Gestational Diabetes Mellitus: The Dual Risk of Small and Large for Gestational Age: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Ethnicity
3.2. Maternal Age
3.3. Maternal Lipid Parameters
3.4. Pre-Pregnancy BMI
3.5. Maternal Diet
3.6. Biochemical Markers and Vascular Adaptation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | Appropriate For Gestational Age |
| BMI | Body Mass Index |
| DNA | Deoxyribonucleic Acid |
| FFA | Free Fatty Acid |
| GDM | Gestational Diabetes Mellitus |
| HDL | High-Density Lipoprotein |
| HR | High Risk |
| IADPSG | International Association Of Diabetes And Pregnancy Study Groups |
| IUGR | Intrauterine Growth Restriction |
| LDL | Low-Density Lipoprotein |
| LGA | Large For Gestational Age |
| LR | Low Risk |
| NHB | Non-Hispanic Black |
| NHW | Non-Hispanic White |
| NTDs | Neural Tube Defects |
| OGTT | Oral Glucose Tolerance Test |
| OR | Odds Ratio |
| pBMI | Pre-Pregnancy Body Mass Index |
| ROS | Reactive Oxygen Species |
| SGA | Small For Gestational Age |
| TG | Triglyceride |
| WHO | World Health Organization |
References
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Abdo, N.; Bettencourt-Silva, R.; Al-Rifai, R. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front. Endocrinol. 2021, 9, 691033. [Google Scholar] [CrossRef] [PubMed]
- Veeraswamy, S.; Vijayam, B.; Gupta, V.; Kapur, A. Gestational diabetes: The public health relevance and approach. Diabetes Res. Clin. Pract. 2012, 97, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Staynova, R.; Vasileva, E.; Yanachkova, V. Gestational diabetes mellitus: A growing economic concern. Folia Medica 2022, 64, 725–732. [Google Scholar] [CrossRef]
- Buchanan, T.; Xiang, A.; Page, K. Gestational Diabetes Mellitus: Risks and Management during and after Pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, H.; Yang, Y.; Tang, Y.; Yang, X. Demographic and Clinical Features of Small-for-Gestational-Age Infants Born to Mothers With Gestational Diabetes Mellitus. Front. Pediatr. 2021, 9, 741793. [Google Scholar] [CrossRef]
- Macfarlane, C.; Tsakalakos, N. The extended Pedersen hypothesis. Clin. Physiol. Biochem. 1988, 6, 68–73. [Google Scholar]
- Schmidt, M.; Duncan, B.; Reichelt, A. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care 2001, 24, 1151–1155. [Google Scholar] [CrossRef]
- McElwain, C.; Tuboly, E.; McCarthy, F.; McCarthy, C. Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef]
- Krishna, R.; Bhat, B. Molecular mechanisms of intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2018, 31, 2634–2640. [Google Scholar] [CrossRef]
- Venkatesh, K.; Lynch, C.; Powe, E.; Costantine, M.; Thung, S.; Gabbe, S.; Grobman, W.A.; Landon, M.B. Risk of Adverse Pregnancy Outcomes Among Pregnant Individuals With Gestational Diabetes by Race and Ethnicity in the United States, 2014–2020. JAMA 2022, 327, 1356–1367. [Google Scholar] [CrossRef]
- Filardi, T.; Gentile, M.; Venditti, V.; Valente, A.; Bleve, E.; Santangelo, C.; Morano, S. The Impact of Ethnicity on Fetal and Maternal Outcomes of Gestational Diabetes. Medicina 2022, 58, 1161. [Google Scholar] [CrossRef]
- Xiang, A.; Black, M.; Li, B.; Martinez, M.; Sacks, D.; Lawrence, J.; Buchanan, T.A.; Jacobsen, S.J. Racial and ethnic disparities in extremes of fetal growth after gestational diabetes mellitus. Diabetologia 2015, 58, 272–281. [Google Scholar] [CrossRef]
- Nielsen, K.; Andersen, G.; Damm, P.; Andersen, A. Migration, Gestational Diabetes, and Adverse Pregnancy Outcomes: A Nationwide Study of Singleton Deliveries in Denmark. J. Clin. Endocrinol. Metab. 2021, 106, e5075–e5087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tian, M.; Zhang, P.; Du, L.; Ma, X.; Zhang, Y.; Tang, Z. Risk of adverse pregnancy outcomes in pregnant women with gestational diabetes mellitus by age: A multicentric cohort study in Hebei, China. Sci. Rep. 2014, 14, 807. [Google Scholar] [CrossRef]
- Lu, L.; He, L.; Hu, J.; Li, J. Association between very advanced maternal age women with gestational diabetes mellitus and the risks of adverse infant outcomes: A cohort study from the NVSS 2014–2019. BMC Pregnancy Childbirth 2023, 23, 158. [Google Scholar] [CrossRef]
- Krstevska, B.; Jovanovska, S.; Krstevkska, S.; Nakova, V.; Serafimoski, V. Maternal lipids may predict fetal growth in type 2 Diabetes Mellitus and Gestational Diabetes Mellitus pregnancies. Prilozi 2016, 37, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Montero, M.; Souza, F.; Jordao, M.; Oliveira, M.; Mattar, R.; Dib, S.A.; Dualib, P.M.; de Almeida-Pititto, B. High-Density Lipoproteins-Cholesterol (HDL-C) in Women With Gestational Diabetes (GDM): A Predictor for Large Gestational Age (LGA) Babies. Cureus 2024, 16, e65546. [Google Scholar] [CrossRef]
- Simeonova, S.; Krstevska, B.; Velkoska-Nakova, V.; Hadji Lega, M.; Samardjiski, I.; Serafimoski, V.; Livrinova, V.; Todorovska, I.; Sima, A. Effect of lipid parameters on foetal growth in gestational diabetes mellitus pregnancies. Prilozi 2014, 35, 131–136. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, L.; Jin, J.; Miao, H.; Liu, G.; Guo, Y. Impact of maternal lipid profiles on offspring birth size in late pregnancy among women with and without gestational diabetes. Lipids Health Dis. 2025, 24, 43. [Google Scholar] [CrossRef]
- Drever, H.; Davidson, S.; Callaway, L.; Sekar, R.; De Jersey, S. Factors associated with higher risk of small-for-gestational-age infants in women treated for gestational diabetes. Aust. N. Z. J. Obstet. Gynaecol. 2023, 63, 714–720. [Google Scholar] [CrossRef]
- Yang, J.; Qian, J.; Qu, Y.; Zhan, Y.; Yue, H.; Ma, H.; Li, X.; Man, D.; Wu, H.; Huang, P.; et al. Pre-pregnancy body mass index and risk of maternal or infant complications with gestational diabetes mellitus as a mediator: A multicenter, longitudinal cohort study in China. Diabetes Res. Clin. Pract. 2023, 198, 110619. [Google Scholar] [CrossRef]
- Kondracki, A.; Valente, M.; Ibrahimou, B.; Bursac, Z. Risk of large for gestational age births at early, full and late term in relation to pre-pregnancy body mass index: Mediation by gestational diabetes status. Paediatr. Perinat. Epidemiol. 2022, 36, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Chen Xu, J.; Coelho, A. Association between Body Mass Index and Gestational Weight Gain with Obstetric and Neonatal Complications in Pregnant Women with Gestational Diabetes. Acta Med. Port. 2022, 35, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yu, T.; Chen, J.; Zheng, X.; Xheng, L.; Yan, J. Relationship between maternal pre-pregnancy BMI and neonatal birth weight in pregnancies with gestational diabetes mellitus: A retrospective cohort study. Front. Med. 2025, 6, 1478907. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kobayashi, S.; Ikeda-Araki, A.; Ito, S.; Miyashita, C.; Kimura, T.; Hirata, T.; Tamakoshi, A.; Mayama, M.; Noshiro, K.; et al. Association between pre-pregnancy body mass index and gestational weight gain and perinatal outcomes in pregnant women diagnosed with gestational diabetes mellitus: The Japan Environment and Children’s Study. J. Diabetes Investig. 2022, 13, 889–899. [Google Scholar] [CrossRef]
- Hu, H.; Feng, P.; Yu, Q.; Zhu, W.; Xu, H.; Wu, D.; Wu, L.; Yin, J.; Li, H. The mediating role of gestational diabetes mellitus in the associations of maternal prepregnancy body mass index with neonatal birth weight. J. Diabetes 2022, 14, 26–33. [Google Scholar] [CrossRef]
- Chen, H.; Wu, C.; Hsieh, C.; Kuo, F.; Sun, C.; Wang, S.; Chen, M.-L.; Wu, M.-T. Relationship of maternal body weight and gestational diabetes mellitus with large-for-gestational-age babies at birth in Taiwan: The TMICS cohort. Taiwan. J. Obs. Gynecol. 2022, 61, 234–242. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, Y.; Li, T.; Fan, Y.; Zhang, Q.; Cheng, H. Effects of obesity indices/GDM on the pregnancy outcomes in Chinese women: A retrospective cohort study. Front. Endocrinol. 2022, 18, 1029978. [Google Scholar] [CrossRef]
- Bruno, R.; Petrella, E.; Bertarini, V.; Pedrielli, G.; Neri, I.; Facchinetti, F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern. Child. Nutr. 2017, 13, e12333. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Tranidou, A.; Chroni, V.; Tsakiridis, I.; Magriplis, E.; Dagklis, T.; Chourdakis, M. Association of Maternal Diet with Infant Birthweight in Women with Gestational Diabetes Mellitus. Nutrients 2023, 15, 4545. [Google Scholar] [CrossRef]
- Siargkas, A.; Tranidou, A.; Magriplis, E.; Tsakiridis, I.; Apostolopoulou, A.; Xenidis, T.; Pazaras, N.; Chourdakis, M.; Dagklis, T. Impact of Maternal Macronutrient Intake on Large for Gestational Age Neonates’ Risk Among Women with Gestational Diabetes Mellitus: Results from the Greek BORN2020 Cohort. Nutrients 2025, 17, 269. [Google Scholar] [CrossRef]
- Xie, C.; Zheng, Q.; Jiang, X.; Liao, Y.; Gao, X.; Zhu, Y.; Li, J.; Liu, R. Association of maternal dietary cholesterol intake during the second and third trimesters of pregnancy and blood glucose and pregnancy outcome in women with gestational diabetes mellitus: A prospective cohort study. Front. Nutr. 2024, 12, 1449000. [Google Scholar] [CrossRef]
- Messika, A.; Toledano, Y.; Hadar, E.; Shmuel, E.; Tauman, R.; Shamir, R.; Froy, O. Relationship among chrononutrition, sleep, and glycemic control in women with gestational diabetes mellitus: A randomized controlled trial. Am. J. Obs. Gynecol. MFM 2022, 4, 100660. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Sha, T.; Gao, X.; He, Q.; Wu, X.; Tian, Q.; Yang, F.; Tang, C.; Wu, X.; Xie, Q.; et al. The Associations between the Duration of Folic Acid Supplementation, Gestational Diabetes Mellitus, and Adverse Birth Outcomes based on a Birth Cohort. Int. J. Environ. Res. Public Health 2019, 16, 4511. [Google Scholar] [CrossRef] [PubMed]
- Soubasi, V.; Petridou, S.; Sarafidis, K.; Tsantali, C.; Diamanti, E.; Buonocore, G.; Drossou-Agakidou, V. Association of increased maternal ferritin levels with gestational diabetes and intra-uterine growth retardation. Diabetes Metab. 2010, 36, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.; Reichelt, A.; Schmitt, L.; Boff, R.; Oppermann, M.; Camargo, J.; Silveiro, S.P.; Rosenfeld, C.S. Vitamin D Deficiency Increases the Risk of Adverse Neonatal Outcomes in Gestational Diabetes. PLoS ONE 2016, 11, e0164999. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiao, M.; Han, N.; Yang, W.; Bao, H.; Ren, Z. The Influence of Maternal Vitamin E Concentrations in Different Trimesters on Gestational Diabetes and Large-for-Gestational-Age: A Retrospective Study in China. Nutrients 2022, 14, 1629. [Google Scholar] [CrossRef]
- Pang, T.; Zhou, X.; Li, P.; Ma, H.; Shen, X.; Wan, Y.; Guo, X.-L.; Liu, Z.-P.; Chen, G.-D. Associations of early pregnancy serum uric acid levels with risk of gestational diabetes and birth outcomes: A retrospective cohort study. BMC Endocr. Disord. 2023, 23, 252. [Google Scholar] [CrossRef]
- Kim, S.; Song, Y.; Kim, S.; Cho, Y.; Kim, K. Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus. Diabetes Metab. J. 2022, 46, 140–148. [Google Scholar] [CrossRef]
- Chatzakis, C.; Lausegger, S.; Sembrera, E.; Vargas, S.; Nicolaides, K.; Charakida, M. Maternal vascular dysfunction in gestational diabetes is associated with birth of small neonates. Diabetes Res. Clin. Pract. 2025, 221, 112032. [Google Scholar] [CrossRef]
- Haertle, L.; El Hajj, N.; Dittrich, M.; Muller, T.; Nanda, I.; Lehnen, H.; Haaf, T. Epigenetic sigrantures of gestational diabetes mellitus on cord blood methylation. Clin. Epigenetics 2017, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Jaddoe, V.; Hofman, A.; Steegers-Theunissen, R.; Steegers, E. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: The Generation R Study. Br. J. Nutr. 2009, 102, 777–785. [Google Scholar] [CrossRef]
- Bai, W.; Wang, H.; Fang, R.; Lin, M.; Qin, Y. Evaluating the effect of gestational diabetes mellitus on macrosomia based on the characteristics of oral glucose tolerance test. Clin. Chim. Acta 2023, 544, 117362. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.; Frey, H.; Lynch, C.; Klebanoff, M.; Thung, S.; Costantine, M.; Landon, M.B.; Venkatesh, K.K. Association between Diabetes in Pregnancy and Shoulder Dystocia by Infant Birth Weight in an Era of Cesarean Delivery for Suspected Macrosomia. Am. J. Perinatol. 2023, 40, 929–936. [Google Scholar] [CrossRef]
- Kekki, M.; Tihtonen, K.; Salonen, A.; Koukkula, T.; Gissler, M.; Laivuori, H.; Huttunen, T.T. Severe birth injuries in neonates and associated risk factors for injury in mothers with different types of diabetes in Finland. Int. J. Gynaecol. Obs. 2022, 159, 195–203. [Google Scholar] [CrossRef]
- Al-Shwyiat, R.; Radwan, A. Fetal anomalies in gestational diabetes mellitus and risk of fetal anomalies in relation to pre-conceptional blood sugar and glycosylated hemoglobin. J. Mother. Child. 2023, 26, 73–77. [Google Scholar]
- Allen, V.; Armson, B. Teratogenicity associated with pre-existing and gestational diabetes. J. Obstet. Gynaecol. Can. 2007, 29, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Millis, J. Malformations in infants of diabetic mothers. Birth Defects Res. 2010, 88, 769–778. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Saeedi, M.; Cao, Y.; Fadl, H.; Gustafson, H.; Simmons, D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2021, 172, 108642. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Lkshmi, R.; Sharma, R.; Swami, S. EE285 Economic Burden of Gestational Diabetes: A Global Targeted Literature Review. Value Health 2024, 27, S110. [Google Scholar] [CrossRef]
- Ehlers, E.; Talton, O.; Schust, D.; Schulz, L. Placental structural abnormalities in gestational diabetes and when they develop: A scoping review. Placenta 2021, 116, 58–66. [Google Scholar] [CrossRef]
- Afsar, S.; Turan, G.; Sonmez, A.; Usta, C.; Usta, A. Fetal vascular malperfusion score is linked with developing preeclampsia in women with gestational diabetes mellitus: A retrospective cohort study. Rev. Assoc. Med. Bras 2023, 69, e20230795. [Google Scholar] [CrossRef]
- Roy, B. Pathophysiological Mechanisms of Diabetes-Induced Macrovascular and Microvascular Complications: The Role of Oxidative Stress. Med. Sci. 2025, 13, 87. [Google Scholar] [CrossRef]
- Arcot, A.; Walker, R.; Gallagher, K.; Goldstein, J.; Gernand, A. Gestational diabetes mellitus and vascular malperfusion lesions in the placenta: A systematic review and meta-analysis. Int. J. Gynecol. Obs. 2025, 00, 1–13. [Google Scholar] [CrossRef]
- Salameh, M.; Oniya, O.; Chamseddine, R.; Konje, J. Maternal Obesity, Gestational Diabetes, and Fetal Macrosomia: An Incidental or a Mechanistic Relationship? Matern. Fetal Med. 2021, 5, 27–30. [Google Scholar] [CrossRef]
- Puga, F.; Duarte, D.; Silva, V.; Pereira, M.; Garrido, S.; Vilaverde, J.; Moreira, M.S.; Pichel, F.; Pinto, C.; Dores, J. Maternal Hypertriglyceridemia in Gestational Diabetes: A New Risk Factor? Nutrients 2024, 16, 1577. [Google Scholar] [CrossRef]
- Barbour, L.; Mccurdy, C.; Hernandes, T.; Kirwan, J.; Catalano, P.; Friedman, J. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30, 112–119. [Google Scholar] [CrossRef]
- Nahavandi, S.; Price, S.; Sumithran, P.; Ekinci, E. Exploration of the shared pathophysiological mechanisms of gestational diabetes and large for gestational age offspring. World J. Diabetes 2019, 10, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.; Graf, K.; Kulbacka, I.; Kjos, S.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal Lipids as Strong Determinants of Fetal Environment and Growth in Pregnancies With Gestational Diabetes Mellitus Free. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Preda, S.D.; Mota, M.; Iliescu, D.G.; Zorila, L.G.; Comanescu, A.C.; Mitrea, A.; Clenciu, D.; Mota, E.; Vladu, I.M. Dyslipidemia in Pregnancy: A Systematic Review of Molecular Alterations and Clinical Implications. Biomedicines 2024, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Zhang, F. Association between maternal lipid levels during pregnancy and delivery of small for gestational age: A systematic review and meta-analysis. Front. Pediatr. 2022, 10, 934505. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Q.P.Y.; Jiang, X.; Li, J.; Liu, R.; Huang, L. Association between prepregnancy body mass index or gestational weight gain and adverse pregnancy outcomes among Chinese women with gestational diabetes mellitus: A systematic review and meta-analysis. BMJ Open 2024, 14, e075226. [Google Scholar] [CrossRef]
- Chia, A.; Chen, L.; Lai, J.; Wong, C.; Neelakantan, N.; Van Dam, R.; Chong, M.F.-F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 19, 685–695. [Google Scholar] [CrossRef]
- De-Regil, L.; Fernandez-Gaxiola, A.; Dowswell, T.; Pena-Rosas, J. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane 2010. [Google Scholar] [CrossRef]
- Lassi, Z.; Salam, R.; Haider, B.; Bhutta, Z. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane 2013. [Google Scholar] [CrossRef]
- Iqbal, S.; Ekmekcioglu, C. Maternal and neonatal outcomes related to iron supplementation or iron status: A summary of meta-analyses. J. Matern. Fetal Neonatal Med. 2019, 32, 1528–1540. [Google Scholar] [CrossRef]
- Lucchetta, R.; Lemos, I.; Gini, A.; Cavicchioli, S.; Forgerini, M. Deficiency and Insufficiency of Vitamin D in Women of Childbearing Age: A Systematic Review and Meta-analysis. Rev. Bras. Ginecol. Obs./RBGO Gynecol. Obstet. 2022, 44, 409–424. [Google Scholar] [CrossRef]
- Sharifipour, F.; Abedi, P.; Ciahkal, S.; Jahanfar, S.; Mohaghegh, Z.; Zahedian, M. Serum vitamin E level and gestational diabetes mellitus: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2020, 19, 1787–1795. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef]
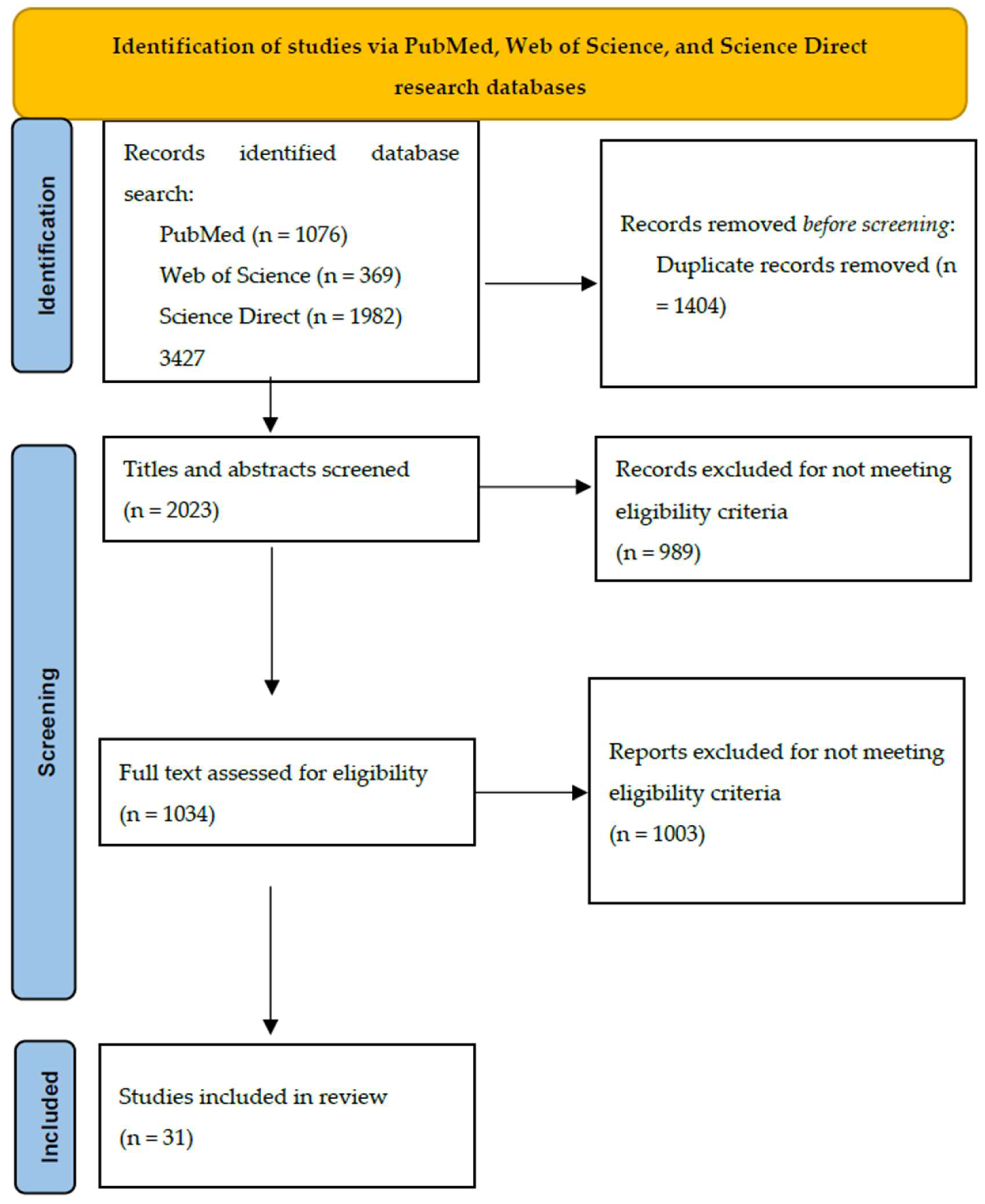
| PubMed | Web of Science | ScienceDirect | |
|---|---|---|---|
| Gestational diabetes mellitus AND small for gestational age/low birth weight/intrauterine growth restriction | 295 | 227 | 1249 |
| Gestational diabetes mellitus AND large for gestational age/macrosomia | 781 | 142 | 733 |
| Total | 1076 | 369 | 1982 |
| 3427 | |||
| Author, Year | Population | Outcome | SGA, Intrauterine Growth Restriction, Low Birth Weight | LGA, Macrosomia | |
|---|---|---|---|---|---|
| 1 | Venkatesh 2022 [11] | 1,560,822 | race/ethnicity | The rate of SGA significantly increased for Asian/Pacific Islander and Hispanic individuals. Black individuals were also associated a higher risk of SGA. | The rate of LGA significantly decreased for all subgroups. American Indian individuals had a higher risk of LGA and macrosomia. |
| 2 | Filardi 2022 [12] | 399 | ethnicity | SGA was noted in 14.3% of the high-risk group (Asian, African, and Hispanic), vs. 4.8% of the low-risk population (Caucasian). | LGA was noted in 21.4% of the high-risk population, vs. 7.2% of the low-risk population. |
| 3 | Xiang 2015 [13] | 29,544 | race/ethnicity | The SGA risks were 11.3% in the Filipino population, 10.4% in the Asian Indian population, 9.5% in the NHW population, 9.4% in the Vietnamese population, 8.9% in the Hispanic population, 7.5% in the NHB population, and 5.6% in the PI population. Chinese, Korean, and Japanese women registered the lowest risks for SGA. After adjustments, none of the other racial/ethnic groups had a statistically significant risk of SGA compared to NHW. | The highest LGA risk was reported in the NHB population (17.2%), followed by PI (16.2%), Hispanic (14.5%), NHW (13.1%), Asian Indian (12.8%), and Filipino (11.6%). Chinese, Korean, and Japanese women registered the lowest risks for LGA. After adjustments, only NHB had a significantly increased risk of LGA compared to NHW. |
| 4 | Nielsen 2021 [14] | 710,413 | ethnicity/migration | Among Danish women, SGA proved to have lower odds. Forty to fifty percent reduced odds of SGA were noted in populations from countries such as the Former Yugoslavia, Turkey, Somalia, Afghanistan, Sri Lanka, Pakistan, and other non-Western countries. | The presence of GDM doubled the odds of LGA among women from all countries; this was not statistically significant among women from India or China. The odds for LGA were significantly lower among women from India, Lebanon, Pakistan, Iraq, Turkey, and Somalia compared to Danish women. |
| 5 | Zhang 2014 [15] | 24,551 | maternal age | The lowest rate of SGA was recorded in the 30–34 group (15.4%), and the highest rate was noted in the 40–44 group, 19.7%. | The 20–24 group had the lowest rate of LGA (14.9%), while the 35–39 and 40–44 groups had the highest rates: 21.1% and 21.2%, respectively. |
| 6 | Lu 2023 [16] | 52,544 | maternal age | No associations were found between GDM and SGA in women over 45 years old. GDM was associated with a significantly reduced risk of low birth weight. | N/A |
| 7 | Krstevska 2016 [17] | 243 | lipid parameters | DM2 group had a statistically significant higher rate of SGA (20%) compared to GDM group (7.5%), likely due to the higher percentage of preterm delivery. | Linear multiple regression analysis demonstrated that triglycerides, LDL-C, and total cholesterol were independent predictors of LGA. BMI was not independent predictor for LGA. |
| 8 | Pereira 2024 [18] | 659 | lipid parameters | N/A | Lower levels of HDL cholesterol were noted for women with LGA infants compared to the two other groups, as well as for women with AGA infants compared to women with SGA infants. Mothers with LGA infants had statistically significant greater pre-pregnancy BMI than mothers who had given birth to AGA and SGA infants, respectively (32.1 vs. 29.8 vs. 28.7). |
| 9 | Simeonova 2014 [19] | 200 | lipid parameters | Maternal triglyceride levels and HbA1c in the second trimester were significantly higher in the SGA group than in the AGA group (3.8 ± 1.9 vs. 3.1 ± 1.1 mmol/L and 6.8 ± 0.8 vs. 5.5 ± 0.8%, p < 0.05). | Maternal triglyceride levels and HbA1c in the second trimester were higher, and HDL-C was significantly lower, in the LGA group than in the AGA group (3.8 ± 1.8 vs. 3.1 ± 1.1 mmol/L, 6.1 ± 1.1 vs. 5.5 ± 0.8%, and 1.3 ± 0.4 vs. 1.6 ± 0.4 mmol/L, p < 0.05). Maternal triglycerides were independent predictors for delivering LGA newborns in GDM women. |
| 10 | Peng 2025 [20] | 10,490 | lipid parameters | There was no association between maternal lipid levels in women with GDM and the risk of SGA. | Compared with women with GDM with TG levels below the 10th percentile, those with TG levels over the 90th percentile had increased risks of LGA offspring and macrosomia; this risk was stronger than that in women without GDM. |
| 11 | Drever 2023 [21] | 308 | pregestational BMI | Women with SGA offsprings had a significantly lower median pre-pregnancy BMI (21.5 vs. 24.4). The absolute risk of having an SGA infant in women with a low pBMI was 27.3% compared to 7.9% in those who had a normal to high BMI. | N/A |
| 12 | Yang 2023 [22] | 6174 | pregestational BMI | Underweight women had a high risk for low birth weight and SGA. | Compared to women with a normal pBMI, women with obesity had a higher risk for macrosomia and LGA; 4.61% and 5.02%, respectively, of the associations were mediated by GDM. |
| 13 | Kondracki 2022 [23] | 3,229,783 | pregestational BMI | N/A | The highest prevalence of GDM was among pregestational overweight/obesity that also had the highest rates of LGA births at term. |
| 14 | Chen Xu 2022 [24] | 13,467 | pregestational BMI | Pregestational overweight and obesity reduced the risk for SGA. Obesity alone was associated with a decreased risk of low-birth-weight newborns. | Obesity alone was associated with a higher risk of macrosomia and LGA. |
| 15 | Liao 2025 [25] | 791 | pregestational BMI | The rate of the SGA babies increased with higher pre-pregnancy BMI. Neonatal birth weight displayed a decrease as maternal pBMI increased when maternal pBMI was greater than 27.78 kg/m2. | The percentage of LGA babies was higher in women with overweight or obesity compared to those of normal weight. Neonatal birth weight displayed a significantly increasing trend with increasing maternal pBMI when maternal pBMI was less than 27.78 kg/m2. |
| 16 | Saito 2022 [26] | 85,228 | pregestational BMI | The highest percentage of SGA was 12% in the underweight GDM group, but the OR of SGA was slightly elevated in the overweight/obese GDM group compared with the overweight/obese non-GDM group. | The incidence of LGA positively correlated with BMI. |
| 17 | Hu 2022 [27] | 17,260 | pregestational BMI | Compared to the maternal normal-weight group, the underweight group had 1.64 times the risk to have SGA neonates. | The overweight and obese groups had 1.79 and 2.76 times the risks of having LGA newborns. |
| 18 | Chen 2022 [28] | 1428 | BMI | N/A | Compared to those without GDM, subjects with GDM were 7.55 times more likely to deliver LGA babies. Women with both pre-pregnancy and pregnancy overweightness/obesity were 3.64 times more likely to deliver LGA. |
| 19 | Song 2022 [29] | 15,065 | pregestational BMI | pBMI over 24 kg/m2 was associated with a decreased risk of SGA. | pBMI over 24 kg/m2 had a higher risk of LGA. |
| 20 | Bruno 2017 [30] | 131 | maternal diet | The incidence of SGA was not different between the two groups (control and intervention). | Women who followed a hypocaloric, low-glycemic, low-saturated fat diet and physical activity recommendations had a significantly lower rate of LGA and macrosomia. |
| 21 | Apostolopoulou 2023 [31] | 90 | maternal diet | Higher fat intake compared to non-SGA during period B was associated with an increased risk for SGA neonates; lower intakes of carbohydrates, fiber intake, magnesium, and copper intake during period B were significantly associated with a decreased risk for SGA neonates. | N/A |
| 22 | Siargkas 2025 [32] | 117 | macronutrient intake | N/A | In normal-BMI women, higher dietary fiber and vegetable protein intake before pregnancy was significantly associated with an increased risk of LGA; the risk from vegetable protein persisted in early pregnancy. During early pregnancy, a higher percentage of total carbohydrate and vegetable protein intake was linked to increased LGA; low intake of saturated fatty acids reduced the odds of LGA. |
| 23 | Xie 2024 [33] | 400 | chol intake | In the second trimester, GDM women with high cholesterol intake had lower risks of SGA. | In the second trimester, GDM women with high cholesterol intake had higher risks of macrosomia and LGA. In the third trimester, GDM women with high cholesterol intake had lower risks of macrosomia and LGA. |
| 24 | Messika 2022 [34] | 103 | nutrition, sleep | N/A | The intervention had no effect on the proportion of large-for-gestational-age newborns. |
| 25 | Cheng 2019 [35] | 950 | folic acid supplementation | FA supplementation for ≥3 months before pregnancy was associated with an increased risk of GDM and decreased risk of SGA birth. | In the group of FA supplementation for ≥3 months during pregnancy, GDM was associated with an increased risk of macrosomia. |
| 26 | Soubasi 2010 [36] | 63 | ferritin | The rate of IUGR was significantly higher in the group with neonates whose mothers had high ferritin levels. | N/A |
| 27 | Weinert 2016 [37] | 184 | vitamin D | Vitamin D deficiency associated higher rates of SGA. After adjustment, relative risk for SGA was 4.32. | N/A |
| 28 | Zhou 2022 [38] | 19,647 | vitamin E | N/A | Maternal vitamin E concentrations in the first and second trimesters were positively associated with GDM and LGA |
| 29 | Pang 2023 [39] | 18,250 | uric acid | Serum uric acid showed a linear correlation with SGA. | Hyperuricemia is associated with a higher incidence of LGA. No significant associations were found between UA and macrosomia |
| 30 | Kim 2022 [40] | 710 | postprandial free fatty acids | N/A | Levels of 2h-FFA were higher in women who delivered LGA newborns than in those who delivered non-LGA newborns. Fasting FFA was not significantly different between the two groups. |
| 31 | Chatzakis 2025 [41] | 11,132 | vascular assessment | Total peripheral resistance, ophthalmic artery peak systolic velocity, and uterine artery pulsatility index were positively correlated with SGA. SGA groups had the highest uterine artery pulsatility index percentiles, carotid-femoral pulse wave velocity, and ophthalmic artery peak systolic velocity ratios. Compared to AGA groups, cardiac output was lower in the SGA groups. | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotă, A.; Petca, A. Gestational Diabetes Mellitus: The Dual Risk of Small and Large for Gestational Age: A Narrative Review. Med. Sci. 2025, 13, 144. https://doi.org/10.3390/medsci13030144
Fotă A, Petca A. Gestational Diabetes Mellitus: The Dual Risk of Small and Large for Gestational Age: A Narrative Review. Medical Sciences. 2025; 13(3):144. https://doi.org/10.3390/medsci13030144
Chicago/Turabian StyleFotă, Andreea, and Aida Petca. 2025. "Gestational Diabetes Mellitus: The Dual Risk of Small and Large for Gestational Age: A Narrative Review" Medical Sciences 13, no. 3: 144. https://doi.org/10.3390/medsci13030144
APA StyleFotă, A., & Petca, A. (2025). Gestational Diabetes Mellitus: The Dual Risk of Small and Large for Gestational Age: A Narrative Review. Medical Sciences, 13(3), 144. https://doi.org/10.3390/medsci13030144






