Understanding Preeclampsia: Cardiovascular Pathophysiology, Histopathological Insights and Molecular Biomarkers
Abstract
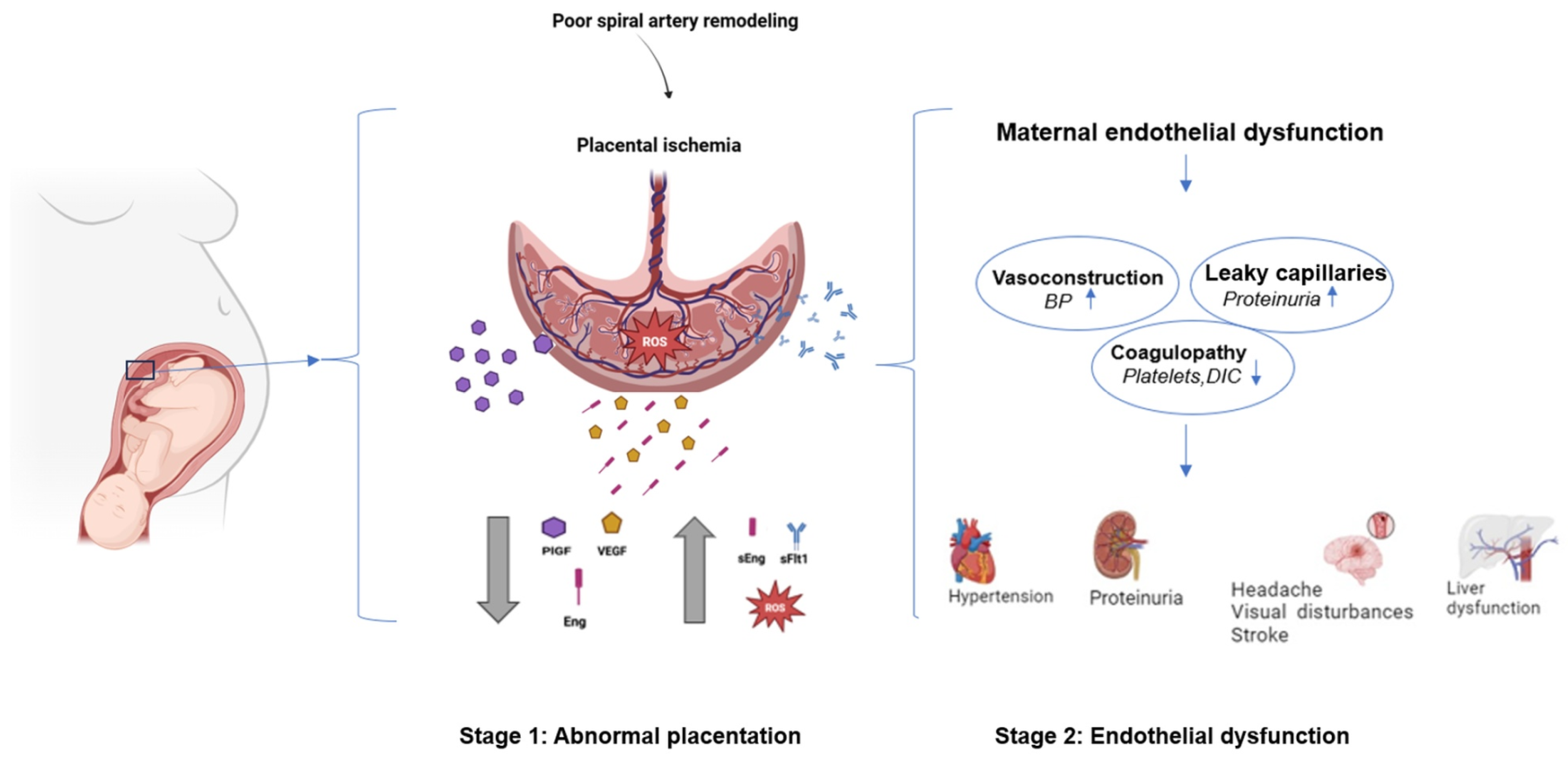
1. Introduction
2. Results
2.1. Cardiovascular Pathophysiology of PE
2.1.1. Abnormal Spiral Artery Remodeling
2.1.2. Endothelial Dysfunction and Vascular Inflammation
2.1.3. Arterial Stiffness: Another Key Feature of the Maternal Cardiovascular Maladaptation in PE
2.1.4. Retinal Vascular Alterations
2.1.5. Hemodynamic Changes and Cardiac Function
2.1.6. Systemic Vascular Injury Beyond the Cardiovascular System
2.2. Histopathological Insights: Placental and Vascular Lesions
2.2.1. Inadequate Spiral Artery Remodeling and Acute Atherosis
2.2.2. Villous Maldevelopment and Inflammatory Cell Infiltrates
2.2.3. Glomerular Endotheliosis, Cardiac and Cerebral Changes
2.3. Molecular and Cellular Mechanisms of PE
2.3.1. Angiogenic Imbalance: A Central Pathway in Disease Progression
2.3.2. Oxidative Stress: Fueling Vascular Injury
2.3.3. Immune Dysregulation: A Breakdown of Tolerance
2.3.4. Vascular Dysfunction: NO, ADMA, and Angiotensin Sensitivity
2.3.5. Neuroendocrine Stress: The Role of Cortisol
| Mechanism | Key Features | Key Molecules/Markers | Clinical Relevance | Reference No. |
|---|---|---|---|---|
| Angiogenic Imbalance | Disruption between pro- and anti-angiogenic factors | increased: sFlt-1, sEng decreased: PlGF, | sFlt-1/PlGF ratio > 38 is predictive of PE; sEng elevated in severe PE and HELLP | [5,25,27,28] |
| Oxidative Stress | Increased ROS from placental ischemia; impaired antioxidant defenses | increased: MDA, 3-nitrotyrosine, ADMA decreased: SOD, GPx | Promotes endothelial dysfunction and reduces nitric oxide availability; therapeutic target | [26,27,28] |
| Immune Dysregulation | Pro-inflammatory shift and breakdown of maternal–fetal tolerance. | increased: TNF-α, IL-6, NETs, AT1-AA, Th17, miR-210/miR-155 decreased: Tregs, | Leads to inflammation, poor placentation, and vascular dysfunction; potential for immunotherapy | [65,66,67,68,69] |
| Vascular Dysfunction | Endothelial injury and abnormal vasoregulation. | increased: ADMA, Thromboxane, AT1-AA decreased: eNOS, prostacyclin, | Results in vasoconstriction, hypertension, and platelet aggregation | [24,32,33,34] |
| Epigenetics and Transcriptomics | Changes in gene expression and DNA methylation in placental tissue | increased: Hypermethylation of PGF, NOS3; leptin, INHBA, sFlt-1; miR-210, miR-155 | Molecular markers of placental stress and dysfunction; potential for early diagnostics | [23,25,68,69] |
2.4. Biomarkers and Molecular Diagnostics of PE
2.4.1. Acknowledged Clinical Biomarkers: Angiogenic Factors
2.4.2. Promising Biomarkers: Expanding the Diagnostic Toolkit
2.4.3. Biophysical and Imaging Markers
| Biomarker | Change in PE | Clinical/Pathophysiological Significance | Evidence (Reference No.) |
|---|---|---|---|
| sFlt-1/PlGF ratio | increased: sFlt-1, decreased: PlGF | Indicates anti-angiogenic imbalance and placental dysfunction; widely used in diagnosis. | [27,74,75] |
| ADMA (Asymmetric Dimethylarginine) | increased | Endogenous NOS inhibitor; impairs endothelial function via reduced nitric oxide. | [29,30,31] |
| PP13 (Placental Protein 13) | decreased | Marker of impaired trophoblast invasion and early placental development. | [76,77,78] |
| miRNAs (e.g., miR-210, miR-155) | increased/decreased (type-dependent) | Reflect hypoxia and immune dysregulation; miR-210 is hypoxia-associated. | [68,69,70] |
| NGAL (Neutrophil Gelatinase-Associated Lipocalin) | increased | Early marker of renal tubular stress in PE. | [92,93,94,95,96] |
| Neprilysin (sNEP) | increased | Degrades vasoactive peptides; may influence vascular tone in PE. | [53,97,98] |
| Inflammatory cytokines (TNF-α, IL-6) | increased | Reflect systemic inflammation; TNF-α induces anti-angiogenic factors. | [68,69,70] |
| Oxidative stress markers (MDA, 8-isoprostane) | increased | Oxidative stress markers linked to placental ischemia. | [86,87,88,89,90,91] |
| Uric acid | increased | Associated with renal dysfunction and oxidative stress. | [86,87] |
| Vitamin D [25(OH)D] | decreased | Deficiency may impair angiogenesis and immune regulation. | [79,80,81,82] |
| PAPP-A (Pregnancy-Associated Plasma Protein A) | decreased | Early screening marker; linked to placental dysfunction. | [101,102,103] |
| Retinal microvasculature (CRAE/CRVE) | increased CRAE decreased CRVE | Non-invasive indicator of systemic microvascular dysfunction. | [40,41,42,48,50] |
| Pulse wave velocity (PWV) | increased | Indicates increased arterial stiffness before clinical PE onset. | [33,34,35,36] |
2.5. Risk Factors and High-Risk Populations in PE
2.5.1. Established Clinical Risk Factors
| Risk Factor | Relative Risk (RR)/Effect Estimate | Comment | Reference No. |
|---|---|---|---|
| History of PE | RR 8–9× | Recurrence rate up to 30–50% after two prior episodes | [4,5,6] |
| Chronic Hypertension | RR ~5× | Established independent risk factor | [6,24,90,104] |
| Chronic Kidney Disease | Not quantified | Consistently associated with increased risk | [6,24,92] |
| Type 1 and 2 Diabetes Mellitus | RR ~3–4× | Pre-existing DM carries higher risk than GDM | [1,6,105] |
| Gestational Diabetes | Modest increase | Risk lower than in pregestational DM | [1,6,105] |
| Autoimmune Disorders (e.g., APS, SLE) | Up to 17% incidence | Especially high in antiphospholipid syndrome | [1,6,106] |
| Nulliparity | RR ~2× | Baseline risk for first pregnancy | [1,5,6,25,26] |
| New Paternity | RR ~2× | Risk comparable to first pregnancy with new partner | [1,5,6,25,26] |
| Obesity (BMI > 30) | RR ~2–3× | Risk increases proportionally with BMI | [1,4,5,6,107] |
| Multiple Gestation | RR ~2–3× | Dose-dependent with number of fetuses | [1,5,6,25,26,108] |
| Advanced Maternal Age (>35, esp. >40) | Increased (not quantified) | Possibly related to endothelial aging | [1,4,5,6,109] |
| Family History (mother/sibling) | RR ~2× | Suggests genetic and shared environmental factor | [1,5,105] |
| Assisted Reproduction (IVF, donor egg) | Elevated risk (not quantified) | Possibly due to immune and placental factors | [1,4,5,6,110] |
| Ethnicity (e.g., Black women) | Increased (qualitative) | Even after adjusting for comorbidities | [1,4,5,6] |
| Low Socioeconomic Status | Increased (qualitative) | May reflect access to care, chronic stress | [1,4,5,6] |
| Psychosocial Stress | Increased (qualitative) | Elevated hair cortisol linked with early PE | [1,5,6,25] |
| Smoking During Pregnancy | Complex/conflicting | Not protective; not recommended | [111,112,113,114,115] |
2.5.2. Additional Risk Modifiers
2.5.3. High-Risk Stratification in Clinical Guidelines
2.5.4. Integration of Machine Learning and Digital Tools in PE Risk Prediction
2.6. Prevention Strategies for PE
2.6.1. Low-Dose Aspirin Prophylaxis
2.6.2. Lifestyle and Behavioral Interventions
3. Discussion
4. Materials and Methods
4.1. Literature Search Strategy
- Cardiovascular mechanisms (e.g., “endothelial dysfunction”, “arterial stiffness”, “hemodynamic”, “heart disease”),
- Placental and histopathological features (e.g., “spiral arteries”, “malperfusion”, “acute atherosis”),
- Molecular biomarkers (e.g., “sFlt-1”, “PlGF”, “microRNA”, “metabolomics”, “hair cortisol”).
4.2. Inclusion and Exclusion Criteria
- Original research (clinical, basic science, and translational studies),
- Systematic reviews and meta-analyses,
- Clinical guidelines or consensus statements relevant to PE’s cardiovascular, placental, or biomarker aspects.
- Case reports,
- Abstracts without full-text availability,
- Non-peer-reviewed literature,
- Studies published in languages other than English.
- In vitro or animal experiments.
4.3. Study Selection and Review Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACOG | American College of Obstetricians and Gynecologists |
| ADMA | Asymmetric Dimethylarginine |
| AI | Artificial Intelligence |
| AMA | Advanced Maternal Age |
| APS | Antiphospholipid Syndrome |
| ASPRE | Aspirin for Evidence-Based PE Prevention (trial) |
| AT1-AA | Angiotensin II Type 1 Receptor Autoantibody |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CD46 | Cluster of Differentiation 46 |
| CNS | Central Nervous System |
| CRAE | Central Retinal Artery Equivalent |
| CRH | Corticotropin-Releasing Hormone |
| CRVE | Central Retinal Venular Equivalent |
| CVD | Cardiovascular Disease |
| COX-1 | Cyclooxygenase-1 |
| DDAH | Dimethylarginine Dimethylaminohydrolase |
| DIC | Disseminated Intravascular Coagulation |
| eNOS | Endothelial Nitric Oxide Synthase |
| EOPE | Early-Onset PE |
| FGR | Fetal Growth Restriction |
| FMD | Flow-Mediated Dilation |
| FMF | Fetal Medicine Foundation |
| GPx | Glutathione Peroxidase |
| HELLP | Hemolysis, Elevated Liver enzymes, Low Platelet count |
| HPA | Hypothalamic–Pituitary–Adrenal |
| IL-6 | Interleukin 6 |
| INHBA | Inhibin Subunit Beta A |
| ISSHP | International Society for the Study of Hypertension in Pregnancy |
| IVF | In Vitro Fertilization |
| MAP | Mean Arterial Pressure |
| MDA | Malondialdehyde |
| MeSH | Medical Subject Headings |
| ML | Machine Learning |
| miRNA | MicroRNA |
| MRI | Magnetic Resonance Imaging |
| NET | Neutrophil Extracellular Trap |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NO | Nitric Oxide |
| NOS3 | Nitric Oxide Synthase 3 |
| OCT-A | Optical Coherence Tomography Angiography |
| PAPP-A | Pregnancy-Associated Plasma Protein A |
| PCOS | Polycystic Ovary Syndrome |
| PE | PE |
| PIERS-ML | Preeclampsia Integrated Estimate of Risk with Machine Learning |
| PlGF | Placental Growth Factor |
| PP13 | Placental Protein 13 |
| PRES | Posterior Reversible Encephalopathy Syndrome |
| PWV | Pulse Wave Velocity |
| ROS | Reactive Oxygen Species |
| sEng | Soluble Endoglin |
| sFlt-1 | Soluble fms-like Tyrosine Kinase-1 |
| sNEP | Soluble Neprilysin |
| SLE | Systemic Lupus Erythematosus |
| SOD | Superoxide Dismutase |
| TGF-β | Transforming Growth Factor Beta |
| Th17 | T-helper 17 Cells |
| TNF-α | Tumor Necrosis Factor Alpha |
| Treg | Regulatory T Cell |
| USPSTF | United States Preventive Services Task Force |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization |
References
- American College of Obstetricians Gynecologists (ACOG). ACOGPractice Bulletin No 222: Gestational Hypertension, P.E. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. PE: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Sibai, B.M. Diagnosis and management of gestational hypertension and PE. Obstet. Gynecol. 2003, 102, 181–192. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A critical review of early-onset and late-onset PE. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef]
- Kovo, M.; Schreiber, L.; Ben-Haroush, A.; Gold, E.; Golan, A.; Bar, J. The placental component in early-onset and late-onset PE in relation to fetal growth restriction. Prenat. Diagn. 2012, 32, 632–637. [Google Scholar] [CrossRef]
- Kovo, M.; Schreiber, L.; Elyashiv, O.; Ben-Haroush, A.; Abraham, G.; Bar, J. Pregnancy outcome and placental findings in pregnancies complicated by fetal growth restriction with and without PE. Reprod. Sci. 2015, 22, 316–321. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” areassociated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of PE: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Placental stress and pre-eclampsia: A revised view. Placenta 2009, 30 (Suppl. SA), S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.K.; Benagiano, M.; D’Elios, M.M.; Brosens, I.; Benagiano, G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 2019, 221, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.B.; Khong, T.Y.; Brosens, I.; De Wolf, F.; Sheppard, B.L.; Bonnar, J. The placental bed biopsy: Review from three European centers. Am. J. Obstet. Gynecol. 1986, 155, 401–412. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in PE. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S895–S906. [Google Scholar] [CrossRef]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in PE and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Melchiorre, K.; Giorgione, V.; Thilaganathan, B. The placenta and PE: Villain or victim? Am. J. Obstet. Gynecol. 2022, 226, S954–S962. [Google Scholar] [CrossRef]
- Masini, G.; Foo, L.F.; Tay, J.; Wilkinson, I.B.; Valensise, H.; Gyselaers, W.; Lees, C.C. PE has two phenotypes which require different treatment strategies. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S1006–S1018. [Google Scholar] [CrossRef]
- Tay, J.; Foo, L.; Masini, G.; Bennett, P.R.; McEniery, C.M.; Wilkinson, I.B.; Lees, C.C. Early and late PE are characterized by high cardiac output, but in the presence of fetal growth restriction, cardiac output is low: Insights from a prospective study. Am. J. Obstet. Gynecol. 2018, 218, 517.E1–517.E12. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J. Reprod. Immunol. 2007, 76, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol. 2012, 64, 309–320. [Google Scholar]
- Moghaddas Sani, H.; Zununi Vahed, S.; Ardalan, M. PE: A close look at renal dysfunction. Biomed. Pharmacother. 2019, 109, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.S.; Ryan, M.J.; LaMarca, B.B.; Sedeek, M.; Murphy, S.R.; Granger, J.P. Pathophysiology of hypertension during PE: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H541–H550. [Google Scholar] [CrossRef] [PubMed]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Zeisler, H.; Hund, M.; Verlohren, S. The sFlt-1:PlGF Ratio in Women with Suspected PE. N. Engl. J. Med. 2016, 374, 1785–1786. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Gielis, J.; Van Craenenbroeck, E.; Cos, P.; Spaanderman, M.; Gyselaers, W.; Cornette, J.; Jacquemyn, Y. Oxidative stress and endothelial function in normal pregnancy versus pre-eclampsia, a combined longitudinal and case control study. BMC Pregnancy Childbirth 2018, 18, 60. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Xie, Y.; Wang, Y.; Dong, L.; Li, H.; Zhu, T. Circulating asymmetric dimethylarginine and the risk of PE: A meta-analysis based on 1338 participants. Oncotarget 2017, 8, 43944–43952. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Liu, R.; Xu, N.; Yan, S.B.; Chen, Y.; Li, T.H. The role and mechanism of asymmetric dimethylarginine in fetal growth restriction via interference with endothelial function and angiogenesis. J. Assist. Reprod. Genet. 2020, 37, 1083–1095. [Google Scholar] [CrossRef]
- Böger, R.H.; Diemert, A.; Schwedhelm, E.; Lüneburg, N.; Maas, R.; Hecher, K. The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of PE. Gynecol. Obstet. Investig. 2010, 69, 1–13. [Google Scholar] [CrossRef]
- Osman, M.W.; Nath, M.; Breslin, E.; Khalil, A.; Webb, D.R.; Robinson, T.G.; Mousa, H.A. Association between arterial stiffness and wave reflection with subsequent development of placental-mediated diseases during pregnancy: Findings of a systematic review and meta-analysis. J. Hypertens. 2018, 36, 1005–1014. [Google Scholar] [CrossRef]
- Tan, I.; Butlin, M.; Avolio, A. Does increase in arterial stiffness and wave reflection precede development of placental-mediated complications in pregnancy? J. Hypertens. 2018, 36, 1029–1031. [Google Scholar] [CrossRef]
- Wykrętowicz, M.; Krauze, T.; Guzik, P.; Piskorski, J.; Markwitz, W.; Wykrętowicz, A.; Wysocki, H. Arterial stiffness, central hemodynamics and wave reflection in normal pregnancy and control nonpregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 49–52. [Google Scholar] [CrossRef]
- Hausvater, A.; Giannone, T.; Sandoval, Y.H.; Doonan, R.J.; Antonopoulos, C.N.; Matsoukis, I.L.; Petridou, E.T.; Daskalopoulou, S.S. The association between PE and arterial stiffness. J. Hypertens. 2012, 30, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Huang, Y.; Nie, H.; Yan, J.; Ruan, L.; Zhang, C. The association between pulse wave velocity and pregnancy-associated diseases: A systematic review and meta-analysis. Heliyon 2024, 10, e29281. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Gomez, Y.H.; Gorgui, J.; El-Messidi, A.; Gagnon, R.; Abenhaim, H.A.; Rahme, E.; Daskalopoulou, S.S. Arterial stiffness for the early prediction of pre-eclampsia compared with blood pressure, uterine artery Doppler and angiogenic biomarkers: A prospective cohort study. BJOG 2023, 130, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Mangos, G.; Roberts, L.M.; Brown, M.A.; Pettit, F.; O’Sullivan, A.J.; Crowley, R.; Youssef, G.; Davis, G.K. PE-Associated Cardiovascular Risk Factors 6 Months and 2 Years After Pregnancy: The P4 Study. Hypertension 2024, 81, 851–860. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Milic, N.M.; Milin-Lazovic, J.S.; Garovic, V.D. Impaired Flow-Mediated Dilation Before, During, and After PE: A Systematic Review and Meta-Analysis. Hypertension 2016, 67, 415–423. [Google Scholar] [CrossRef]
- Kirollos, S.; Skilton, M.; Patel, S.; Arnott, C. A Systematic Review of Vascular Structure and Function in Pre-eclampsia: Non-invasive Assessment and Mechanistic Links. Front. Cardiovasc. Med. 2019, 6, 166. [Google Scholar] [CrossRef]
- Lupton, S.J.; Chiu, C.L.; Hodgson, L.A.; Tooher, J.; Ogle, R.; Wong, T.Y.; Hennessy, A.; Lind, J.M. Changes in retinal microvascular caliber precede the clinical onset of PE. Hypertension 2013, 62, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Pillay, R.; Wong, T.Y.; Makin, J.D.; Pattinson, R.C. The effect of pre-eclampsia on retinal microvascular caliber at delivery and post-partum. Obstet. Med. 2018, 11, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.V.; Goh, Y.Q.; Rojas-Carabali, W.; Cifuentes-González, C.; Cheung, C.Y.; Arora, A.; de-la-Torre, A.; Gupta, V.; Agrawal, R. Association between retinal vessels caliber and systemic health: A comprehensive review. Surv. Ophthalmol. 2025, 70, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Marincowitz, C.; Webster, I.; Westcott, C.; Goswami, N.; de Boever, P.; Seidel, G.; Strijdom, H. Vascular health assessment with flow-mediated dilatation and retinal image analysis: A pilot study in an adult population from Cape Town. Cardiovasc. J. Afr. 2021, 32, 133–140. [Google Scholar] [CrossRef]
- Saloň, A.; De Boever, P.; Goswami, N. Microvascular Changes during Viral Infections: A Systematic Review of Studies Using Retinal Vessel Diameter Assessments. Biomedicines 2024, 12, 1488. [Google Scholar] [CrossRef]
- Benschop, L.; Schalekamp-Timmermans, S.; Roeters van Lennep, J.E.; Jaddoe, V.W.V.; Wong, T.Y.; Cheung, C.Y.; Steegers, E.A.P.; Ikram, M.K. Gestational hypertensive disorders and retinal microvasculature: The Generation R Study. BMC Med. 2017, 15, 153. [Google Scholar] [CrossRef]
- Singh, S.; Chauhan, S.S.; Ranjan, R. A cross-sectional study on the incidence of retinal changes and its correlation with variables like blood pressure, liver function tests, kidney function tests, proteinuria, and pedal edema in patients of pregnancy-induced hypertension in a rural setting. Indian J. Ophthalmol. 2022, 70, 3335–3340, Erratum in Indian J. Ophthalmol. 2023, 71, 675. [Google Scholar] [CrossRef]
- Phillipos, J.; Luong, T.V.; Chang, D.; Varadarajan, S.; Howat, P.; Hodgson, L.; Colville, D.; Savige, J. Retinal small vessel narrowing in women with gestational diabetes, pregnancy-associated hypertension, or small-for-gestational age babies. Front. Med. 2023, 10, 1265555. [Google Scholar] [CrossRef]
- Zhou, T.; Gu, S.; Shao, F.; Li, P.; Wu, Y.; Xiong, J.; Wang, B.; Zhou, C.; Gao, P.; Hua, X. Prediction of PE from retinal fundus images via deep learning in singleton pregnancies: A prospective cohort study. J. Hypertens. 2024, 42, 701–710. [Google Scholar] [CrossRef]
- Malik, V.; Agrawal, N.; Prasad, S.; Talwar, S.; Khatuja, R.; Jain, S.; Sehgal, N.P.; Malik, N.; Khatuja, J.; Madan, N. Prediction of PE Using Machine Learning: A Systematic Review. Cureus 2024, 16, e76095. [Google Scholar] [CrossRef]
- Abu Samra, K. The eye and visual system in the PE/eclampsia syndrome: What to expect? Saudi J. Ophthalmol. 2013, 27, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.M.; Blace, N. Ophthalmic Pathology of PE; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hasani, K.K.; Kulovic-Sissawo, A.; Saloň, A.; Stern, C.; Mayer-Pickel, K.; Cervar-Zivkovic, M.; Goswami, N.; Fluhr, H.; Hiden, U. Association of Circulating Neprilysin with BMI, Cardiovascular Health, and Kidney Function in High-Risk Pregnancies: A Pilot Study. Biomedicines 2024, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Louw, M.C.; Adeyemo, A.O.; Makin, J.; Pattinson, R.C. Cardiac diastolic function after recovery from pre-eclampsia. Cardiovasc. J. Afr. 2018, 29, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Company Calabuig, A.M.; Nunez, E.; Georgiopoulos, G.; Nicolaides, K.H.; Charakida, M.; De Paco Matallana, C. Three-dimensional echocardiography and strain cardiac imaging in women with pre-eclampsia with follow-up to 6 months postpartum. Ultrasound Obstet. Gynecol. 2023, 62, 852–859. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.M. Acute Atherosis of the Uterine Spiral Arteries: Clinicopathologic Implications. J. Pathol. Transl. Med. 2015, 49, 462–471. [Google Scholar] [CrossRef]
- Pitz Jacobsen, D.; Fjeldstad, H.E.; Johnsen, G.M.; Fosheim, I.K.; Moe, K.; Alnæs-Katjavivi, P.; Dechend, R.; Sugulle, M.; Staff, A.C. Acute Atherosis Lesions at the Fetal-Maternal Border: Current Knowledge and Implications for Maternal Cardiovascular Health. Front. Immunol. 2021, 12, 791606. [Google Scholar] [CrossRef]
- Stillman, I.E.; Karumanchi, S.A. The glomerular injury of PE. J. Am. Soc. Nephrol. 2007, 18, 2281–2284. [Google Scholar] [CrossRef]
- Cornelis, T.; Odutayo, A.; Keunen, J.; Hladunewich, M. The kidney in normal pregnancy and PE. Semin. Nephrol. 2011, 31, 4–14. [Google Scholar] [CrossRef]
- Kim, Y.M.; Chaemsaithong, P.; Romero, R.; Shaman, M.; Kim, C.J.; Kim, J.S.; Qureshi, F.; Jacques, S.M.; Ahmed, A.I.; Chaiworapongsa, T.; et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J. Matern. Fetal Neonatal Med. 2015, 28, 2001–2009. [Google Scholar] [CrossRef]
- Hecht, J.L.; Ordi, J.; Carrilho, C.; Ismail, M.R.; Zsengeller, Z.K.; Karumanchi, S.A.; Rosen, S. The pathology of eclampsia: An autopsy series. Hypertens. Pregnancy 2017, 36, 259–268. [Google Scholar] [CrossRef]
- Bartynski, W.S. Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. AJNR Am. J. Neuroradiol. 2008, 29, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Fragoso, M.B.T.; Bueno, N.B.; Goulart, M.O.F.; de Oliveira, A.C.M. Oxidative stress markers in preeclamptic placentas: A systematic review with meta-analysis. Placenta 2020, 99, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of PE: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Vadillo-Ortega, F.; Perichart-Perera, O.; Espino, S.; Avila-Vergara, M.A.; Ibarra, I.; Ahued, R.; Godines, M.; Parry, S.; Macones, G.; Strauss, J.F. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: Randomised controlled trial. BMJ 2011, 342, d2901. [Google Scholar] [CrossRef]
- Hernández González, L.L.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Mayoral Andrade, G.; Martínez Cruz, M.; Ramos-Martínez, E.; Pérez-Campos Mayoral, E.; Cruz Hernández, V.; Antonio García, I.; Matias-Cervantes, C.A.; et al. Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of PE. Pharmaceuticals 2024, 17, 605. [Google Scholar] [CrossRef]
- Clark, C.R.; Khalil, R.A. Regulation of vascular angiotensin II type 1 and type 2 receptor and angiotensin-(1-7)/MasR signaling in normal and hypertensive pregnancy. Biochem. Pharmacol. 2024, 220, 115963. [Google Scholar] [CrossRef]
- Eghbal-Fard, S.; Yousefi, M.; Heydarlou, H.; Ahmadi, M.; Taghavi, S.; Movasaghpour, A.; Jadidi-Niaragh, F.; Yousefi, B.; Dolati, S.; Hojjat-Farsangi, M.; et al. The imbalance of Th17/Treg axis involved in the pathogenesis of PE. J. Cell. Physiol. 2019, 234, 5106–5116. [Google Scholar] [CrossRef]
- Ahmadi, M.; Yousefi, M.; Abbaspour-Aghdam, S.; Dolati, S.; Aghebati-Maleki, L.; Eghbal-Fard, S.; Khabbazi, A.; Rostamzadeh, D.; Alipour, S.; Shabani, M.; et al. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet’s disease. J. Cell Physiol. 2019, 234, 3985–3994. [Google Scholar] [CrossRef]
- van Esch, J.J.A.; Bolte, A.C.; Spaanderman, M.E.A.; Vandenbussche, F.P.H.A.; de Weerth, C.; Beijers, R. Maternal anxiety forecasts shorter prolongation of pregnancies complicated by early-onset PE. Arch. Gynecol. Obstet. 2023, 308, 1703–1711. [Google Scholar] [CrossRef]
- van Esch, J.J.A.; Bolte, A.C.; Vandenbussche, F.P.H.A.; Schippers, D.H.; de Weerth, C.; Beijers, R. Differences in hair cortisol concentrations and reported anxiety in women with preeclampsia versus uncomplicated pregnancies. Pregnancy Hypertens. 2020, 21, 200–202. [Google Scholar] [CrossRef]
- Khoury, J.E.; Giles, L.; Kaur, H.; Johnson, D.; Gonzalez, A.; Atkinson, L. Associations between psychological distress and hair cortisol during pregnancy and the early postpartum: A meta-analysis. Psychoneuroendocrinology 2023, 147, 105969. [Google Scholar] [CrossRef]
- Karteris, E.; Vatish, M.; Hillhouse, E.W.; Grammatopoulos, D.K. PE is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J. Clin. Endocrinol. Metab. 2005, 90, 3680–3687. [Google Scholar] [CrossRef]
- Agrawal, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Meta-Analysis and Systematic Review to Assess the Role of Soluble FMS-Like Tyrosine Kinase-1 and Placenta Growth Factor Ratio in Prediction of PE: The SaPPPhirE Study. Hypertension 2018, 71, 306–316. [Google Scholar] [CrossRef]
- Sovio, U.; Gaccioli, F.; Cook, E.; Hund, M.; Charnock-Jones, D.S.; Smith, G.C. Prediction of PE Using the Soluble fms-Like Tyrosine Kinase 1 to Placental Growth Factor Ratio: A Prospective Cohort Study of Unselected Nulliparous Women. Hypertension 2017, 69, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Odibo, A.O.; Zhong, Y.; Goetzinger, K.R.; Odibo, L.; Bick, J.L.; Bower, C.R.; Nelson, D.M. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta 2011, 32, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Piskun, A.; Dmytro, K.; Honcharenko, O.; Rud, V.; Klimas, L. Placental biomarkers: PP13, VEGF in diagnostics of early and late PE. Wiad. Lek. 2022, 75, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; van den Berg, C.; Alfirevic, Z.; O’Brien, S.; Röthlisberger, M.; Baker, P.N.; Kenny, L.C.; Kublickiene, K.; Duvekot, J.J. Early Pregnancy Biomarkers in Pre-Eclampsia: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2015, 16, 23035–23056. [Google Scholar] [CrossRef]
- Tashie, W.; Fondjo, L.A.; Owiredu, W.K.B.A.; Ephraim, R.K.D.; Asare, L.; Adu-Gyamfi, E.A.; Seidu, L. Altered Bioavailability of Nitric Oxide and L-Arginine Is a Key Determinant of Endothelial Dysfunction in Preeclampsia. BioMed Res. Int. 2020, 2020, 3251956. [Google Scholar] [CrossRef]
- Németh, B.; Murányi, E.; Hegyi, P.; Mátrai, P.; Szakács, Z.; Varjú, P.; Hamvas, S.; Tinusz, B.; Budán, F.; Czimmer, J.; et al. Asymmetric dimethylarginine levels in preeclampsia—Systematic review and meta-analysis. Placenta 2018, 69, 57–63. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947, Erratum in J. Clin. Endocrinol. Metab. 2025, 110, e916. [Google Scholar] [CrossRef]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G.; et al. Vitamin D supplementation and incident PE: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef]
- Moghib, K.; Ghanm, T.I.; Abunamoos, A.; Rajabi, M.; Moawad, S.M.; Mohsen, A.; Kasem, S.; Elsayed, K.; Sayed, M.; Dawoud, A.I.; et al. Efficacy of vitamin D supplementation on the incidence of PE: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2024, 24, 852. [Google Scholar] [CrossRef] [PubMed]
- Khaing, W.; Vallibhakara, S.A.; Tantrakul, V.; Vallibhakara, O.; Rattanasiri, S.; McEvoy, M.; Attia, J.; Thakkinstian, A. Calcium and Vitamin D Supplementation for Prevention of PE: A Systematic Review and Network Meta-Analysis. Nutrients 2017, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, E.; Afshari, M.; Khazaei, S. The association between PE and the risk of metabolic syndrome after delivery: A meta-analysis. J. Matern. Fetal Neonatal Med. 2021, 34, 3253–3258. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, S.A.; Roberts, J.M. Uric acid as a pathogenic factor in PE. Placenta 2008, 29 (Suppl. SA), S67–S72. [Google Scholar] [CrossRef]
- Mazloomi, S.; Alimohammadi, S.; Khodadadi, I.; Ghiasvand, T.; Shafiee, G. Evaluation of methylenetetrahydrofolate reductase (MTHFR) activity and the levels of homocysteine and malondialdehyde (MDA) in the serum of women with PE. Clin. Exp. Hypertens. 2020, 42, 590–594. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Lv, Y.; Ding, H. Dissecting the Roles of Lipids in PE. Metabolites 2022, 12, 590. [Google Scholar] [CrossRef]
- Hosier, H.; Lipkind, H.S.; Rasheed, H.; DeWan, A.T.; Rogne, T. Dyslipidemia and Risk of PE: A Multiancestry Mendelian Randomization Study. Hypertension 2023, 80, 1067–1076. [Google Scholar] [CrossRef]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial dysfunction and PE: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef]
- Holmes, V.A.; McCance, D.R. Could antioxidant supplementation prevent pre-eclampsia? Proc. Nutr. Soc. 2005, 64, 491–501. [Google Scholar] [CrossRef]
- Kelly, C.B.; Hookham, M.B.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Garg, S.K.; Scardo, J.A.; Hammad, S.M.; Menard, M.K.; et al. Subclinical First Trimester Renal Abnormalities Are Associated with PE in Normoalbuminuric Women with Type 1 Diabetes. Diabetes Care 2018, 41, 120–127. [Google Scholar] [CrossRef]
- Karampas, G.; Eleftheriades, M.; Panoulis, K.; Rizou, M.; Haliassos, A.; Hassiakos, D.; Vitoratos, N.; Rizos, D. Maternal serum levels of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in pregnancies with PE and those with a small for gestational age neonate: A longitudinal study. Prenat. Diagn. 2014, 34, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Moyake, N.; Buchmann, E.; Crowther, N.J. Neutrophil gelatinase-associated lipocalin as a diagnostic marker of acute kidney injury in pre-eclampsia. J. Obstet. Gynaecol. Res. 2016, 42, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, R.; Hossain, N.; Butt, S.; Bhellar, Z.; Fatima, E.; Imtiaz, S.; Moosa, P.G.; Abbas, K.; Jafri, S.B.; Khan, S. Efficacy of multiple Biomarkers: NGAL, KIM1, Cystatin C and IL18 in predicting pregnancy related acute kidney injury. Pak. J. Med. Sci. 2023, 39, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Y.; Gu, X.; Cooper, D.B.; Lewis, D.F. Evidence of kidney injury in PE: Increased maternal and urinary levels of NGAL and KIM-1 and their enhanced expression in proximal tubule epithelial cells. Front. Med. 2023, 10, 1130112. [Google Scholar] [CrossRef]
- Atta, S.; Mekky, R.; Ibrahim, M.; Abdallah, M.M.; Elbaz, M.A.H.; Radwan, E. Increased Expression of Neprilysin Is Associated with Inflammation in PE. Reprod. Sci. 2024, 31, 1385–1390. [Google Scholar] [CrossRef]
- Gill, M.; Motta-Mejia, C.; Kandzija, N.; Cooke, W.; Zhang, W.; Cerdeira, A.S.; Bastie, C.; Redman, C.; Vatish, M. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in PE. Hypertension 2019, 73, 1112–1119. [Google Scholar] [CrossRef]
- Cao, L.; He, B.; Zhou, Y.; Chen, T.; Gao, Y.; Yao, B. Utility of uterine artery Doppler ultrasound imaging in predicting PE during pregnancy: A meta-analysis. Med. Ultrason. 2024, 26, 197–204. [Google Scholar] [CrossRef]
- Shahid, N.; Masood, M.; Bano, Z.; Naz, U.; Hussain, S.F.; Anwar, A.; Hashmi, A.A. Role of Uterine Artery Doppler Ultrasound in Predicting Pre-Eclampsia in High-Risk Women. Cureus 2021, 13, e16276. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm PE. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE trial: Performance of screening for preterm pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 492–495, Erratum in Ultrasound Obstet. Gynecol. 2017, 50, 807. [Google Scholar] [CrossRef] [PubMed]
- von Dadelszen, P.; Payne, B.; Li, J.; Ansermino, J.M.; Broughton Pipkin, F.; Côté, A.M.; Douglas, M.J.; Gruslin, A.; Hutcheon, J.A.; Joseph, K.S.; et al. Prediction of adverse maternal outcomes in pre-eclampsia: Development and validation of the fullPIERS model. Lancet 2011, 377, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kametas, N.A.; Nzelu, D.; Nicolaides, K.H. Chronic hypertension and superimposed PE: Screening and diagnosis. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S1182–S1195. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.E.; Myers, J.; Seed, P.T.; Sparkes, J.; Lowe, J.; Hunter, R.M.; Shennan, A.H.; Chappell, L.C.; PARROT trial group. Placental growth factor testing to assess women with suspected pre-eclampsia: A multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 2019, 393, 1807–1818. [Google Scholar] [CrossRef]
- Mayer-Pickel, K.; Nanda, M.; Gajic, M.; Cervar-Zivkovic, M. PE and the Antiphospholipid Syndrome. Biomedicines 2023, 11, 2298. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Ness, R.B.; Markovic, N.; Roberts, J.M. The risk of PE rises with increasing prepregnancy body mass index. Ann. Epidemiol. 2005, 15, 475–482. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, N.; Shen, H. A Review of Research Progress of Pregnancy with Twins with PE. Risk Manag. Healthc. Policy 2021, 14, 1999–2010. [Google Scholar] [CrossRef]
- Ye, X.; Baker, P.N.; Tong, C. The updated understanding of advanced maternal age. Fundam. Res. 2023, 4, 1719–1728. [Google Scholar] [CrossRef]
- Augusto, J.; Margarida Póvoa, A. PE risk in oocyte donation versus double gamete donation pregnancies: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 219–227. [Google Scholar] [CrossRef]
- England, L.; Zhang, J. Smoking and risk of PE: A systematic review. Front. Biosci. 2007, 12, 2471–2483. [Google Scholar] [CrossRef]
- Wei, J.; Liu, C.X.; Gong, T.T.; Wu, Q.J.; Wu, L. Cigarette smoking during pregnancy and PE risk: A systematic review and meta-analysis of prospective studies. Oncotarget 2015, 6, 43667–43678. [Google Scholar] [CrossRef]
- Kharkova, O.A.; Grjibovski, A.M.; Krettek, A.; Nieboer, E.; Odland, J.Ø. First-trimester smoking cessation in pregnancy did not increase the risk of PE/eclampsia: A Murmansk County Birth Registry study. PLoS ONE 2017, 12, e0179354. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, H.; Wright, C.; Schubert, P.; Boyd, T.K.; Roberts, D.J.; Brink, L.; Nel, D.; Groenewald, C. Associations of maternal smoking and drinking with fetal growth and placental abruption. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Savitz, D.A.; Luther, E.R. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am. J. Epidemiol. 1996, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.K.; Goudar, S.S.; Kodkany, B.S.; Metgud, M.; Somannavar, M.; Okitawutshu, J.; Lokangaka, A.; Tshefu, A.; Bose, C.L.; Mwapule, A.; et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 285–293. [Google Scholar] [CrossRef]
- WHO. WHO Recommendation: Calcium Supplementation During Pregnancy for the Prevention of Pre-Eclampsia and Its Complications; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Duley, L.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014, 6, CD001059. [Google Scholar] [CrossRef]
- Ushida, T.; Tano, S.; Matsuo, S.; Fuma, K.; Imai, K.; Kajiyama, H.; Kotani, T. Dietary supplements and prevention of preeclampsia. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2025, 48, 1444–1457. [Google Scholar] [CrossRef]
- Jaiswal, V.; Joshi, A.; Jha, M.; Hanif, M.; Arora, A.; Gupta, S.; Shah, M.; Deb, N.; Peng Ang, S.; Aujla, S.; et al. Association between calcium supplementation and gestational hypertension, and preeclampsia: A Meta-analysis of 26 randomized controlled trials. Curr. Probl. Cardiol. 2024, 49, 102217. [Google Scholar] [CrossRef]
- Woo Kinshella, M.L.; Sarr, C.; Sandhu, A.; Bone, J.N.; Vidler, M.; Moore, S.E.; Elango, R.; Cormick, G.; Belizan, J.M.; Hofmeyr, G.J.; et al. Calcium for pre-eclampsia prevention: A systematic review and network meta-analysis to guide personalised antenatal care. BJOG 2022, 129, 1833–1843. [Google Scholar] [CrossRef]
- Villar, J.; Abdel-Aleem, H.; Merialdi, M.; Mathai, M.; Ali, M.M.; Zavaleta, N.; Purwar, M.; Hofmeyr, J.; Nguyen, T.N.; Campódonico, L.; et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am. J. Obstet. Gynecol. 2006, 194, 639–649. [Google Scholar] [CrossRef]
- Syngelaki, A.; Sequeira Campos, M.; Roberge, S.; Andrade, W.; Nicolaides, K.H. Diet and exercise for preeclampsia prevention in overweight and obese pregnant women: Systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2019, 32, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Taliento, C.; Piccolotti, I.; Sabattini, A.; Tormen, M.; Cappadona, R.; Greco, P.; Scutiero, G. Effect of Physical Activity during Pregnancy on the Risk of Hypertension Disorders and Gestational Diabetes: Evidence Generated by New RCTs and Systematic Reviews. J. Clin. Med. 2024, 13, 2198. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xia, B.; Yuan, Y.; Wang, Y.; Wang, Y. Low-molecular-weight heparin in addition to low-dose aspirin for preventing preeclampsia and its complications: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1073148. [Google Scholar] [CrossRef]
- Heidari, B.; Lerman, A.; Lalia, A.Z.; Lerman, L.O.; Chang, A.Y. Effect of Metformin on Microvascular Endothelial Function in Polycystic Ovary Syndrome. Mayo Clin. Proc. 2019, 94, 2455–2466. [Google Scholar] [CrossRef]
- Costantine, M.M.; West, H.; Wisner, K.L.; Caritis, S.; Clark, S.; Venkataramanan, R.; Stika, C.S.; Rytting, E.; Wang, X.; Ahmed, M.S.; et al. A randomized pilot clinical trial of pravastatin versus placebo in pregnant patients at high risk of PE. Am. J. Obstet. Gynecol. 2021, 225, 666.E1–666.E15. [Google Scholar] [CrossRef]
- Akbar, M.I.A.; Azis, M.A.; Riu, D.S.; Wawengkang, E.; Ernawati, E.; Bachnas, M.A.; Sulistyowati, S.; Dachlan, E.G.; Mose, J.C.; Dekker, G. INOVASIA Study: A Multicenter Randomized Clinical Trial of Pravastatin to Prevent PE in High-Risk Patients. Am. J. Perinatol. 2024, 41, 1203–1211. [Google Scholar] [CrossRef]
- Provinciatto, H.; Barbalho, M.E.; Almeida, J.; Provinciatto, A.; Philip, C.E. The role of pravastatin in preventing PE in high-risk pregnant women: A meta-analysis with trial sequential analysis. Am. J. Obstet. Gynecol. MFM 2024, 6, 101260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutllovci Hasani, K.; Ajeti, N.; Goswami, N. Understanding Preeclampsia: Cardiovascular Pathophysiology, Histopathological Insights and Molecular Biomarkers. Med. Sci. 2025, 13, 154. https://doi.org/10.3390/medsci13030154
Kutllovci Hasani K, Ajeti N, Goswami N. Understanding Preeclampsia: Cardiovascular Pathophysiology, Histopathological Insights and Molecular Biomarkers. Medical Sciences. 2025; 13(3):154. https://doi.org/10.3390/medsci13030154
Chicago/Turabian StyleKutllovci Hasani, Kaltrina, Nurxhan Ajeti, and Nandu Goswami. 2025. "Understanding Preeclampsia: Cardiovascular Pathophysiology, Histopathological Insights and Molecular Biomarkers" Medical Sciences 13, no. 3: 154. https://doi.org/10.3390/medsci13030154
APA StyleKutllovci Hasani, K., Ajeti, N., & Goswami, N. (2025). Understanding Preeclampsia: Cardiovascular Pathophysiology, Histopathological Insights and Molecular Biomarkers. Medical Sciences, 13(3), 154. https://doi.org/10.3390/medsci13030154






