.; Reyes-Copa, G.; Panoulas, V. Right ventricular failure: Current strategies and future development. Front. Cardiovasc. Med. 2023, 10, 998382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ventetuolo, C.E.; Klinger, J.R. Management of acute right ventricular failure in the intensive care unit. Ann. Am. Thorac. Soc. 2014, 11, 811–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hameed, A.; Condliffe, R.; Swift, A.J.; Alabed, S.; Kiely, D.G.; Charalampopoulos, A. Assessment of Right Ventricular Function-a State of the Art. Curr. Heart Fail. Rep. 2023, 20, 194–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voelkel, N.F.; Quaife, R.A.; Leinwand, L.A.; Barst, R.J.; McGoon, M.D.; Meldrum, D.R.; Dupuis, J.; Long, C.S.; Rubin, L.J.; Smart, F.W.; et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006, 114, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology, 14th ed.; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Dell’Italia, L.J. The right ventricle: Anatomy, physiology, and clinical importance. Curr. Probl. Cardiol. 1991, 16, 653–720. [Google Scholar] [CrossRef] [PubMed]
- Greyson, C.R. Pathophysiology of right ventricular failure. Crit. Care Med. 2008, 36 (Suppl. 1), S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Pouleur, H.; Lefèvre, J.; Van Mechelen, H.; Charlier, A.A. Free-wall shortening and relaxation during ejection in the canine right ventricle. Am. J. Physiol. 1980, 239, H601–H613. [Google Scholar] [CrossRef] [PubMed]
- Maughan, W.L.; Shoukas, A.A.; Sagawa, K.; Weisfeldt, M.L. Instantaneous pressure-volume relationship of the canine right ventricle. Circ. Res. 1979, 44, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hurford, W.E.; Zapol, W.M. The right ventricle and critical illness: A review of anatomy, physiology, and clinical evaluation of its function. Intensive Care Med. 1988, 14 (Suppl. 2), 448–457. [Google Scholar] [CrossRef] [PubMed]
- Weyman, A.E.; Wann, S.; Feigenbaum, H.; Dillon, J.C. Mechanism of abnormal septal motion in patients with right ventricular volume overload: A cross-sectional echocardiographic study. Circulation 1976, 54, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Jardin, F.; Gueret, P.; Prost, J.F.; Farcot, J.C.; Ozier, Y.; Bourdarias, J.P. Two-dimensional echocardiographic assessment of left ventricular function in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1984, 129, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Thandavarayan, R.A.; Chitturi, K.R.; Guha, A. Pathophysiology of Acute and Chronic Right Heart Failure. Cardiol. Clin. 2020, 38, 149–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.; Landi, A.; Barco, S.; Cassina, T.; Merlani, P.; Milzi, A.; Petrino, R.; Rigamonti, E.; Sürder, D.; Zucconi, E.; et al. Contemporary management of acute pulmonary embolism. Int. J. Cardiol. 2025, 436, 133422. [Google Scholar] [CrossRef] [PubMed]
- Martinez Licha, C.R.; McCurdy, C.M.; Maldonado, S.M.; Lee, L.S. Current Management of Acute Pulmonary Embolism. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 65–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bowers, T.R.; O’Neill, W.W.; Pica, M.; Goldstein, J.A. Patterns of coronary compromise resulting in acute right ventricular ischemic dysfunction. Circulation 2002, 106, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A. Stunned and Hibernating Myocardium: Where Are We Nearly 4 Decades Later? J. Am. Heart Assoc. 2020, 9, e015502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merlo, A.; Cirelli, C.; Vizzardi, E.; Fiorendi, L.; Roncali, F.; Marino, M.; Merlo, M.; Senni, M.; Sciatti, E. Right Ventricular Dysfunction before and after Cardiac Surgery: Prognostic Implications. J. Clin. Med. 2024, 13, 1609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harjola, V.P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, B.; Borgdorff, M.A.; Smit-van Oosten, A.; Takens, J.; Boersma, B.; Nederhoff, M.G.; Elzenga, N.J.; van Gilst, W.H.; De Windt, L.J.; Berger, R.M. Differential responses of the right ventricle to abnormal loading conditions in mice: Pressure vs. volume load. Eur. J. Heart Fail. 2011, 13, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Maughan, W.L.; Kallman, C.H.; Shoukas, A. The effect of right ventricular filling on the pressure-volume relationship of ejecting canine left ventricle. Circ. Res. 1981, 49, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Waymack, J.R. Acute Cardiac Tamponade. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Mattei, A.; Strumia, A.; Benedetto, M.; Nenna, A.; Schiavoni, L.; Barbato, R.; Mastroianni, C.; Giacinto, O.; Lusini, M.; Chello, M.; et al. Perioperative Right Ventricular Dysfunction and Abnormalities of the Tricuspid Valve Apparatus in Patients Undergoing Cardiac Surgery. J. Clin. Med. 2023, 12, 7152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Álvarez, P.; Carrasco, R.; Romero-Dapueto, C.; Castillo, R.L. Transfusion-Related Acute Lung Injured (TRALI): Current Concepts. Open Respir. Med. J. 2015, 9, 92–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zagorski, J.; Sanapareddy, N.; Gellar, M.A.; Kline, J.A.; Watts, J.A. Transcriptional profile of right ventricular tissue during acute pulmonary embolism in rats. Physiol. Genom. 2008, 34, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Bernstein, D. Molecular Mechanisms of Right Ventricular Failure. Circulation 2015, 132, 1734–1742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watts, J.A.; Zagorski, J.; Gellar, M.A.; Stevinson, B.G.; Kline, J.A. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J. Mol. Cell Cardiol. 2006, 41, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Zhao, M.; Hu, D.Q.; Fajardo, G.; Katznelson, E.; Punn, R.; Spin, J.M.; Chan, F.P.; Bernstein, D. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1314–H1327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shults, N.V.; Kanovka, S.S.; Ten Eyck, J.E.; Rybka, V.; Suzuki, Y.J. Ultrastructural Changes of the Right Ventricular Myocytes in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2019, 8, e011227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva Gonçalves Bós, D.; Van Der Bruggen, C.E.E.; Kurakula, K.; Sun, X.Q.; Casali, K.R.; Casali, A.G.; Rol, N.; Szulcek, R.; Dos Remedios, C.; Guignabert, C.; et al. Contribution of Impaired Parasympathetic Activity to Right Ventricular Dysfunction and Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Circulation 2018, 137, 910–924. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.E.; Selzman, C.H.; McKellar, S.H. Right Ventricular Involution: Big Changes in Small Hearts. J. Surg. Res. 2019, 243, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Clapham, K.R.; Singh, I.; Capuano, I.S.; Rajagopal, S.; Chun, H.J. MEF2 and the Right Ventricle: From Development to Disease. Front. Cardiovasc. Med. 2019, 6, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmuck, E.G.; Hacker, T.A.; Schreier, D.A.; Chesler, N.C.; Wang, Z. Beneficial effects of mesenchymal stem cell delivery via a novel cardiac bioscaffold on right ventricles of pulmonary arterial hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1005–H1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, J.J.; Huston, J.; Kutty, S.; Hatton, N.D.; Bowman, L.; Tian, L.; Herr, J.E.; Johri, A.M.; Archer, S.L. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can. J. Cardiol. 2015, 31, 391–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.Q.; Abbate, A.; Bogaard, H.J. Role of cardiac inflammation in right ventricular failure. Cardiovasc. Res. 2017, 113, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Satoh, K.; Kikuchi, N.; Miyata, S.; Suzuki, K.; Omura, J.; Shimizu, T.; Kobayashi, K.; Kobayashi, K.; Fukumoto, Y.; et al. Crucial role of rho-kinase in pressure overload-induced right ventricular hypertrophy and dysfunction in mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Avazmohammadi, R.; Hill, M.; Simon, M.; Sacks, M. Transmural remodeling of right ventricular myocardium in response to pulmonary arterial hypertension. APL Bioeng. 2017, 1, 016105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendes-Ferreira, P.; Santos-Ribeiro, D.; Adão, R.; Maia-Rocha, C.; Mendes-Ferreira, M.; Sousa-Mendes, C.; Leite-Moreira, A.F.; Brás-Silva, C. Distinct right ventricle remodeling in response to pressure overload in the rat. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H85–H95. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Foster, E.; Kuehl, K.; Alpert, J.; Brabeck, S.; Crumb, S.; Davidson, W.R., Jr.; Earing, M.G.; Ghoshhajra, B.B.; Karamlou, T.; et al. American Heart Association Council on Clinical Cardiology. Congenital heart disease in the older adult: A scientific statement from the American Heart Association. Circulation 2015, 131, 1884–1931, Erratum in Circulation 2015, 131, e510. https://doi.org/10.1161/CIR.0000000000000216. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Sandica, E.; Prietz, S.; Klopsch, C.; Ugurlucan, M.; Kaminski, A.; Abdija, S.; Lorenzen, B.; Boltze, J.; Nitzsche, B.; et al. Autologous umbilical cord blood mononuclear cell transplantation preserves right ventricular function in a novel model of chronic right ventricular volume overload. Cell Transplant. 2009, 18, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.A.; Kent, R.L.; Uboh, C.E.; Fernandez, E.; Thompson, E.W.; Cooper, G., 4th. Structural analysis of pressure versus volume overload hypertrophy of cat right ventricle. Am. J. Physiol. 1985, 249 Pt 2, H371–H379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ferrara, F.; Contaldi, C.; Bossone, E. Right Ventricular Size and Function in Chronic Heart Failure: Not to Be Forgotten. Heart Fail. Clin. 2019, 15, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T.; et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sutendra, G.; Dromparis, P.; Paulin, R.; Zervopoulos, S.; Haromy, A.; Nagendran, J.; Michelakis, E.D. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J. Mol. Med. 2013, 91, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Douglas, I.S.; Archer, S.L.; Bogaard, H.J.; Chesler, N.C.; Haddad, F.; Hemnes, A.R.; Kawut, S.M.; Kline, J.A.; Kolb, T.M.; et al. American Thoracic Society Assembly on Pulmonary Circulation. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e15–e43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Calcutteea, A.; Chung, R.; Lindqvist, P.; Hodson, M.; Henein, M.Y. Differential right ventricular regional function and the effect of pulmonary hypertension: Three-dimensional echo study. Heart 2011, 97, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.T.; Gan, C.T.; Zwanenburg, J.J.; Boonstra, A.; Allaart, C.P.; Götte, M.J.; Vonk-Noordegraaf, A. Interventricular mechanical asynchrony in pulmonary arterial hypertension: Left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J. Am. Coll. Cardiol. 2008, 51, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Rain, S.; Handoko, M.L.; Trip, P.; Gan, C.T.; Westerhof, N.; Stienen, G.J.; Paulus, W.J.; Ottenheijm, C.A.; Marcus, J.T.; Dorfmüller, P.; et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013, 128, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Soós, P.; Bährle, S.; Radovits, T.; Weigang, E.; Kékesi, V.; Merkely, B.; Hagl, S. Adaptation of the right ventricle to an increased afterload in the chronically volume overloaded heart. Ann. Thorac. Surg. 2006, 82, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Agger, P.; Ilkjær, C.; Laustsen, C.; Smerup, M.; Frandsen, J.R.; Ringgaard, S.; Pedersen, M.; Partridge, J.B.; Anderson, R.H.; Hjortdal, V. Changes in overall ventricular myocardial architecture in the setting of a porcine animal model of right ventricular dilation. J. Cardiovasc. Magn. Reson. 2017, 19, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van De Bruaene, A.; Buys, R.; Vanhees, L.; Delcroix, M.; Voigt, J.U.; Budts, W. Regional right ventricular deformation in patients with open and closed atrial septal defect. Eur. J. Echocardiogr. 2011, 12, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Dragulescu, A.; Grosse-Wortmann, L.; Redington, A.; Friedberg, M.K.; Mertens, L. Differential effect of right ventricular dilatation on myocardial deformation in patients with atrial septal defects and patients after tetralogy of Fallot repair. Int. J. Cardiol. 2013, 168, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.M.; Haber, I.; Wald, R.; Valente, A.M.; Powell, A.J.; Geva, T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation 2009, 119, 1370–1377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masih, R.; Paudyal, V.; Basnet, Y.M.; Sunesara, S.; Sharma, M.; Surani, S. Revisiting Acute Decompensated Right Ventricle Failure in Pulmonary Arterial Hypertension. Open Respir. Med. J. 2025, 19, e18743064359315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vieillard-Baron, A.; Prigent, A.; Repessé, X.; Goudelin, M.; Prat, G.; Evrard, B.; Charron, C.; Vignon, P.; Geri, G. Right ventricular failure in septic shock: Characterization, incidence and impact on fluid responsiveness. Crit. Care 2020, 24, 630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alipour Symakani, R.S.; van Genuchten, W.J.; Zandbergen, L.M.; Henry, S.; Taverne, Y.J.H.J.; Merkus, D.; Helbing, W.A.; Bartelds, B. The right ventricle in tetralogy of Fallot: Adaptation to sequential loading. Front. Pediatr. 2023, 11, 1098248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vijan, A.; Daha, I.C.; Delcea, C.; Dan, G.A. The complex interplay between right ventricular dysfunction and atrial fibrillation—A narrative review. Rom. J. Intern. Med. 2023, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, W.; Emaminia, A. Tricuspid Regurgitation and Right Heart Failure: “It All Begins and Ends with the RV”. JACC Heart Fail. 2020, 8, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Skouri, H.N.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Paganini, E.; Tang, W.H. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? J. Am. Coll. Cardiol. 2008, 51, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Henzler, T.; Roeger, S.; Meyer, M.; Schoepf, U.J.; Nance, J.W., Jr.; Haghi, D.; Kaminski, W.E.; Neumaier, M.; Schoenberg, S.O.; Fink, C. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur. Respir. J. 2012, 39, 919–926. [Google Scholar] [CrossRef] [PubMed]
- van Veldhuisen, D.J.; Anker, S.D.; Ponikowski, P.; Macdougall, I.C. Anemia and iron deficiency in heart failure: Mechanisms and therapeutic approaches. Nat. Rev. Cardiol. 2011, 8, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Klersy, C.; Magrini, G.; D’Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Viganò, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Humbert, M.; Jaïs, X.; Ioos, V.; Hamid, A.M.; Provencher, S.; Garcia, G.; Parent, F.; Hervé, P.; Simonneau, G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005, 111, 3105–3111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhao, Z.; Liu, J.; Liu, M.; Xie, F. Efficacy and safety of iloprost in the treatment of pulmonary arterial hypertension: A systematic review and meta-analysis. Heart Lung. 2024, 64, 36–45. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, S.; Zhou, K.; Jin, Y.; He, L.; Xu, W.; Lao, C.; Liu, G.; Han, S. Sildenafil for pulmonary hypertension in neonates: An updated systematic review and meta-analysis. Pediatr. Pulmonol. 2021, 56, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Lv, Y.; Cui, Y.; Wang, P.; He, X. Bosentan in the treatment of persistent pulmonary hypertension in newborns: A systematic review and meta-analysis. Cardiol. Young 2024, 34, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.S.; Doerfler, S.; Shelburne, N.; Kennedy, K.; Whitson, J.; Dahhan, T.; Fortin, T.; Rajagopal, S. Experience in Transitioning from Parenteral Prostacyclins to Selexipag in Pulmonary Arterial Hypertension. J. Cardiovasc. Pharmacol. 2020, 75, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Boucly, A.; Gerges, C.; Savale, L.; Jaïs, X.; Jevnikar, M.; Montani, D.; Sitbon, O.; Humbert, M. Pulmonary arterial hypertension. Presse Med. 2023, 52, 104168. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.M.; Fradin, J.J.; Russ, D.H.; Warner, E.D.; Brailovsky, Y.; Rajapreyar, I. Post-Capillary Pulmonary Hypertension: Clinical Review. J. Clin. Med. 2024, 13, 625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731, Erratum in Eur. Heart. J. 2023, 44, 1312. https://doi.org/10.1093/eurheartj/ehad005. [Google Scholar] [CrossRef] [PubMed]
- Cordina, R.L.; Celermajer, D.S. Therapeutic approaches in adults with congenital heart disease-associated pulmonary arterial hypertension. Eur. Respir. Rev. 2010, 19, 300–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vongpatanasin, W.; Brickner, M.E.; Hillis, L.D.; Lange, R.A. The Eisenmenger syndrome in adults. Ann. Intern. Med. 1998, 128, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Beghetti, M.; Gatzoulis, M.A.; Granton, J.; Berger, R.M.; Lauer, A.; Chiossi, E.; Landzberg, M. Bosentan Randomized Trial of Endothelin Antagonist Therapy-5 (BREATHE-5) Investigators. Bosentan therapy in patients with Eisenmenger syndrome: A multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006, 114, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Sharma, M.; Ramakrishnan, S.; Yusuf, J.; Gupta, M.D.; Bhamri, N.; Trehan, V.; Tyagi, S. Phosphodiesterase-5 inhibitor in Eisenmenger syndrome: A preliminary observational study. Circulation 2006, 114, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.M.; Cook, S.; Festa, P.; Ko, H.H.; Krishnamurthy, R.; Taylor, A.M.; Warnes, C.A.; Kreutzer, J.; Geva, T. Multimodality imaging guidelines for patients with repaired tetralogy of fallot: A report from the AmericanSsociety of Echocardiography: Developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J. Am. Soc. Echocardiogr. 2014, 27, 111–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Poh, A.L.; Tang, W.H.W. Novel Insights and Treatment Strategies for Right Heart Failure. Curr. Heart Fail. Rep. 2018, 15, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, L.; Bi, X.; Li, X.; Cong, H. Right ventricular-arterial uncoupling as an independent prognostic factor in acute heart failure with preserved ejection fraction accompanied with coronary artery disease. Chin. Med. J. 2023, 136, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anastasiou, V.; Papazoglou, A.S.; Moysidis, D.V.; Daios, S.; Barmpagiannos, K.; Gossios, T.; Efthimiadis, G.K.; Karamitsos, T.; Ziakas, A.; Kamperidis, V. The prognostic impact of right ventricular-pulmonary arterial coupling in heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2024, 29, 13–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639, Erratum in Eur. Heart J. 2024, 45, 53. https://doi.org/10.1093/eurheartj/ehad613. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Hwang, S.J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoendermis, E.S.; Liu, L.C.; Hummel, Y.M.; van der Meer, P.; de Boer, R.A.; Berger, R.M.; van Veldhuisen, D.J.; Voors, A.A. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur. Heart J. 2015, 36, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.P.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.V.; et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jenkins, D.; Mayer, E.; Screaton, N.; Madani, M. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur. Respir. Rev. 2012, 21, 32–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, S.; Bonios, M.; Ben Gal, T.; Gustafsson, F.; Abdelhamid, M.; Adamo, M.; Bayes-Genis, A.; Böhm, M.; Chioncel, O.; Cohen-Solal, A.; et al. Right heart failure with left ventricular assist devices: Preoperative, perioperative and postoperative management strategies. A clinical consensus statement of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 2304–2322. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, A.; Paone, G.; Brewer, R.J.; Nemeh, H.W.; Borgi, J.; Morgan, J.A. Outcomes of patients with right ventricular failure on milrinone after left ventricular assist device implantation. ASAIO J. 2015, 61, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sabato, L.A.; Salerno, D.M.; Moretz, J.D.; Jennings, D.L. Inhaled Pulmonary Vasodilator Therapy for Management of Right Ventricular Dysfunction after Left Ventricular Assist Device Placement and Cardiac Transplantation. Pharmacotherapy 2017, 37, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Choudhri, A.F.; Moazami, N.; Rose, E.A.; Smith, C.R.; Levin, H.R.; Smerling, A.J.; Oz, M.C. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann. Thorac. Surg. 1998, 65, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.; Farag, M.; Brcic, A.; Zubarevich, A.; Schamroth, J.; Kreusser, M.M.; Karck, M.; Ruhparwar, A.; Schmack, B. Temporary right ventricular circulatory support following right ventricular infarction: Results of a groin-free approach. ESC Heart Fail. 2020, 7, 2853–2861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheel, P.J., 3rd; Giuliano, K.; Tichnell, C.; James, C.; Murray, B.; Tandri, H.; Carter, D.; Fehr, T.; Umapathi, P.; Vaishnav, J.; et al. Heart transplantation strategies in arrhythmogenic right ventricular cardiomyopathy: A tertiary ARVC centre experience. ESC Heart Fail. 2022, 9, 1008–1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilstrap, L.G.; Dominici, F.; Wang, Y.; El-Sady, M.S.; Singh, A.; Di Carli, M.F.; Falk, R.H.; Dorbala, S. Epidemiology of Cardiac Amyloidosis-Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ. Heart Fail. 2019, 12, e005407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mandoli, G.E.; De Carli, G.; Pastore, M.C.; Cameli, P.; Contorni, F.; D’Alessandro, M.; Bargagli, E.; Mondillo, S.; Cameli, M. Right cardiac involvement in lung diseases: A multimodality approach from diagnosis to prognostication. J. Intern. Med. 2021, 289, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Chioncel, O.; Pagnesi, M.; Bayes-Genis, A.; Abdelhamid, M.; Anker, S.D.; Antohi, E.L.; Badano, L.; Ben Gal, T.; Böhm, M.; et al. Epidemiology, pathophysiology, diagnosis and management of chronic right-sided heart failure and tricuspid regurgitation. A clinical consensus statement of the Heart Failure Association (HFA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur. J. Heart Fail. 2024, 26, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Iturriagagoitia, A.; Vanderheyden, M.; Budts, W.; Vercauter, P. Right Heart Failure in a Patient with Critical Pulmonary Stenosis, Absent Right Pulmonary Artery, and Lung Cancer. Am. J. Case Rep. 2022, 23, e937305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raizada, V.; Sato, K.; Alashi, A.; Kumar, A.; Kwon, D.; Ramchand, J.; Dillenbeck, A.; Zumwalt, R.E.; Vangala, A.S.; Earley, T.D.; et al. Depressed right ventricular systolic function in heart failure due to constrictive pericarditis. ESC Heart Fail. 2021, 8, 3119–3129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoit, B.D. Pathophysiology of the Pericardium. Prog. Cardiovasc. Dis. 2017, 59, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Perl, M.; Perelman-Gvili, M.; Sirota Dorfman, S.; Baruch, G.; Rothschild, E.; Beer, G.; Arbel, Y.; Arnold, J.H.; Rozenbaum, Z.; Banai, S.; et al. Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy. Life 2022, 12, 291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surkova, E.; Muraru, D.; Iliceto, S.; Badano, L.P. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int. J. Cardiol. 2016, 214, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Pazzano, A.S.; Klersy, C.; Scelsi, L.; Raineri, C.; Camporotondo, R.; D’Armini, A.; Visconti, L.O. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am. J. Cardiol. 2011, 107, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Frémont, B.; Pacouret, G.; Jacobi, D.; Puglisi, R.; Charbonnier, B.; de Labriolle, A. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: Results from a monocenter registry of 1416 patients. Chest 2008, 133, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Anavekar, N.S.; Skali, H.; Bourgoun, M.; Ghali, J.K.; Kober, L.; Maggioni, A.P.; McMurray, J.J.; Velazquez, E.; Califf, R.; Pfeffer, M.A.; et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am. J. Cardiol. 2008, 101, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Recusani, F.; Klersy, C.; Sebastiani, R.; Laudisa, M.L.; Campana, C.; Gavazzi, A.; Tavazzi, L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am. J. Cardiol. 2000, 85, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Tei, C.; Dujardin, K.S.; Hodge, D.O.; Bailey, K.R.; McGoon, M.D.; Tajik, A.J.; Seward, S.B. Doppler echocardiographic index for assessment of global right ventricular function. J. Am. Soc. Echocardiogr. 1996, 9, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Singbal, Y.; Vollbon, W.; Huynh, L.T.; Wang, W.Y.; Ng, A.C.; Wahi, S. Exploring Noninvasive Tricuspid dP/dt as a Marker of Right Ventricular Function. Echocardiography 2015, 32, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.N.; Schäfer, M.; Li, L.; Craft, M.; Ivy, D.D.; Kutty, S. Right Atrial Deformation in Predicting Outcomes in Pediatric Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2017, 10, e006250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alenezi, F.; Mandawat, A.; Il’Giovine, Z.J.; Shaw, L.K.; Siddiqui, I.; Tapson, V.F.; Arges, K.; Rivera, D.; Romano, M.M.D.; Velazquez, E.J.; et al. Clinical Utility and Prognostic Value of Right Atrial Function in Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2018, 11, e006984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasselberg, N.E.; Kagiyama, N.; Soyama, Y.; Sugahara, M.; Goda, A.; Ryo-Koriyama, K.; Batel, O.; Chakinala, M.; Simon, M.A.; Gorcsan, J., 3rd. The Prognostic Value of Right Atrial Strain Imaging in Patients with Precapillary Pulmonary Hypertension. J. Am. Soc. Echocardiogr. 2021, 34, 851–861.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yan, Y.; Jiang, W.; Wang, Z.; Wu, C.; Wu, Q.; Deng, Y.; Du, Y.; Yang, Z.; Zhang, Z.; et al. Impaired right atrial function preceding right ventricular systolic dysfunction: Clinical utility and long-term prognostic value in pulmonary hypertension. Insights Imaging 2025, 16, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Vanermen, H.; Maisano, F.; Guidotti, A.; La Canna, G.; Alfieri, O. The growing clinical importance of secondary tricuspid regurgitation. J. Am. Coll. Cardiol. 2012, 59, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Dannenberg, V.; König, A.; Geller, W.; Binder, T.; Hengstenberg, C.; Goliasch, G. Prognostic Value of Echocardiographic Right Ventricular Function Parameters in the Presence of Severe Tricuspid Regurgitation. J. Clin. Med. 2021, 10, 2266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tadic, M.; Nita, N.; Schneider, L.; Kersten, J.; Buckert, D.; Gonska, B.; Scharnbeck, D.; Reichart, C.; Belyavskiy, E.; Cuspidi, C.; et al. The Predictive Value of Right Ventricular Longitudinal Strain in Pulmonary Hypertension, Heart Failure, and Valvular Diseases. Front. Cardiovasc. Med. 2021, 8, 698158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamada-Harimura, Y.; Seo, Y.; Ishizu, T.; Nishi, I.; Machino-Ohtsuka, T.; Yamamoto, M.; Sugano, A.; Sato, K.; Sai, S.; Obara, K.; et al. Incremental Prognostic Value of Right Ventricular Strain in Patients with Acute Decompensated Heart Failure. Circ. Cardiovasc. Imaging 2018, 11, e007249. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Larose, E.; Ganz, P.; Reynolds, H.G.; Dorbala, S.; Di Carli, M.F.; Brown, K.A.; Kwong, R.Y. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, Q.J.; Witschey, W.R.; Fang-Yen, C.M.; Arkles, J.S.; Barker, A.J.; Forfia, P.R.; Han, Y. Altered Right Ventricular Kinetic Energy Work Density and Viscous Energy Dissipation in Patients with Pulmonary Arterial Hypertension: A Pilot Study Using 4D Flow MRI. PLoS ONE 2015, 10, e0138365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Liu, M.; Zhang, P.Y.; Dai, J.Z.; Ma, H.Y.; Tao, X.C.; Xie, W.M.; Wan, J.; Jing, A. Analysis of right ventricular flow with 4-dimensional flow cardiovascular magnetic resonance imaging in patients with pulmonary arterial hypertension. Quant. Imaging Med. Surg. 2021, 11, 3655–3665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoepf, U.J.; Goldhaber, S.Z.; Costello, P. Spiral computed tomography for acute pulmonary embolism. Circulation 2004, 109, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sendon, J.; Coma-Canella, I.; Gamallo, C. Sensitivity and specificity of hemodynamic criteria in the diagnosis of acute right ventricular infarction. Circulation 1981, 64, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Hakgor, A.; Yilmaz, B.; Erkanli, K.; Cakal, B.; Yazar, A.; Yildiz, Y.; Boztosun, B.; Karaca, I.O. Right Atrial Pressure/Pulmonary Capillary Wedge Pressure Ratio Predicts In-Hospital Mortality in Left Ventricular Assist Device Recipients. J. Clin. Med. 2025, 14, 4784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beneyto, M.; Martins, R.; Galand, V.; Kindo, M.; Schneider, C.; Sebestyen, A.; Boignard, A.; Sebbag, L.; Pozzi, M.; Genet, T.; et al. Right Ventriculoarterial Coupling Surrogates and Long-Term Survival in LVAD Recipients: Results of the ASSIST-ICD Multicentric Registry. J. Card. Fail. 2025, 31, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.F.; Schulze, P.C.; Kato, T.S.; Bacchetta, M.; Thirapatarapong, W.; Bartels, M.N. Right ventricular stroke work index as a negative predictor of mortality and initial hospital stay after lung transplantation. J. Heart Lung Transplant. 2013, 32, 603–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thenappan, T.; Prins, K.W.; Pritzker, M.R.; Scandurra, J.; Volmers, K.; Weir, E.K. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2016, 13, 276–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kempton, H.; Hungerford, S.; Muller, D.W.; Hayward, C.S. Pulmonary arterial compliance as a measure of right ventricular loading in mitral regurgitation. Int. J. Cardiol. Heart Vasc. 2024, 53, 101472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naeije, R.; Vachiery, J.L.; Yerly, P.; Vanderpool, R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur. Respir. J. 2013, 41, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.I.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.M.M.H.; Bogers, A.J.J.C.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018, 137, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.E.; Karahan, M.; Aygun, E.; Kocabeyoglu, S.S.; Akdi, M.; Kervan, U. Prediction of right ventricular failure after continuous flow left ventricular assist device implantation. J. Card. Surg. 2020, 35, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Taleb, I.; Kyriakopoulos, C.P.; Fong, R.; Ijaz, N.; Demertzis, Z.; Sideris, K.; Wever-Pinzon, O.; Koliopoulou, A.G.; Bonios, M.J.; Shad, R.; et al. Machine Learning Multicenter Risk Model to Predict Right Ventricular Failure After Mechanical Circulatory Support: The STOP-RVF Score. JAMA Cardiol. 2024, 9, 272–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sert, S.; Selçuk, N.; Yıldırımtürk, Ö.; Orhan, G. Prognostic value of TAPSE/PASP ratio in right ventricular failure after left ventricular assist device implantation: Experience from a tertiary center. Turk. Gogus Kalp Damar Cerrahisi Derg. 2022, 30, 334–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rako, Z.A.; Kremer, N.; Yogeswaran, A.; Richter, M.J.; Tello, K. Adaptive versus maladaptive right ventricular remodelling. ESC Heart Fail. 2023, 10, 762–775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanderpool, R.R.; Pinsky, M.R.; Naeije, R.; Deible, C.; Kosaraju, V.; Bunner, C.; Mathier, M.A.; Lacomis, J.; Champion, H.C.; Simon, M.A. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015, 101, 37–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bowcock, E.; Huang, S.; Yeo, R.; Walisundara, D.; Duncan, C.F.; Pathan, F.; Strange, G.; Playford, D.; Orde, S. The value of right ventricular to pulmonary arterial coupling in the critically ill: A National Echocardiography Database of Australia (NEDA) substudy. Ann. Intensive Care 2024, 14, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keranov, S.; Widmann, L.; Jafari, L.; Liebetrau, C.; Keller, T.; Troidl, C.; Kriechbaum, S.; Voss, S.; Bauer, P.; Richter, M.J.; et al. GDF-15 and soluble ST2 as biomarkers of right ventricular dysfunction in pulmonary hypertension. Biomark. Med. 2022, 16, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure-The Diagnostic, Prognostic and Therapeutic Potential-Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paulin, R.; Sutendra, G.; Gurtu, V.; Dromparis, P.; Haromy, A.; Provencher, S.; Bonnet, S.; Michelakis, E.D. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ. Res. 2015, 116, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, Y.; Cheng, H.; Guo, Z.; Yu, X.; Tuohuti, A.; Li, G. Correlation between serum GDF-15 level and pulmonary vascular morphological changes and prognosis in patients with pulmonary arterial hypertension. Front. Cardiovasc. Med. 2023, 10, 1085122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakar, S.N.; Jia, S.; Smith, S.J. Right ventricular failure management. Curr. Opin. Cardiol. 2019, 34, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biswas, S.; Malik, A.H.; Bandyopadhyay, D.; Gupta, R.; Goel, A.; Briasoulis, A.; Fonarow, G.C.; Lanier, G.M.; Naidu, S.S. Meta-analysis Comparing the Efficacy of Dobutamine Versus Milrinone in Acute Decompensated Heart Failure and Cardiogenic Shock. Curr. Probl. Cardiol. 2023, 48, 101245. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Szwed, P.; Gasecka, A.; Rafique, Z.; Pruc, M.; Filipiak, K.J.; Jaguszewski, M.J. Milrinone or dobutamine in patients with heart failure: Evidence from meta-analysis. ESC Heart Fail. 2022, 9, 2049–2050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groeneveldt, J.A.; de Man, F.S.; Westerhof, B.E. The right treatment for the right ventricle. Curr. Opin. Pulm. Med. 2019, 25, 410–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hyldebrandt, J.A.; Bøgh, N.; Omann, C.; Agger, P. Norepinephrine and dobutamine improve cardiac index equally by supporting opposite sides of the heart in an experimental model of chronic pulmonary hypertension. Intensive Care Med. Exp. 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arrigo, M.; Huber, L.C.; Winnik, S.; Mikulicic, F.; Guidetti, F.; Frank, M.; Flammer, A.J.; Ruschitzka, F. Right Ventricular Failure: Pathophysiology, Diagnosis and Treatment. Card. Fail. Rev. 2019, 5, 140–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graham, F.J.; Guha, K.; Cleland, J.G.; Kalra, P.R. Treating iron deficiency in patients with heart failure: What, why, when, how, where and who. Heart 2024, 110, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- King, N.E.; Brittain, E. Emerging therapies: The potential roles SGLT2 inhibitors, GLP1 agonists, and ARNI therapy for ARNI pulmonary hypertension. Pulm. Circ. 2022, 12, e12028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mustapic, I.; Bakovic, D.; Susilovic Grabovac, Z.; Borovac, J.A. Impact of SGLT2 Inhibitor Therapy on Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. J. Clin. Med. 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çamcı, S.; Yılmaz, E. Effects of Sodium-Glucose Co-Transporter-2 Inhibition on Pulmonary Arterial Stiffness and Right Ventricular Function in Heart Failure with Reduced Ejection Fraction. Medicina 2022, 58, 1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alkhunaizi, F.A.; Burkhoff, D.; Brener, M.I. Right-Sided Mechanical Circulatory Support—A Hemodynamic Perspective. Curr. Heart Fail. Rep. 2022, 19, 334–345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyns, B.; Ector, J.; Rega, F.; Droogne, W.; Vanhaecke, J.; Vanhemelrijck, J.; Griffith, B.; Dowling, R.; Zucker, M.; Burkhoff, D. First human use of partial left ventricular heart support with the Circulite synergy micro-pump as a bridge to cardiac transplantation. Eur. Heart J. 2008, 29, 2582. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.M.; De By, T.M.; Reichenspurner, H.; Deuse, T. Isolated permanent right ventricular assist device implantation with the HeartWare continuous-flow ventricular assist device: First results from the European Registry for Patients with Mechanical Circulatory Support. Eur. J. Cardiothorac. Surg. 2015, 48, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.; Starck, C.; Falk, V.; Eulert-Grehn, J.J. Mechanical circulatory support: Technical tips for the implantation of a right ventricular assist device. JTCVS Open 2021, 8, 37–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shishehbor, M.H.; Moazami, N.; Tong, M.Z.; Unai, S.; Tang, W.H.; Soltesz, E.G. Cardiogenic shock: From ECMO to Impella and beyond. Cleve Clin. J. Med. 2017, 84, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, P.A.; Fadel, R.A.; Giustino, G.; Jabri, A.; Basir, M.B.; Cowger, J.; Alaswad, K.; O’Neill, B.; Gonzalez, P.E.; Gyzm, G.G.; et al. Hemodynamic Effects and Clinical Outcomes of Left Atrial Veno-Arterial Extracorporeal Membrane Oxygenation (LAVA-ECMO) in Cardiogenic Shock. Am. J. Cardiol. 2025, 236, 79–85. [Google Scholar] [CrossRef] [PubMed]
- D’Ettore, N.; Cardinale, A.; Maj, G.; Bertolin, S.; Audo, A.; Montisci, A.; Gallo, A.; Cavozza, C.; Pappalardo, F. ECPella 5+ in Patients with Cardiogenic Shock: Potential for Improved Outcomes. J. Cardiothorac. Vasc. Anesth. 2025, 39, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.M.; Capoccia, M.; Maybauer, D.M.; Lorusso, R.; Swol, J.; Maybauer, M.O. The ProtekDuo dual-lumen cannula for temporary acute mechanical circulatory support in right heart failure: A systematic review. Perfusion 2023, 38 (Suppl. 1), 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lo Coco, V.; De Piero, M.E.; Massimi, G.; Chiarini, G.; Raffa, G.M.; Kowalewski, M.; Maessen, J.; Lorusso, R. Right ventricular failure after left ventricular assist device implantation: A review of the literature. J. Thorac. Dis. 2021, 13, 1256–1269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bermudez, C.A.; Lagazzi, L.; Crespo, M.M. Prolonged Support Using a Percutaneous OxyRVAD in a Patient with End-Stage Lung Disease, Pulmonary Hypertension, and Right Cardiac Failure. ASAIO J. 2016, 62, e37–e40. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Morris, D.L.; Tang, D.; Batsides, G.; Kirtane, A.; Hanson, I.; Meraj, P.; Kapur, N.K.; O’Neill, W. Outcomes of patients with right ventricular failure requiring short-term hemodynamic support with the Impella RP device. J. Heart Lung Transplant. 2018, 37, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, I.; DeWolf, J.; Sevillano, M.; Schnell, L.; Bezerra, H.; Rinde-Hoffman, D. Utilization of Impella RP Flex for Right Ventricular Recovery in Cardiogenic Shock: A Single-Center Experience. Catheter Cardiovasc Interv. 2025, 106, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Bhama, J.K.; Kormos, R.L.; Toyoda, Y.; Teuteberg, J.J.; McCurry, K.R.; Siegenthaler, M.P. Clinical experience using the Levitronix CentriMag system for temporary right ventricular mechanical circulatory support. J. Heart Lung Transplant. 2009, 28, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.; Blank, N.; Kaki, A.; Alraies, M.C.; Grines, C.L.; Kajy, M.; Hasan, R.; Mohamad, T.; Schreiber, T. Mechanical circulatory support for acute right ventricular failure in the setting of pulmonary embolism. J. Interv. Cardiol. 2018, 31, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, D.; Schulze, P.C.; Ferrari, M.W. Hemodynamic Performance of a Novel Right Ventricular Assist Device (PERKAT). ASAIO J. 2017, 63, 123–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, M.W.; Schulze, P.C.; Kretzschmar, D. Acute right heart failure: Future perspective with the PERKAT RV pulsatile right ventricular support device. Ther. Adv. Cardiovasc. Dis. 2020, 14, 1753944719895902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Usman, A.A.; Spelde, A.E.; Olia, S.E.; Cevasco, M.; Bermudez, C.; Haddle, J.; Ibrahim, M.; Szeto, W.; Vernick, W.; Gutsche, J. First-in-man successful use of the SPECTRUM percutaneous dual-stage right ventricle and right atrium to pulmonary artery ventricular assist device. J. Card. Surg. 2022, 37, 3403–3407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marasco, S.F.; McLean, J.; Kure, C.E.; Rix, J.; Lake, T.; Linton, A.; Farag, J.; Zhu, M.Z.L.; Doi, A.; Bergin, P.J.; et al. HeartMate 3 implantation with an emphasis on the biventricular configuration. Artif. Organs 2024, 48, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Vanderpluym, C.J.; Fynn-Thompson, F.; Blume, E.D. Ventricular assist devices in children: Progress with an orphan device application. Circulation 2014, 129, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Melton, N.; Soleimani, B.; Dowling, R. Current Role of the Total Artificial Heart in the Management of Advanced Heart Failure. Curr. Cardiol. Rep. 2019, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Mohacsi, P.; Leprince, P. The CARMAT total artificial heart. Eur. J. Cardiothorac. Surg. 2014, 46, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, G.; Anyanwu, A.; Zucchetta, F.; Gerosa, G. SynCardia: The total artificial heart. Ann. Cardiothorac. Surg. 2014, 3, 612–620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salna, M.; Garan, A.R.; Kirtane, A.J.; Karmpaliotis, D.; Green, P.; Takayama, H.; Sanchez, J.; Kurlansky, P.; Yuzefpolskaya, M.; Colombo, P.C.; et al. Novel percutaneous dual-lumen cannula-based right ventricular assist device provides effective support for refractory right ventricular failure after left ventricular assist device implantation. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Aghili, N.; Bader, Y.; Vest, A.R.; Kiernan, M.S.; Kimmelstiel, C.; DeNofrio, D.; Kapur, N.K. Biventricular circulatory support using 2 axial flow catheters for cardiogenic shock without the need for surgical vascular access. Circ. Cardiovasc. Interv. 2016, 9, e003636. [Google Scholar] [CrossRef]
- Kapur, N.K.; Jumean, M.; Ghuloom, A.; Aghili, N.; Vassallo, C.; Kiernan, M.S.; DeNofrio, D.; Pham, D.T. First Successful Use of 2 Axial Flow Catheters for Percutaneous Biventricular Circulatory Support as a Bridge to a Durable Left Ventricular Assist Device. Circ. Heart Fail. 2015, 8, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Steahr, G.; Wilmer, C.I.; Raval, N.Y. A novel percutaneous mechanical biventricular bridge to recovery in severe cardiac allograft rejection. J. Heart Lung Transplant. 2010, 29, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, D.C.; Mutlu, D.; Lendel, V.; Ates, I.; Kulaksizoglu, S.; Cilingiroglu, M. BiPella: Mini Review on a Novel Method to Treat Cardiogenic Shock Patients. Acta Cardiol. Sin. 2021, 37, 312–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, R.W.; Smith, N.J.; Shepherd, J.F.; Turbati, M.S.; Teng, B.Q.; Brazauskas, R.; Joyce, D.L.; Joyce, L.D.; Durham, L., 3rd; Rossi, P.J. Peripherally inserted concomitant surgical right and left ventricular support, the Propella, is associated with low rates of limb ischemia, with mortality comparable with peripheral venoarterial extracorporeal membrane oxygenation. Surgery 2023, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Dipchand, A.I.; Goldfarb, S.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report—2015; Focus Theme: Early Graft Failure. J. Heart Lung Transplant. 2015, 34, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Marsboom, G.; Archer, S.L. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J. Mol. Med. 2010, 88, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardehali, H.; Sabbah, H.N.; Burke, M.A.; Sarma, S.; Liu, P.P.; Cleland, J.G.; Maggioni, A.; Fonarow, G.C.; Abel, E.D.; Campia, U.; et al. Targeting myocardial substrate metabolism in heart failure: Potential for new therapies. Eur. J. Heart Fail. 2012, 14, 120–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liles, J.T.; Hoyer, K.; Oliver, J.; Chi, L.; Dhalla, A.K.; Belardinelli, L. Ranolazine reduces remodeling of the right ventricle and provoked arrhythmias in rats with pulmonary hypertension. J. Pharmacol. Exp. Ther. 2015, 353, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Campbell, R.; Scheuermann-Freestone, M.; Taylor, R.; Gunaruwan, P.; Williams, L.; Ashrafian, H.; Horowitz, J.; Fraser, A.G.; Clarke, K.; et al. Metabolic modulation with perhexiline in chronic heart failure: A randomized, controlled trial of short-term use of a novel treatment. Circulation 2005, 112, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Bayeva, M.; Gheorghiade, M.; Ardehali, H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 2013, 61, 599–610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosca, M.G.; Tandler, B.; Hoppel, C.L. Mitochondria in cardiac hypertrophy and heart failure. J. Mol. Cell Cardiol. 2013, 55, 31–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Hachem, S.; Zhang, K. Chronic Therapy with Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs with Advanced Heart Failure. Circ. Heart Fail. 2016, 9, e002206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.H.; Cowan, D.B.; Nathan, M.; Poutias, D.; Stamm, C.; del Nido, P.J.; McGowan, F.X., Jr. Myocardial hypertrophy overrides the angiogenic response to hypoxia. PLoS ONE 2008, 3, e4042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Bogaard, H.J.; Natarajan, R.; Henderson, S.C.; Long, C.S.; Kraskauskas, D.; Smithson, L.; Ockaili, R.; McCord, J.M.; Voelkel, N.F. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009, 120, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ. Res. 2014, 114, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.; Teijeiro-Paradis, R.; Kochan, A.; Billia, F.; Douflé, G.; Magder, S.; Mendelson, A.A.; McGuinty, C.; Granton, J. The Etiology and Management of Critical Acute Right Heart Failure. Can. J. Cardiol. 2025, 41, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A. Right heart failure in acute respiratory distress syndrome: An unappreciated albeit a potential target for intervention in the management of the disease. Indian J. Crit. Care Med. 2015, 19, 606–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guido, T.; Giovanni, T.; Elena, G.; Anna, Z.; Michele, Z.; Stefano, F. Cardiogenic shock in general intensive care unit: A nationwide prospective analysis of epidemiology and outcome. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, M.; Makar, C.; Wissa, A.; Le, T.; Eghbali, M.; Umar, S. Oxidative Stress and Its Implications in the Right Ventricular Remodeling Secondary to Pulmonary Hypertension. Front. Physiol. 2019, 10, 1233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, M.; Donhauser, E.; Maske, T.; Bischof, C.; Dumitrescu, D.; Rudolph, V.; Klinke, A. Mitochondrial Integrity Is Critical in Right Heart Failure Development. Int. J. Mol. Sci. 2023, 24, 11108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, A.; Li, H.; Zhang, Y.; Liu, J.; Gao, Y.; Qian, M.; Lin, Y.; Yi, L.; Zhang, L.; Li, Y.; et al. Three-Dimensional Echocardiography Assessment of Right Ventricular Volumes and Function: Technological Perspective and Clinical Application. Diagnostics 2022, 12, 806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raissi-Dehkordi, N.; Raissi-Dehkordi, N.; Xu, B. Contemporary applications of artificial intelligence and machine learning in echocardiography. npj Cardiovasc. Health 2025, 2, 30. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, K.; Wen, Y.; Wang, P.; Hu, Y.; Lai, Y.; Wang, Y.; Zhao, K.; Tang, S.; Zhang, A.; et al. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat. Med. 2024, 30, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Prognostic Implications of Staging Right Heart Failure in Patients with Significant Secondary Tricuspid Regurgitation. JACC Heart Fail. 2020, 8, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Levitt, C.V.; Williams, C.A.; Ahari, J.; Pourmand, A. Approach to Decompensated Right Heart Failure in the Acute Setting. J. Clin. Med. 2024, 13, 869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeFilippis, E.M.; Topkara, V.K.; Kirtane, A.J.; Takeda, K.; Naka, Y.; Garan, A.R. Mechanical Circulatory Support for Right Ventricular Failure. Card. Fail. Rev. 2022, 8, e14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haller, C.; Friedberg, M.K.; Laflamme, M.A. The role of regenerative therapy in the treatment of right ventricular failure: A literature review. Stem Cell Res. Ther. 2020, 11, 502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makkaoui, N.; Prasad, V.; Bagchi, P.; Carmona, T.; Li, K.; Latham, O.L.; Zhang, Y.; Lee, J.; Furdui, C.M.; Maxwell, J.T. Cell-based therapies reverse the heart failure-altered right ventricular proteome towards a pre-disease state. Stem Cell Res. Ther. 2024, 15, 420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.S.; Choi, J.R.; Kim, J.B. Gene Therapy for Cardiovascular Disease: Clinical Perspectives. Yonsei Med. J. 2024, 65, 557–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

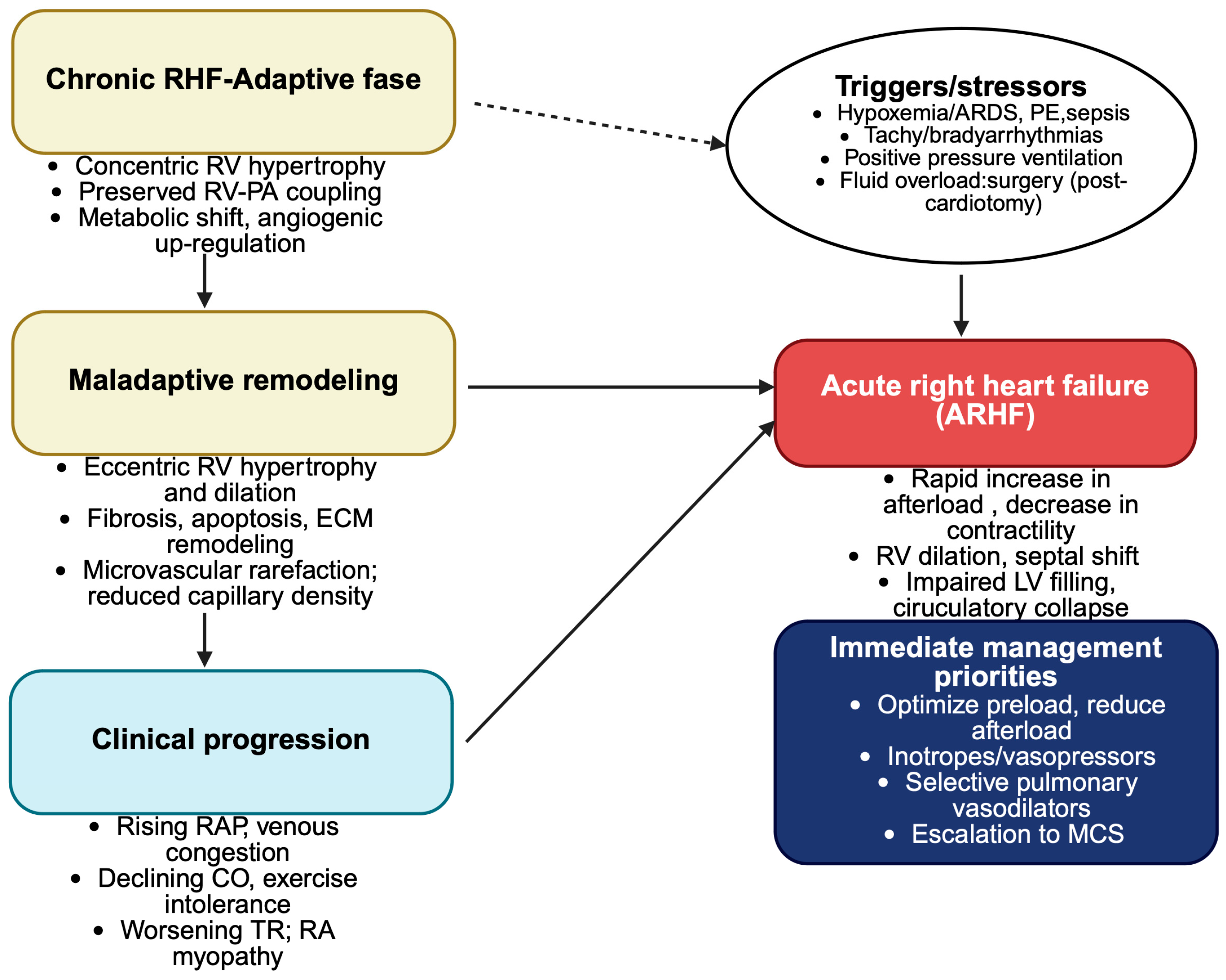



| Acute Right Heart Failure (ARHF) | Chronic Right Heart Failure (CRHF) | |
|---|---|---|
| Onset | Sudden, hours to days | Gradual, months to years |
| Predominant mechanisms | Abrupt pressure overload (massive PE, PH crisis, ARDS); Acute contractile dysfunction (RV infarction, myocarditis); Acute volume overload (severe TR, iatrogenic fluid overload); Mechanical compromise (tamponade, tension pneumothorax); Arrhythmic precipitants | Sustained pressure overload (PH, CTEPH, left-sided HF, chronic lung disease); Chronic volume overload (congenital heart disease, TR, PR, ASD); Progressive contractile dysfunction (ARVC, ischemia infiltrative disease); Constrictive pericarditis, systemic RV, pacing-induced dyssynchrony |
| RV remodeling | Acute dilatation, wall stress increase, impaired contractility | Adaptive hypertrophy, maladaptive dilatation, fibrosis, impaired RV-PA coupling |
| Hemodynamics | Rapid rise in RAP and PAP, reduced LV preload due to septal shift, low cardiac output, risk of circulatory collapse | Progressive increase in RAP, reduced CO, eventual fall in PAP in end-stage failure |
| Compensatory response | Minimal, overwhelmed within hours | Initially effective (hypertrophy, metabolic shift, angiogenic upregulation), later maladaptive (fibrosis, apoptosis, ECM remodeling, neurohormonal activation) |
| Clinical features | Acute hypotension, shock, syncope, acute systemic congestion, low output | Chronic venous congestion (edema, ascites, hepatomegaly), fatigue, exercise intolerance, cachexia |
| Extra-cardiac consequences | Acute renal/hepatic dysfunction due to low output and congestion | Congestive nephropathy, cardiac cirrhosis, gut edema, sarcopenia/cachexia |
| Prognosis | High in-hospital mortality if untreated (6–14%) | Progressive functional decline; morbidity and mortality depend on etiology (e.g., PH, systemic RV) |
| Mechanism | Acute RHF-Causes | Chronic RHF-Causes |
|---|---|---|
| Pressure overload | Acute pulmonary embolism (massive/submassive) Acute pulmonary hypertensive crisis (post-cardiotomy, post-transplant) Severe hypoxic pulmonary vasoconstriction (ARDS, pneumonia, asthma exacerbation) Acute pulmonary vasoconstriction from vasoactive mediators (thromboxane A2, serotonin) Acute aortic dissection involving RCA Sepsis with acute PH, TRALI | PAH (idiopathic, heritable, BMPR2 mutations) Secondary/post-capillary PH (left-sided HF, mitral/aortic valve disease) CTPH COPD, interstitial lung disease, sleep-disordered breathing, obesity, hypoventilation Chronic hypoxemia (high altitude, hepatopulmonary/portopulmonary hypertension, HIV-associated vasculopathy) |
| Volume overload | Acute severe tricuspid regurgitation (endocarditis, chordal rupture, device lead trauma) Acute pulmonary regurgitation (post-surgery, trauma) Iatrogenic acute fluid overload | Repaired congenital heart disease (TOF, pulmonary atresia, HLHS) Chronic tricuspid or pulmonary regurgitation Atrial septal defect, anomalous pulmonary venous return Systemic-to-pulmonary shunts High output states (large AV fistula, severe anemia, thyrotoxicosis) Chronic sequelae after valve replacement or congenital repair |
| Contractile dysfunction | Acute RV myocardial infarction (proximal RCA occlusion) Acute myocarditis Myocardial stunning (post-cardiotomy, ECMO weaning) Ischemia-reperfusion injury Acute pericardial tamponade or tension pneumothorax (mechanical compromise) Tachyarrhythmias (AF with RVR, VT) or severe bradyarrhythmias | Chronic ischemic RV disease (CAD, microvascular dysfunction) Arrhythmogenic RV cardiomyopathy Infiltrative cardiomyopathies (amyloidosis, sarcoidosis) Storage diseases (hemochromatosis) Chemotherapy/radiation-induced cardiotoxicity Chronic myocarditis Constrictive pericarditis, restrictive cardiomyopathy Systemic RV (congenitally corrected TGA, d-TGA after atrial switch) Chronic arrhythmias (AF with RA dilation, pacing-induced dyssynchrony, conduction delay) |
| Diagnostic Modality | ARHF | CRHF | Comments |
|---|---|---|---|
| Clinical assessment | Critical for prompt identification of volume overload and hemodynamic compromise | Key for longitudinal monitoring of symptom progression and functional status | Remain cornerstone for diagnosis and follow-up |
| TTE | First-line imaging to assess RV size, function (TAPSE, FAC) and valvular competence | Serial monitoring of RV remodeling, function and TR severity | Non-invasive central tool in both setting |
| RH catheterization | Indicated in unstable patients or unclear etiology for precise hemodynamic guidance | Reserved for selected cases (PH severity, RAP, advanced therapy candidacy). | Invasive but pivotal in complex or refractory cases |
| CMR | Limited utility in acute setting due to logistical constraints | Gold standard for RV volumes, function and tissue characterization | High reproducibility and accuracy in chronic evaluation |
| Biomarkers | Adjunctive diagnosis and prognosis; useful for differential etiologies | Used for risk stratification and therapy monitoring | Integrates with clinical and imaging data refine diagnosis and prognosis |
| CPET | Rarely feasible in acute decompensation | Valuable for functional capacity assessment and elucidation exercise intolerance | Employed primarily for prognostication and therapeutic evaluation in stable chronic RHF |
| Pulmonary imaging (CT, lung-US) | Critical for exclusion or confirmation of acute causes such as PE | Not routinely used in CRHF management | Useful for differential diagnosis in acute settings |
| Device | Access/Configuration | Flow (L/min) | Main Indication | Advantages | Limitation | Duration |
|---|---|---|---|---|---|---|
| V-A ECMO | Femoral/axillary veno-arterial circuit | >3–5 | Refractory cardiopulmonary failure, ARHF with hypoxemia, biventricular shock | Full cardiopulmonary support, rapid deployment | LV distension, limb ischemia, bleeding, Harlequin syndrome (FF V-A ECMO) | Days-Weeks |
| TandemHeart RVAD | RA drainage with PA return (percutaneous) | 3–5 | Isolated RV failure, post-cardiotomy, post-LVAD, PE | Direct RV bypass, optional oxygenator | Invasive cannulation, bleeding, anticoagulation | Days-Weeks |
| ProtekDuo RVAD | Single site RIJ dual lumen cannula (RA to PA) | 3–5 | Isolated RV failure ± hypoxemia | Single venous access, allows ambulation | Cannula migration | Days-Weeks |
| Impella RP/RP Flex | Femoral (RP) or IJ (RP Flex), RA to PA | 3–5 | Acute isolated RV failure post MI, post-LVAD, post-surgery | Rapid deployment, no extracorporeal circuit | Hemolysis, valve trauma, short-term only | Days |
| CentriMag surgical RVAD | RA or RV to PA (surgical) | >10 | Severe RV shock, post-LVAD, transplant PGD | High flows, stable support | Invasive, bleeding, infection | Days-weeks |
| Durable RVAD (off-label LVAD) | RA or RV inflow, PA outflow | >4–6 | Chronic RV failure, BiVAD, bridge to transplant | Long-term support | Off-label, suction risk, surgical complexity | Weeks-months (potentially longer) |
| Device | Access/Configuration | Flow (L/min) | Main Indication | Advantages | Limitations | Duration |
|---|---|---|---|---|---|---|
| BiPella | Impella 5.0/5.5 via axillary artery or Impella CP vie femoral artery + Impella RP via femoral/IJV | 3–5 each | Acute biventricular CS | Minimally invasive, percutaneous, rapid deployment, stepwise explant possible | Emolysis, cost | Days-weeks |
| Propella | Impella 5.0/5.5 + ProtekDuo with centrifugal pump | 3–5 each | Acute biventricular CS with hypoxemia | Single arterial + single venous access | Cannula migration, extracorporeal circuit required | Days-weeks |
| ECPELLA | V-A ECMO + Impella(for LV unloading) | >4–5 | Refractory biventriculr shock | Full cardiopulmonary support, LV unloading reduces distension | Invasive, bleeding, limb ischemia, LV suction risk | Days-Weeks |
| Durable BiVAD | Dual LVAD (one in RV configuration) | 4–6 each | Chronic biventricular failure, brige to Tx | Long-term support feasible, bridge to Tx | Off-label RV use, surgical complexity | Weeks-months (longer in selected) |
| BerlinHeart(EXCOR) in BiVAD configuration | Surgical paracorporeal pulsatile pumps | >7–10 | Pediatric and adult chronic biventricular failure | Long-term support, bridge to Tx, pulsatile flow | Invasive, infection risk, cumbersome | Months/Years |
| TAH | Surgical biventricular replacement | >9–10 | End-stage biventricular failure, bridge to transplant | Full systemic and pulmonary replacement | Size/device complications, specialized centers | Months (bridge to transplant) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, D.E.; Pirri, C. Right Heart Failure in Critical and Chronic Care: Current Concepts, Challenges and Mechanical Support Strategies. Med. Sci. 2025, 13, 210. https://doi.org/10.3390/medsci13040210
Torre DE, Pirri C. Right Heart Failure in Critical and Chronic Care: Current Concepts, Challenges and Mechanical Support Strategies. Medical Sciences. 2025; 13(4):210. https://doi.org/10.3390/medsci13040210
Chicago/Turabian StyleTorre, Debora Emanuela, and Carmelo Pirri. 2025. "Right Heart Failure in Critical and Chronic Care: Current Concepts, Challenges and Mechanical Support Strategies" Medical Sciences 13, no. 4: 210. https://doi.org/10.3390/medsci13040210
APA StyleTorre, D. E., & Pirri, C. (2025). Right Heart Failure in Critical and Chronic Care: Current Concepts, Challenges and Mechanical Support Strategies. Medical Sciences, 13(4), 210. https://doi.org/10.3390/medsci13040210







