Transcranial Direct Current Stimulation in Episodic Migraine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials †
Abstract
1. Introduction
2. Method
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
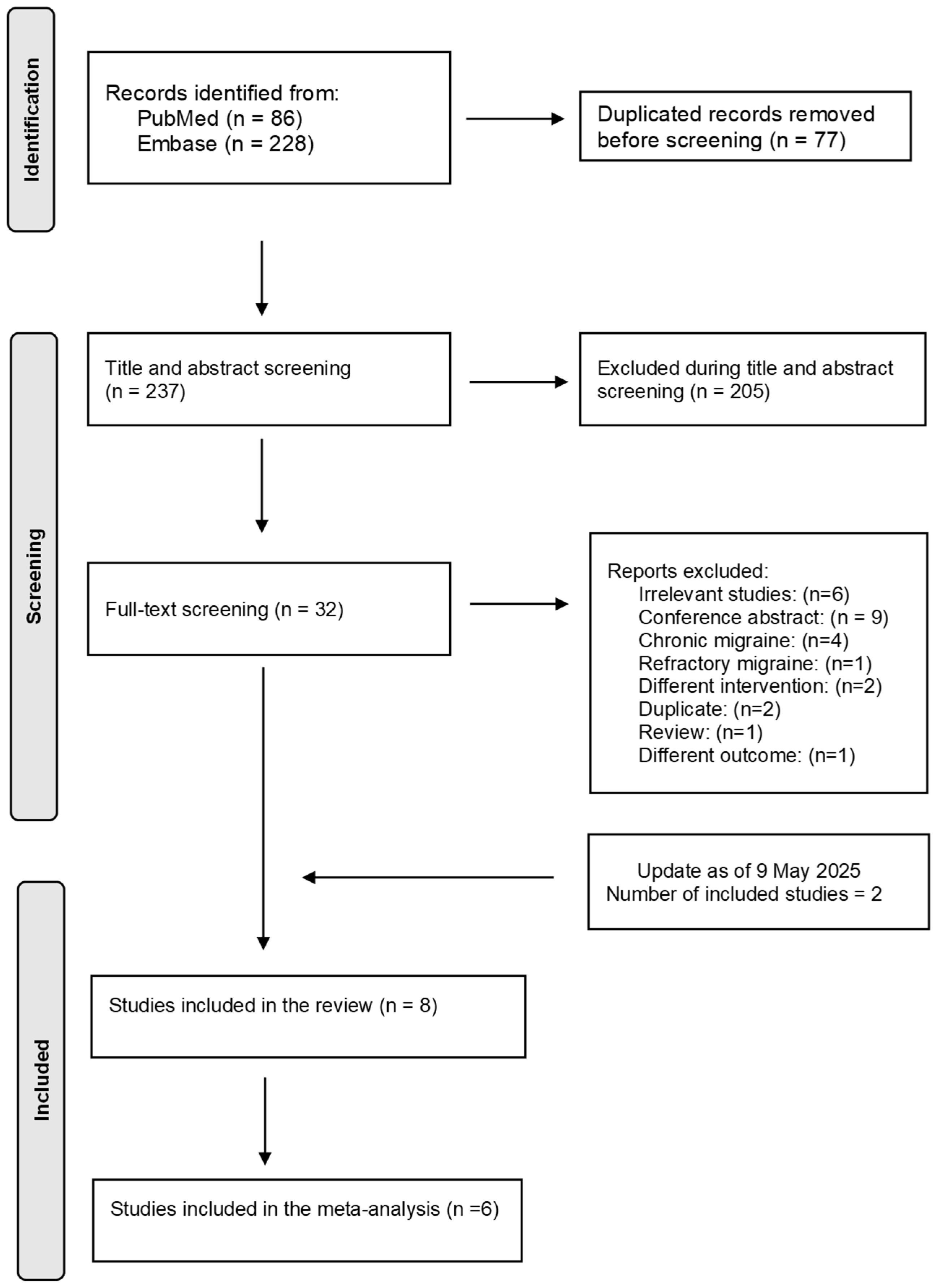
2.3. Study Selection and Data Extraction
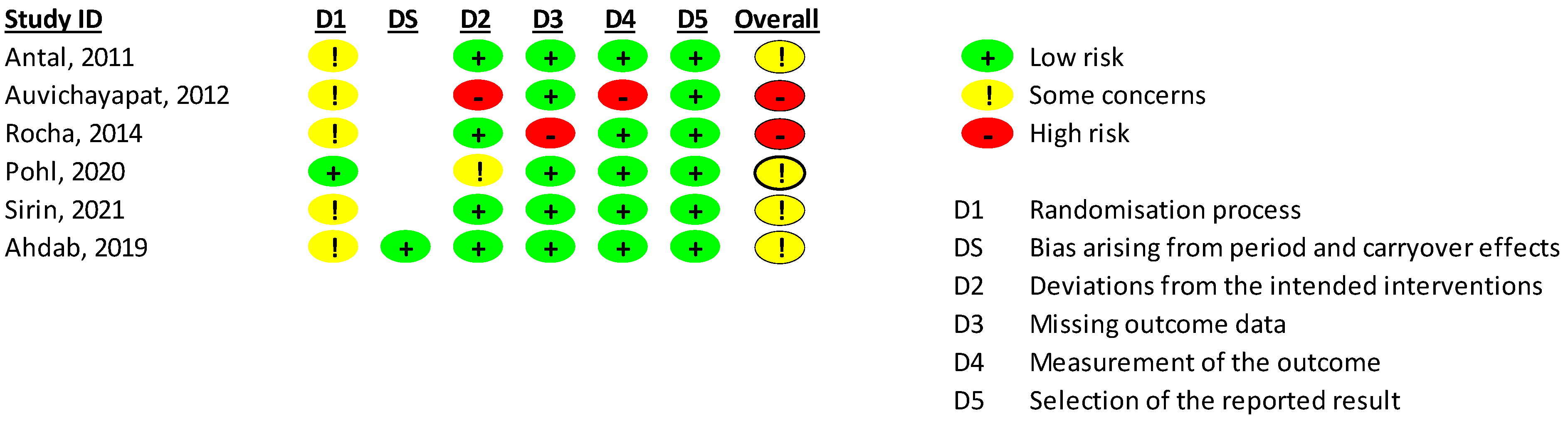
2.4. Risk of Bias Assessment
2.5. Outcomes
2.6. Data Analysis
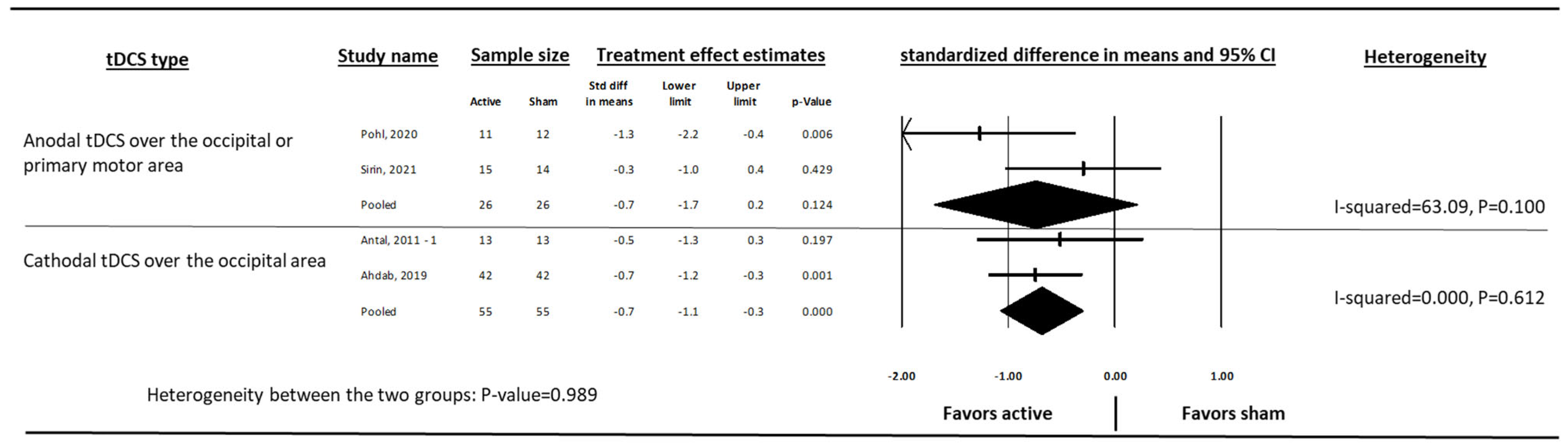
3. Results
4. Discussion
4.1. Strengths, Limitations and Future Directions
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| tDCS | Transcranial direct current stimulation |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses. |
| RCT | Randomized clinical trials |
| ICHD | International Classification of Headache Disorders. |
| RoB | Risk-of-bias. |
| SD | standard deviation |
| 95% CI | 95% confidence intervals |
| CMA | Comprehensive Meta-Analysis |
| Oz | Occipital cortex |
| Cz | Vertex |
| M1 | Primary motor. |
| DLPFC | Dorsolateral prefrontal cortex |
References
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef]
- Global, regional, and national burden of migraine and tension-type headache, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Silberstein, S.D. Migraine. Lancet 2004, 363, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Buse, D.C.; Ashina, H.; Pozo-Rosich, P.; Peres, M.F.P.; Lee, M.J.; Terwindt, G.M.; Halker Singh, R.; Tassorelli, C.; Do, T.P.; et al. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet 2021, 397, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Haghdoost, F.; Togha, M. Migraine management: Non-pharmacological points for patients and health care professionals. Open Med. 2022, 17, 1869–1882. [Google Scholar] [CrossRef]
- Ailani, J.; Burch, R.C.; Robbins, M.S. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache 2021, 61, 1021–1039. [Google Scholar] [CrossRef]
- Puledda, F.; Shields, K. Non-Pharmacological Approaches for Migraine. Neurotherapeutics 2018, 15, 336–345. [Google Scholar] [CrossRef]
- Reuter, U.; McClure, C.; Liebler, E.; Pozo-Rosich, P. Non-invasive neuromodulation for migraine and cluster headache: A systematic review of clinical trials. J. Neurol. Neurosurg. Psychiatry 2019, 90, 796–804. [Google Scholar] [CrossRef]
- Stilling, J.M.; Monchi, O.; Amoozegar, F.; Debert, C.T. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. Headache 2019, 59, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Leale, I.; Di Stefano, V.; Torrente, A.; Alonge, P.; Monastero, R.; Roccella, M.; Brighina, F.; Giustino, V.; Battaglia, G. Telecoaching and Migraine: Digital Approach to Physical Activity in Migraine Management. A Scoping Review. J. Clin. Med. 2025, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. Effects of single versus dual-site High-Definition transcranial direct current stimulation (HD-tDCS) on cortical reactivity and working memory performance in healthy subjects. Brain Stimul. 2018, 11, 1033–1043. [Google Scholar] [CrossRef]
- Aloi, D.; Della Rocchetta, A.I.; Ditchfield, A.; Coulborn, S.; Fernández-Espejo, D. Therapeutic use of transcranial direct current stimulation in the rehabilitation of prolonged disorders of consciousness. Front. Neurol. 2021, 12, 632572. [Google Scholar] [CrossRef]
- Živanović, M.; Paunović, D.; Konstantinović, U.; Vulić, K.; Bjekić, J.; Filipović, S.R. The effects of offline and online prefrontal vs parietal transcranial direct current stimulation (tDCS) on verbal and spatial working memory. Neurobiol. Learn. Mem. 2021, 179, 107398. [Google Scholar] [CrossRef]
- Viganò, A.; D’Elia, T.S.; Sava, S.L.; Auvé, M.; De Pasqua, V.; Colosimo, A.; Di Piero, V.; Schoenen, J.; Magis, D. Transcranial Direct Current Stimulation (tDCS) of the visual cortex: A proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J. Headache Pain 2013, 14, 23. [Google Scholar] [CrossRef]
- Ahdab, R.; Mansour, A.G.; Khazen, G.; El-Khoury, C.; Sabbouh, T.M.; Salem, M.; Yamak, W.; Ayache, S.S.; Riachi, N. Cathodal Transcranial Direct Current Stimulation of the Occipital cortex in Episodic Migraine: A Randomized Sham-Controlled Crossover Study. J. Clin. Med. 2019, 9, 60. [Google Scholar] [CrossRef]
- Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020215735 (accessed on 1 October 2020).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Chang, Y.; Phillips, M.R.; Guymer, R.H.; Thabane, L.; Bhandari, M.; Chaudhary, V. The 5 min meta-analysis: Understanding how to read and interpret a forest plot. Eye 2022, 36, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Hasırcı Bayır, B.R.; Aksu, S.; Gezegen, H.; Karaaslan, Z.; Yüceer, H.; Cerrahoğlu Şirin, T.; Küçükali, C.; Kurt, A.; Karamürsel, S.; Yılmaz, V.; et al. Effects of Transcranial Direct Current Stimulation on Clinical Outcomes, Calcitonin Gene-Related Peptide, and Pituitary Adenylate Cyclase-Activating Polypeptide-38 Levels in Menstrual Migraine. Neuromodulation 2024, 27, 835–846. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Kim, D.J.; Lim, M.; Nascimento, T.D.; Scott, P.J.H.; Smith, Y.R.; Koeppe, R.A.; Zubieta, J.K.; Kaciroti, N. Effect of High-Definition Transcranial Direct Current Stimulation on Headache Severity and Central µ-Opioid Receptor Availability in Episodic Migraine. J. Pain. Res. 2023, 16, 2509–2523. [Google Scholar] [CrossRef]
- Antal, A.; Kriener, N.; Lang, N.; Boros, K.; Paulus, W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 2011, 31, 820–828. [Google Scholar] [CrossRef]
- Auvichayapat, P.; Janyacharoen, T.; Rotenberg, A.; Tiamkao, S.; Krisanaprakornkit, T.; Sinawat, S.; Punjaruk, W.; Thinkhamrop, B.; Auvichayapat, N. Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial. J. Med. Assoc. Thai 2012, 95, 1003–1012. [Google Scholar]
- Rocha, S.; Melo, L.; Boudoux, C.; Foerster, Á.; Araújo, D.; Monte-Silva, K. Transcranial direct current stimulation in the prophylactic treatment of migraine based on interictal visual cortex excitability abnormalities: A pilot randomized controlled trial. J. Neurol. Sci. 2015, 349, 33–39. [Google Scholar] [CrossRef]
- Pohl, H.; Moisa, M.; Jung, H.H.; Brenner, K.; Aschmann, J.; Riederer, F.; Ruff, C.C.; Schoenen, J.; Luechinger, R.; Widmer, L.; et al. Long-Term Effects of Self-Administered Transcranial Direct Current Stimulation in Episodic Migraine Prevention: Results of a Randomized Controlled Trial. Neuromodulation 2021, 24, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Şirin, T.C.; Aksu, S.; Hasirci Bayir, B.R.; Ulukan, Ç.; Karamürsel, S.; Kurt, A.; Baykan, B. Is Allodynia a Determinant Factor in the Effectiveness of Transcranial Direct Current Stimulation in the Prophylaxis of Migraine? Neuromodulation 2021, 24, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ornello, R.; Caponnetto, V.; Ratti, S.; D’Aurizio, G.; Rosignoli, C.; Pistoia, F.; Ferrara, M.; Sacco, S.; D’Atri, A. Which is the best transcranial direct current stimulation protocol for migraine prevention? A systematic review and critical appraisal of randomized controlled trials. J. Headache Pain 2021, 22, 144. [Google Scholar] [CrossRef]
- Moisset, X.; Pereira, B.; Ciampi de Andrade, D.; Fontaine, D.; Lantéri-Minet, M.; Mawet, J. Neuromodulation techniques for acute and preventive migraine treatment: A systematic review and meta-analysis of randomized controlled trials. J. Headache Pain 2020, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Xia, Z.; Charvet, L.; Xiao, F.; Datta, A.; Androulakis, X.M. A Systematic Review and Meta-Analysis on the Efficacy of Repeated Transcranial Direct Current Stimulation for Migraine. J. Pain. Res. 2021, 14, 1171–1183. [Google Scholar] [CrossRef]
- Hong, P.; Liu, Y.; Wan, Y.; Xiong, H.; Xu, Y. Transcranial direct current stimulation for migraine: A systematic review and meta-analysis of randomized controlled trials. CNS Neurosci. Ther. 2022, 28, 992–998. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, D.; Remohí-Balanza, D.; Ávila-López, V.; Muñoz Fernández, A.C.; Vicente de Frutos, G.; Martín Pérez, S.; Martín Pérez, I.M.; Alonso Pérez, J.L.; Villafañe, J.H.; Sosa Reina, M.D. Effectiveness of Transcranial Direct Current Stimulation for Migraine Treatment: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Top. Geriatr. Rehabil. 2025, 41, 29–45. [Google Scholar] [CrossRef]
- Andrade, S.M.; de Brito Aranha, R.E.L.; de Oliveira, E.A.; de Mendonça, C.; Martins, W.K.N.; Alves, N.T.; Fernández-Calvo, B. Transcranial direct current stimulation over the primary motor vs prefrontal cortex in refractory chronic migraine: A pilot randomized controlled trial. J. Neurol. Sci. 2017, 378, 225–232. [Google Scholar] [CrossRef]
- Dawood Rahimi, M.; Taghi Kheirkhah, M.; Salehi Fadardi, J. Efficacy of tDCS in chronic migraine: A multiprotocol randomized controlled trial. Clin. Neurophysiol. 2023, 150, 119–130. [Google Scholar] [CrossRef]
- Rahimi, M.D.; Fadardi, J.S.; Saeidi, M.; Bigdeli, I.; Kashiri, R. Effectiveness of cathodal tDCS of the primary motor or sensory cortex in migraine: A randomized controlled trial. Brain Stimul. 2020, 13, 675–682. [Google Scholar] [CrossRef]
- Tassorelli, C.; Diener, H.C.; Silberstein, S.D.; Dodick, D.W.; Goadsby, P.J.; Jensen, R.H.; Magis, D.; Pozo-Rosich, P.; Yuan, H.; Martinelli, D.; et al. Guidelines of the International Headache Society for clinical trials with neuromodulation devices for the treatment of migraine. Cephalalgia 2021, 41, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Haghdoost, F.; Padala, D.; Salam, A.; Delcourt, C.; Rodgers, A. Effect of transcranial direct current stimulation compared to sham in episodic migraine: Systematic review and meta-analysis of randomized controlled trials. J. Headache Pain 2021, 22, 126. [Google Scholar]



| PICOs | Explanation |
|---|---|
| Population (P) | Adult participants (age ≥ 18 years) with episodic migraine |
| Intervention (I) | Transcranial direct current stimulation (both cathodal and anodal tDCS) |
| Comparator (C) | Sham * |
| Outcome (O) | Difference in change from baseline in migraine days per month and migraine attacks per month |
| Study design (s) | Randomized controlled trials |
| Study Name | Country | Sample Size, Female (%), Age (Mean) | Reported Frequency Outcome | Study Duration, Duration of Each Session, Number of Interventions, Intervention Intensity | tDCS Lead Locations | ||||
|---|---|---|---|---|---|---|---|---|---|
| Monthly Migraine Days | Monthly Migraine Attacks | Headache Days Per Month | Cathode | Anode | tDCS Type (Cathodal vs. Anodal) | ||||
| Antal, 2011 [29] | Germany | n = 26, 88%, 33 years | X | X | 3 weeks, 15 min, 3 times per week, 1 mA | Oz (Primary visual cortex) | Cz (vertex) | Cathodal tDCS | |
| Auvichayapat, 2012 [30] | Thailand | n = 37, 70%, 32 years | X | 3 weeks, 20 min, 20 consecutive days, 1 mA | contralateral supraorbital | M1 (C3) | Anodal tDCS | ||
| Rocha, 2014 [31] | Brazil | n = 15, 97%, 24 years | X | 3 weeks, 20 min, 3 times per week, 2 mA | Oz (Primary visual cortex) | Cz (vertex) | Cathodal tDCS | ||
| Ahdab, 2019 * [20] | Lebanon | n = 42, 83%, 36 years | X | 1 week (2 weeks outcome measure), 20 min, 3 consecutive days, 2 mA | occipital cortex (O1/O2) | opposite supraorbital | Cathodal tDCS | ||
| Pohl, 2020 [32] | Switzerland | n = 23, 95%, 37 years | X | X | 4 weeks, 20 min, 28 consecutive days, 1 mA | Cz (vertex) | Oz (Primary visual cortex) | Anodal tDCS | |
| Sirin, 2021 [33] | Turkey | n = 29, 59%, 41 years | X | X | 1 week (4 weeks outcome measure), 20 min, 3 consecutive days, 2 mA | contralateral supraorbital | M1 (C3) | Anodal tDCS | |
| DaSilva, 2023 [28] # | USA | n = 25, 88%, 30 years | X | 2 weeks, 20 min, 10 consecutive days, 2 mA | FC3 and FC5 | C3 and C5 | Both anodal and cathodal electrodes, centered on M1 | ||
| Hasırcı Bayır, 2024 [27] # | Turkey | n = 58 (n = 22 with non-menstrual migraine) **, 100%, 30 years | X | X | 1 week (4 weeks outcome measure), 20 min, 3 consecutive days, 2 mA | contralateral supraorbital | left dorsolateral prefrontal cortex (F3) | Anodal tDCS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghdoost, F.; Salam, A.; Seyed-Kolbadi, F.Z.; Padala, D.; Delcourt, C.; Rodgers, A. Transcranial Direct Current Stimulation in Episodic Migraine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2025, 13, 84. https://doi.org/10.3390/medsci13030084
Haghdoost F, Salam A, Seyed-Kolbadi FZ, Padala D, Delcourt C, Rodgers A. Transcranial Direct Current Stimulation in Episodic Migraine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medical Sciences. 2025; 13(3):84. https://doi.org/10.3390/medsci13030084
Chicago/Turabian StyleHaghdoost, Faraidoon, Abdul Salam, Fatemeh Zahra Seyed-Kolbadi, Deepika Padala, Candice Delcourt, and Anthony Rodgers. 2025. "Transcranial Direct Current Stimulation in Episodic Migraine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Medical Sciences 13, no. 3: 84. https://doi.org/10.3390/medsci13030084
APA StyleHaghdoost, F., Salam, A., Seyed-Kolbadi, F. Z., Padala, D., Delcourt, C., & Rodgers, A. (2025). Transcranial Direct Current Stimulation in Episodic Migraine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medical Sciences, 13(3), 84. https://doi.org/10.3390/medsci13030084







