Advances in Adoptive Cell Therapies in Cancer: From Mechanistic Breakthroughs to Clinical Frontiers and Overcoming Barriers
Abstract
1. Introduction
2. Fundamentals of CAR-T-Cell Therapy
2.1. Generations of CAR-T-Cell Therapy
2.1.1. First Generation
2.1.2. Second Generation
2.1.3. Third Generation
2.1.4. Fourth Generation
2.1.5. Fifth Generation
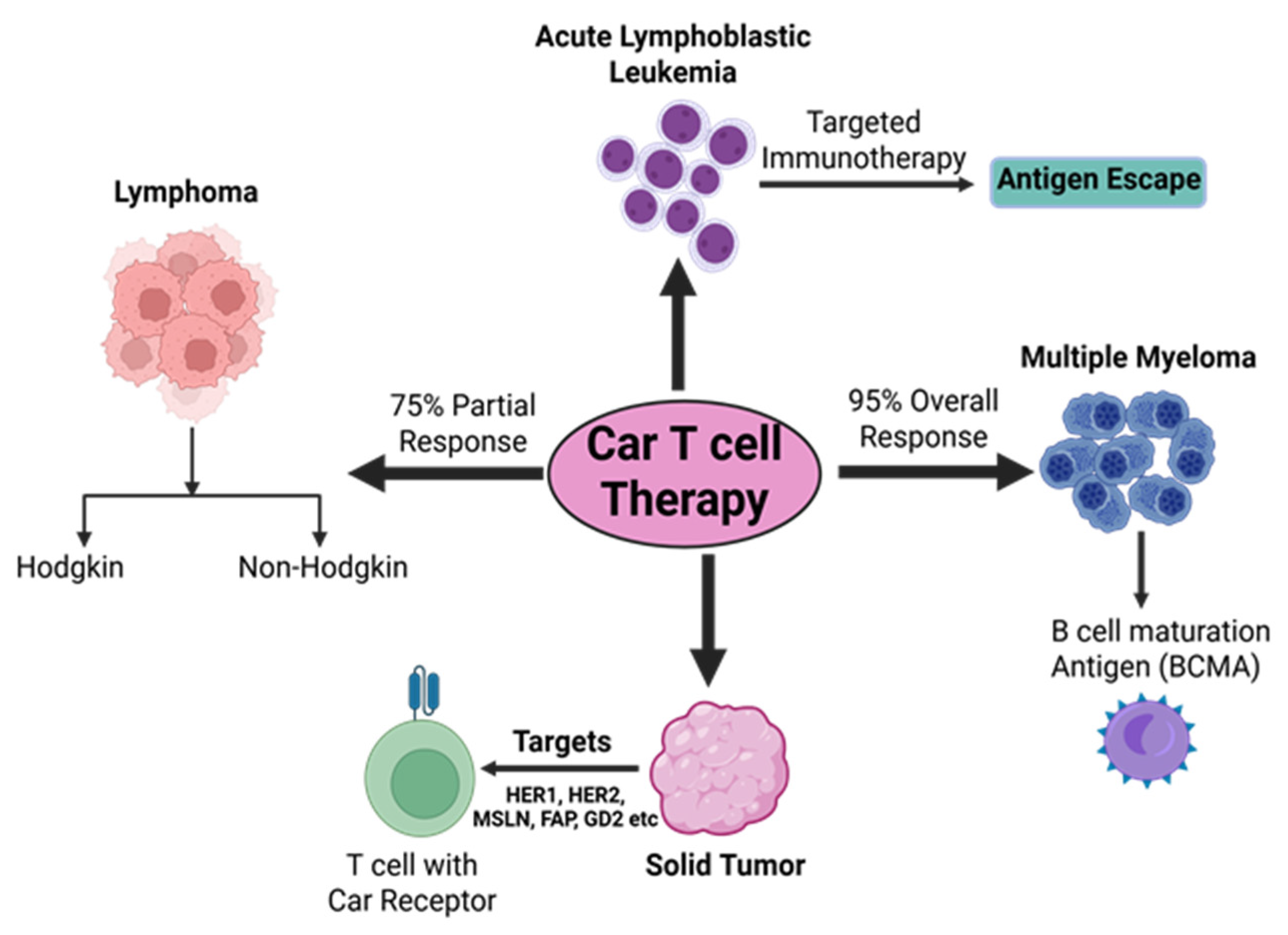
2.2. CAR-T-Cell Therapy for Different Malignancies
2.2.1. Lymphoma
2.2.2. Chronic Lymphocytic Leukemia
2.2.3. Acute Lymphoblastic Leukemia
2.2.4. Multiple Myeloma
2.2.5. Breast Cancer
2.2.6. CAR-T-Cell Therapies in Solid Tumors
2.3. CAR-T-Cell Therapies: Limitations and Potential Strategies
2.3.1. Antigen Escape
2.3.2. T-Cell Exhaustion
2.3.3. Severe Adverse Effects
2.3.4. On Target Off-Tumor Effects
2.3.5. Tumor Infiltration
2.3.6. Overcoming Solid Tumor Barriers in CAR-T-Cell Therapy
2.3.7. Integration of Artificial Intelligence and Machine Learning in CAR-T Cell Development
2.3.8. CAR-T-Cell Therapy—Economic and Logistical Challenges
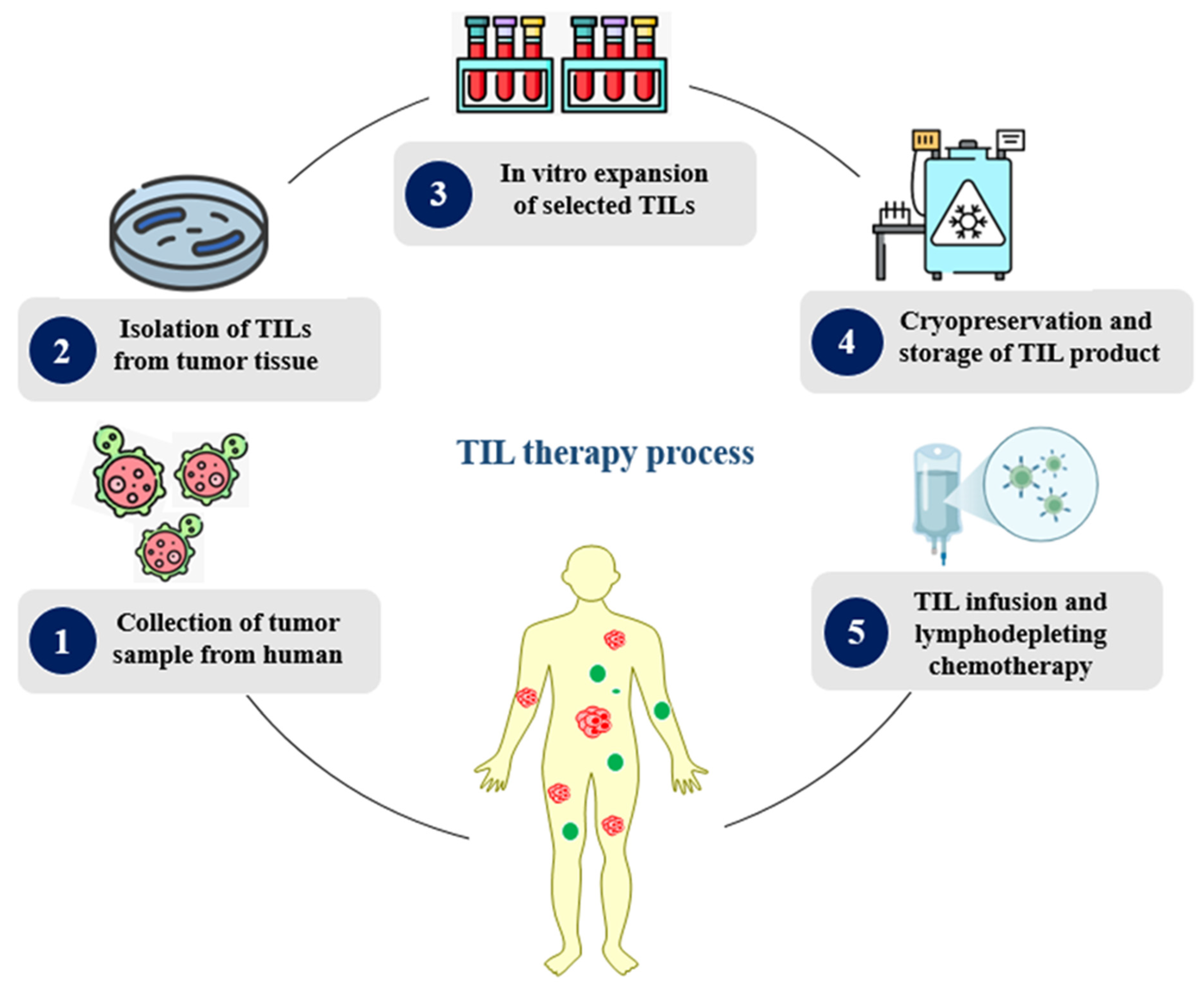
3. Fundamentals of Tumor Infiltration Lymphocyte Therapy (TIL)
3.1. TIL Therapy for Cancer
3.1.1. Solid Tumors
3.1.2. Non-Small-Cell Lung Cancer (NSCLC)
3.1.3. Cervical Cancer
3.1.4. Breast Cancer
4. TCR-Engineered T Cells
4.1. TCR-Targeted Tumor Antigens
4.1.1. Tumor Associated Antigens (TAA)
4.1.2. Cancer-Germline Antigens (CGAs)
4.1.3. Tumor-Specific Antigens (TSAs)
4.2. Challenges of TCR T-Cell Therapies
4.2.1. Toxicity Prediction
4.2.2. Resistance
4.2.3. Tumor Microenvironment
4.2.4. TCR-Engineered T-Cell Therapy—Economic and Logistical Challenges
4.3. Integration of Artificial Intelligence and Machine Learning in TCR-Engineered T Cells
4.3.1. Epitope Prediction and Prioritization
4.3.2. TCR–pMHC Interaction Modeling
4.3.3. Personalized Target Discovery
5. FDA-Approved ACTs
5.1. Tisagenlecleucel
5.2. Axicabtagene Ciloleucel
5.3. Brexucabtagene Autoleucel
5.4. Lisocabtagene Maraleucel
5.5. Idecabtagene Vicleucel
5.6. Ciltacabtagene Autoleucel
5.7. Obecabtagene Autoleucel
5.8. Lifileucel
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. Recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10. [Google Scholar] [CrossRef]
- Abshire, D.; Lang, M.K. The evolution of radiation therapy in treating cancer. Semin. Oncol. Nurs. 2018, 34, 151–157. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Tu, J.; Tang, M.; Ashrafizadeh, M.; Nabavi, N.; Sethi, G.; Zhao, P.; Liu, S. Advances in cancer immunotherapy: Historical perspectives, current developments, and future directions. Mol. Cancer 2025, 24, 136. [Google Scholar] [CrossRef]
- Li, X.; Yamazaki, T.; Ebara, M.; Shirahata, N.; Hanagata, N. Nanoengineered coordination polymers boost cancer immunotherapy. Mater. Today 2023, 67, 127–150. [Google Scholar] [CrossRef]
- Uscanga-Palomeque, A.C.; Chávez-Escamilla, A.K.; Alvizo-Báez, C.A.; Saavedra-Alonso, S.; Terrazas-Armendáriz, L.D.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C.; Alcocer-González, J.M. CAR-T cell therapy: From the shop to cancer therapy. Int. J. Mol. Sci. 2023, 24, 15688. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Q.; Ren, X. Novel strategies for cancer immunotherapy: Counter-immunoediting therapy. J. Hematol. Oncol. 2023, 16, 38. [Google Scholar] [CrossRef]
- Abbasi, M.H.; Riaz, A.; Khawar, M.B.; Farooq, A.; Majid, A.; Sheikh, N. CAR-T-cell therapy: Present progress and future strategies. Biomed. Res. Ther. 2022, 9, 4920–4929. [Google Scholar] [CrossRef]
- Parsonidis, P.; Papasotiriou, I. Adoptive cellular transfer immunotherapies for cancer. Cancer Treat. Res. Commun. 2022, 32, 100575. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Rosenberg, S.A. Adoptive cell transfer therapy. Semin. Oncol. 2007, 34, 524–531. [Google Scholar] [CrossRef][Green Version]
- Hazboun, N. Adoptive cellular immunotherapy for solid tumors. Int. J. Tumor Ther. 2020, 9, 1–4. [Google Scholar][Green Version]
- Parums, D.V. A Review of CAR T Cells and Adoptive T-Cell Therapies in Lymphoid and Solid Organ Malignancies. Med. Sci. Monit. 2025, 31, e948125. [Google Scholar] [CrossRef]
- Mitra, A.; Barua, A.; Huang, L.; Ganguly, S.; Feng, Q.; He, B. From bench to bedside: The history and progress of CAR T cell therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef]
- Miliotou, A.N.; Papadopoulou, L.C. CAR T-cell therapy: A new era in cancer immunotherapy. Curr. Pharm. Biotechnol. 2018, 19, 5–18. [Google Scholar] [CrossRef]
- de Saint Basile, G.; Menasche, G.; Fischer, A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat. Rev. Immunol. 2010, 10, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Martin, S.J. Mechanisms of granule-dependent killing. Cell Death Differ. 2007, 15, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, D.A. T-cell antigen receptor signal transduction. Immunology 2002, 105, 369–374. [Google Scholar] [PubMed]
- Savoldo, B.; Grover, N.; Dotti, G. CAR T Cells for Hematological Malignancies. J. Clin. Investig. 2024, 134, e177160. [Google Scholar] [CrossRef]
- Odstrcil, M.S.; Lee, C.J.; Sobieski, C.; Weisdorf, D.; Couriel, D. Access to CAR T-cell therapy: Focus on diversity, equity and inclusion. Blood Rev. 2024, 63, 101136. [Google Scholar]
- Abate-Daga, D.; Davila, M.L. CAR models: Next-generation CAR modifications for enhanced T-cell function. Mol. Ther. Oncolytics 2016, 3, 16014. [Google Scholar] [CrossRef]
- Labanieh, L.; Mackall, C.L. CAR immune cells: Design principles, resistance and the next generation. Nature 2023, 614, 635–648. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kitaura, M.; Tsunei, A.; Kusabuka, H.; Ogaki, E.; Okada, N. Structure of the signal transduction domain in second-generation CAR regulates the input efficiency of CAR signals. Int. J. Mol. Sci. 2021, 22, 2476. [Google Scholar] [CrossRef]
- Doherty, K. Obe-Cel may mark additional treatment option for R/R B-ALL. Suppl. Featur. Publ. 2022, 1. Available online: https://www.onclive.com/view/obe-cel-may-mark-additional-treatment-option-for-r-r-b-all?utm_source=chatgpt.com (accessed on 7 June 2025).
- Zheng, Z.; Li, S.; Liu, M.; Chen, C.; Zhang, L.; Zhou, D. Fine-tuning through generations: Advances in structure and production of CAR-T therapy. Cancers 2023, 15, 3476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.-L.; Schmitt, A.; Neuber, B.; Hückelhoven-Krauss, A.; Kunz, A.; Wang, L.; Gern, U.; Michels, B.; Sellner, L.; Hofmann, S.; et al. Third-generation CAR T cells targeting CD19 are associated with an excellent safety profile and might improve persistence of CAR T cells in treated patients. Blood 2019, 134, 51. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Khan, S.H.; Choi, Y.; Veena, M.; Lee, J.K.; Shin, D.S. Advances in CAR T Cell Therapy: Antigen Selection, Modifications, and Current Trials for Solid Tumors. Front. Immunol. 2024, 15, 1489827. [Google Scholar] [CrossRef]
- Farooq, A.; Abbasi, M.H.; Khawar, M.B.; Sheikh, N. Overview of the advantages and disadvantages of chimeric antigen receptor T cell therapy in the tumor microenvironment. Biomed. Res. Ther. 2022, 9, 5341–5350. [Google Scholar] [CrossRef]
- Sanomachi, T.; Katsuya, Y.; Nakatsura, T.; Koyama, T. Next-Generation CAR-T and TCR-T Cell Therapies for Solid Tumors: Innovations, Challenges, and Global Development Trends. Cancers 2025, 17, 1945. [Google Scholar] [CrossRef]
- Asmamaw Dejenie, T.; Tiruneh, G.; Medhin, M.; Dessie Terefe, G.; Tadele Admasu, F.; Wale Tesega, W.; Chekol Abebe, E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum. Vaccines Immunother. 2022, 18, 2114254. [Google Scholar] [CrossRef]
- Hjalgrim, H.; Engels, E.A. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: A review of the epidemiological evidence. J. Intern. Med. 2008, 264, 537–548. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Hanrahan, E.O.; Daly, P.A. Non-Hodgkin lymphoma: An update. Lancet Oncol. 2004, 5, 341–353. [Google Scholar] [CrossRef]
- Denlinger, N.; Bond, D.; Jaglowski, S. CAR T-cell therapy for B-cell lymphoma. Curr. Probl. Cancer 2022, 46, 100826. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.; Carpenter, R.O.; Stetler-Stevenson, M.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Geyer, M.B.; Brentjens, R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: Interpreting clinical outcomes to date. Blood 2016, 127, 3312–3320. [Google Scholar] [CrossRef]
- Miller, B.C.; Maus, M.V. CD19-targeted CAR T cells: A new tool in the fight against B cell malignancies. Oncol. Res. Treat. 2015, 38, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef]
- Shadman, M.; Gopal, A.K.; Smith, S.D.; Lynch, R.C.; Ujjani, C.S.; Turtle, C.J.; Greenbaum, A.; Redman, M.; Yeung, C.; Sersch, M.; et al. CD20 targeted CAR-T for high-risk B-cell non-Hodgkin lymphomas. Blood 2019, 134, 3235. [Google Scholar] [CrossRef]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.-F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef]
- Ramos, C.A.; Bilgi, M.; Gerken, C.; Dakhova, O.; Mei, Z.; Wu, M.-F.; Grilley, B.; Gee, A.P.; Rooney, C.M.; Dotti, G.; et al. CD30-chimeric antigen receptor (CAR) T cells for therapy of Hodgkin lymphoma (HL). Biol. Blood Marrow Transplant. 2019, 25, S63. [Google Scholar] [CrossRef]
- Lemal, R.; Tournilhac, O. State-of-the-art for CAR T-cell therapy for chronic lymphocytic leukemia in 2019. J. Immunother. Cancer 2019, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.B.; Rivière, I.; Sénéchal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Palomba, M.L.; Halton, E.; et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight 2019, 4, e122627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Xiao, W.; Li, W.; Wang, L.; Yang, S.; Wang, W.; Xu, L.; Liao, S.; Liu, W.; et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. Hematol. Oncol. 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.B.; Rivière, I.; Sénéchal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Halton, E.; Lamanna, N.; et al. Autologous CD19-targeted CAR T cells in patients with residual CLL following initial purine analog-based therapy. Mol. Ther. 2018, 26, 1896–1905. [Google Scholar] [CrossRef]
- Zah, E.; Lin, M.-Y.; Silva-Benedict, A.; Jensen, M.C.; Chen, Y.Y. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016, 4, 498–508. [Google Scholar] [CrossRef]
- Han, D.; Xu, Z.; Zhuang, Y.; Ye, Z.; Qian, Q. Current progress in CAR-T cell therapy for hematological malignancies. J. Cancer 2021, 12, 326–334. [Google Scholar] [CrossRef]
- Sheth, V.S.; Gauthier, J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021, 56, 552–566. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and management of multiple myeloma: A review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Barlogie, B.; Shaughnessy, J.; Tricot, G.; Jacobson, J.; Zangari, M.; Anaissie, E.; Walker, R.; Crowley, J. Treatment of multiple myeloma. Blood 2004, 103, 20–32. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma: Diagnosis and treatment. Mayo Clin Proc. 2016, 91, 101–119. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liu, J.-W.; Lu, C.; Wei, J.-F. CAR-T cell therapy for breast cancer: From basic research to clinical application. Int. J. Biol. Sci. 2022, 18, 2609–2626. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, S.; Zhang, X.; Gong, Z.; Wang, S. Non-Conventional Allogeneic Anti-BCMA Chimeric Antigen Receptor-Based Immune Cell Therapies for Multiple Myeloma Treatment. Cancers 2023, 15, 567. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Brentjens, R.J. CD19-targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 2016, 14, 802–808. [Google Scholar] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.E.; et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Curigliano, G.; Piccart, M. Emerging treatments in HER2-positive advanced breast cancer: Keep raising the bar. Cell Rep. Med. 2024, 5, 101575. [Google Scholar] [CrossRef]
- Xin, Q.; Chen, Y.; Sun, X.; Li, R.; Wu, Y.; Huang, X. CAR-T therapy for ovarian cancer: Recent advances and future directions. Biochem. Pharmacol. 2024, 221, 116349. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Q.; Liang, X.; Chen, Z.; Zhang, X.; Zhou, X.; Li, M.; Tu, H.; Liu, Y.; Tu, S.; et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front. Immunol. 2019, 10, 2664. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef]
- Green, D.J.; Pont, M.; Sather, B.D.; Cowan, A.J.; Turtle, C.J.; Till, B.G.; Nagengast, A.M.; Libby, E.N., III; Becker, P.S.; Coffey, D.G.; et al. Fully human BCMA targeted chimeric antigen receptor T cells administered in a defined composition demonstrate potency at low doses in advanced stage high-risk multiple myeloma. Blood 2018, 132 (Suppl. 1), 1011. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Bartoszewska, E.; Tota, M.; Kisielewska, M.; Skowron, I.; Sebastianka, K.; Stefaniak, O.; Molik, K.; Rubin, J.; Kraska, K.; Choromańska, A. Overcoming antigen escape and T-cell exhaustion in CAR-T therapy for leukemia. Cells 2024, 13, 1596. [Google Scholar] [CrossRef]
- Wilkie, S.; Picco, G.; Foster, J.; Davies, D.M.; Julien, S.; Cooper, L.; Arif, S.; Mather, S.J.; Taylor-Papadimitriou, J.; Burchell, J.M.; et al. Retargeting of human T cells to tumor-associated MUC1: The evolution of a chimeric antigen receptor. J. Immunol. 2008, 180, 4901–4909. [Google Scholar] [CrossRef]
- Murad, J.P.; Kozlowska, A.K.; Lee, H.J.; Ramamurthy, M.; Chang, W.-C.; Yazaki, P.; Colcher, D.; Shively, J.; Cristea, M.; Forman, S.J.; et al. Effective targeting of TAG72+ peritoneal ovarian tumors via regional delivery of CAR-engineered T cells. Front. Immunol. 2018, 9, 2268. [Google Scholar] [CrossRef]
- Wang, H.; Kaur, G.; Sankin, A.I.; Chen, F.; Guan, F.; Zang, X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J. Hematol. Oncol. 2019, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Caruana, I.; Savoldo, B.; Hoyos, V.; Weber, G.; Liu, H.; Kim, E.S.; Ittmann, M.M.; Marchetti, D.; Dotti, G. Heparanase Promotes Tumor Infiltration and Antitumor Activity of CAR-Redirected T Lymphocytes. Nat. Med. 2015, 21, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Gu, J.; Xu, H. Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors. Mol. Cancer 2018, 17, 7. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Neelapu, S.S. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019, 37, 48–52. [Google Scholar] [CrossRef]
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA approval summary: Tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef]
- Kotch, C.; Barrett, D.; Teachey, D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev. Clin. Immunol. 2019, 15, 813–822. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, Y.; Zhou, W.; Wang, F.; Yan, F.; Gao, H.; Wang, W. Infusion and delivery strategies to maximize the efficacy of CAR-T cell immunotherapy for cancers. Exp. Hematol. Oncol. 2024, 13, 70. [Google Scholar] [CrossRef]
- Du, H.; Hirabayashi, K.; Ahn, S.; Kren, N.P.; Montgomery, S.A.; Wang, X.; Tiruthani, K.; Mirlekar, B.; Michaud, D.; Greene, K.; et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell 2019, 35, 221–237. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Koneru, M.; O’cEarbhaill, R.; Pendharkar, S.; Spriggs, D.R.; Brentjens, R.J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16 ecto directed chimeric antigen receptors for recurrent ovarian cancer. Transl. Med. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Chekmasova, A.A.; Rao, T.D.; Nikhamin, Y.; Park, K.J.; Levine, D.A.; Spriggs, D.R.; Brentjens, R.J. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin. Cancer Res. 2010, 16, 3594–3606. [Google Scholar] [CrossRef] [PubMed]
- Hege, K.M.; Bergsland, E.K.; Fisher, G.A.; Nemunaitis, J.J.; Warren, R.S.; McArthur, J.G.; Lin, A.A.; Schlom, J.; June, C.H.; Sherwin, S.A. Safety, Tumor Trafficking, and Immunogenicity of Chimeric Antigen Receptor (CAR)-T Cells Specific for TAG-72 in Colorectal Cancer. J. Immunother. Cancer 2017, 5, 22. [Google Scholar] [CrossRef]
- Hou, A.J.; Chen, L.C.; Chen, Y.Y. Navigating CAR-T cells through the solid-tumor microenvironment. Nat. Rev. Drug Discov. 2021, 20, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Greta Maria Paola Giordano Attianese; Ash, S.; Irving, M. Coengineering specificity, safety, and function into T cells for cancer immunotherapy. Immunol. Rev. 2023, 320, 166–198. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Y. Phase I clinical trial using a unique immunotherapeutic combination of MUC1-targeted CAR-T cells with PD-1-knockout in the treatment of patients with advanced esophageal cancer. J. Clin. Oncol. 2023, 41, e16061. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, Z.; Zhao, X.; Zhao, Z.; Song, W.; Yang, X. Combination strategies to improve the efficacy of CAR T cell therapy in hematologic malignancies. Front. Immunol. 2020, 11, 616. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M. Use of CAR-T cell therapy, PD-1 blockade, and their combination for the treatment of hematological malignancies. Clin. Immunol. 2020, 214, 108382. [Google Scholar] [CrossRef]
- Andersen, R.; Donia, M.; Westergaard, M.C.W.; Pedersen, M.; Hansen, M.; Svane, I.M. Tumor infiltrating lymphocyte therapy for ovarian cancer and renal cell carcinoma. Hum. Vaccines Immunother. 2015, 11, 2790–2795. [Google Scholar] [CrossRef]
- Monberg, T.J.; Borch, T.H.; Svane, I.M.; Donia, M. TIL therapy: Facts and hopes. Clin. Cancer Res. 2023, 29, 3275–3283. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: Progressions and challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zheng, H.; Yang, S.; Hua, Y.; Huang, N.; Kleeff, J.; Liao, Q.; Wu, W. Adoptive cellular immunotherapy for solid neoplasms beyond CAR-T. Mol. Cancer 2023, 22, 28. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Wan, X. Challenges and new technologies in adoptive cell therapy. J. Hematol. Oncol. 2023, 16, 97. [Google Scholar] [CrossRef]
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive cellular therapy in solid tumor malignancies: Review of the literature and challenges ahead. J. Immunother. Cancer 2021, 9, e002723. [Google Scholar] [CrossRef]
- Betof Warner, A.; Corrie, P.G.; Hamid, O. Tumor-infiltrating lymphocyte therapy in melanoma: Facts to the future. Clin. Cancer Res. 2023, 29, 1835–1854. [Google Scholar] [CrossRef]
- Metts, J.; Rodriguez-Valentin, M.; Hensel, J.; Alfaro, A.; Snyder, C.W.; Binitie, O.; Chebli, C.; Monforte, H.; Pilon-Thomas, S.; Mullinax, J. Expansion of tumor-infiltrating and marrow-infiltrating lymphocytes from pediatric malignant solid tumors. Cytotherapy 2024, 27, 29–35. [Google Scholar] [CrossRef]
- Zhou, Y.; Espenel, S.; Achkar, S.; Leary, A.; Gouy, S.; Chargari, C. Combined modality including novel sensitizers in gynecological cancers. Int. J. Gynecol. Cancer 2022, 32, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Bian, Y.; Ji, H. TIL therapy in lung cancer: Current progress and perspectives. Adv. Sci. 2024, 11, e2409356. [Google Scholar] [CrossRef] [PubMed]
- Creelan, B.C.; Wang, C.; Teer, J.K.; Toloza, E.M.; Yao, J.; Kim, S.; Landin, A.M.; Mullinax, J.E.; Saller, J.J.; Saltos, A.N.; et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: A phase 1 trial. Nat. Med. 2021, 27, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.; Lee, S. Tumor-Infiltrating Lymphocyte Therapy: A New Frontier. Biol. Blood Marrow Transplant. 2025, 31, S599–S609. [Google Scholar] [CrossRef]
- Zacharakis, N.; Huq, L.M.; Seitter, S.J.; Kim, S.P.; Gartner, J.J.; Sindiri, S.; Hill, V.K.; Li, Y.F.; Paria, B.C.; Ray, S.; et al. Breast cancers are immunogenic: Immunologic analyses and a phase II pilot clinical trial using mutation-reactive autologous lymphocytes. J. Clin. Oncol. 2022, 40, 1741–1754. [Google Scholar] [CrossRef]
- Sorkhabi, A.D.; Khosroshahi, L.M.; Sarkesh, A.; Mardi, A.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Baradaran, B. The current landscape of CAR T-cell therapy for solid tumors: Mechanisms, research progress, challenges, and counter-strategies. Front. Immunol. 2023, 14, 1113882. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, L.; Chen, J. Current advances and challenges in CAR T-cell therapy for solid tumors: Tumor-associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wei, W.; Li, Y. TCR engineered T cells for solid tumor immunotherapy. Exp. Hematol. Oncol. 2022, 11, 38. [Google Scholar] [CrossRef]
- Chen, L.; Qiao, D.; Wang, J.; Tian, G.; Wang, M. Cancer immunotherapy with lymphocytes genetically engineered with T cell receptors for solid cancers. Immunol. Lett. 2019, 216, 51–62. [Google Scholar] [CrossRef]
- Biernacki, M.A.; Brault, M.; Bleakley, M. T-cell receptor-based immunotherapy for hematologic malignancies. Cancer J. 2019, 25, 179–190. [Google Scholar] [CrossRef]
- He, W.; Cui, K.; Farooq, M.A.; Huang, N.; Zhu, S.; Jiang, D.; Zhang, X.; Chen, J.; Liu, Y.; Xu, G. TCR-T cell therapy for solid tumors: Challenges and emerging solutions. Front. Pharmacol. 2025, 16, 1493346. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Bover, M.; Herrera-Juarez, M.; Rey-Cardenas, M.; Paz-Ares, L.; A Lopez-Martin, J.; Haanen, J. Efficacy of Tcell receptor-based adoptive cell therapy in cutaneous melanoma: A meta-analysis. Oncologist 2023, 28, e406–e415. [Google Scholar] [CrossRef] [PubMed]
- Leidner, R.; Silva, N.S.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.-P.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef]
- Schultz-Thater, E.; Piscuoglio, S.; Iezzi, G.; Le Magnen, C.; Zajac, P.; Carafa, V.; Terracciano, L.; Tornillo, L.; Spagnoli, G.C. MAGE-A10 is a nuclear protein frequently expressed in high percentages of tumor cells in lung, skin and urothelial malignancies. Int. J. Cancer 2011, 129, 1137–1148. [Google Scholar] [CrossRef]
- Anderson, K.G.; Voillet, V.; Bates, B.M.; Chiu, E.Y.; Burnett, M.G.; Garcia, N.M.; Oda, S.K.; Morse, C.B.; Stromnes, I.M.; Drescher, C.W.; et al. Engineered adoptive T-cell therapy prolongs survival in a preclinical model of advanced-stage ovarian cancer. Cancer Immunol. Res. 2019, 7, 1412–1425. [Google Scholar] [CrossRef]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef]
- Xu, Y.; Morales, A.J.; Cargill, M.J.; Towlerton, A.M.H.; Coffey, D.G.; Warren, E.H.; Tykodi, S.S. Preclinical development of T-cell receptor-engineered T-cell therapy targeting the 5T4 tumor antigen on renal cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 1979–1993. [Google Scholar] [CrossRef]
- Miyahara, Y.; Naota, H.; Wang, L.; Hiasa, A.; Goto, M.; Watanabe, M.; Kitano, S.; Okumura, S.; Takemitsu, T.; Yuta, A.; et al. Determination of cellularly processed HLA-A2402-restricted novel CTL epitopes derived from two cancer germ line genes, MAGE-A4 and SAGE. Clin. Cancer Res. 2005, 11, 5581–5589. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; E Dudley, M.; Nathan, D.-A.N.; A Feldman, S.; Davis, J.L.; A Morgan, R.; Merino, M.J.; Sherry, R.M.; et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef]
- Kawakami, Y.; Eliyahu, S.; Delgado, C.H.; Robbins, P.F.; Rivoltini, L.; Topalian, S.L.; Miki, T.; A Rosenberg, S. Cloning of the Gene Coding for a Shared Human Melanoma Antigen Recognized by Autologous T Cells Infiltrating Into Tumor. Proc. Natl. Acad. Sci. USA 1994, 91, 3515–3519. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Schreurs, M.W.; de Boer, A.J.; Kawakami, Y.; A Rosenberg, S.; Adema, G.J.; Figdor, C.G. Melanocyte Lineage-Specific Antigen Gp100 Is Recognized by Melanoma-Derived Tumor-Infiltrating Lymphocytes. J. Exp. Med. 1994, 179, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Gottardi, E.; De Micheli, D.; Serra, A.; Volpe, G.; Messa, F.; Rege-Cambrin, G.; Guerrasio, A.; Divona, M.; Coco, F.L.; et al. Quantitative Assessment of WT1 Expression by Real Time Quantitative PCR may be a Useful Tool for Monitoring Minimal Residual Disease in Acute Leukemia Patients. Leukemia 2002, 16, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ogawa, H.; Sonoda, Y.; Kimura, T.; Sakabe, H.; Oka, Y.; Miyake, S.; Tamaki, H.; Oji, Y.; Yamagami, T.; et al. Aberrant Overexpression of the Wilms Tumor Gene (WT1) in Human Leukemia. Blood 1997, 89, 1405–1412. [Google Scholar] [CrossRef]
- Shafer, P.; Kelly, L.M.; Hoyos, V. Cancer therapy with TCR-engineered T cells: Current strategies, challenges, and prospects. Front. Immunol. 2022, 13, 835762. [Google Scholar] [CrossRef]
- Cho, H.-I.; Kim, U.-H.; Shin, A.-R.; Won, J.-N.; Lee, H.-J.; Sohn, H.-J.; Kim, T.-G. A Novel Epstein–Barr Virus-Latent Membrane Protein-1-Specific T-Cell Receptor for TCR Gene Therapy. Br. J. Cancer 2018, 118, 534–545. [Google Scholar] [CrossRef]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves first CAR T therapy. Nat. Rev. Drug Discov. 2017, 16, 669–670. [Google Scholar] [CrossRef]
- O’Leary, M.C.; Lu, X.; Huang, Y.; Lin, X.; Mahmood, I.; Przepiorka, D.; Gavin, D.; Lee, S.; Liu, K.; George, B.; et al. FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin. Cancer Res. 2019, 25, 1142–1146. [Google Scholar] [CrossRef]
- Bouchkouj, N.; Kasamon, Y.L.; de Claro, R.A.; George, B.; Lin, X.; Lee, S.; Blumenthal, G.M.; Bryan, W.; McKee, A.E.; Pazdur, R. FDA approval summary: Axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin. Cancer Res. 2019, 25, 1702–1708. [Google Scholar] [CrossRef]
- Elsawy, M.; Chavez, J.C.; Avivi, I.; Larouche, J.-F.; Wannesson, L.; Cwynarski, K.; Osman, K.; Davison, K.; Rudzki, J.D.; Dahiya, S.; et al. Patient-reported outcomes (PROs) in ZUMA-7, a phase 3, randomized, open-label study evaluating the efficacy of axicabtagene ciloleucel (axi-cel) versus standard-of-care (SOC) therapy in patients with relapsed/refractory large B-cell lymphoma (LBCL). Biol. Blood Marrow Transplant. 2022, 28, S190–S191. [Google Scholar] [CrossRef]
- Tisa, L.J. CEL for Third Indication, r/r Follicular Lymphoma. Innov Multi Care. May 2022. Available online: https://www.ajmc.com/view/fda-approves-tisa-cel-for-third-indication-r-r-follicular-lymphoma?utm_source=chatgpt.com (accessed on 31 May 2025).
- Bouchkouj, N.; Lin, X.; Wang, X.; Przepiorka, D.; Xu, Z.; Purohit-Sheth, T.; Theoret, M. FDA approval summary: Brexucabtagene autoleucel for treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Oncologist 2022, 27, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.; William, B.M.; Munoz, J.; Salles, G.A.; Casulo, C.; Munshi, P.N.; Maloney, D.G.; De Vos, S.; et al. Outcomes in ZUMA-5 with axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory indolent non-Hodgkin lymphoma (iNHL) who had the high-risk feature of progression within 24 months from initiation of first anti-CD20–containing chemoimmunotherapy (POD24). J. Clin. Oncol. 2021, 39, 7515. [Google Scholar]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.D.; Ghobadi, A.; O Oluwole, O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Kharfan-Dabaja, M.A.; Yassine, F.; Moustafa, M.A.; Iqbal, M.; Murthy, H. Lisocabtagene maraleucel in relapsed or refractory diffuse large B cell lymphoma: What is the evidence? Hematol. Oncol. Stem Cell Ther. 2022, 15, 168–175. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Sharma, P.; Kanapuru, B.; George, B.; Lin, X.; Xu, Z.; Bryan, W.W.; Pazdur, R.; Theoret, M.R. FDA approval summary: Idecabtagene vicleucel for relapsed or refractory multiple myeloma. Clin. Cancer Res. 2022, 28, 1759–1764. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Jacobson, C.A.; Ghobadi, A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Five-Year Follow-Up of ZUMA-1 Supports the Curative Potential of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma. Blood 2023, 141, 2307–2315. [Google Scholar] [CrossRef]
- Natrajan, K.; Kaushal, M.; George, B.; Kanapuru, B.; Theoret, M.R. FDA approval summary: Ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. Clin. Cancer Res. 2024, 30, 2865–2871. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Shiferaw, M.Y.; Admasu, F.T.; Dejenie, T.A. Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front. Immunol. 2022, 13, 991092. [Google Scholar] [CrossRef]
- Hoffman, M. FDA Approves Obe-Cel for Adults with r/r B-Cell Precursor Acute Lymphoblastic Leukemia. FDA News Release. 2023. Available online: https://www.cgtlive.com/view/fda-approves-obe-cel-adults-relapsed-refractory-b-cell-precursor-acute-lymphoblastic-leukemia (accessed on 4 May 2024).
- Roddie, C.; Sandhu, K.S.; Tholouli, E.; Shaughnessy, P.; Barba, P.; Guerreiro, M.N.; Yallop, D.; Abedi, M.; Chaganti, S.; Ghobadi, A.; et al. Safety and efficacy of obecabtagene autoleucel (Obe-Cel, AUTO1), a fast-off rate CD19 CAR, in relapsed/refractory adult B-cell acute lymphoblastic leukemia (r/r B-ALL): Top line results of the pivotal FELIX study. Blood 2023, 142, 550–561. [Google Scholar] [CrossRef]
- Keam, S.J. Lifileucel: First approval. Mol. Diagn. Ther. 2024, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 2021, 39, 2656–2666. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Ali, A.; DiPersio, J.F. ReCARving the future: Bridging CAR T-cell therapy gaps with synthetic biology, engineering, and economic insights. Front. Immunol. 2024, 15, 1432799. [Google Scholar] [CrossRef]

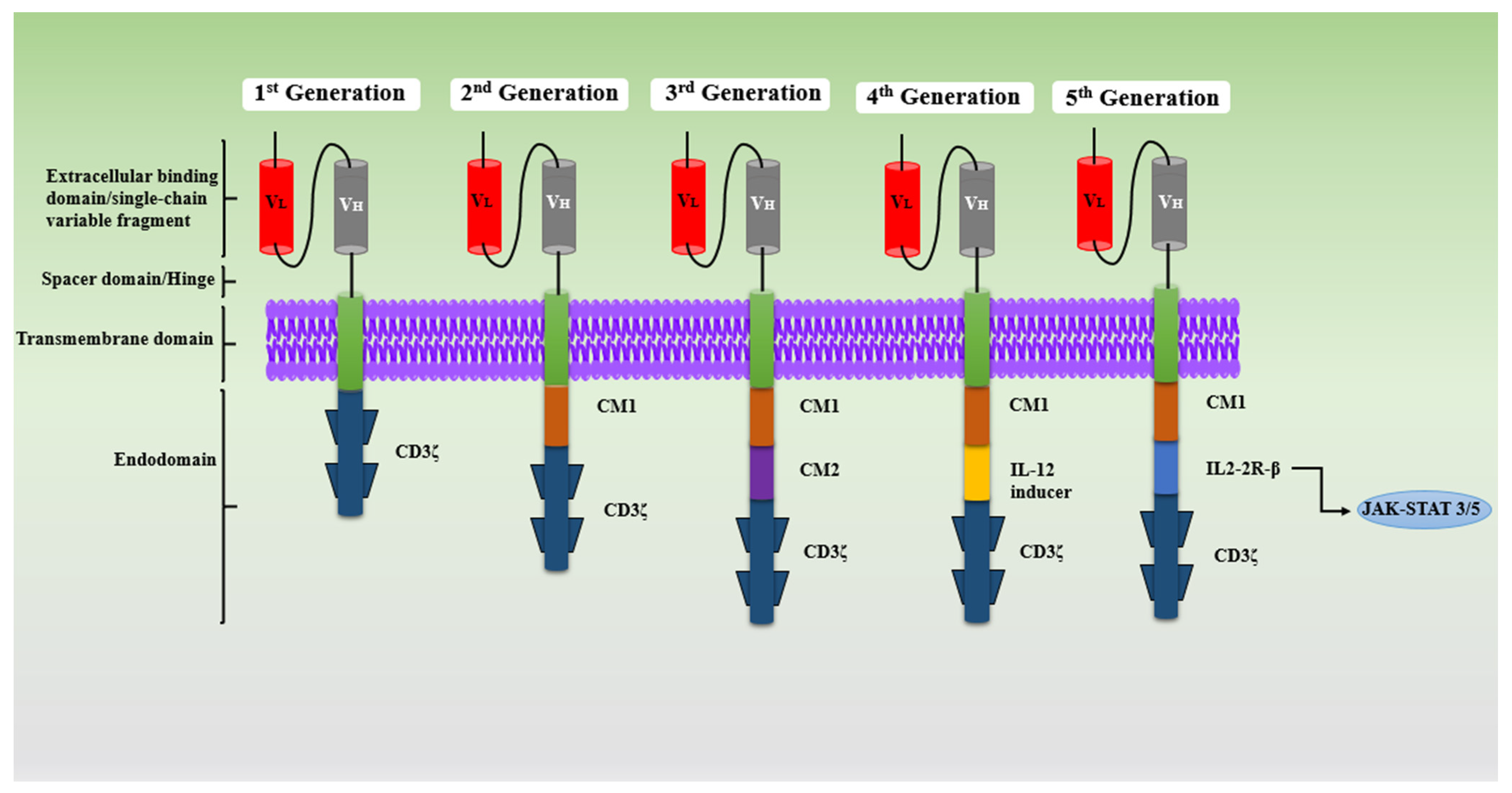

| CAR Generation | Components | Key Features | Pros | Cons | References |
|---|---|---|---|---|---|
| First | scFv, spacer, CD3ζ, a transmembrane | Single signaling domain (CD3ζ) | Basic design, first proof-of-concept | Weak proliferation and activation, poor cytokine release, limited efficacy | [21,22,23,24,25,26,27,28] |
| Second | costimulatory molecule (CD28, CD134 etc.), CD3ζ, transmembrane. | Added costimulatory domain (CD28 or 4-1BB) | Improved T-cell proliferation, activation and survival; better persistence | Causes immunosuppressive tumor microenvironment (TME) due to single costimulatory molecule | [19,20,21,22] |
| Third | CD3ζ, costimulatory molecule (CD27, CD28, CD134 etc.); CD3ζ-CD28-CD134, CD3ζ-CD28-CD137 and CD3ζ TLR2-CD28 | Dual costimulatory domains (CD28 + 4-1BB, or others) | Increase cytokine release, better antitumor activity | Rapid exhaustion of CAR-T cells; Toxicity due to over activation of costimulatory molecule, complex design | [25,26] |
| Fourth (TRUCKs) | costimulatory molecule (CD28, CD134 etc.), CD3ζ, transmembrane, Cytokine costimulatory molecule (IL-2, IL-5, IL-15) | Cytokine-secreting CARs (e.g., IL-12) | Activate innate response and enhance immune response, regulate TME modulation, | Increased side effect and high risk of offsite target | [27,28] |
| Fifth | costimulatory molecule (CD28, CD134 etc.), CD3ζ, transmembrane, TF binding motif and cytoplasmic receptor | Incorporates cytokine receptor signaling | Enhances immune response, better persistence and function | Still under early-stage research pregress, potential unknown risks | [30] |
| Target Antigens | Cancer | Treatment | Phase | Clinicaltrials.gov Identifier | Status |
|---|---|---|---|---|---|
| HER | R/R HER2-positive solid tumors | CAR-T cells (CCT303-406) | Phase I | NCT04511871 | Active, not recruiting |
| HER2-positive local advanced/metastatic solid tumors | Hypoxia-stimulated CAR HER2 T cells | Phase I, II | NCT05681650 | Recruiting | |
| Brain and/or Leptomeningeal metastases | HER2-targeted CAR-T cells | Phase I | NCT03696030 | Recruiting | |
| MSLN | MSLN-positive ovarian cancer | TCR-like CAR-T cells | Phase I | NCT05963100 | Recruiting |
| MSLN-positive advanced malignant solid tumors | Anti-MSLN CAR-T cells | Phase I | NCT05783089 | Not yet recruiting | |
| Pancreatic cancer | MSLN targeted CAR-T cells | Phase I | NCT05779917 | Recruiting | |
| Advanced/Metastatic solid tumors | TAK-103 | Phase I | NCT05164666 | Active, not recruiting | |
| Advanced, locally advanced (inoperable) metastatic MSLN-expressing cancers | ZW171 | Phase I | NCT06523803 | Recruiting | |
| GD2 | High Risk and/or R/R Neuroblastoma | GD2 targeted CAR-T cells | Phase I, II | NCT03373097 | Recruiting |
| Lung Cancer | GD2 CAR-T cells + IL-15 + iCaspase9 | Phase I | NCT05620342 | Recruiting | |
| R/R Neuroblastoma | GD-2 CAR-T cell + iCaspase9 | Phase I | NCT01822652 | Active, not recruiting | |
| MUC 1 | MUC1 Positive Advanced Solid Tumors | Anti-CTLA-4/PD-1 expressing MUC1 CAR-T cells | Phase I/II | NCT03179007 | Unknown status |
| Advanced/Metastatic epithelial-derived solid tumors | P-MUC1C-ALLO1 CAR-T cells | Phase I | NCT05239143 | Recruiting | |
| MUC 16 | R/R Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | Anti MUC16 CAR-T cell (27T51) | Phase I | NCT06469281 | Recruiting |
| EGFR | Advanced adult NSCLC | CXCR5-modified EGFR CAR-T cells | Phase I | NCT05060796 | Recruiting |
| R/R Solid tumor in children and young adults | EGFR806-specific CAR-T cell | Phase I | NCT03618381 | Recruiting |
| Category | Challenges | Strategies | Examples | Reference |
|---|---|---|---|---|
| Toxicities associated CAR-T Therapy | High toxicity rates limit first-line use | - Toxicity Management: Tocilizumab, corticosteroids, IL-6 blockade - Engineering CARs to reduce toxicity: Modify CAR-T-cell structures to reduce toxicity (e.g., cytokine secretion). | - Most common in 2nd-gen CD19 CAR-T therapy - CRS, ICANS, renal failure, MAS, splenomegaly - Higher-grade CRS in FDA-approved therapies (tisagenlecleucel, axicabtagene) | [62] |
| Antigen Escape | Loss of expression at antigens targets causes tumor resistance | - Targeting Multiple Antigens; construct Tandem CARs | - Develop dual-directed CAR-T cells (e.g., CD19/CD22, CD19/BCMA). Construct CARs with two different ScFvs targeting multiple antigens simultaneously. | [63] |
| On-Target/Off-Tumor Effects | Normal tissue damage due to shared antigen expression | - Careful antigen selection - Clinical trials with more tumor-specific targets (e.g., TAG72 CARs for ovarian cancer. | - Solid tumor antigens also present on healthy tissue - Risk of toxicity when selecting broadly expressed targets like B7H3, MUC1, MUC16, TAG72 | [64,65] |
| Tumor Microenvironment (TME) | Immunosuppression and poor infiltration reduce CAR-T efficacy | - Combine with PD-1 inhibitors (e.g., NCT05659628, NCT03706326) - Gene editing, e.g., Siglec15 or cMet-CARs - CARs resistant to TME suppression | Presence of checkpoint proteins, suppressor cytokines, regulatory T cells - High cell density and low vascularity in solid tumors | [66,67] |
| Trafficking and Infiltration | CAR-T cells face barriers reaching and penetrating solid tumors | - Local delivery (intrapleural/intraventricular) - CARs with heparinase/FAP to degrade ECM - CARs expressing CXCR1/CXCR2 receptors | - Physical barriers (tumor stroma, ECM with HSPG) - Inefficient chemokine signaling | [68,69] |
| Indication | Therapy | Phase | Sponsor | Clinical Trial |
|---|---|---|---|---|
| R/R Multiple Myeloma | ||||
| Anti-GPRC5D CAR-T Cells | I/II | XuYan | NCT05749133 | |
| BCMA-targeted CAR-T cells | Chongqing Precision Biotech Co., Ltd. | NCT04272151 | ||
| BCMA-targeted CAR-T Cells | I | University of California, San Francisco | NCT05577000 | |
| CAR-T cells (Retreatment) | I | Second Affiliated Hospital of Xi’an Jiaotong University | NCT03672253 | |
| BCMA-TGF-BETA CAR-T Cells | I | Medical College of Wisconsin | NCT05976555 | |
| BCMA-targeted CAR-T Cells | I | Second Affiliated Hospital, School of Medicine, Zhejiang University | NCT04706936 | |
| BCMA-targeted CAR-T Cells | I/II | Chongqing Precision Biotech Co., Ltd. | NCT04271644 | |
| CS1-targeted CAR-T Cells | I | Zhejiang University | NCT04541368 | |
| Acute Myeloid Leukemia | ||||
| CLL-1-, CD33- and/or CD123-specific CAR-gene-engineered T cells | I/II | Shenzhen Geno-Immune Medical Institute | NCT04010877 | |
| Anti-CLL1 CAR-T cells | I | 920th Hospital of Joint Logistics Support Force of People’s Liberation Army of China | NCT04923919 | |
| CD123-targeted CAR-T Cells | I/II | Chongqing Precision Biotech Co., Ltd. | NCT04265963 | |
| Hematological Malignancies | ||||
| GLPG CAR-T cells | III | Galapagos NV | NCT06652633 | |
| CAR-T Autologous T-cell injection | NA | Hebei Senlang Biotechnology Inc., Ltd. | NCT05618041 | |
| CAR-T cells | I | Mingzhi Zhang | NCT06647329 | |
| Acute Lymphoblastic Leukemia | ||||
| Anti-CD7 CAR-T cells | I | National University Hospital, Singapore | NCT05043571 | |
| CD19 and CD22 CAR sequential therapy | I/II | Chongqing Precision Biotech Co., Ltd. | NCT04265963 | |
| CD5/CD7-specific CAR-T cells (BAH246) | I/II | Essen Biotech | NCT06420076 | |
| CD19/CD22 CAR-T cells | NA | Beijing GoBroad Hospital | NCT06343090 | |
| R/R B-lineage | CAR-T cell | I | National University Hospital, Singapore | NCT05038696 |
| R/R T-cell malignancies | ||||
| CD4 CAR-T cells | I | iCell Gene Therapeutics | NCT04162340 | |
| Humanized CD7 CAR-T cells | II | The First Affiliated Hospital of Soochow University | NCT05059912 | |
| CD4-specific CAR-T Cells | I | iCell Gene Therapeutics | NCT04162340 | |
| R/R B-cell Malignancies | ||||
| CD19+-Targeted CAR-T cells | I/II | Chongqing Precision Biotech Co., Ltd. | NCT04271410 | |
| Cord-Blood-Derived CAR-T cells | I | Henan Cancer Hospital | NCT03881774 | |
| R/R Immune Thrombocytopenia | ||||
| Anti-BCMA CAR-T cells | II | The First Affiliated Hospital of Soochow University | NCT05315778 | |
| Refractory SLE | ||||
| CD19-BCMA CAR-T cells | I | RenJi Hospital | NCT05858684 | |
| Recurrent Glioblastoma | ||||
| Tris-CAR-T cells | Phase | Beijing Tiantan Hospital | NCT05577091 | |
| Advanced Malignant Solid Tumors | ||||
| TIL Therapy | I | Shanghai Juncell Therapeutics | NCT05417750 | |
| TIL Therapy | I | Shanghai Juncell Therapeutics | NCT06488950 | |
| TIL Therapy | I | Hervor Therapeutics | NCT06334783 | |
| TIL Therapy | I | AgonOx, Inc. | NCT05902520 | |
| TIL Therapy | I | Shanghai Juncell Therapeutics | NCT04967833 | |
| Pediatric High Risk Solid Tumors | TIL Therapy | I | Johns Hopkins All Children’s Hospital | NCT06047977 |
| Advanced Melanoma | ||||
| TIL Therapy | II | Shanghai Juncell Therapeutics | NCT06703398 | |
| TIL Therapy | I/II | Shanghai Juncell Therapeutics | NCT06120712 | |
| TIL Therapy | I | Shanghai Juncell Therapeutics | NCT05098184 | |
| Advanced Gynecologic Tumors | ||||
| TIL Therapy | I | Shanghai Juncell Therapeutics | NCT05098171 | |
| Membrane-Bound Cytokine-Modified TIL | I | Shanghai Juncell Therapeutics | NCT05468307 | |
| R/R Gynecologic Tumors | TIL Therapy | I | Shanghai 10th People’s Hospital | NCT04766320 |
| NSCLC’s | ||||
| TIL Therapy | Ib | Shanghai Juncell Therapeutics | NCT06473961 | |
| TIL Therapy | II | University Hospital, Basel, Switzerland | NCT06455917 | |
| Type of Cancer | Target Antigen | Clinical Trial Number | Reference |
|---|---|---|---|
| Melanoma | Melanoma Antigen Recognized by T cells-1 (MART-1) | NCT00910650 | [104] |
| New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1) | NCT01967823 | ||
| Melanoma-Associated Antigen (MAGE)-A3 | NCT02111850 | ||
| Pancreatic Cancer | KRAS G12D | NCT03935893 | [105] |
| Human Papilloma Virus (HPV)-associated epithelial cancer | E6 and E7 antigens of HPVs | NCT02858310 | [106] |
| Non-small-cell lung cancer | MAGE-A10 | NCT02592577 | [107] |
| Ovarian cancer | Mesothelin | - | [108] |
| Sarcoma | NY-ESO-1 | - | [109] |
| Renal Cell Carcinoma | TNF-related apoptosis-inducing ligand (TRAIL) | NCT00923390 | [110] |
| Esophageal Cancer | MAGE-A4 | - | [111] |
| Colorectal Cancer | Carcinoembryonic antigen (CEA) | - | [112] |
| Characteristic | CAR-T-Cell Therapy | TIL Therapy | TCR Therapy |
|---|---|---|---|
| Target | Surface proteins (e.g., CD19, BCMA) | Neoantigens or tumor-specific antigens | Intracellular peptides bound to MHC |
| Specificity | Low | High | High |
| MHC Dependency | No | Yes | Yes |
| Tumor Types Treated | Hematological malignancies (e.g., B-cell leukemia, lymphoma) | Solid tumor, including melanoma, ovarian, cervical, etc. | Solid tumors and hematologic malignancies |
| Time to Prepare | Several weeks (complex engineering) | Several weeks (tumor extraction and expansion) | Several weeks (engineering and selection of TCRs) |
| Toxicity | CRS, neurotoxicity | IL-2-related toxicity. Hypotension, nausea | Off-target effects, CRS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabbani, S.A.; El-Tanani, M.; El-Tanani, Y.; Kumar, R.; Sharma, S.; Khan, M.A.; Parvez, S.; Aljabali, A.A.A.; Matalka, M.I.; Rizzo, M. Advances in Adoptive Cell Therapies in Cancer: From Mechanistic Breakthroughs to Clinical Frontiers and Overcoming Barriers. Med. Sci. 2025, 13, 190. https://doi.org/10.3390/medsci13030190
Rabbani SA, El-Tanani M, El-Tanani Y, Kumar R, Sharma S, Khan MA, Parvez S, Aljabali AAA, Matalka MI, Rizzo M. Advances in Adoptive Cell Therapies in Cancer: From Mechanistic Breakthroughs to Clinical Frontiers and Overcoming Barriers. Medical Sciences. 2025; 13(3):190. https://doi.org/10.3390/medsci13030190
Chicago/Turabian StyleRabbani, Syed Arman, Mohamed El-Tanani, Yahia El-Tanani, Rakesh Kumar, Shrestha Sharma, Mohammad Ahmed Khan, Suhel Parvez, Alaa A. A. Aljabali, Mohammad I. Matalka, and Manfredi Rizzo. 2025. "Advances in Adoptive Cell Therapies in Cancer: From Mechanistic Breakthroughs to Clinical Frontiers and Overcoming Barriers" Medical Sciences 13, no. 3: 190. https://doi.org/10.3390/medsci13030190
APA StyleRabbani, S. A., El-Tanani, M., El-Tanani, Y., Kumar, R., Sharma, S., Khan, M. A., Parvez, S., Aljabali, A. A. A., Matalka, M. I., & Rizzo, M. (2025). Advances in Adoptive Cell Therapies in Cancer: From Mechanistic Breakthroughs to Clinical Frontiers and Overcoming Barriers. Medical Sciences, 13(3), 190. https://doi.org/10.3390/medsci13030190








