Reactive Oxygen and Nitrogen Species in Myocardial Infarction: Mechanistic Insights and Clinical Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Analysis
2.2.1. Sample Collection Procedures
2.2.2. Measurements Procedures for ROS
2.2.3. Measurements Procedures for RNS
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Hematological Tests and Lipid Profile
3.3. Reactive Oxygen and Nitrogen Species Serum Levels
3.4. Correlation Between the ROS and RNS and Biochemical Markers/Echocardiographic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljefree, N.; Ahmed, F. Prevalence of Cardiovascular Disease and Associated Risk Factors among Adult Population in the Gulf Region: A Systematic Review. Adv. Public Health 2015, 2015, 235101. [Google Scholar] [CrossRef]
- Alzahrani, A.; Mufti, H.; Alswat, A.; Altirkistani, B.; Aljehani, M.; Jazzar, A.; Alutaibi, F.; Abushouk, A.; Al Rahimi, J.; Al Kashkari, W.; et al. The Impact of Viability Assessment Using Cardiac MRI and Echocardiogram on the Outcome of Revascularization in Patients with Multi-Vessels Coronary Artery Disease and Moderate to Severe Ischemic Cardiomyopathy. Saudi Med. J. 2023, 44, 373–378. [Google Scholar] [CrossRef]
- Alshahrani, H.; McConkey, R.; Wilson, J.; Youssef, M.; Fitzsimons, D. Female Gender Doubles Pre-Hospital Delay Times for Patients Experiencing ST Segment Elevation Myocardial Infarction in Saudi Arabia. Eur. J. Cardiovasc. Nurs. 2013, 13, 399–407. [Google Scholar] [CrossRef]
- Moldovan, N.I.; Anghelina, M.; Varadharaj, S.; Butt, O.I.; Wang, T.; Yang, F.; Moldovan, L.; Zweier, J.L. Reoxygenation-Derived Toxic Reactive Oxygen/Nitrogen Species Modulate the Contribution of Bone Marrow Progenitor Cells to Remodeling After Myocardial Infarction. J. Am. Heart Assoc. 2025, 3, e000471. [Google Scholar] [CrossRef]
- Wróbel-Nowicka, K.; Wojciechowska, C.; Jacheć, W.; Zalewska, M.; Romuk, E. The Role of Oxidative Stress and Inflammatory Parameters in Heart Failure. Medicina 2024, 60, 760. [Google Scholar] [CrossRef]
- Kuster, G.M.; Häuselmann, S.P.; Rosc-Schlüter, B.I.; Lorenz, V.; Pfister, O. Reactive Oxygen/Nitrogen Species and the Myocardial Cell Homeostasis: An Ambiguous Relationship. Antioxid. Redox Signal. 2010, 13, 1899–1910. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Li, H.; Chai, S.; Zhang, L.; Fan, T.; Hu, Z.; Feng, Y. The Relationship between Cardiac Oxidative Stress, Inflammatory Cytokine Response, Cardiac Pump Function, and Prognosis Post-Myocardial Infarction. Sci. Rep. 2024, 14, 8985. [Google Scholar] [CrossRef] [PubMed]
- Vara, D.; Pula, G. Reactive Oxygen Species: Physiological Roles in the Regulation of Vascular Cells. Curr. Mol. Med. 2014, 14, 1103–1125. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Hamza, A.-B. Reactive Oxygen and Nitrogen Species in Patients with Rheumatoid Arthritis as Potential Biomarkers for Disease Activity and the Role of Antioxidants. Free Radic. Biol. Med. 2016, 97, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Munno, M.; Mallia, A.; Greco, A.; Modafferi, G.; Banfi, C.; Eligini, S. Radical Oxygen Species, Oxidized Low-Density Lipoproteins, and Lectin-like Oxidized Low-Density Lipoprotein Receptor 1: A Vicious Circle in Atherosclerotic Process. Antioxidants 2024, 13, 583. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Enzymatic Antioxidant System in Vascular Inflammation and Coronary Artery Disease. World J. Exp. Med. 2015, 5, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, H.E.; Plastino, J.A.; Escudero, E.M.; Mangal, B.; Brown, J.; Pérez, N.G. The Effect of Xanthine Oxidase Inhibition Upon Ejection Fraction in Heart Failure Patients: La Plata Study. J. Card. Fail. 2006, 12, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.; Radosavljevic, J.; Pavlovic, D.; Cvetkovic, V.; Miladinovic, T.; Milinkovic, D.; Stojanovic, M.; Stojanovic, D. Oxidative Stress in Acute Myocardial Infarction Patients. Exp. Appl. Biomed. Res. 2024, 25, 51–56. [Google Scholar] [CrossRef]
- Aladağ, N.; Asoğlu, R.; Ozdemir, M.; Asoğlu, E.; Derin, A.R.; Demir, C.; Demir, H. Oxidants and Antioxidants in Myocardial Infarction (MI): Investigation of Ischemia Modified Albumin, Malondialdehyde, Superoxide Dismutase and Catalase in Individuals Diagnosed with ST Elevated Myocardial Infarction (STEMI) and Non-STEMI (NSTEMI). J. Med. Biochem. 2021, 40, 286–294. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Zhou, M.; Diwu, Z.; Panchuk-Voloshina, N.; Haugland, R.P. A Stable Nonfluorescent Derivative of Resorufin for the Fluorometric Determination of Trace Hydrogen Peroxide: Applications in Detecting the Activity of Phagocyte NADPH Oxidase and Other Oxidases. Anal. Biochem. 1997, 253, 162–168. [Google Scholar] [CrossRef]
- Manevich, Y.; Held, K.D.; Biaglow, J.E. Coumarin-3-Carboxylic Acid as a Detector for Hydroxyl Radicals Generated Chemically and by Gamma Radiation. Radiat. Res. 1997, 148, 580–591. [Google Scholar] [CrossRef]
- Benov, L.; Sztejnberg, L.; Fridovich, I. Critical Evaluation of the Use of Hydroethidine as a Measure of Superoxide Anion Radical. Free Radic. Biol. Med. 1998, 25, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: Photochemical Behavior in Solution and in a Mammalian Cell. Photochem. Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of Novel Fluorescence Probes That Can Reliably Detect Reactive Oxygen Species and Distinguish Specific Species*210. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an Oxidation-Sensitive Fluorescent Lipid Peroxidation Probe: (Micro)Spectroscopic Characterization and Validation of Methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Detection and Imaging of Nitric Oxide with Novel Fluorescent Indicators: Diaminofluoresceins. Anal. Chem. 1998, 70, 2446–2453. [Google Scholar] [CrossRef]
- Nussler, A.K.; Glanemann, M.; Schirmeier, A.; Liu, L.; Nüssler, N.C. Fluorometric Measurement of Nitrite/Nitrate by 2,3-Diaminonaphthalene. Nat. Protoc. 2006, 1, 2223–2226. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Publishing Co.: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular Reactive Oxygen Species (ROS) in Cardiovascular Pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K.K. Reactive Oxygen Species in the Vasculature. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef]
- Barry-Lane, P.A.; Patterson, C.; van der Merwe, M.; Hu, Z.; Holland, S.M.; Yeh, E.T.H.; Runge, M.S. P47phox Is Required for Atherosclerotic Lesion Progression in ApoE–/– Mice. J. Clin. Investig. 2001, 108, 1513–1522. [Google Scholar] [CrossRef][Green Version]
- Zheng, H.; Xu, Y.; Liehn, E.A.; Rusu, M. Vitamin C as Scavenger of Reactive Oxygen Species during Healing after Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 3114. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Hardy, M.; Podsiadly, R.; Cheng, G.; Zielonka, J. Recent Developments in Detection of Superoxide Radical Anion and Hydrogen Peroxide: Opportunities, Challenges, and Implications in Redox Signaling. Arch. Biochem. Biophys. 2017, 617, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Babuin, L.; Jaffe, A.S. Troponin: The Biomarker of Choice for the Detection of Cardiac Injury. Can. Med. Assoc. J. 2005, 173, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.D.; Newman, K.P.; Weber, K.T.; Ramanathan, K.B.; McGee, J.E. Oxidative Stress and Cardiomyocyte Necrosis With Elevated Serum Troponins: Pathophysiologic Mechanisms. Am. J. Med. Sci. 2011, 342, 129–134. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Chen, W.; Xu, S.; Feng, X.; Zhang, L. Natural Products: The Role and Mechanism in Low-Density Lipoprotein Oxidation and Atherosclerosis. Phytother. Res. 2021, 35, 2945–2967. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, D.; Xu, R.-X.; Guo, Y.-L.; Zhu, C.-G.; Wu, N.-Q.; Zhang, Y.; Li, S.; Gao, Y.; Liu, G.; et al. Low-Density Lipoprotein-Associated Variables and the Severity of Coronary Artery Disease: An Untreated Chinese Cohort Study. Biomarkers 2018, 23, 647–653. [Google Scholar] [CrossRef]
- Rajesh, K.G.; Surekha, R.H.; Mrudula, S.K.; Prasad, Y.; Sanjib, K.S.; Prathiba, N. Oxidative and Nitrosative Stress in Association with DNA Damage in Coronary Heart Disease. Singapore Med. J. 2011, 52, 283–288. [Google Scholar]
- Rho, Y.H.; Chung, C.P.; Oeser, A.; Solus, J.F.; Gebretsadik, T.; Shintani, A.; Raggi, P.; Milne, G.L.; Stein, C.M. Interaction between Oxidative Stress and High-Density Lipoprotein Cholesterol Is Associated with Severity of Coronary Artery Calcification in Rheumatoid Arthritis. Arthritis Care Res. 2010, 62, 1473–1480. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; Giugliano, R.P.; Burton, P.B.J.; Murphy, S.A.; McCabe, C.H.; Gibson, C.M.; Braunwald, E. Association of Hemoglobin Levels with Clinical Outcomes in Acute Coronary Syndromes. Circulation 2005, 111, 2042–2049. [Google Scholar] [CrossRef]
- Luna-Marco, C.; de Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 Receptor Agonists on Mitochondrial Function, Inflammatory Markers and Leukocyte-Endothelium Interactions in Type 2 Diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Madaudo, C.; Coppola, G.; Parlati, A.L.; Corrado, E. Discovering Inflammation in Atherosclerosis: Insights from Pathogenic Pathways to Clinical Practice. Int. J. Mol. Sci. 2024, 25, 6016. [Google Scholar] [CrossRef]
- Diaba-Nuhoho, P.; Mittag, J.; Brunssen, C.; Morawietz, H.; Brendel, H. The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue. Antioxidants 2024, 13, 503. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic Cardiomyopathy Revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.F.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The Cardioprotective Effect of an Aqueous Extract of Fermented Rooibos (Aspalathus Linearis) on Cultured Cardiomyocytes Derived from Diabetic Rats. Phytomedicine 2014, 21, 595–601. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-Induced Oxidative Stress and Heart Disease-Cardioprotective Effects of Rooibos Flavonoids and Phenylpyruvic Acid-2-O-β-D-Glucoside. Nutr. Metab. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, P.; Kallikourdis, M.; Emig, R.; Prabhu, S.D. Myocardial Inflammation in Heart Failure With Reduced and Preserved Ejection Fraction. Circ. Res. 2024, 134, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Akki, A.; Zhang, M.; Murdoch, C.; Brewer, A.; Shah, A.M. NADPH Oxidase Signaling and Cardiac Myocyte Function. J. Mol. Cell. Cardiol. 2009, 47, 15–22. [Google Scholar] [CrossRef]
- Borchi, E.; Bargelli, V.; Stillitano, F.; Giordano, C.; Sebastiani, M.; Nassi, P.A.; d’Amati, G.; Cerbai, E.; Nediani, C. Enhanced ROS Production by NADPH Oxidase Is Correlated to Changes in Antioxidant Enzyme Activity in Human Heart Failure. Biochim. Biophys. Acta-Mol. Basis Dis. 2010, 1802, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, M.; Li, T.; Su, Z.; Zhu, Z.; Dou, C.; Liu, Y.; Pei, H.; Yang, J.; Ye, H.; et al. Honokiol Ameliorates Post-Myocardial Infarction Heart Failure Through Ucp3-Mediated Reactive Oxygen Species Inhibition. Front. Pharmacol. 2022, 13, 811682. [Google Scholar]
- Xu, X.; Jiang, T.; Li, Y.; Kong, L. Endostatin Attenuates Heart Failure via Inhibiting Reactive Oxygen Species in Myocardial Infarction Rats. Biosci. Rep. 2021, 41, BSR20200787. [Google Scholar] [CrossRef] [PubMed]


| Variables | Participants (n = 146) | p-Value a | |
|---|---|---|---|
| MI Patients (n = 86) (%) | Control (n = 60) (%) | ||
| Age: | 0.48 (NS) | ||
| Median | 57.0 | 53.0 | |
| IQR | 15.5 | 10.5 | |
| Gender: | 0.49 (NS) | ||
| Male | 66 (76.7%) | 43 (71.7%) | |
| Female | 20 (23.3%) | 17 (28.3%) | |
| Smoking: | 0.14 (NS) | ||
| No | 50 (58.1%) | 42 (70%) | |
| Yes | 36 (41.9%) | 18 (30%) | |
| Family History of CAD: | 0.90 (NS) | ||
| No | 71 (82.1%) | 50(83.3%) | |
| Yes | 15 (17.9%) | 10 (16.7%) | |
| History of DM: | |||
| No | 39 (45.3%) | 0 (0%) | N/A |
| Yes | 47 (54.7%) | 0 (0%) | N/A |
| History of hypertension: | |||
| No | 37 (43%) | 0 (0%) | N/A |
| Yes | 49 (57%) | 0 (0%) | N/A |
| Parameters | MI Patients (n = 86) Median ± IQR | Control (n = 60) Median ± IQR | p-Value * |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 125 (110–140) | 117 (110–125) | 0.001 (HS) |
| Diastolic blood pressure (mmHg) | 73 (68–80) | 75 (70–80) | 0.62 (NS) |
| BMI | 28 (25–32) | 27 (24–30) | 0.13 (NS) |
| Glucose Level, random (mg/dL) | 151 (120–200) | 117 (110–135) | 0.004 (S) |
| Total cholesterol (mmol/L) | 5 (4.2–6) | 3.04 (2.8–3.5) | <0.001(HS) |
| LDL-cholesterol (mmol/L) | 3 (2.2–3.8) | 2 (1.6–2.4) | <0.001 (HS) |
| HDL-cholesterol (mmol/L) | 1 (0.9–1.1) | 1.16 (1.1–1.2) | <0.001 (HS) |
| Triglycerides (mmol/L) | 1 (0.8–1.8) | 1.04 (0.8–1.4) | 0.04 (S) |
| Hb (g/dL) | 14 (12.5–15.7) | 11 (10.7–11.9) | <0.001 (HS) |
| RBCs (106/uL) | 5 (4.5–5.6) | 4.8 (4.4–5.1) | 0.08 (NS) |
| Total WBCs (103/uL) | 10 (8–13) | 7.3 (6.4–8) | <0.001 (HS) |
| Platelets (103/uL) | 262 (220–310) | 212.5 (200–230) | <0.001 (HS) |
| Troponin (ng/mL) | 15.72 + 4.2 (15.5) | - | N/A |
| LV-EF (%) | 45 (40–50) | - | N/A |
| MI Patients (n = 86) Median ± IQR | Control (n = 60) Median ± IQR | p-Value * | |
|---|---|---|---|
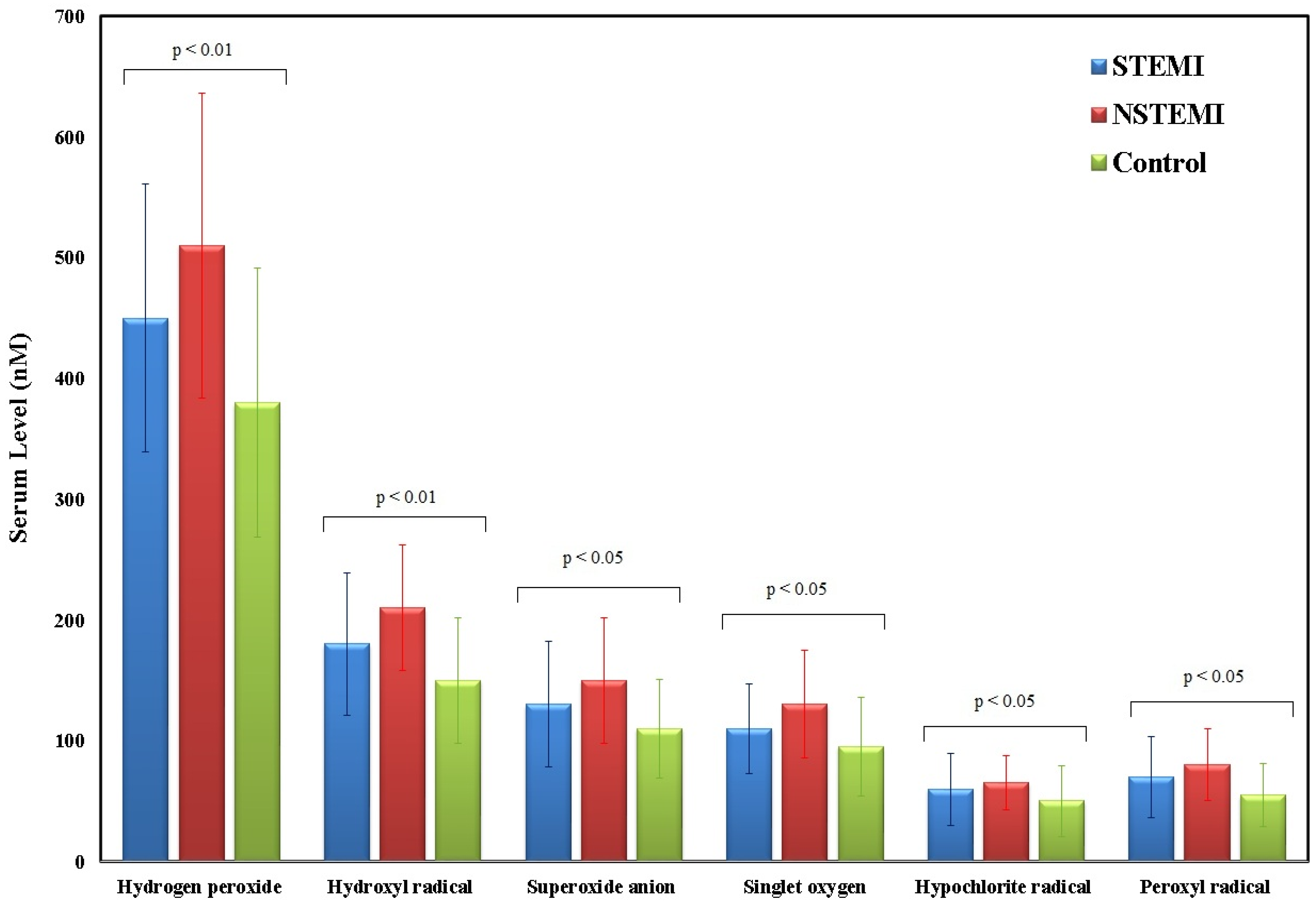
| Reactive oxygen species | |||
| Hydrogen peroxide (nM) | 531 (420–620) | 352 (280–450) | 0.008 (S) |
| Hydroxyl radical (nM) | 217 (180–260) | 154 (120–200) | ≤0.001 (HS) |
| Superoxide anion (nM) | 161 (120–200) | 103.5 (80–130) | ≤0.001(HS) |
| Singlet oxygen (nM) | 135 (100–170) | 91.5 (70–120) | 0.005 (S) |
| Hypochlorite radical (nM) | 62.5 (40–85) | 36 (20–65) | 0.01 (S) |
| Peroxyl radical (nM) | 74.5 (50–100) | 45.5 (30–70) | 0.002 (S) |
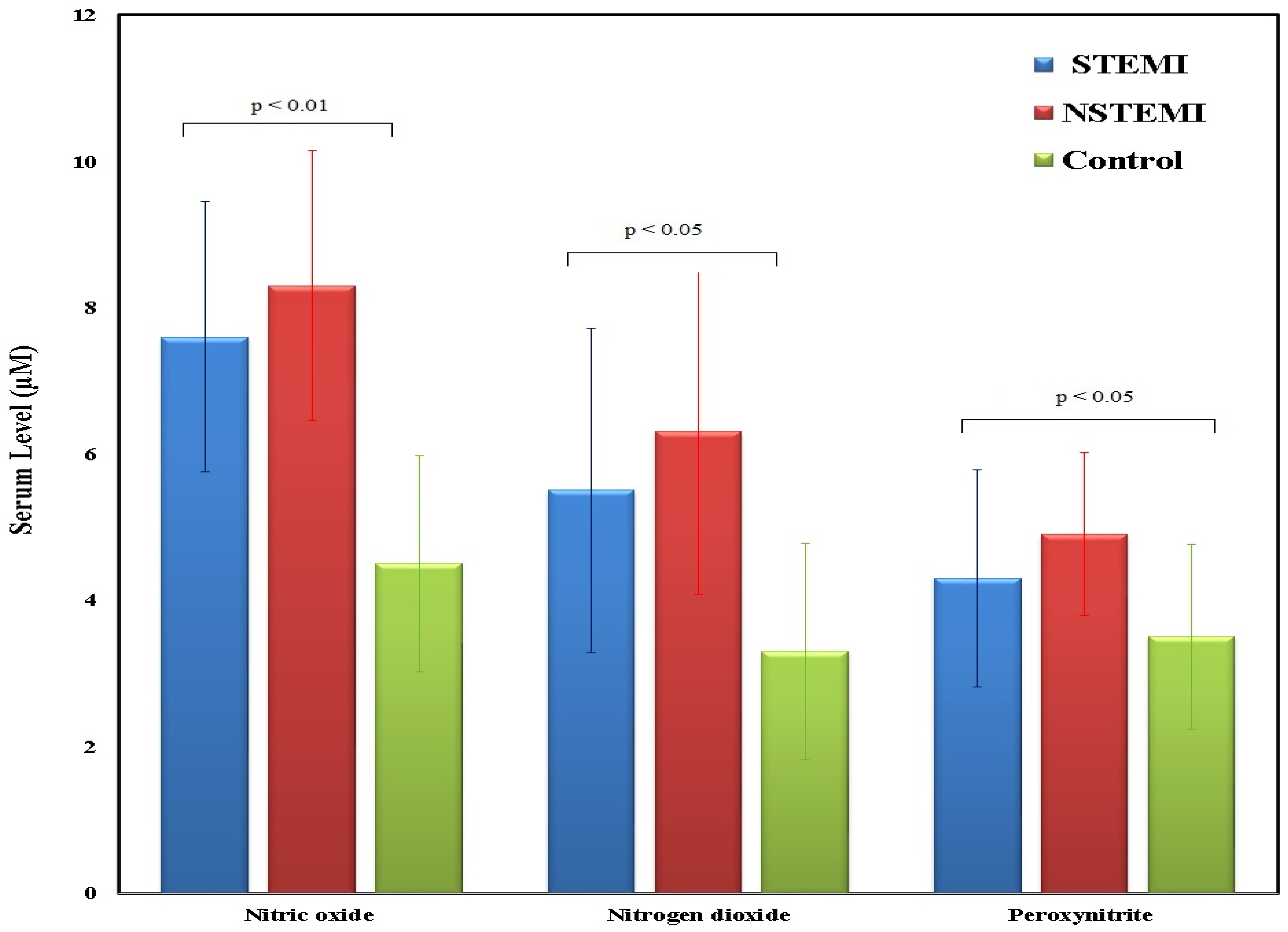
| Reactive nitrogen species | |||
| Nitric oxide (µM) | 12.80 (10–15) | 8.7 (6.5–11) | ≤0.001 (HS) |
| Nitrogen dioxide(µM) | 6.45 (5.5–7.5) | 6.15 (4.5–7.2) | 0.315(NS) |
| Peroxynitrite (µM) | 6.85 (6–7.5) | 4.95 (4–6) | ≤0.001 (HS) |
| Reactive Species | LDL-C | Total Cholesterol | Glucose Level (Random) | Troponin | LV-EF% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Reactive oxygen species | ||||||||||
| Hydrogen peroxide (nM) | 0.610 | <0.001(HS) | 0.492 | <0.001(HS) | −0.06 | 0.48(NS) | 0.121 | 0.27(NS) | 0.19 | 0.08(NS) |
| Hydroxyl radical (nM) | 0.647 | <0.001(HS) | 0.627 | <0.001(HS) | −0.05 | 0.49(NS) | 0.169 | 0.12(NS) | 0.19 | 0.07(NS) |
| Superoxide anion (nM) | 0.602 | <0.001(HS) | 0.576 | <0.001(HS) | −0.110 | 0.19(NS) | 0.146 | 0.18(NS) | 0.22 | 0.05(S) |
| Singlet oxygen (nM) | 0.444 | <0.001(HS) | 0.484 | <0.001(HS) | −0.111 | 0.18(NS) | 0.121 | 0.27(NS) | 0.16 | 0.14(NS) |
| Hypochlorite radical (nM) | 0.343 | <0.001(HS) | 0.425 | <0.001(HS) | −0.14 | 0.08(NS) | 0.09 | 0.37(NS) | 0.08 | 0.46(NS) |
| Peroxyl radical (nM) | 0.371 | <0.001(HS) | 0.465 | <0.001(HS) | −0.10 | 0.21(NS) | 0.09 | 0.37(NS) | 0.08 | 0.44(NS) |
| Reactive nitrogen species | ||||||||||
| Nitric oxide (µM) | 0.321 | <0.001(HS) | 0.337 | <0.001(HS) | 0.123 | 0.18(NS) | −0.259 | 0.05(S) | 0.15 | 0.73(NS) |
| Nitrogen dioxide(µM) | 0.134 | 0.148(NS) | 0.045 | 0.626(NS) | 0.068 | 0.46(NS) | −0.322 | 0.01(S) | 0.08 | 0.52(NS) |
| Peroxynitrite (µM) | 0.320 | <0.001(HS) | 0.270 | 0.003(S) | 0.10 | 0.27(NS) | −0.261 | 0.05(S) | 0.11 | 0.42(NS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, H.M.; Ahmed, S.A.; Alsaedi, A.M.; Almaramhy, W.H.; Alraddadi, M.K.; Albadrani, M.S.; Alhejaily, I.M.; Mohammad, F.A.; Ghaith, A.M.; Youssef, A.A. Reactive Oxygen and Nitrogen Species in Myocardial Infarction: Mechanistic Insights and Clinical Correlations. Med. Sci. 2025, 13, 152. https://doi.org/10.3390/medsci13030152
Ismail HM, Ahmed SA, Alsaedi AM, Almaramhy WH, Alraddadi MK, Albadrani MS, Alhejaily IM, Mohammad FA, Ghaith AM, Youssef AA. Reactive Oxygen and Nitrogen Species in Myocardial Infarction: Mechanistic Insights and Clinical Correlations. Medical Sciences. 2025; 13(3):152. https://doi.org/10.3390/medsci13030152
Chicago/Turabian StyleIsmail, Hussein M., Sameh A. Ahmed, Ahmed M. Alsaedi, Waleed H. Almaramhy, Man K. Alraddadi, Muhannad S. Albadrani, Ibraheam M. Alhejaily, Faisal A. Mohammad, Anas M. Ghaith, and Ali A. Youssef. 2025. "Reactive Oxygen and Nitrogen Species in Myocardial Infarction: Mechanistic Insights and Clinical Correlations" Medical Sciences 13, no. 3: 152. https://doi.org/10.3390/medsci13030152
APA StyleIsmail, H. M., Ahmed, S. A., Alsaedi, A. M., Almaramhy, W. H., Alraddadi, M. K., Albadrani, M. S., Alhejaily, I. M., Mohammad, F. A., Ghaith, A. M., & Youssef, A. A. (2025). Reactive Oxygen and Nitrogen Species in Myocardial Infarction: Mechanistic Insights and Clinical Correlations. Medical Sciences, 13(3), 152. https://doi.org/10.3390/medsci13030152







