National Trends in Admissions, Treatments, and Outcomes for Dilated Cardiomyopathy (2016–2021)
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: Endorsed by the World Heart Federation. J. Am. Coll. Cardiol. 2013, 62, 2046–2072, Erratum in J. Am. Coll. Cardiol. 2014, 63, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Tavazzi, L.; Serio, A.; Grasso, M.; Favalli, V.; Bellazzi, R.; Tajik, J.A.; Bonow, R.O.; Fuster, V.; et al. The MOGE(S) classification of cardiomyopathy for clinicians. J. Am. Coll. Cardiol. 2014, 64, 304–318, Erratum in J. Am. Coll. Cardiol. 2014, 64, 1186. [Google Scholar] [CrossRef]
- Felker, G.M.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.L.; Baughman, K.L.; Kasper, E.K. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.E.; Stevenson, L.W.; Fonarow, G.C.; Hamilton, M.A.; Moriguchi, J.D. Prediction of improvement in recent oncet cardiomyopathy after referral for heart transplantation. J. Am. Coll. Cardiol 1994, 23, 553–559. [Google Scholar] [CrossRef]
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999, 353, 9–13. [CrossRef]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032, Erratum in Circulation 2022, 145, e1033; Erratum in Circulation 2022, 146, e185; Erratum in Circulation 2023, 147, e674. [Google Scholar] [CrossRef] [PubMed]
- Spinarova, L.; Spinar, J. Pharmacotherapy of dilated cardiomyopathy. Curr. Pharm. Des. 2015, 21, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Curtis, A.B.; Fonarow, G.C.; Lee, K.; Little, W.; Tang, A.; Levya, F.; Momomura, S.-I.; Manrodt, C.; Bergemann, T.; et al. Cardiac resynchronization therapy in chronic heart failure with moderately reduced left ventricular ejection fraction: Lessons from the Multicenter InSync Randomized Clinical Evaluation MIRACLE EF study. Int. J. Cardiol. 2016, 202, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Alsamman, M.; Prashad, A.; Abdelmaseih, R.; Khalid, T.; Prashad, R. Update on Wearable Cardioverter Defibrillator: A Comprehensive Review of Literature. Cardiol. Res. 2022, 13, 185–189. [Google Scholar] [CrossRef]
- Masarone, D.; Kittleson, M.M.; Falco, L.; Martucci, M.L.; Catapano, D.; Brescia, B.; Petraio, A.; De Feo, M.; Pacileo, G. The ABC of Heart Transplantation—Part 1: Indication, Eligibility, Donor Selection, and Surgical Technique. J. Clin. Med. 2023, 12, 5217. [Google Scholar] [CrossRef]
- Blumer, V.; Kanwar, M.K.; Barnett, C.F.; Cowger, J.A.; Damluji, A.A.; Farr, M.; Goodlin, S.J.; Katz, J.N.; McIlvennan, C.K.; Sinha, S.S.; et al. Cardiogenic Shock in Older Adults: A Focus on Age-Associated Risks and Approach to Management: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1051–e1065. [Google Scholar] [CrossRef]
- Givertz, M.M.; DeFilippis, E.M.; Colvin, M.; Darling, C.E.; Elliott, T.; Hamad, E.; Hiestand, B.C.; Martindale, J.L.; Pinney, S.P.; Shah, K.B.; et al. HFSA/SAEM/ISHLT clinical expert consensus document on the emergency management of patients with ventricular assist devices. J. Heart Lung Transplant. 2019, 38, 677–698. [Google Scholar] [CrossRef]
- Birks, E.J.; Tansley, P.D.; Hardy, J.; George, R.; Bowles, C.T.; Burke, M.; Banner, N.R.; Khaghani, A.; Yacoub, M.H. Left ventricular assist device and drug therapy for the reversal of heart failure. N. Engl. J. Med. 2006, 355, 1873–1884. [Google Scholar] [CrossRef]
- Birks, E.J.; George, R.S.; Hedger, M.; Bahrami, T.; Wilton, P.; Bowles, C.T.; Webb, C.; Bougard, R.; Amrani, M.; Yacoub, M.H.; et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation 2011, 123, 381–390. [Google Scholar] [CrossRef]
- Dandel, M. Cardiological Challenges Related to Long-Term Mechanical Circulatory Support for Advanced Heart Failure in Patients with Chronic Non-Ischemic Cardiomyopathy. J. Clin. Med. 2023, 12, 6451. [Google Scholar] [CrossRef]
- Kelty, C.E.; Dickinson, M.G.; Leacche, M.; Jani, M.; Shrestha, N.K.; Lee, S.; Acharya, D.; Rajapreyar, I.; Sadler, R.C.; McNeely, E.; et al. Increased disparities in waitlist and post-heart transplantation outcomes according to socioeconomic status with the new heart transplant allocation system. J. Heart Lung Transplant. 2023, 43, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Bergan, N.; Prachee, I.; Curran, L.; McGurk, K.A.; Lu, C.; de Marvao, A.; Bai, W.; Halliday, B.P.; Gregson, J.; O’rEgan, D.P.; et al. Systematic Review, Meta-Analysis, and Population Study to Determine the Biologic Sex Ratio in Dilated Cardiomyopathy. Circulation 2025, 151, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Kinnamon, D.D.; Haas, G.J.; Hofmeyer, M.; Kransdorf, E.; Ewald, G.A.; Morris, A.A.; Owens, A.; Lowes, B.; Stoller, D.; et al. Genetic Architecture of Dilated Cardiomyopathy in Individuals of African and European Ancestry. JAMA 2023, 330, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Ntusi, N.A.; Sliwa, K. Impact of Racial and Ethnic Disparities on Patients With Dilated Cardiomyopathy: JACC Focus Seminar 7/9. J. Am. Coll. Cardiol. 2021, 78, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
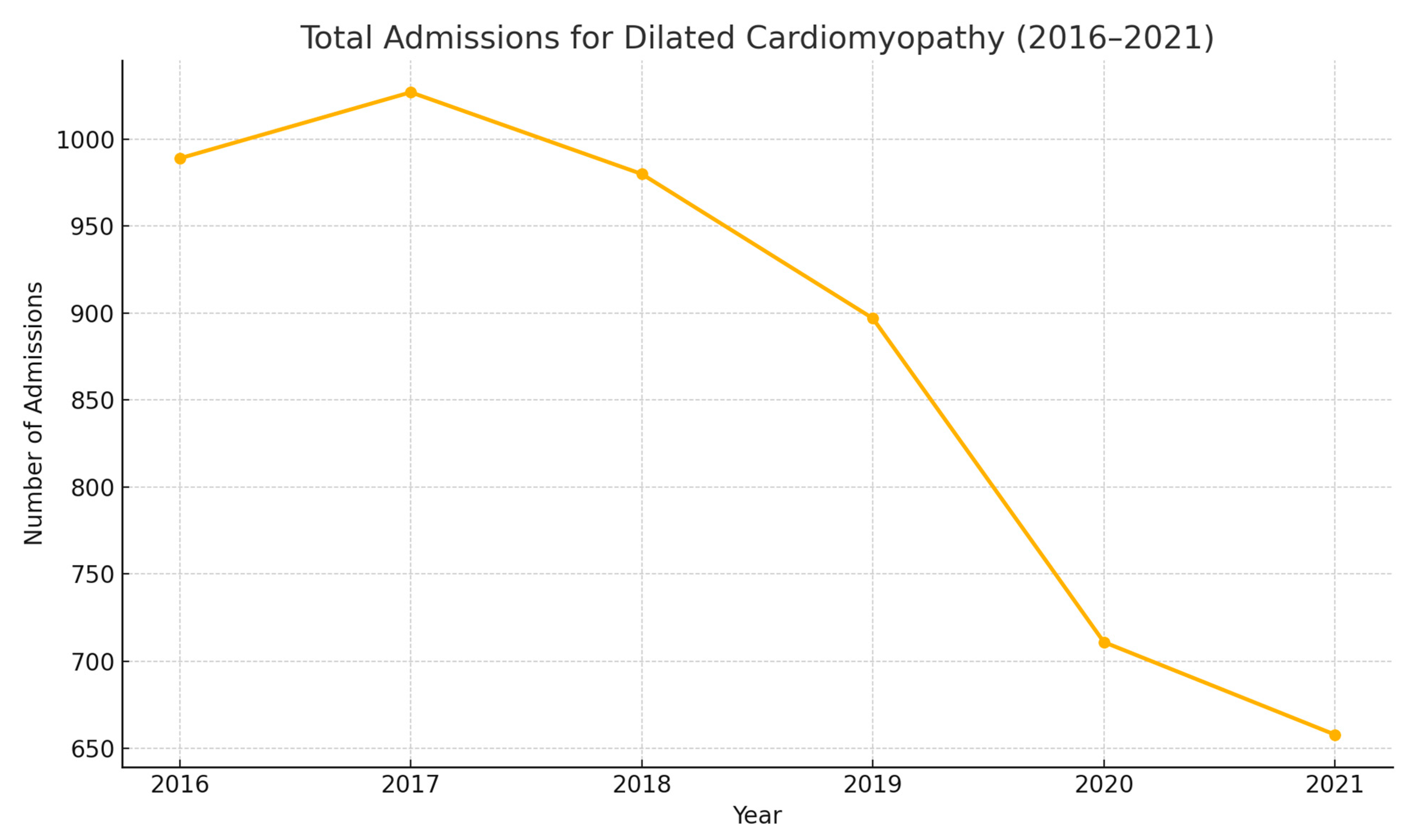
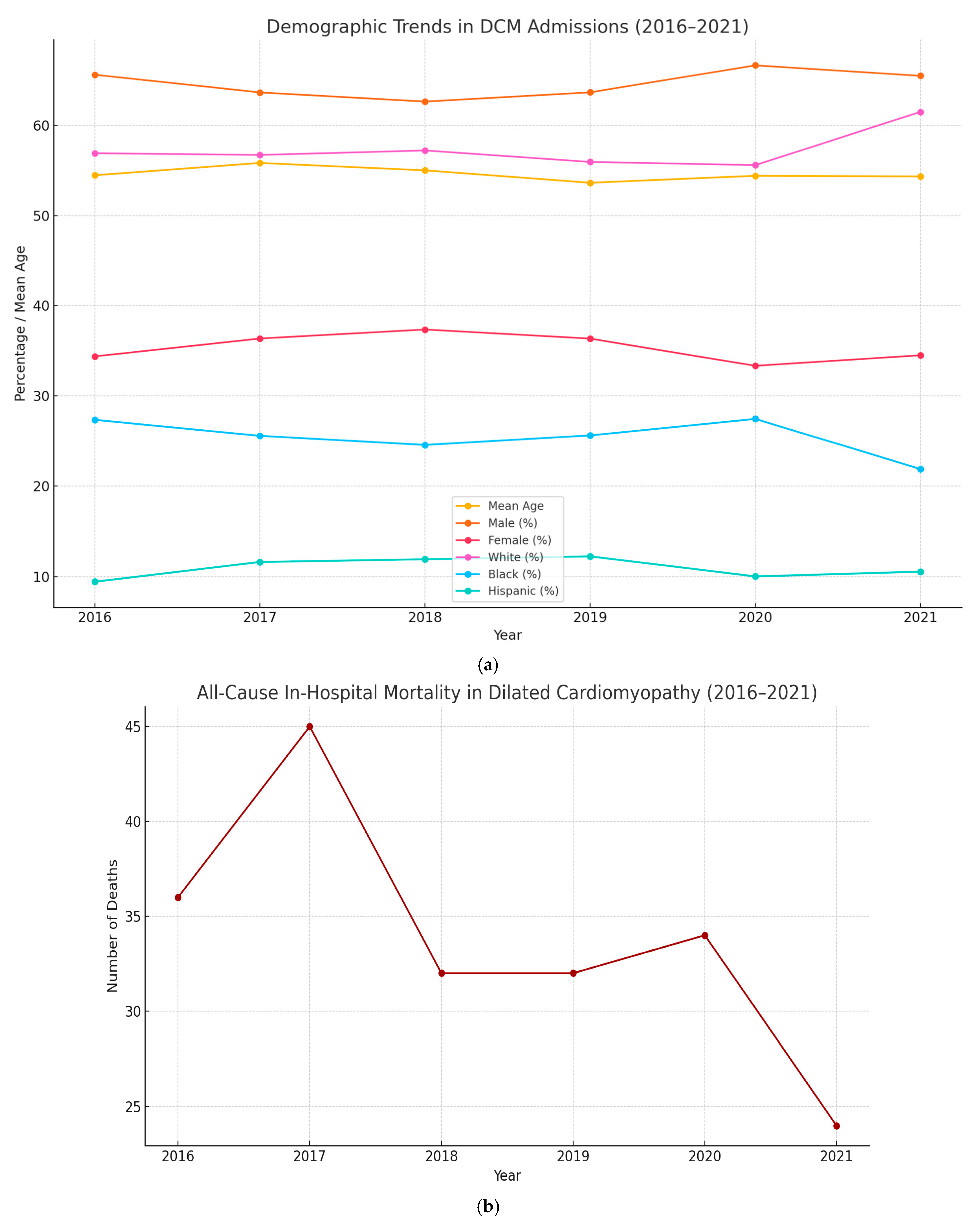


| Year | Total Number of Admissions for Dilated Cardiomyopathy | Mean Age | Sex | Race | All-Cause In-Hospital Mortality | Mean of Total Hospital Charges (USD) | Mean Length of Stay (Days) | Belonging to Lowest Quarter of Monthly Income (%) |
|---|---|---|---|---|---|---|---|---|
| 2016 | 989 | 54.47 (53.28–55.66) | Male: 649 (65.62%) Female: 340 (34.38%) | White: 531 (56.91%) | 36 (3.64%) | 190,235.4 | 7.95 (6.83–9.06) | 317 (32.92%) |
| Black: 255 (27.33%) | ||||||||
| Hispanic: 88 (9.43%) | ||||||||
| 2017 | 1027 | 55.83 (54.67–56.98) | Male: 653 (63.65%) Female: 373 (36.35%) | White: 557 (56.72%) | 45 (4.38%) | 203,101.7 | 8.13 (7.09–9.17) | 340 (34.10%) |
| Black: 251 (25.56%) | ||||||||
| Hispanic: 114 (11.61%) | ||||||||
| 2018 | 980 | 55.01 (53.84– 56.19) | Male: 614 (62.65%) Female: 366 (37.35%) | White: 543 (57.22%) | 32 (3.27%) | 212,764.4 | 8.09 (7.01–9.16) | 327 (34.28%) |
| Black: 233 (24.55%) | ||||||||
| Hispanic: 113 (11.91%) | ||||||||
| 2019 | 897 | 53.64 (52.37–54.91) | Male: 571 (63.66%) Female: 326 (36.34%) | White: 485 (55.94%) | 32 (3.57%) | 213,565.3 | 8.15 (7.23–9.08) | 331 (37.44%) |
| Black: 222 (25.61%) | ||||||||
| Hispanic: 106 (12.23%) | ||||||||
| 2020 | 711 | 54.41 (52.94–55.89) | Male: 474 (66.67%) Female: 237 (33.33%) | White: 383 (55.59%) | 34 (4.78%) | 241,843.1 | 9.37 (7.80–10.93) | 236 (33.67%) |
| Black: 189 (27.43%) | ||||||||
| Hispanic: 69 (10.01%) | ||||||||
| 2021 | 658 | 54.34 (52.76–55.91) | Male: 431 (65.50%) Female: 227 (34.50%) | White: 385 (61.50%) | 24 (3.65%) | 311,854. 5 | 9.77 (8.10–11.45) | 192 (29.63%) |
| Black: 137 (21.88%) | ||||||||
| Hispanic: 66 (10.54%) |
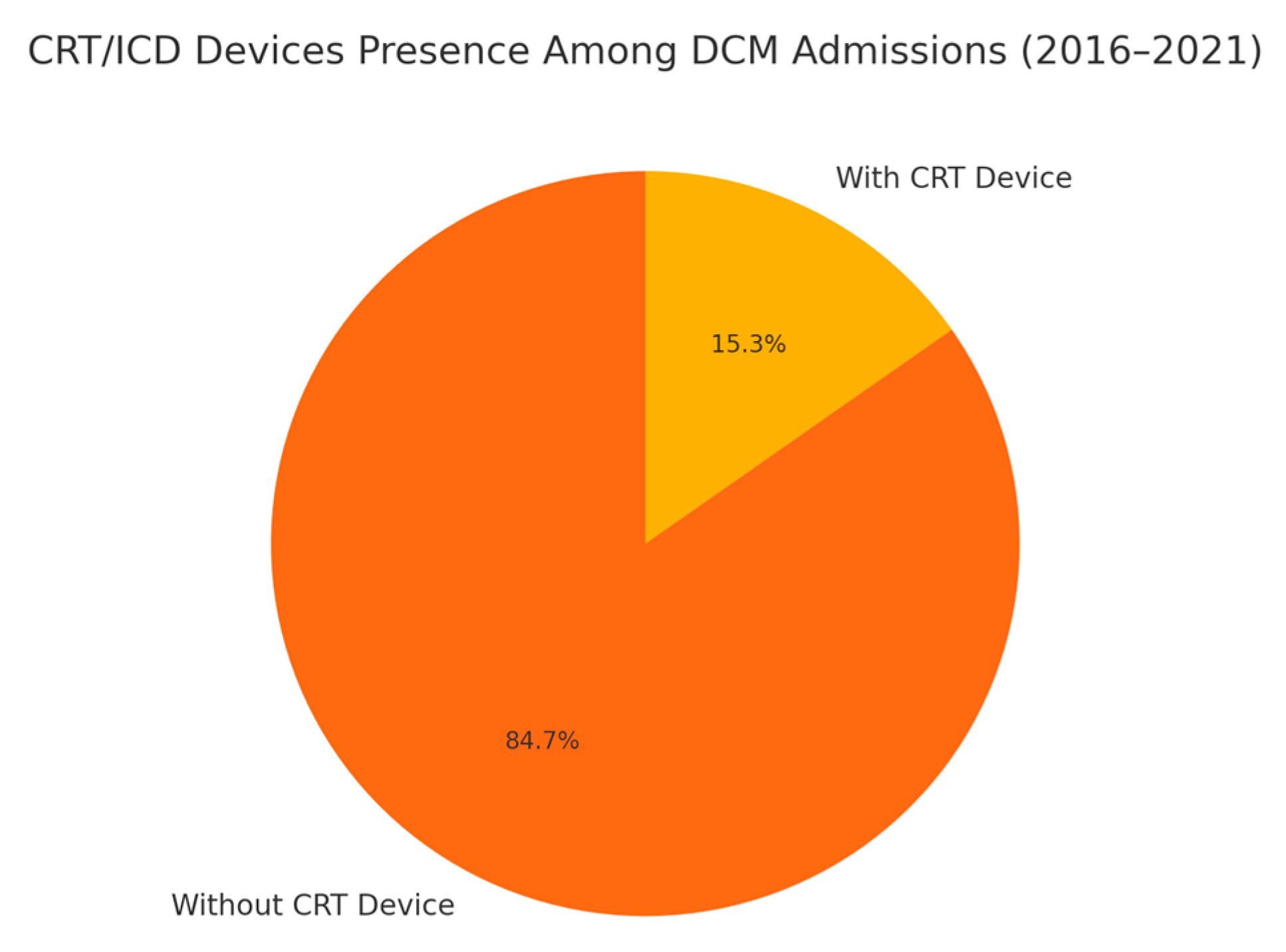
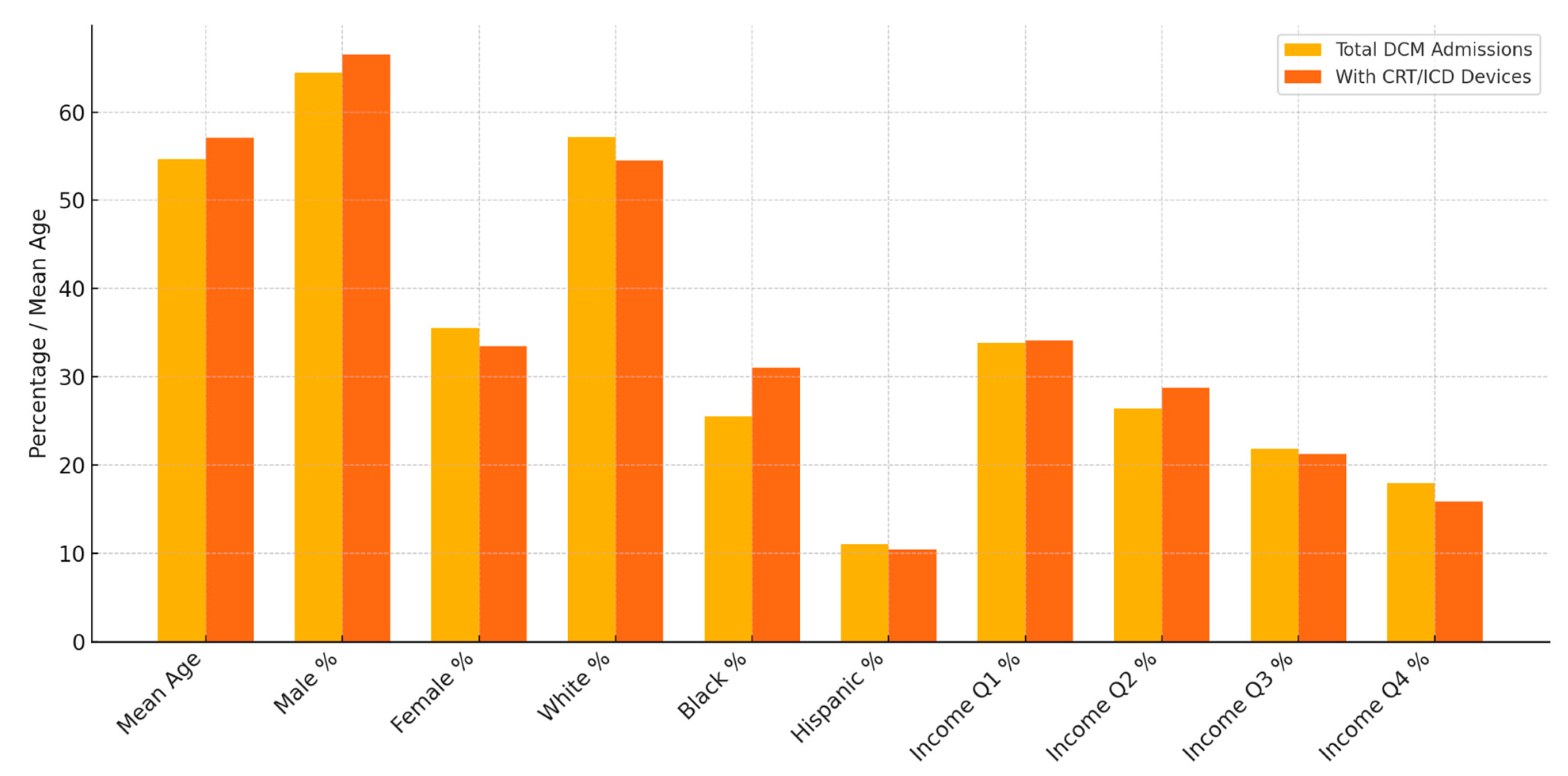
| Total DCM Admissions | DCM Admissions with CRT/ICD Devices | Comparison of Differences | |
|---|---|---|---|
| Mean Age | 54.67 (54.15–55.19) | 57.11 (56.09–58.14) | Two-sample t-test: Mean difference: −2.866, p value: 0.0001 |
| Sex | Male: 3392 (64.47%) | Male: 534 (66.50%) | Pearson’s chi-squared test: 1.698, p value: 0.192 |
| Female: 1869 (35.53%) | Female: 269 (33.50%) | ||
| Race | White: 2884 (57.15%) | White: 418 (54.50%) | Pearson’s chi-squared test: 20.46, p value: 0.001 |
| Black: 1287 (25.51%) | Black: 238 (31.03%) | ||
| Hispanic: 556 (11.02%) | Hispanic: 80 (10.43%) | ||
| Quarterly Income (Quartile) | 1st: 1743 (33.86%) | 1st: 266 (34.15%) | Pearson’s chi-squared test: 4.225, p value: 0.238 |
| 2nd:1358 (26.38%) | 2nd: 224 (28.75%) | ||
| 3rd: 1123 (21.82%) | 3rd: 165 (21.28%) | ||
| 4th: 923 (17.93%) | 4th: 124 (15.92%) |
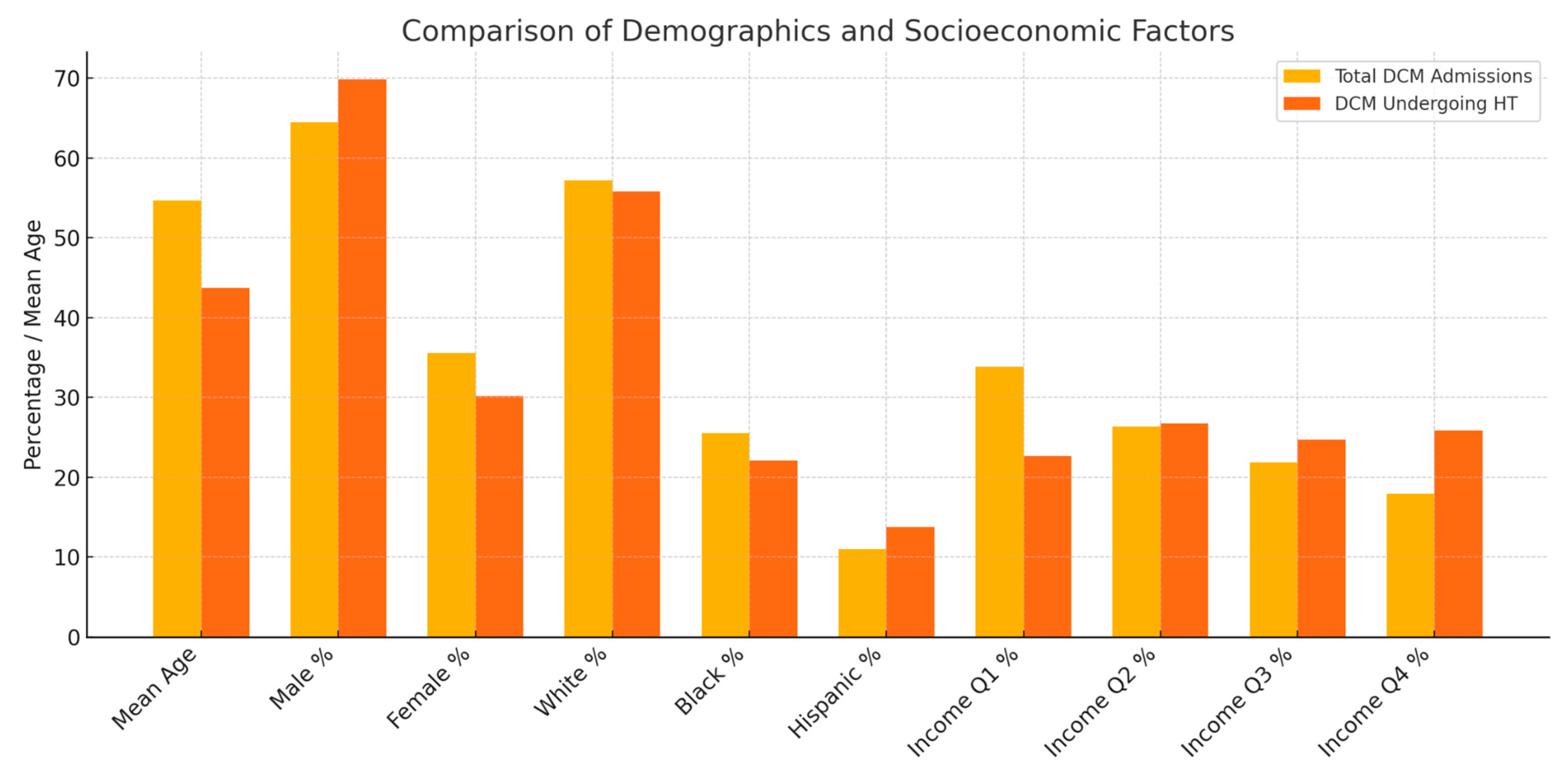
| Total DCM Admissions | DCM Admissions Undergoing HT | Comparison of Differences | |
|---|---|---|---|
| Mean Age | 54.67 (54.15–55.19) | 43.71 (41.45–45.97) | Two-sample t-test: Mean difference: 12.33, p value < 0.0001 |
| Sex | Male: 3392 (64.47%) | Male: 245 (69.80%) | Pearson’s chi-squared test: 4.615, p value: 0.032 |
| Female:1869 (35.53%) | Female: 106 (30.20%) | ||
| Race | White: 2884 (57.15%) | White: 182 (55.83%) | Pearson’s chi-squared test: 8.077, p value: 0.152 |
| Black: 1287 (25.51%) | Black: 72 (22.09%) | ||
| Hispanic: 556 (11.02%) | Hispanic: 45 (13.80%) | ||
| Quarterly Income (Quartile) | 1st: 1743 (33.86%) | 1st: 78 (22.67%) | Pearson’s chi-squared test: 29.15, p value: 0.00 |
| 2nd: 1358 (26.38%) | 2nd: 92 (26.74%) | ||
| 3rd: 1123 (21.82%) | 3rd: 85 (24.71%) | ||
| 4th: 923 (17.93%) | 4th: 89 (25.87%) |
| Total DCM Admissions | DCM Admissions Undergoing LVAD Placements | Comparison of Differences | |
|---|---|---|---|
| Mean Age | 54.67 (54.15–55.19) | 46.39 (43.65–49.13) | Two-sample t-test: Mean difference: 9.89, p value: 0.000 |
| Sex | Male: 3392 (64.47%) | Male: 139 (63.18%) | Pearson’s chi-squared test: 0.456, p value: 0.499 |
| Female: 1869 (35.53%) | Female: 81 (36.82%) | ||
| Race | White: 2884 (57.15%) | White: 110 (52.63%) | Pearson’s chi-squared test: 7.573, p value: 0.181 |
| Black: 1287 (25.51%) | Black: 54 (25.84%) | ||
| Hispanic: 556 (11.02%) | Hispanic: 27 (12.92%) | ||
| Quarterly Income (Quartile) | 1st: 1743 (33.86%) | 1st: 61 (28.37%) | Pearson’s chi-squared test: 8.0416, p value: 0.045 |
| 2nd: 1358 (26.38%) | 2nd: 55 (25.58%) | ||
| 3rd: 1123 (21.82%) | 3rd: 42 (19.53%) | ||
| 4th: 923 (17.93%) | 4th: 57 (26.51%) |
| Total DCM Admissions | DCM Admissions That Died During the Hospital Stay | Comparison of Differences | |
|---|---|---|---|
| Mean Age | 54.67 (54.15–55.19) | 57.33 (54.57–60.08) | Two-sample t-test: Mean difference: 2.75, p value: 0.0465 |
| Sex | Male: 3392 (64.47%) | Male: 152 (74.88%) | Pearson’s chi-squared test: 9.9875 p value: 0.002 |
| Female: 1869 (35.53%) | Female: 51 (25.12%) | ||
| Race | White: 2884 (57.15%) | White: 108 (54.82%) | Pearson’s chi-squared test: 8.509 p value: 0.130 |
| Black: 1287 (25.51%) | Black: 51 (25.89%) | ||
| Hispanic: 556 (11.02%) | Hispanic: 20 (10.15%) | ||
| Quarterly Income (Quartile) | 1st: 1743 (33.86%) | 1st: 65 (33.68%) | Pearson’s chi-squared test: 0.0616 p value: 0.996 |
| 2nd: 1358 (26.38%) | 2nd: 52 (26.94%) | ||
| 3rd: 1123 (21.82%) | 3rd: 41 (21.24%) | ||
| 4th: 923 (17.93%) | 4th: 35 (18.13%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varughese, V.J.; Mohamed, A.; Nagesh, V.K.; Atoot, A. National Trends in Admissions, Treatments, and Outcomes for Dilated Cardiomyopathy (2016–2021). Med. Sci. 2025, 13, 83. https://doi.org/10.3390/medsci13030083
Varughese VJ, Mohamed A, Nagesh VK, Atoot A. National Trends in Admissions, Treatments, and Outcomes for Dilated Cardiomyopathy (2016–2021). Medical Sciences. 2025; 13(3):83. https://doi.org/10.3390/medsci13030083
Chicago/Turabian StyleVarughese, Vivek Joseph, Abdifitah Mohamed, Vignesh Krishnan Nagesh, and Adam Atoot. 2025. "National Trends in Admissions, Treatments, and Outcomes for Dilated Cardiomyopathy (2016–2021)" Medical Sciences 13, no. 3: 83. https://doi.org/10.3390/medsci13030083
APA StyleVarughese, V. J., Mohamed, A., Nagesh, V. K., & Atoot, A. (2025). National Trends in Admissions, Treatments, and Outcomes for Dilated Cardiomyopathy (2016–2021). Medical Sciences, 13(3), 83. https://doi.org/10.3390/medsci13030083






