Psychic and Cognitive Impacts of Cardiovascular Disease: Evidence from an Observational Study and Comparison by a Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Setting
2.2. Measures
2.3. Statistical Analysis
2.4. Results of Observational Study
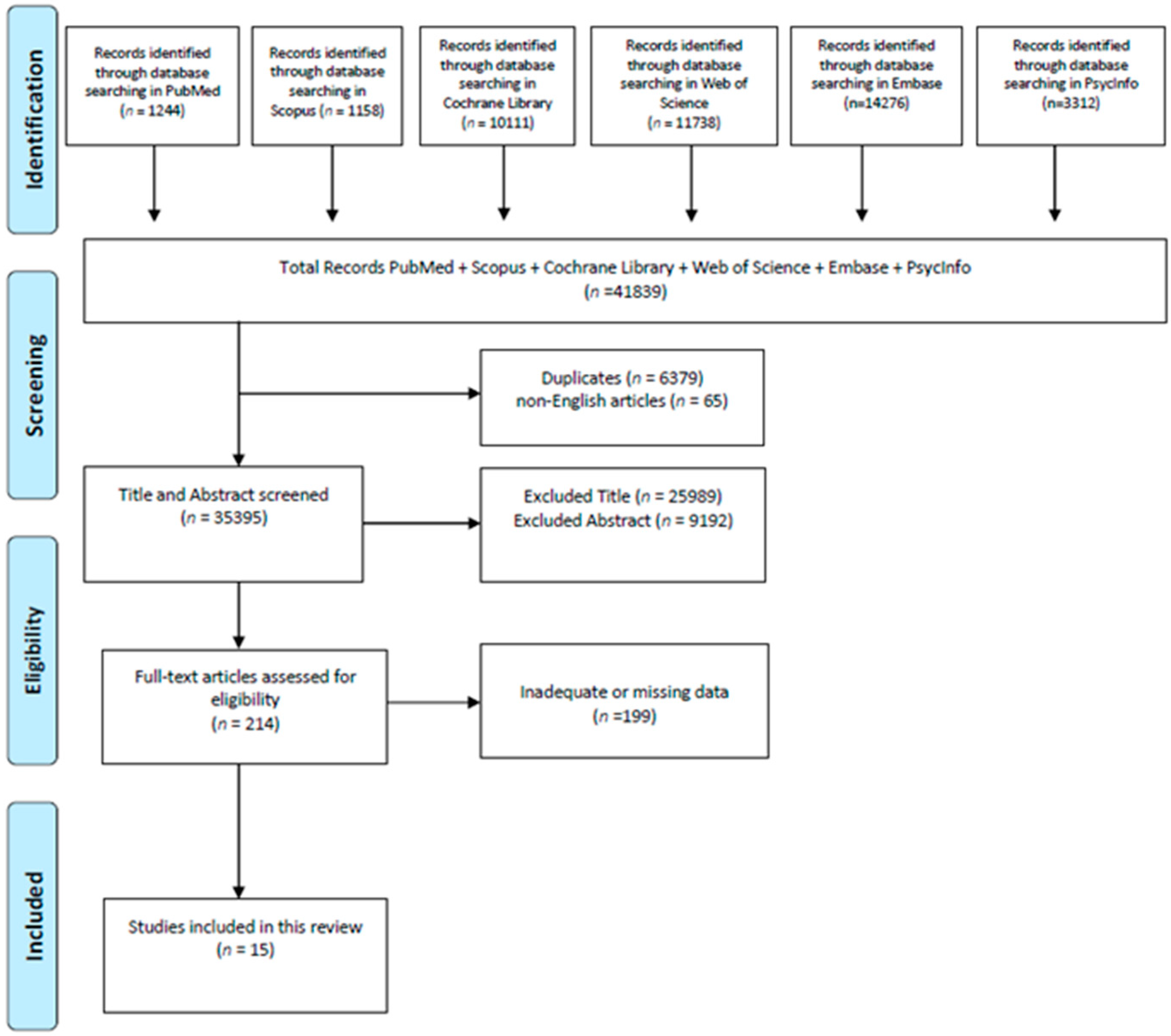
3. Materials and Methods of the Systematic Review
3.1. Search Strategy
3.2. Inclusion Criteria
3.3. Exclusion Criteria
3.4. Study Selection and Quality Assessment
4. Results
4.1. Overall Results
4.2. Cognitive Assessment Results
4.3. Depression Assessment Results
4.4. Anxiety Assessment Results
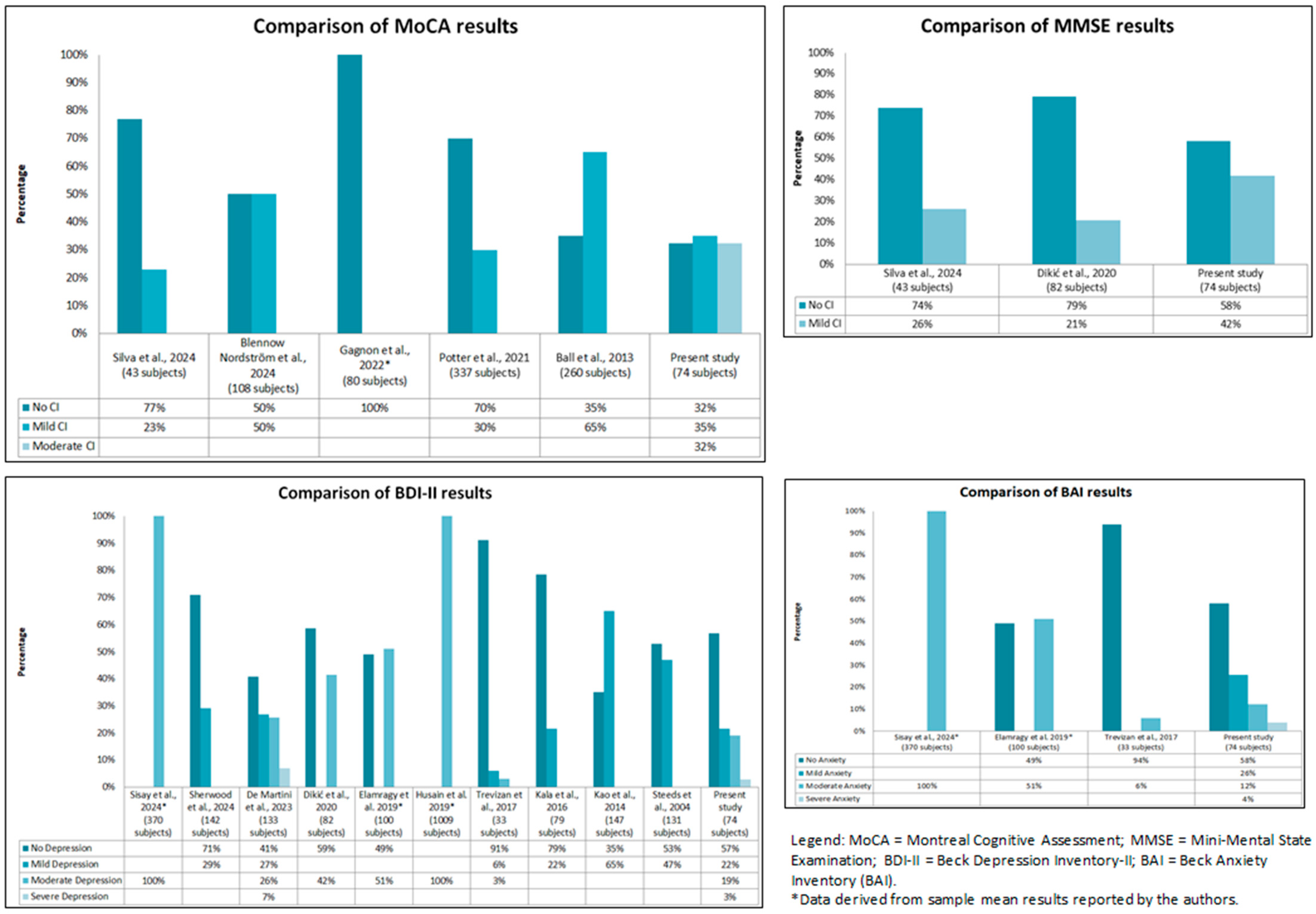
4.5. Comparison Between the Present Observational Study with the Literature
5. Discussion
5.1. General Results
5.2. Cognitive Impairments
5.3. Anxiety and Depression
5.4. Strengths and Limitations
5.5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamanouchi, D. Molecular Insights into Cardiovascular Disease: Unraveling Pathways for Diagnosis and Treatment. Int. J. Mol. Sci. 2025, 26, 2067. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.P. Genetic Markers of Cardiovascular Disease. Curr. Probl. Cardiol. 2024, 49, 102588. [Google Scholar] [CrossRef] [PubMed]
- Dankar, R.; Wehbi, J.; Refaat, M.M. Tailoring Treatment in Cardiovascular Diseases: The Role of Targeted Therapies. Pharmaceutics 2024, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Cousineau, J.P.; Dawe, A.M.; Alpaugh, M. Investigating the Interplay between Cardiovascular and Neurodegenerative Disease. Biology 2024, 13, 764. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.C.; Celano, C.M.; Beach, S.R.; Motiwala, S.R.; Januzzi, J.L. Depression and cardiac disease: Epidemiology, mechanisms, and diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 2013, 695925. [Google Scholar] [CrossRef]
- Civieri, G.; Abohashem, S.; Grewal, S.S.; Aldosoky, W.; Qamar, I.; Hanlon, E.; Choi, K.W.; Shin, L.M.; Rosovsky, R.P.; Bollepalli, S.C.; et al. Anxiety and Depression Associated with Increased Cardiovascular Disease Risk Through Accelerated Development of Risk Factors. JACC Adv. 2024, 3, 101208. [Google Scholar] [CrossRef]
- Jha, M.K.; Qamar, A.; Vaduganathan, M.; Charney, D.S.; Murrough, J.W. Screening and Management of Depression in Patients with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1827–1845. [Google Scholar] [CrossRef]
- Madaudo, C.; Coppola, G.; Parlati, A.L.M.; Corrado, E. Discovering Inflammation in Atherosclerosis: Insights from Pathogenic Pathways to Clinical Practice. Int. J. Mol. Sci. 2024, 25, 6016. [Google Scholar] [CrossRef]
- Işık, B.; Erbaş, O. Depression and Cardiovascular Disease: A mutual relationship. J. Exp. Basic Med. Sci. 2023, 4, 21–27. [Google Scholar] [CrossRef]
- Plante, G.E. Depression and cardiovascular disease: A reciprocal relationship. Metab. Clin. Exp. 2005, 54, 45–48. [Google Scholar] [CrossRef]
- Warriach, Z.I.; Patel, S.; Khan, F.; Ferrer, G.F. Association of Depression with Cardiovascular Diseases. Cureus 2022, 14, e26296. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. Mental Stress and Cardiovascular Health-Part I. J. Clin. Med. 2022, 11, 3353. [Google Scholar] [CrossRef] [PubMed]
- Kwek, S.Q.; Yeo, T.M.; Teo, J.Y.C.; Seah, C.W.A.; Por, K.N.J.; Wang, W. Effectiveness of therapist-supported internet-based cognitive behavioural therapy interventions on depression, anxiety and quality of life among patients with cardiovascular disease: A systematic review and meta-analysis. Eur. J. Cardiovasc. Nurs. 2025, 00, 1–11. [Google Scholar] [CrossRef]
- Eifert, G.H.; Thompson, R.N.; Zvolensky, M.J.; Edwards, K.; Frazer, N.L.; Haddad, J.W.; Davig, J. The cardiac anxiety questionnaire: Development and preliminary validity. Behav. Res. Ther. 2000, 38, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Qin, Z.; Yang, Y.; Gao, T.; Ren, H.; Hu, Y.; Cao, R.; Liang, L.; Li, C.; Tong, Q. Influence of Life Satisfaction on Quality of Life: Mediating Roles of Depression and Anxiety Among Cardiovascular Disease Patients. Clin. Nurs. Res. 2021, 30, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Wedegärtner, S.M.; Langheim, E.; Kleinschmidt, J.; Köllner, V. Heart-Focused Anxiety Affects Behavioral Cardiac Risk Factors and Quality of Life: A Follow-Up Study Using a Psycho-Cardiological Rehabilitation Concept. Front. Psychiatry 2022, 13, 836750. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Pietrzykowski, Ł.; Kosobucka-Ozdoba, A.; Michalski, P.; Kasprzak, M.; Ratajczak, J.; Rzepka-Cholasińska, A.; Siódmiak, J.; Grzelakowska, K.; Kubica, A. The Impact of Anxiety and Depression Symptoms on Cardiovascular Risk Factor Control in Patients Without a History of Atherosclerotic Cardiovascular Disease. Vasc. Health Risk Manag. 2024, 20, 301–311. [Google Scholar] [CrossRef]
- Micali, G.; Corallo, F.; Pagano, M.; Giambò, F.M.; Duca, A.; D’Aleo, P.; Anselmo, A.; Bramanti, A.; Garofano, M.; Mazzon, E.; et al. Artificial Intelligence and Heart-Brain Connections: A Narrative Review on Algorithms Utilization in Clinical Practice. Healthcare 2024, 12, 1380. [Google Scholar] [CrossRef]
- Cannon, J.A.; Moffitt, P.; Perez-Moreno, A.C.; Walters, M.R.; Broomfield, N.M.; McMurray, J.J.V.; Quinn, T.J. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J. Card. Fail. 2017, 23, 464–475. [Google Scholar] [CrossRef]
- Pagano, M.; Corallo, F.; D’Aleo, P.; Duca, A.; Bramanti, P.; Bramanti, A.; Cappadona, I. A Set of Possible Markers for Monitoring Heart Failure and Cognitive Impairment Associated: A Review of Literature from the Past 5 Years. Biomolecules 2024, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Loke, Y.K.; Hale, R.; Potter, J.F.; Myint, P.K. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology 2011, 76, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Marzona, I.; O’Donnell, M.; Teo, K.; Gao, P.; Anderson, C.; Bosch, J.; Yusuf, S. Increased risk of cognitive and functional decline in patients with atrial fibrillation: Results of the ONTARGET and TRANSCEND studies. CMAJ Can. Med. Assoc. J. 2012, 184, E329–E336. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, S.; Ay, H.; Gollub, R.L.; Lee, H.; Retzepi, K.; Mansour, M.; Ruskin, J.N. Association between atrial fibrillation and silent cerebral infarctions: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 161, 650–658. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, E.J. A Correlative Relationship Between Heart Failure and Cognitive Impairment: A Narrative Review. J. Korean Med. Sci. 2023, 38, e334. [Google Scholar] [CrossRef]
- Wolters, F.J.; Segufa, R.A.; Darweesh, S.K.L.; Bos, D.; Ikram, M.A.; Sabayan, B.; Hofman, A.; Sedaghat, S. Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 1493–1504. [Google Scholar] [CrossRef]
- Van Nieuwkerk, A.C.; Delewi, R.; Wolters, F.J.; Muller, M.; Daemen, M.; Biessels, G.J.; Heart-Brain Connection Consortium. Cognitive Impairment in Patients with Cardiac Disease: Implications for Clinical Practice. Stroke 2023, 54, 2181–2191. [Google Scholar] [CrossRef]
- Chiatto, L.M.; Corallo, F.; Calabrò, R.S.; Cardile, D.; Pagano, M.; Cappadona, I. A systematic review about the importance of neuropsychological features in heart failure: Is at heart the only failure? Neurol. Sci. 2024, 45, 3611–3624. [Google Scholar] [CrossRef]
- Cappadona, I.; Ielo, A.; Pagano, M.; Anselmo, A.; Micali, G.; Giambò, F.M.; Duca, A.; D’Aleo, P.; Costanzo, D.; Carcione, G.; et al. Observational protocol on neuropsychological disorders in cardiovascular disease for holistic prevention and treatment. Future Cardiol. 2025, 21, 349–358. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Pezzotti, P.; Scalmana, S.; Mastromattei, A.; Di Lallo, D.; Progetto Alzheimer Working Group. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: A prospective observational study. BMC Fam. Pract. 2008, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II; APA PsycTests: Washington, DC, USA, 1996. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. 2021, 372, n71. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.D.; Sampaio, C.P.B.M.; Guimarães, N.E.D.S.; Moreno, L.P.; Pontes, G.S.; Ferreira, E.J.F.; Figueiredo Neto, J.A. Valutazione della funzione cognitiva nei pazienti anziani con insufficienza cardiaca. Rev. Da Assoc. Medica Bras. 2024, 70, e20240429. [Google Scholar] [CrossRef]
- Blennow Nordström, E.; Evald, L.; Mion, M.; Segerström, M.; Vestberg, S.; Ullén, S.; Heimburg, K.; Gregersen Oestergaard, L.; Grejs, A.M.; Keeble, T.R.; et al. Combined use of the Montreal Cognitive Assessment and Symbol Digit Modalities Test improves neurocognitive screening accuracy after cardiac arrest: A validation sub-study of the TTM2 trial. Resuscitation 2024, 202, 110361. [Google Scholar] [CrossRef]
- Sisay, T.; Mulate, M.; Hailu, T.; Belete, T.M. The prevalence of depression and anxiety among cardiovascular patients at University of Gondar specialized hospital using beck’s depression inventory II and beck anxiety inventory: A cross-sectional study. Heliyon 2024, 10, e24079. [Google Scholar] [CrossRef]
- Sherwood, A.; Blumenthal, J.A.; Mentz, R.J.; Koch, G.G.; Rogers, J.G.; Chang, P.P.; Chien, C.; Adams, K.F., Jr.; Rose-Jones, L.J.; Jensen, B.C.; et al. Depressive symptoms are associated with clinical outcomes in heart failure with reduced ejection fraction. ESC Heart Fail. 2024, 11, 2627–2636. [Google Scholar] [CrossRef]
- De Martini, G.A.; Grisante, D.L.; Gonçalves, A.L.P.; D’Agostino, F.; Lopes, J.L.; Santos, V.B.; Lopes, C.T. Relationships between Depressive Symptoms, Appetite, and Quality of Life in Heart Failure. West. J. Nurs. Res. 2023, 45, 416–424. [Google Scholar] [CrossRef]
- Gagnon, C.; Saillant, K.; Olmand, M.; Gayda, M.; Nigam, A.; Bouabdallaoui, N.; Rouleau, J.L.; Desjardins-Crépeau, L.; Bherer, L. Performances on the Montreal Cognitive Assessment Along the Cardiovascular Disease Continuum. Arch. Clin. Neuropsychol. 2022, 37, 117–124. [Google Scholar] [CrossRef]
- Potter, E.L.; Ramkumar, S.; Wright, L.; Marwick, T.H. Associations of subclinical heart failure and atrial fibrillation with mild cognitive impairment: A cross-sectional study in a subclinical heart failure screening programme. BMJ Open 2021, 11, e045896. [Google Scholar] [CrossRef]
- Dikić, A.; Radmilo, L.; Živanović, Ž.; Keković, G.; Sekulić, S.; Kovačić, Z.; Radmilo, R. Cognitive impairment and depression after acute myocardial infarction: Associations with ejection fraction and demographic characteristics. Acta Neurol. Belg. 2021, 121, 1615–1622. [Google Scholar] [CrossRef]
- Elamragy, A.A.; Abdelhalim, A.A.; Arafa, M.E.; Baghdady, Y.M. Anxiety and depression relationship with coronary slow flow. PLoS ONE 2019, 14, e0221918. [Google Scholar] [CrossRef]
- Husain, M.I.; Chaudhry, I.B.; Husain, M.O.; Abrol, E.; Junejo, S.; Saghir, T.; Ur Rahman, R.; Soomro, K.; Bassett, P.; Khan, S.A.; et al. Depression and congestive heart failure: A large prospective cohort study from Pakistan. J. Psychosom. Res. 2019, 120, 46–52. [Google Scholar] [CrossRef]
- Trevizan, F.B.; Miyazaki, M.C.O.S.; Silva, Y.L.W.; Roque, C.M.W. Quality of Life, Depression, Anxiety and Coping Strategies after Heart Transplantation. Braz. J. Cardiovasc. Surg. 2017, 32, 162–170. [Google Scholar] [CrossRef][Green Version]
- Kala, P.; Hudakova, N.; Jurajda, M.; Kasparek, T.; Ustohal, L.; Parenica, J.; Sebo, M.; Holicka, M.; Kanovsky, J. Depression and Anxiety after Acute Myocardial Infarction Treated by Primary PCI. PLoS ONE 2016, 11, e0152367. [Google Scholar] [CrossRef]
- Kao, C.W.; Chen, T.Y.; Cheng, S.M.; Lin, W.S.; Friedmann, E.; Thomas, S.A. Gender differences in the predictors of depression among patients with heart failure. Eur. J. Cardiovasc. Nurs. 2014, 13, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.; Carrington, M.J.; Stewart, S.; SAFETY Investigators. Mild cognitive impairment in high-risk patients with chronic atrial fibrillation: A forgotten component of clinical management? Heart 2013, 99, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Steeds, R.P.; Bickerton, D.; Smith, M.J.; Muthusamy, R. Assessment of depression following acute myocardial infarction using the Beck depression inventory. Heart 2004, 90, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, W.; King, R.; Zhang, Z.; Walley, J.; Gong, W.; Yu, M.; Wei, X. Process Evaluation of a Clustered Randomized Control Trial of a Comprehensive Intervention to Reduce the Risk of Cardiovascular Events in Primary Health Care in Rural China. Int. J. Environ. Res. Public Health 2020, 17, 4156. [Google Scholar] [CrossRef] [PubMed]
- Kalantarzadeh, M.; Yousefi, H.; Alavi, M.; Maghsoudi, J. Adherence Barriers to Treatment of Patients with Cardiovascular Diseases: A Qualitative Study. Iran. J. Nurs. Midwifery Res. 2022, 27, 317–324. [Google Scholar] [CrossRef]
- De Boer, L.; Poos, J.M.; Van Den Berg, E.; De Houwer, J.F.H.; Swartenbroekx, T.; Dopper, E.G.P.; Boesjes, P.; Tahboun, N.; Bouzigues, A.; Foster, P.H.; et al. Montreal Cognitive Assessment vs the Mini-Mental State Examination as a Screening Tool for Patients with Genetic Frontotemporal Dementia. Neurology 2025, 104, e213401. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Seldenrijk, A.; Beekman, A.T.; van Hout, H.P.; de Jonge, P.; Penninx, B.W. Cardiovascular disease in persons with depressive and anxiety disorders. J. Affect. Disord. 2010, 125, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lindert, J.; Paul, K.C.; Lachman, M.E.; Ritz, B.; Seeman, T.E. Depression-, Anxiety-, and Anger and Cognitive Functions: Findings from a Longitudinal Prospective Study. Front. Psychiatry 2021, 12, 665742. [Google Scholar] [CrossRef]
- Hamada, T.; Kubo, T.; Kawai, K.; Nakaoka, Y.; Yabe, T.; Furuno, T.; Yamada, E.; Kitaoka, H.; Kochi YOSACOI Study. Frailty in patients with acute decompensated heart failure in a super-aged regional Japanese cohort. ESC Heart Fail. 2021, 8, 2876–2888. [Google Scholar] [CrossRef]
- Yao, L.; Ni, J.; Wei, M.; Li, T.; Long, Z.; Shi, J.; Tian, J. Association of Depression and Cognitive Performance in US Older Adults: A Secondary Analysis of Cross-Sectional Data Using NHANES 2013–2014. Eur. Neurol. 2024, 87, 147–158. [Google Scholar] [CrossRef]
- Park, D.Y.; Jamil, Y.; Babapour, G.; Kim, J.; Campbell, G.; Akman, Z.; Kochar, A.; Sen, S.; Samsky, M.D.; Sikand, N.V.; et al. Association of cardiovascular diseases with cognitive performance in older adults. Am. Heart J. 2024, 273, 10–20. [Google Scholar] [CrossRef]
- Stanek, K.M.; Gunstad, J.; Paul, R.H.; Poppas, A.; Jefferson, A.L.; Sweet, L.H.; Hoth, K.F.; Haley, A.P.; Forman, D.E.; Cohen, R.A. Longitudinal cognitive performance in older adults with cardiovascular disease: Evidence for improvement in heart failure. J. Cardiovasc. Nurs. 2009, 24, 192–197. [Google Scholar] [CrossRef]
- Bahall, M. Prevalence and associations of depression among patients with cardiac diseases in a public health institute in Trinidad and Tobago. BMC Psychiatry 2019, 19, 4. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Wu, S.; Ying, P.; Zhang, Z.; Tan, X.; Zhu, J. Prevalence and management of depressive symptoms in coronary heart disease patients and relationship with cardiovascular prognosis: A prospective cohort study. BMC Psychiatry 2024, 24, 644. [Google Scholar] [CrossRef]
- Williams, J.B.; Alexander, K.P.; Morin, J.F.; Langlois, Y.; Noiseux, N.; Perrault, L.P.; Smolderen, K.; Arnold, S.V.; Eisenberg, M.J.; Pilote, L.; et al. Preoperative anxiety as a predictor of mortality and major morbidity in patients aged >70 years undergoing cardiac surgery. Am. J. Cardiol. 2013, 111, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Gorini, A.; Giuliani, M.; Raggio, L.; Barbieri, S.; Tremoli, E. Depressive and Anxiety Symptoms Screening in Cardiac Inpatients: A Virtuous Italian Approach to Psychocardiology. Int. J. Environ. Res. Public Health 2020, 17, 5007. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Kazeminia, M.; Karami, A.; Salimi, Y.; Ziapour, A.; Janjani, P. Global prevalence of depression, anxiety, and stress in cardiac patients: A systematic review and meta-analysis. J. Affect. Disord. 2023, 324, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; McIntyre, T.; Coelho, R.; Prata, J.; Maciel, M.J. Brief psychological intervention in phase I of cardiac rehabilitation after acute coronary syndrome. Port. J. Cardiol. 2017, 36, 641–649. [Google Scholar] [CrossRef]
- Cully, J.A.; Stanley, M.A.; Deswal, A.; Hanania, N.A.; Phillips, L.L.; Kunik, M.E. Cognitive-behavioral therapy for chronic cardiopulmonary conditions: Preliminary outcomes from an open trial. Prim. Care Companion J. Clin. Psychiatry 2010, 12, PCC.09m00896. [Google Scholar] [CrossRef]
- Li, Y.N.; Buys, N.; Ferguson, S.; Li, Z.J.; Sun, J. Effectiveness of cognitive behavioral therapy-based interventions on health outcomes in patients with coronary heart disease: A meta-analysis. World J. Psychiatry 2021, 11, 1147–1166. [Google Scholar] [CrossRef]
- Griffo, R.; Urbinati, S.; Giannuzzi, P.; Jesi, A.P.; Sommaruga, M.; Sagliocca, L.; Bianco, E.; Tassoni, G.; Iannucci, M.; Sanges, D.; et al. Italian guidelines on cardiac rehabilitation and secondary prevention of cardiovascular disease: Executive summary. G. Ital. Cardiol. 2008, 9, 286–297. [Google Scholar]
- Pagano, M.; Corallo, F.; Anselmo, A.; Giambò, F.M.; Micali, G.; Duca, A.; D’Aleo, P.; Bramanti, A.; Garofano, M.; Bramanti, P.; et al. Optimisation of Remote Monitoring Programmes in Heart Failure: Evaluation of Patient Drop-Out Behaviour and Healthcare Professionals’ Perspectives. Healthcare 2024, 12, 1271. [Google Scholar] [CrossRef]
| Test | Domains | Cutoff | Interpretation | References |
|---|---|---|---|---|
| Montreal Cognitive Assessment (MoCA) | Visuospatial abilities, executive function, attention, language, memory (delayed recall), orientation | 26–30: Normal | No cognitive impairment | [30] |
| 21–25: Mild | Possible MCI | |||
| 11–20: Moderate | Moderate dementia stages | |||
| 0–10: Severe | Severe dementia stages | |||
| Mini-Mental State Examination (MMSE) | Orientation, memory, attention, calculation, language, visuospatial skills | 27–30: Normal | No cognitive impairment | [34] |
| 21–26: Mild | Possible MCI or early dementia | |||
| 11–20: Moderate | Moderate dementia stages | |||
| 0–10: Severe | Severe dementia stages | |||
| Beck Depression Inventory-II (BDI-II) | Depressive symptoms | 0–13: Minimal | No clinically significant depression. | [32] |
| 14–19: Mild | Mild or transient depressive symptoms. | |||
| 20–28: Moderate | Presence of overt depressive symptoms, possible clinical depression. | |||
| 29–63: Severe | Severe symptoms indicative of major clinical depression. | |||
| Beck Anxiety Inventory (BAI) | Anxiety symptoms | 0–7: Minimal | Very mild or absent anxiety. | [33] |
| 8–15: Mild | Mild anxiety symptoms, often consistent with stressful situations. | |||
| 16–25: Moderate | Significant anxiety, which can affect quality of life. | |||
| 26–63: Severe | Intense anxiety, possible clinical anxiety disorder. |
| Sociodemographic Variables | n (%) | |
|---|---|---|
| Gender | ||
| Male | 66 (89.19%) | |
| Female | 8 (10.81%) | |
| Age | ||
| From 45 to 50 years old | 1 (1.35%) | |
| From 51 to 60 years old | 21 (28.38%) | |
| From 61 to 70 years old | 29 (39.19%) | |
| From 71 to 80 years old | 20 (27.03%) | |
| From 81 to 85 years old | 3 (4.05%) | |
| Marital Status | ||
| Single | 10 (13.51%) | |
| Married | 42 (56.76%) | |
| Widowed | 8 (10.81%) | |
| Divorced | 14 (18.92%) | |
| Sons | ||
| Yes | 58 (78.38%) | |
| No | 16 (21.62%) | |
| Educational level | ||
| Primary education (5 years) | 5 (6.76%) | |
| Lower secondary education (3 years) | 16 (21.62%) | |
| Upper secondary education (5 years) | 33 (44.59%) | |
| Bachelor’s degree | 12 (16.22%) | |
| Master’s degree | 7 (9.46%) | |
| Post-graduate specialization | 1 (1.35%) | |
| Economic activity | ||
| Unemployed | 8 (10.81%) | |
| Working | 41 (55.41%) | |
| Retired | 25 (33.78%) | |
| Variable | Whole Sample Mean (SD) | Male Subjects Mean (SD) | Female Subjects Mean (SD) | p-Value |
|---|---|---|---|---|
| Age | 66.15 (8.61) | 65.62 (8.38) | 70.50 9.91 | 0.217 |
| MoCA score | 22.61 (4.24) | 22.80 (4.23) | 21.00 (4.24) | 0.287 |
| MMSE score | 26.76 (2.58) | 26.86 (2.57) | 25.88 (2.70) | 0.356 |
| BDI score | 11.09 (8.74) | 10.56 (8.17) | 15.50 (12.31) | 0.302 |
| BAI score | 8.54 (8.08) | 8.94 (8.27) | 5.25 (5.63) | 0.127 |
| Test | % | |
|---|---|---|
| MoCA | ||
| Normal | 32.43% | |
| Mild | 35.14% | |
| Moderate | 32.43% | |
| MMSE | ||
| Normal | 58.11% | |
| Mild | 41.89% | |
| BDI | ||
| Minimal | 56.76% | |
| Mild | 21.62% | |
| Moderate | 18.92% | |
| Severe | 2.70% | |
| BAI | ||
| Minimal | 58.11% | |
| Mild | 25.68% | |
| Moderate | 12.16% | |
| Severe | 4.05% | |
| Study | Aim | Country | Sample | Study Design | Procedure | Measure | Outcomes | Limitations |
|---|---|---|---|---|---|---|---|---|
| [37] | To compare the MMSE and MoCA tests for the identification of CD in elderly patients with HF. | United States | 43 patients | Cross-sectional observational study | Neuropsychological assessment was performed by a psychologist. Initially, the MMSE test was administered, the results of which were later compared with those obtained from the MoCA test. | MMSE; MoCA | The MoCA identified mild CD (<26) in 23% of the sample, while the MMSE identified mild CD in 26% of the sample. | Lack of sample size calculation. Predominance of low educational level among patients evaluated. |
| [38] | To evaluate the usefulness of clinical assessment tools in neurocognitive screening after OHCA. | Sweden, Denmark, United Kingdom | 108 patients | Retrospective cohort study | Neurocognitive screening was performed as part of the 6-month follow-up. | MoCA; SDMT; TSQ; IQCODE-CA | The MoCA identified a cognitive deficit in 50% of the sample, associated with a total score of <26. | Possible false positives due to nonselective diagnostic criteria. |
| [39] | Analyze the prevalence of depression and anxiety in patients with cardiovascular disease and identify factors associated with these disorders. | Ethiopia | 370 patients | Cross-sectional observational study | Standardized tests were administered as part of the 6-month follow-up. | BDI-II; BAI | The mean score of the BAI was 18.8. The mean score of the BDI-II was 21.05. | Lack of data on confounding variables, which might influence mental health. Results show only an association, not a causal relationship. |
| [40] | To analyze the association between depressive symptoms and the risk of adverse clinical events in patients with HFrEF. | United States | 142 patients | Prospective observational study | Participants underwent medical and psychosocial assessments both at the beginning of the study (baseline) and after 6 months of follow-up. | BDI-II; SCHFI | 29% of the sample had a BDI-II ≥ 14 at baseline. The mean BDI-II score at baseline was 10.3. | The sample had better health status than other populations with HFrEF, with higher levels of physical activity and better self-care behaviors. |
| [41] | To analyze the relationship between depressive symptoms, appetite, and QoL in 86 patients hospitalized for heart failure. | Brazil | 133 patients | Cross-sectional observational study | Patients were recruited on the ward, directly in their rooms. After signing the informed consent, they filled out the questionnaire. | BDI-II | The mean score of the BDI-II was 16.3. Within the sample, 25.6% had moderate depressive symptoms, 26.7% had mild depressive symptoms, and 7% had severe depressive symptoms. | The study design limited the generalizability of the results to the entire Brazilian population. Hospital recruitment may have influenced levels of worry and depression because of patients’ perceptions of their own health status. |
| [42] | To compare cognitive performance in patients with different cardiovascular disease profiles. | Canada | 80 patients | Cross-sectional observational study | Cognitive function assessment was performed by a qualified psychometrician or neuropsychologist at the baseline visit. | MoCA | The average MoCA score in the different cardiovascular diseases was 26.23. | Relatively small sample size. The study design did not allow for the detection of cognitive changes over time. |
| [43] | To examine the prevalence and characteristics of MCI and evaluate its associations with LVD, LA, and AF. | Australia | 337 patients | Cross-sectional observational study | Participants were recruited through primary care and advertisements. A medical history, clinical examination, and cognitive assessment were performed. The analysis was based on data collected at baseline. | MoCA | Thirty percent of the sample scored <26, compatible with MCI. Executive functions (69%) and delayed recall (93%) were the most frequently abnormal domains. | Absence of longitudinal patterns. Relatively small sample. Failure to use brain MRI. |
| [44] | To evaluate the impact of reduced ejection fraction and demographic characteristics on the occurrence of cognitive impairment and depression following myocardial infarction. | Serbia | 82 patients | Prospective study | Three months after the diagnosis of acute myocardial infarction, a review of cognitive function was performed during a follow-up visit, and patients were examined for the presence of depressive symptoms. | BDI-II; MMSE | A total of 20.7% of the sample scored <26, consistent with mild cognitive problems, and 41.5% scored <29 in the BDI-II, compatible with moderate depressive symptoms. | Reduced sample size. The use of the MMSE, a screening tool with low sensitivity for mild cognitive impairment, is a limitation. |
| [45] | To investigate the relationship between psychiatric disorders (anxiety/depression) and CSF. | Egypt | 100 patients | Cross-sectional observational study | Psychiatric interviews were conducted blind to CAG results to assess the severity of anxiety and depression. | BDI-II; BAI | Most patients had moderate to severe levels of anxiety and depression, with scores of <29 on the BDI and <26 on the BAI. | The study design does not allow a causal relationship to be concluded. No follow-up is planned. Study parameters cannot be correlated with long-term outcomes. |
| [46] | To assess the prevalence of depression in a large sample of patients with CHF. | Pakistan | 1009 patients | Prospective observational study | Patients were recruited from the cardiology department. The questionnaire was administered at baseline and at six months. | BDI-II | The estimated average BDI score was about 23.4. | Lack of data on length of hospital stay, baseline severity of congestive heart failure (CHF), and adherence to drug therapy. |
| [47] | To assess psychological disorders, quality of life, and coping strategies in postoperative heart transplant patients. | Brazil | 33 patients | Cross-sectional observational study | Participants completed the questionnaires in one individual session during follow-up visits (usually semiannual for most transplant patients) or during other healthcare procedures. | BDI-II; BAI; WHOQOL-BREF; Ways of Coping Scale; MINI International Neuropsychiatric Interview | A total of 91% of the sample had minimal depressive symptoms compatible with a BDI score < 13, 6% showed mild symptoms with a BDI < 19, 3% showed moderate symptoms with a BDI < 28. 94% had minimal anxiety symptoms with a BAI < 7. Only 6% had mild to moderate symptoms with a BAI < 25. | The study design does not allow causal relationships to be established among the variables analyzed. The evolution of phenomena over time cannot be assessed, limiting longitudinal inferences. The absence of a control group reduces the robustness of the conclusions |
| [48] | To assess the symptoms of depression and anxiety in patients with STEMI treated with primary PCI. | Czech Republic | 79 patients | Prospective observational study | Tests were administered within 24 hours of pPCI, before discharge, and subsequently at 3, 6, and 12 months | BDI-II SAS | Within 24 hours after the procedure, 21.5% had symptoms of mild depression (BDI-II ≥ 14). | Lack of data before the onset of MI. |
| [49] | To determine whether the prevalence of depression in patients with HF differed by gender. | Taiwan | 147 patients | Cross-sectional observational study | Participants were recruited at the hospital, provided written informed consent, and individually completed self-assessment questionnaires | BDI-II | Within the sample, 65% of the males and 65.7% of the females had mild depressive symptoms, with a BDI-II score ≥14. | The cross-sectional design allows for the identification of associations but not causality. The relatively small female sample reduced the statistical power, which excluded potential associations with other variables. |
| [50] | To examine cognitive function in older hospitalized patients with chronic AF. | Australia | 260 patients | Prospective observational study | Cognitive function was assessed during hospitalization using the MoCA. | MoCA | Sixty-five percent of the sample had an MoCA score of 21. Multiple deficits were identified in cognitive domains, particularly in executive functions, visuospatial skills, and short-term memory. | Exclusive use of MoCA for cognitive assessment. Potential selection bias due to exclusion of non-English-speaking patients and the influence of an acute clinical setting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappadona, I.; Anselmo, A.; Cardile, D.; Micali, G.; Giambò, F.M.; Speciale, F.; Costanzo, D.; D'Aleo, P.; Duca, A.; Bramanti, A.; et al. Psychic and Cognitive Impacts of Cardiovascular Disease: Evidence from an Observational Study and Comparison by a Systematic Literature Review. Med. Sci. 2025, 13, 105. https://doi.org/10.3390/medsci13030105
Cappadona I, Anselmo A, Cardile D, Micali G, Giambò FM, Speciale F, Costanzo D, D'Aleo P, Duca A, Bramanti A, et al. Psychic and Cognitive Impacts of Cardiovascular Disease: Evidence from an Observational Study and Comparison by a Systematic Literature Review. Medical Sciences. 2025; 13(3):105. https://doi.org/10.3390/medsci13030105
Chicago/Turabian StyleCappadona, Irene, Anna Anselmo, Davide Cardile, Giuseppe Micali, Fabio Mauro Giambò, Francesco Speciale, Daniela Costanzo, Piercataldo D'Aleo, Antonio Duca, Alessia Bramanti, and et al. 2025. "Psychic and Cognitive Impacts of Cardiovascular Disease: Evidence from an Observational Study and Comparison by a Systematic Literature Review" Medical Sciences 13, no. 3: 105. https://doi.org/10.3390/medsci13030105
APA StyleCappadona, I., Anselmo, A., Cardile, D., Micali, G., Giambò, F. M., Speciale, F., Costanzo, D., D'Aleo, P., Duca, A., Bramanti, A., Garofano, M., Bramanti, P., Corallo, F., & Pagano, M. (2025). Psychic and Cognitive Impacts of Cardiovascular Disease: Evidence from an Observational Study and Comparison by a Systematic Literature Review. Medical Sciences, 13(3), 105. https://doi.org/10.3390/medsci13030105






