Mapping Healthcare Needs: A Systematic Review of Population Stratification Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- A.
- Inclusion criteria
- B.
- Exclusion criteria
- 1.
- Duplicate studies
- 2.
- Systematic reviews
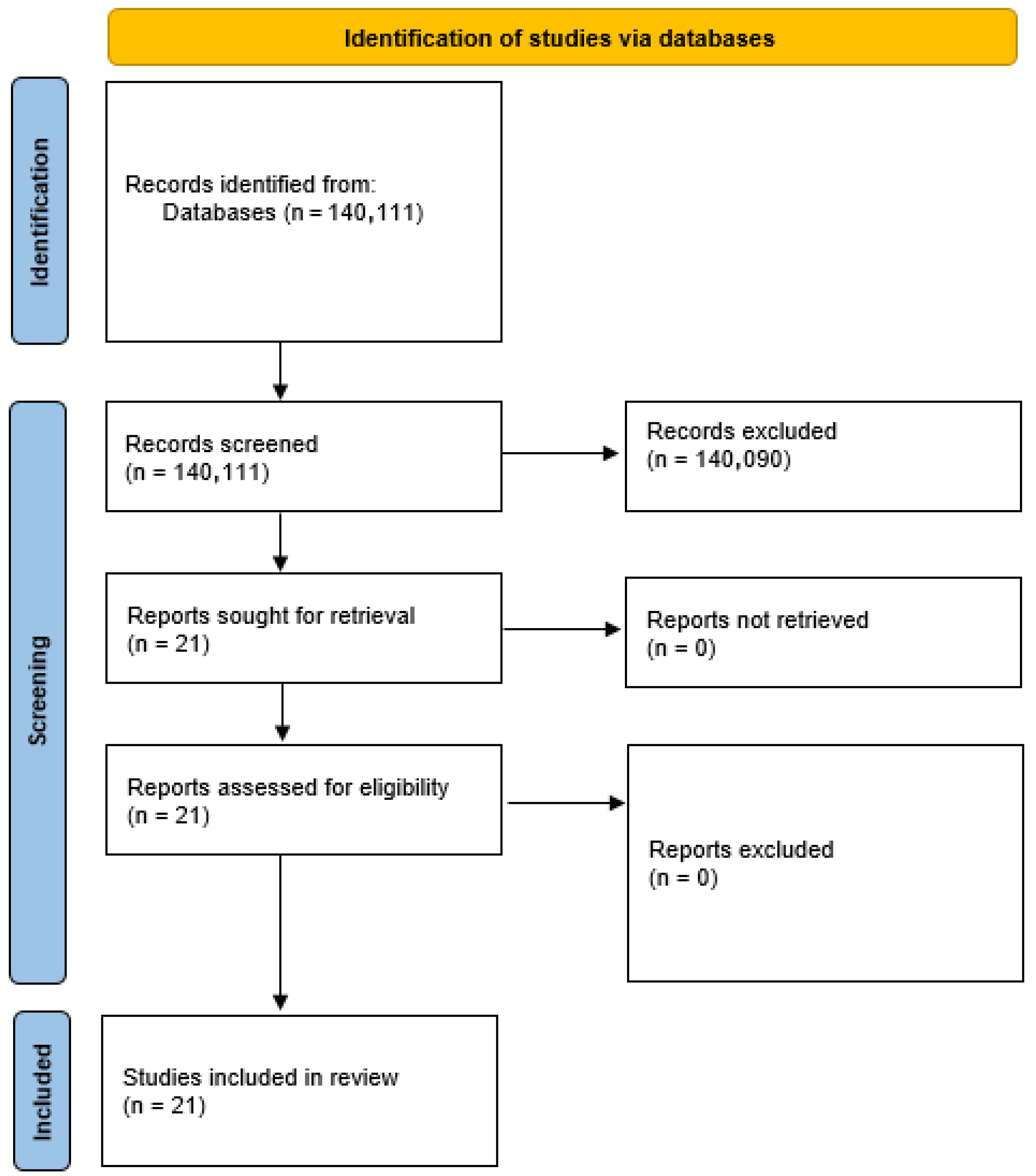
2.2. Selection Process
3. Results
3.1. Models Applied in Italy
3.1.1. Lazio Region
3.1.2. Emilia-Romagna Region
3.1.3. Lombardy Region
3.1.4. Umbria Region
3.1.5. Veneto Region
4. Discussion
4.1. Summary of Main Results
4.2. Generalizability of the Retrieved Results
4.3. Implications for the Daily Practice of Potential Readers [Including Public Health Professionals]
4.4. Limitations
4.5. Validity
- Predictive validity: This was the most common approach, where a tool’s performance was assessed based on its ability to accurately predict future outcomes. Examples from our review include forecasting future healthcare expenditures, predicting hospitalizations or emergency department visits, and assessing mortality risks.
- Construct validity: This approach involved evaluating a tool’s ability to segment a population into distinct, meaningful groups. Validation was demonstrated by showing statistically significant differences in healthcare utilization patterns, demographic profiles, or clinical complexity across the generated segments.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Italy: Public Healthcare Expenditure. 2023. Available online: https://www.statista.com/statistics/793788/public-healthcare-expenditure-in-italy/ (accessed on 20 June 2025).
- Ricciardi, W.; Atella, V.; Cricelli, C.; Serra, F. La Tempesta Perfetta. Ⅱ Possibile Naufragio Del Servizio Sanitario Nazionale: Come Evitarlo? Vita e Pensiero, Ed.; Vita e Pensiero: Milan, Italy, 2015; ISBN 9788834329573. [Google Scholar]
- Lopreite, M.; Mauro, M. The Effects of Population Ageing on Health Care Expenditure: A Bayesian VAR Analysis Using Data from Italy. Health Policy 2017, 121, 663–674. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lynn, J.; Straube, B.M.; Bell, K.M.; Jencks, S.F.; Kambic, R.T. Using Population Segmentation to Provide Better Health Care for All: The “Bridges to Health” Model. Milbank Q. 2007, 85, 185–208. [Google Scholar] [CrossRef] [PubMed]
- De Luca d’Alessandro, E.; Bonacci, S.; Giraldi, G. Aging Populations: The Health and Quality of Life of the Elderly. Clin. Ter. 2011, 162, e13–e18. [Google Scholar]
- Girwar, S.M.; Jabroer, R.; Fiocco, M.; Sutch, S.P.; Numans, M.E.; Bruijnzeels, M.A. A Systematic Review of Risk Stratification Tools Internationally Used in Primary Care Settings. Health Sci. Rep. 2021, 4, e329. [Google Scholar] [CrossRef]
- World Health Organization. Population Health Management in Primary Health Care: A Proactive Approach to Improve Health and Well-Being: Primary Heath Care Policy Paper Series. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2023-7497-47264-69316 (accessed on 20 June 2025).
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-Quality Health Systems in the Sustainable Development Goals Era: Time for a Revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef]
- Delaware State Innovation Models (SIM) Initiative-Delaware Health and Social Services-State of Delaware. Available online: https://dhss.delaware.gov/dhcc/sim.html (accessed on 8 April 2024).
- Corrao, G.; Bonaugurio, A.S.; Chen, Y.X.; Franchi, M.; Lora, A.; Leoni, O.; Pavesi, G.; Bertolaso, G. Improved Prediction of 5-Year Mortality by Updating the Chronic Related Score for Risk Profiling in the General Population: Lessons from the Italian Region of Lombardy. Front. Public. Health 2023, 11, 1173957. [Google Scholar] [CrossRef]
- 3MTM Clinical Risk Groups (CRGs)|3M. Available online: https://www.3m.com/3M/en_US/health-information-systems-us/drive-value-based-care/patient-classification-methodologies/crgs/ (accessed on 8 April 2024).
- Joynt, K.E.; Figueroa, J.F.; Beaulieu, N.; Wild, R.C.; Orav, E.J.; Jha, A.K. Segmenting High-Cost Medicare Patients into Potentially Actionable Cohorts. Healthcare 2017, 5, 62–67. [Google Scholar] [CrossRef]
- Low, L.L.; Kwan, Y.H.; Liu, N.; Jing, X.; Low, E.C.T.; Thumboo, J. Evaluation of a Practical Expert Defined Approach to Patient Population Segmentation: A Case Study in Singapore. BMC Health Serv. Res. 2017, 17, 771. [Google Scholar] [CrossRef]
- Eissens van der Laan, M.R.; van Offenbeek, M.A.G.; Broekhuis, H.; Slaets, J.P.J. A Person-Centred Segmentation Study in Elderly Care: Towards Efficient Demand-Driven Care. Soc. Social. Sci. Med. 2014, 113, 68–76. [Google Scholar] [CrossRef]
- Liu, L.-F.; Tian, W.-H.; Yao, H.-P. Utilization of Health Care Services by Elderly People with National Health Insurance in Taiwan: The Heterogeneous Health Profile Approach. Health Policy. 2012, 108, 246–255. [Google Scholar] [CrossRef]
- Lafortune, L.; Béland, F.; Bergman, H.; Ankri, J. Health State Profiles and Service Utilization in Community-Living Elderly. Med. Care 2009, 47, 286–294. [Google Scholar] [CrossRef]
- Vuik, S.I.; Mayer, E.; Darzi, A. A Quantitative Evidence Base for Population Health: Applying Utilization-Based Cluster Analysis to Segment a Patient Population. Popul. Health Metr. 2016, 14, 44. [Google Scholar] [CrossRef]
- Low, L.L.; Yan, S.; Kwan, Y.H.; Tan, C.S.; Thumboo, J. Assessing the Validity of a Data Driven Segmentation Approach: A 4 Year Longitudinal Study of Healthcare Utilization and Mortality. PLoS ONE 2018, 13, e0195243. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Shen, E.; Shah, N.R.; Glenn, B.A.; Ponce, N.A.; Telesca, D.; Gould, M.K.; Needleman, J. Segmentation of High-Cost Adults in an Integrated Healthcare System Based on Empirical Clustering of Acute and Chronic Conditions. J. Gen. Intern. Med. 2018, 33, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Duminy, L.; Sivapragasam, N.R.; Matchar, D.B.; Visaria, A.; Ansah, J.P.; Blankart, C.R.; Schoenenberger, L. Validation and Application of a Needs-Based Segmentation Tool for Cross-Country Comparisons. Health Serv. Res. 2021, 56, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Dorr, D.A.; Ross, R.; Cohen, D.A.; Kansagara, D.; Ramsey, K.; Sachdeva, B.; Weiner, J.P. Primary Care Practices’ Ability to Predict Future Risk of Expenditures and Hospitalization Using Risk Stratification and Segmentation. BMC Med. Inf. Inform. Decis. Mak. 2021, 21, 104. [Google Scholar] [CrossRef]
- Nobilio, L.; Sforza, S.; Berti, E.; Moro, M. Guida Alla Stratificazione Del Rischio Della Popolazione Residente in Emilia-Romagna Con Dati Amministrativi: L’algoritmo RiskER. Available online: https://assr.regione.emilia-romagna.it/pubblicazioni/rapporti-documenti/report-risker-2021. (accessed on 8 April 2025).
- Hewner, S.; Wu, Y.-W.B.; Castner, J. Comparative Effectiveness of Risk-Stratified Care Management in Reducing Readmissions in Medicaid Adults with Chronic Disease. J. Healthc. Qual. 2016, 38, 3–16. [Google Scholar] [CrossRef]
- Alderwick, H.; Buck, D.; Ham, S.C. Population Health Systems|the King’s Fund. Available online: https://www.kingsfund.org.uk/insight-and-analysis/reports/population-health-systems (accessed on 13 March 2025).
- Hewner, S. Getting HIE “Just Right” for Population-Level Clinical Decision Support; PCAM: Strasbourg, France, 2015. [Google Scholar]
- Schuurmans, H.; Steverink, N.; Lindenberg, S.; Frieswijk, N.; Slaets, J.P.J. Old or Frail: What Tells Us More? J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M962–M965. [Google Scholar] [CrossRef]
- de Jonge, P.; Latour, C.; Huyse, F.J. Interrater Reliability of the INTERMED in a Heterogeneous Somatic Population. J. Psychosom. Res. 2002, 52, 25–27. [Google Scholar] [CrossRef]
- Whitson, H.E.; Johnson, K.J.; Sloane, R.; Cigolle, C.T.; Pieper, C.F.; Landerman, L.R.; Hastings, S.N. Identifying Patterns of Multimorbidity in Older Americans: Application of Latent Class Analysis. J. Am. Geriatr. Soc. 2016, 64, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Fusco, D.; Bontempi, K.; Colais, P.; Davoli, M. Regional Outcome Evaluation Program (P.Re.Val.E.): Reduction of Inequality in Access to Effective Health Care in the Lazio Region of Italy (2012–2015). PLoS ONE 2018, 13, e0194972. [Google Scholar] [CrossRef]
- Johns Hopkins ACG® System. Available online: https://www.hopkinsacg.org/ (accessed on 20 June 2025).
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Chong, J.L.; Lim, K.K.; Matchar, D.B. Population Segmentation Based on Healthcare Needs: A Systematic Review. Syst. Rev. 2019, 8, 202. [Google Scholar] [CrossRef]
- Daumit, G.; Boulware, L.E.; Powe, N.R.; Minkovitz, C.S.; Frick, K.D.; Anderson, L.A.; Janes, G.R.; Lawrence, R.S. A Computerized Tool for Evaluating the Effectiveness of Preventive Interventions. Public. Health Rep. 2001, 116, 244–253. [Google Scholar] [CrossRef]
- Genovese, C.; De Belvis, G.; Rinaldi, M.; Manno, V.; Squeri, R.; La Fauci, V.; Tabbi, P. Quality and Management Care Improvement of Patients with Chronic Kidney Disease: From Data Analysis to the Definition of a Targeted Clinical Pathway in an Italian Region. J. Prev. Med. Hyg. 2018, 59, E305–E310. [Google Scholar] [CrossRef]
- Ministero Della Salute Principali Caratteristiche Diagnosis Related Groups (DRG). Available online: https://www.salute.gov.it/new/it/tema/assistenza-ospedaliera/principali-caratteristiche-diagnosis-related-groups-drg/ (accessed on 8 April 2024).
- AGENAS. Progetto It.DRG, al via Il Sistema “Made in Italy”. Available online: https://www.agenas.gov.it/aree-tematiche/monitoraggio-e-valutazione/monitoraggio-della-spesa-sanitaria?view=article&id=620:progetto-itdrg-sistema-made-italy&catid=116 (accessed on 8 April 2024).
- Ministero Delle Imprese e del Made in Italy. Piano Nazionale Ripresa E Resilienza. Available online: https://www.mimit.gov.it/it/pnrr/piano (accessed on 20 June 2025).
- Mondello, C.; Baldino, G.; Bottari, A.; Sapienza, D.; Perri, F.; Argo, A.; Asmundo, A.; Ventura Spagnolo, E. The Role of PMCT for the Assessment of the Cause of Death in Natural Disaster (Landslide and Flood): A Sicilian Experience. Int. J. Leg. Med. 2021, 136, 237–244. [Google Scholar] [CrossRef]
- Ventura Spagnolo, E.; Mondello, C.; Di Mauro, D.; Vermiglio, G.; Asmundo, A.; Filippini, E.; Alibrandi, A.; Rizzo, G. Analysis on Sarcoglycans Expression as Markers of Septic Cardiomyopathy in Sepsis-Related Death. Int. J. Leg. Med. 2018, 132, 1685–1692. [Google Scholar] [CrossRef]
| Element | Description |
|---|---|
| Population | Patients of all ages with chronic diseases |
| Intervention | Application of the population health management (PHM) technique |
| Comparison | Other available tools or no intervention |
| Outcome | Reduction in healthcare costs (e.g., fewer hospitalizations, better management) |
| Application | Applicable across various clinical settings and chronic patient populations |
| Tool/Model | Method | Stratification Type | Peer Review | Owner | Needs Full EHR | # Segments |
|---|---|---|---|---|---|---|
| Bridges to Health [5] | Expert | Clinical | ✘ | ✘ | ✘ | 8 |
| COMPLEXedex [6] | Expert | Clinical, Lifestyle | ✘ | ✔ | ✔ | 4 |
| SSA—Senior Segmentation Algorithm | Expert | Clinical | ✔ | ✔ | ✔ | 3 |
| Delaware Clustering [10] | Expert | Clinical | ✘ | ✘ | ✔ | 20 |
| Lombardy Region Segmentation [11] | Guided Expert | Clinical, Demographic | ✘ | ✘ | ✔ | 8 |
| 3M™ Clinical Risk Groups [12] | Guided Expert | Clinical, Demographic | ✔ | ✔ | ✔ | 6–269 |
| Medicare Segmentation [13] | Guided Expert | Clinical, Fragility, Demographic | ✔ | ✘ | ✔ | 6 |
| British Columbia Matrix | Guided Expert | Clinical, Demographic | ✘ | ✘ | ✔ | 14 |
| Singapore MOH Segmentation [14] | Expert | Clinical, Utilization | ✔ | ✘ | ✔ | 6 |
| North West London Segmentation | Data + Expert | Clinical, Demographic | ✘ | ✘ | ✔ | 10 |
| ACG—Adjusted Clinical Groups | Data + Expert | Clinical, Demographic | ✔ | ✔ | ✔ | 92 |
| Demand-Driven Model [15] | Data-Driven | Clinical, Functional | ✔ | ✘ | ✘ | 5 |
| LCA (Taiwan NHIS) [16] | Data-Driven | Clinical, Functional, Socio-demographic | ✔ | ✘ | ✘ | 4 |
| LCA (SIPA) [17] | Data-Driven | Clinical, Functional, Socio-demographic | ✔ | ✘ | ✘ | 4 |
| Utilization-Based Segmentation [18] | Data-Driven | Utilization | ✘ | ✘ | ✔ | 8 |
| Utilization/Demographic [19] | Data-Driven | Utilization, Demographic | ✔ | ✘ | ✔ | 5 |
| Davis et al. [20] | Data-Driven | Clinical | ✘ | ✘ | ✔ | 7–53 |
| Whitson et al. [21] | Data + Expert | Probabilistic | ✘ | ✘ | ✔ | 6 |
| Dorr et al. [22] | Data-Driven | Clinical, Probabilistic | ✔ | ✘ | ✘ | 2 |
| RiskER—Emilia-Romagna Region [23] | Data-Driven | Probabilistic | ✘ | ✘ | ✔ | 4 |
| CCSST [21] | Data-Driven | Clinical | ✘ | ✘ | ✘ | 5–20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, G.; Rizzo, C.E.; Nirta, A.; Bartucciotto, L.; Venuto, R.; Fedele, F.; Squeri, R.; Genovese, C. Mapping Healthcare Needs: A Systematic Review of Population Stratification Tools. Med. Sci. 2025, 13, 145. https://doi.org/10.3390/medsci13030145
Genovese G, Rizzo CE, Nirta A, Bartucciotto L, Venuto R, Fedele F, Squeri R, Genovese C. Mapping Healthcare Needs: A Systematic Review of Population Stratification Tools. Medical Sciences. 2025; 13(3):145. https://doi.org/10.3390/medsci13030145
Chicago/Turabian StyleGenovese, Giovanni, Caterina Elisabetta Rizzo, Antonio Nirta, Linda Bartucciotto, Roberto Venuto, Francesco Fedele, Raffaele Squeri, and Cristina Genovese. 2025. "Mapping Healthcare Needs: A Systematic Review of Population Stratification Tools" Medical Sciences 13, no. 3: 145. https://doi.org/10.3390/medsci13030145
APA StyleGenovese, G., Rizzo, C. E., Nirta, A., Bartucciotto, L., Venuto, R., Fedele, F., Squeri, R., & Genovese, C. (2025). Mapping Healthcare Needs: A Systematic Review of Population Stratification Tools. Medical Sciences, 13(3), 145. https://doi.org/10.3390/medsci13030145







