Pleomorphic Adenoma: Extracapsular Dissection vs. Superficial Parotidectomy—An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.2.1. Participants
2.2.2. Intervention
2.2.3. Comparison
2.2.4. Outcomes
2.2.5. Study Design
2.3. Exclusion Criteria
2.4. Data Collection Process
2.5. Data Synthesis and Analysis
2.6. Risk of Bias and Study Quality Assessment
3. Results
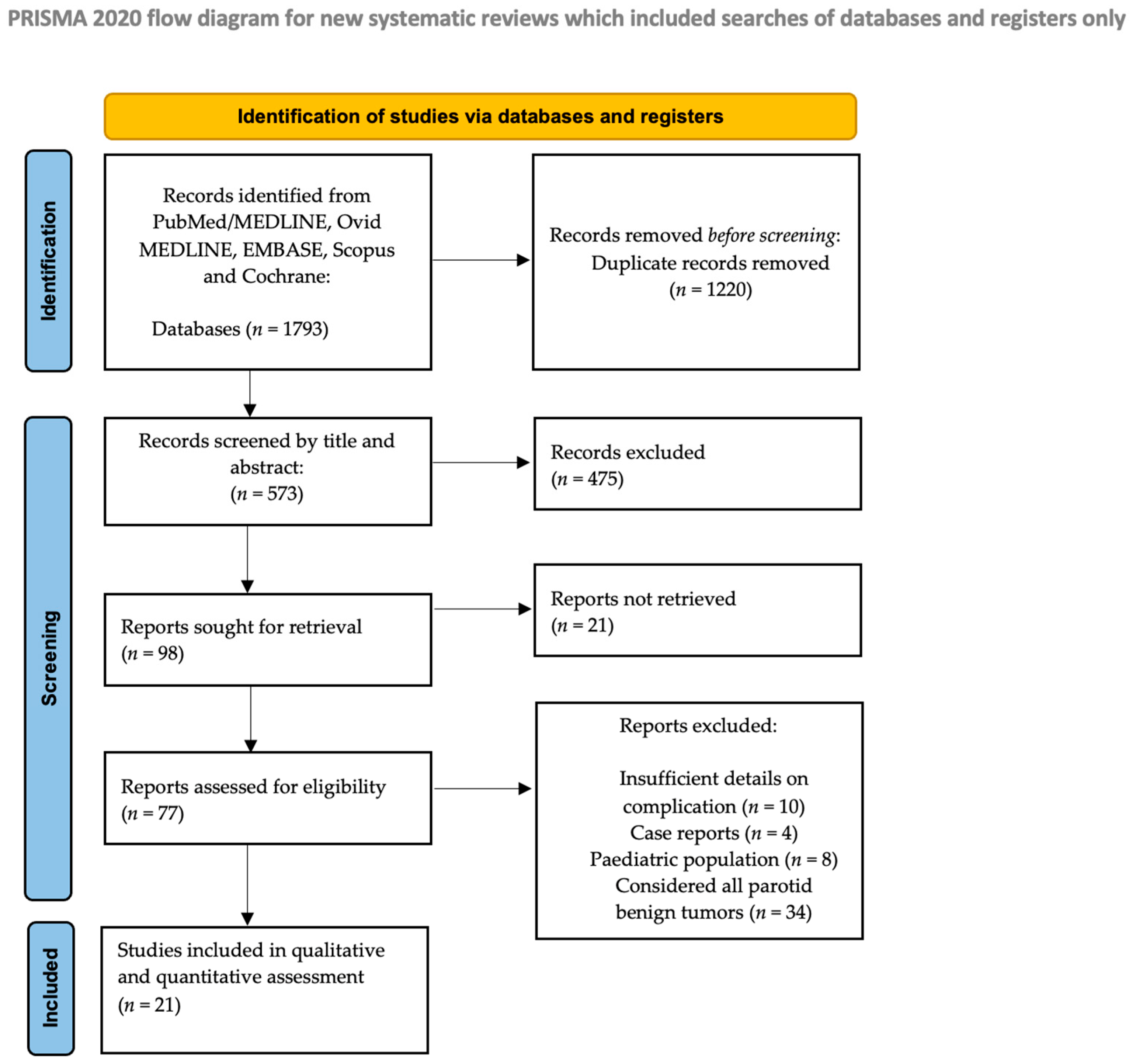
3.1. Studies and Settings
3.2. Description of the Studies
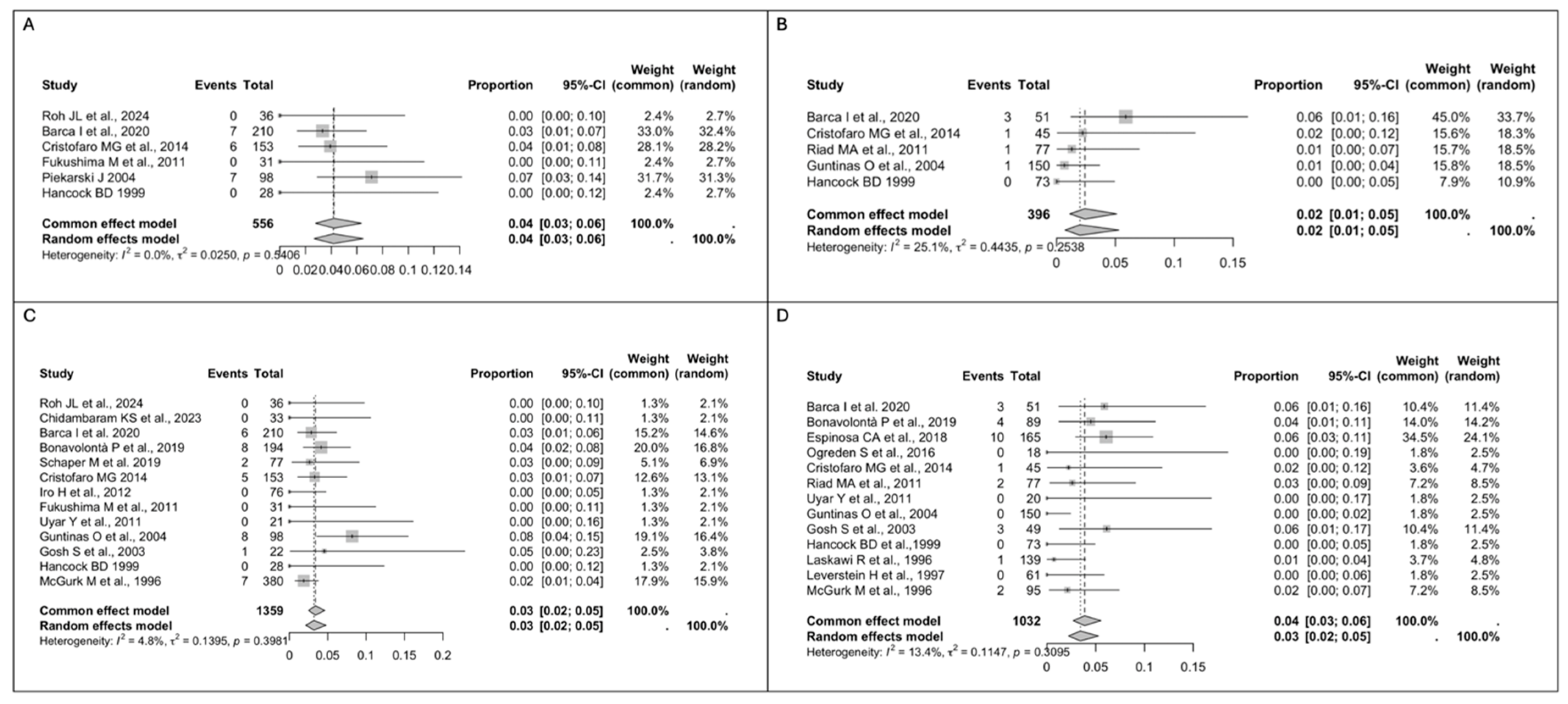
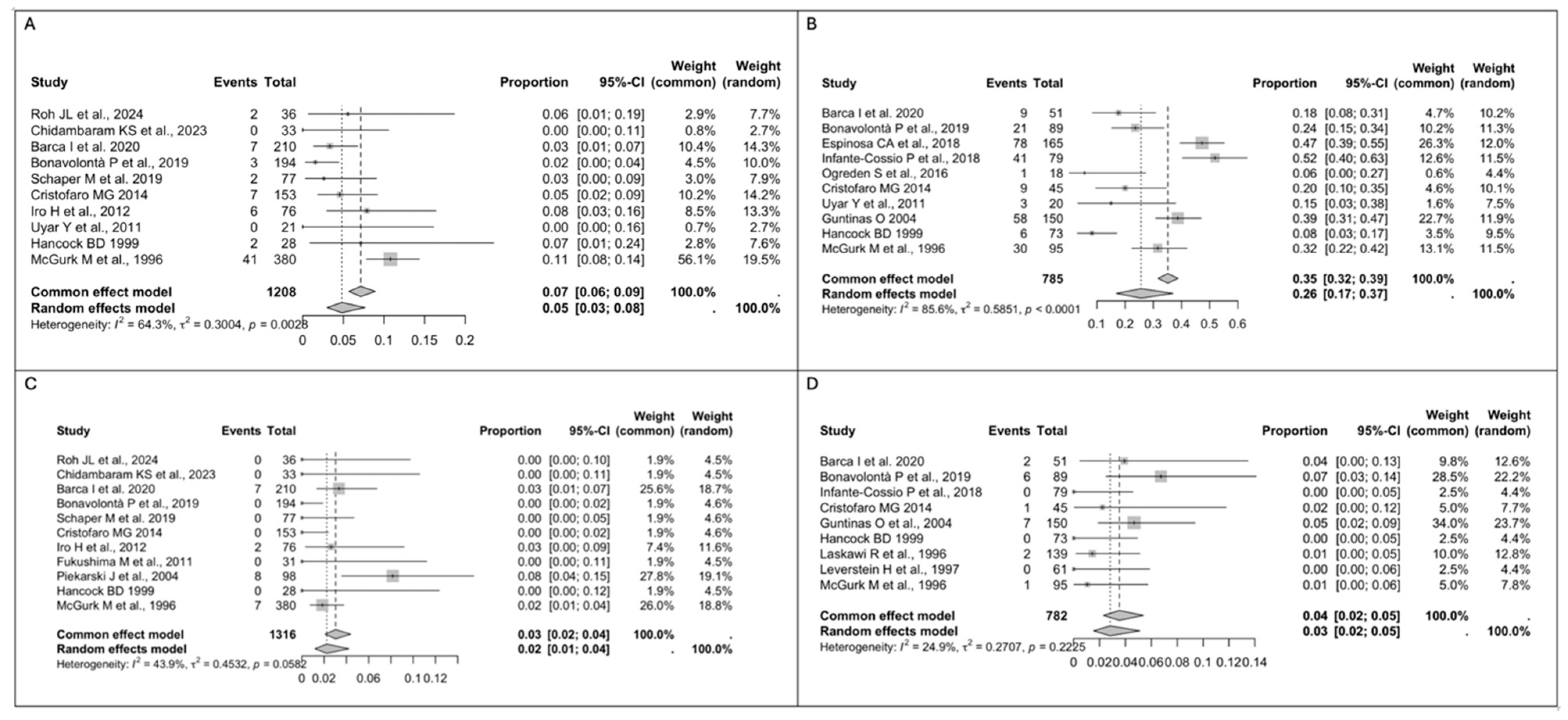
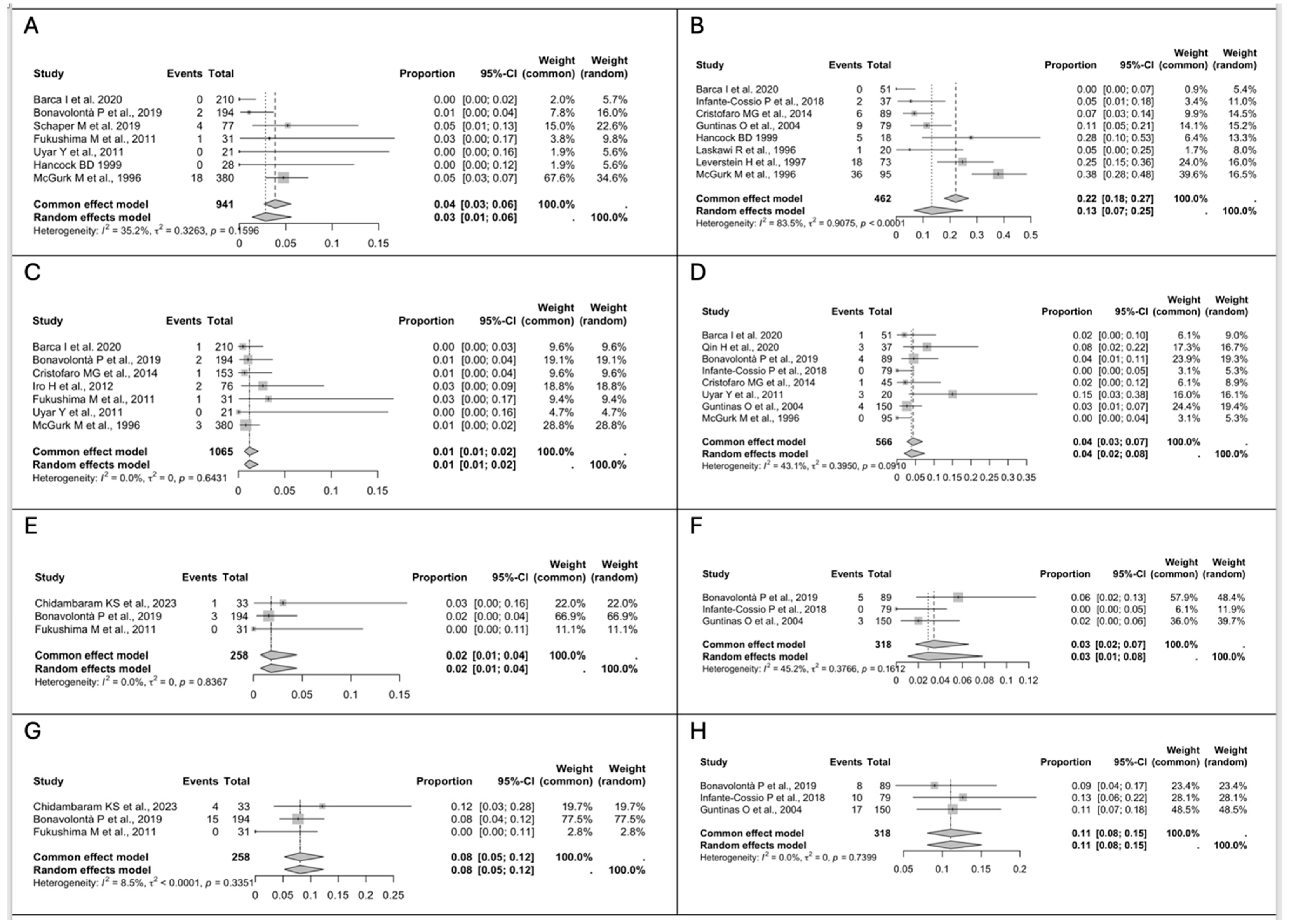
3.3. Study Results
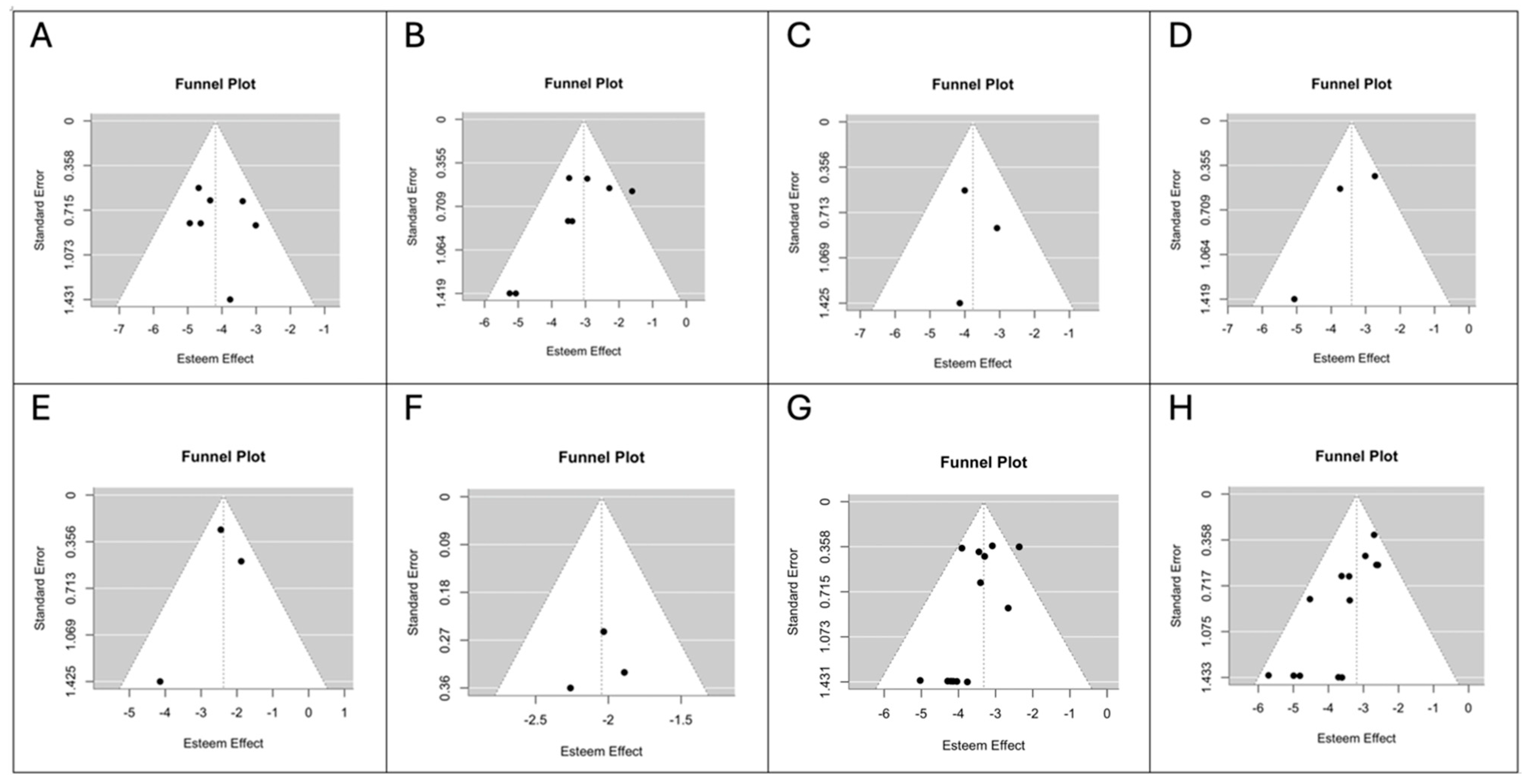
3.4. Risk of Bias Assessment
4. Discussion
5. Conclusions
- (1)
- The risk of recurrence is similar in patients treated with ED and SP.
- (2)
- The transient facial nerve palsy rate is lower after ED, while the permanent facial nerve palsy rate is similar for ED and SP.
- (3)
- Post-operative complications, especially Frey’s syndrome, are more common after SP.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadletz, L.; Grasl, S.; Grasl, M.C.; Perisanidis, C.; Erovic, B.M. Extracapsular dissection versus superficial parotidectomy in benign parotid gland tumors: The Vienna Medical School experience. Head Neck 2017, 39, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.; Testa, D.; Montagnani, S.; Tafuri, D.; Salzano, F.A.; Rocca, A.; Amato, B.; Salzano, G.; Dell’Aversana Orabona, G.; Piombino, P.; et al. Surgical management of pleomorphic adenoma of parotid gland in elderly patients: Role of morphological features. Int. J. Surg. 2014, 12 (Suppl. 2), S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.; Hariharan, A.; Cao, U.M.N.; Mai, C.T.T.; Wang, A.; Khayambashi, P.; Nguyen, B.H.; Safi, L.; Tran, S.D. An Overview on the Histogenesis and Morphogenesis of Salivary Gland Neoplasms and Evolving Diagnostic Approaches. Cancers 2021, 13, 3910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woods, J.E.; Chong, G.C.; Beahrs, O.H. Experience with 1,360 primary parotid tumors. Am. J. Surg. 1975, 130, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.D. Management of pleomorphic adenoma of the parotid. Br. J. Surg. 1988, 75, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Prichard, A.J.; Barton, R.P.; Narula, A.A. Complications of superficial parotidectomy versus extracapsular lumpectomy in the treatment of benign parotid lesions. J. R. Coll. Surg. Edinb. 1992, 37, 155–158. [Google Scholar] [PubMed]
- Witt, R.L.; Rejto, L. Pleomorphic adenoma: Extracapsular dissection versus partial superficial parotidectomy with facial nerve dissection. Del. Med. J. 2009, 81, 119–125. [Google Scholar] [PubMed]
- Donovan, D.T.; Conley, J.J. Capsular significance in parotid tumor surgery: Reality and myths of lateral lobectomy. Laryngoscope 1984, 94, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Stea, G. Conservative surgical treatment of mixed tumours of the parotid gland. J. Maxillofac. Surg. 1975, 3, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Tomoda, K.; Kumazawa, T. The usefulness of partial parotidectomy for benign parotid gland tumors. A retrospective study of 306 cases. Acta Otolaryngol. Suppl. 1993, 500, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yan, G.; Wei, X.; He, X. Superficial parotidectomy versus partial superficial parotidectomy in treating benign parotid tumors. Oncol. Lett. 2015, 9, 887–890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foresta, E.; Torroni, A.; Di Nardo, F.; de Waure, C.; Poscia, A.; Gasparini, G.; Marianetti, T.M.; Pelo, S. Pleomorphic adenoma and benign parotid tumors: Extracapsular dissection vs superficial parotidectomy—Review of literature and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Kick, C.; Klussmann, J.P.; Jungehuelsing, M.; Stennert, E. Pleomorphic adenoma of the parotid gland: A 13-year experience of consequent management by lateral or total parotidectomy. Eur. Arch. Oto-Rhino-Laryngol. 2004, 261, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Albergotti, W.G.; Nguyen, S.A.; Zenk, J.; Gillespie, M.B. Extracapsular dissection for benign parotid tumors: A meta-analysis. Laryngoscope 2012, 122, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Troise, S.; Calabria, F.; Merola, R.; Calvanese, C.; Arena, A.; Abbate, V.; Bonavolontà, P.; Dell’Aversana Orabona, G. Extracapsular Dissection Versus Superficial Parotidectomy for Benign Parotid Tumors: A Bibliometric and Visual Analysis. J. Craniofac. Surg. 2025, 36, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Snow, G.B. The surgical approaches to the treatment of parotid pleomorphic adenomas. In Controversies in the Management of Salivary Gland Disease; McGurk, M., Renehan, A.G., Eds.; Oxford University Press: Oxford, UK, 2001; Chapter 5; p. 58. [Google Scholar]
- Roh, J.L. Extracapsular dissection via single cervical incision for parotid pleomorphic adenoma. Clin. Oral Investig. 2023, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Infante-Cossio, P.; Gonzalez-Cardero, E.; Garcia-Perla-Garcia, A.; Montes-Latorre, E.; Gutierrez-Perez, J.L.; Prats-Golczer, V.E. Complications after superficial parotidectomy for pleomorphic adenoma. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e485–e492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uyar, Y.; Cağlak, F.; Keleş, B.; Yıldırım, G.; Saltürk, Z. Extracapsular dissection versus superficial parotidectomy in pleomorphic adenomas of the parotid gland. Kulak Burun Bogaz Ihtis. Derg. 2011, 21, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Riad, M.A.; Abdel-Rahman, H.; Ezzat, W.F.; Adly, A.; Dessouky, O.; Shehata, M. Variables related to recurrence of pleomorphic adenomas: Outcome of parotid surgery in 182 cases. Laryngoscope 2011, 121, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- McGurk, M.; Renehan, A.; Gleave, E.N.; Hancock, B.D. Clinical significance of the tumour capsule in the treatment of parotid pleomorphic adenomas. Br. J. Surg. 1996, 83, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Leverstein, H.; van der Wal, J.E.; Tiwari, R.M.; van der Waal, I.; Snow, G.B. Surgical management of 246 previously untreated pleomorphic adenomas of the parotid gland. Br. J. Surg. 1997, 84, 399–403. [Google Scholar] [PubMed]
- Laskawi, R.; Schott, T.; Mirzaie-Petri, M.; Schroeder, M. Surgical management of pleomorphic adenomas of the parotid gland: A follow-up study of three methods. J. Oral Maxillofac. Surg. 1996, 54, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.D. Clinically benign parotid tumours: Local dissection as an alternative to superficial parotidectomy in selected cases. Ann. R. Coll. Surg. Engl. 1999, 81, 299–301. [Google Scholar] [PubMed] [PubMed Central]
- Ghosh, S.; Panarese, A.; Bull, P.D.; Lee, J.A. Marginally excised parotid pleomorphic salivary adenomas: Risk factors for recurrence and management. A 12.5-year mean follow-up study of histologically marginal excisions. Clin. Otolaryngol. Allied Sci. 2003, 28, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, J.; Nejc, D.; Szymczak, W.; Wronski, K.; Jeziorski, A. Results of extracapsular dissection of pleomorphic adenoma of parotid gland. J. Oral Maxillofac. Surg. 2004, 62, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Miyaguchi, M.; Kitahara, T. Extracapsular dissection: Minimally invasive surgery applied to patients with parotid pleomorphic adenoma. Acta Otolaryngol. 2011, 131, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Iro, H.; Zenk, J.; Koch, M.; Klintworth, N. Follow-up of parotid pleomorphic adenomas treated by extracapsular dissection. Head Neck 2013, 35, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Cristofaro, M.G.; Allegra, E.; Giudice, A.; Colangeli, W.; Caruso, D.; Barca, I.; Giudice, M. Pleomorphic adenoma of the parotid: Extracapsular dissection compared with superficial parotidectomy—A 10-year retrospective cohort study. Sci. World J. 2014, 2014, 564053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogreden, S.; Ruzgar, S.; Alimoglu, Y.; Eroglu, S.; Taskin, U.; Oktay, M.F. Comparison of Frey Syndrome Rates Following Superficial Parotidectomy and Partial Superficial Parotidectomy for Pleomorphic Adenoma. J. Craniofac Surg. 2016, 27, e469–e471. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.A.; Fernández-Valle, Á.; Lequerica-Fernández, P.; de Villalaín, L.; de Vicente, J.C. Clinicopathologic and Surgical Study of Pleomorphic Adenoma of the Parotid Gland: Analysis of Risk Factors for Recurrence and Facial Nerve Dysfunction. J. Oral Maxillofac. Surg. 2018, 76, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Schapher, M.; Koch, M.; Agaimy, A.; Goncalves, M.; Mantsopoulos, K.; Iro, H. Parotid pleomorphic adenomas: Factors influencing surgical techniques, morbidity, and long-term outcome relative to the new ESGS classification in a retrospective study. J. Craniomaxillofac. Surg. 2019, 47, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Bonavolontà, P.; Dell’Aversana Orabona, G.; Maglitto, F.; Abbate, V.; Committeri, U.; Salzano, G.; Improta, G.; Iaconetta, G.; Califano, L. Postoperative complications after removal of pleomorphic adenoma from the parotid gland: A long-term follow up of 297 patients from 2002 to 2016 and a review of publications. Br. J. Oral Maxillofac. Surg. 2019, 57, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Barca, I.; Cristofaro, M.G. Surgical approach to parotid pleomorphic adenoma: A 15-year retrospective cohort study. Br. J. Oral Maxillofac. Surg. 2020, 58, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, K.S.; Muraleedharan, M.; Keshri, A.; Mayilvaganan, S.; Hameed, N.; Aqib, M.; Kumar, A.; Manogaran, R.S.; Kumar, R. The Outcomes and Surgical Nuances of Minimally Invasive Parotid Surgery for Pleomorphic Adenoma. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 3256–3262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, H.; Zhang, L.; Ren, F.; Yi, M. A comparison of the effects of partial superficial parotidectomy and superficial parotidectomy on the postoperative parotid absorption and secretion functions in the treatment of pleomorphic adenoma. Int. J. Clin. Exp. Med. 2020, 13, 3456–3462. [Google Scholar]
- Eveson, J.W.; Cawson, R.A. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J. Pathol. 1985, 146, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.S.; Attie, J.N. Treatment of Warthin’s tumor by enucleation. Am. J. Surg. 1988, 156, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, G.; Westrin, K.M.; Carlsöö, B.; Silfverswärd, C. Recurrent primary pleomorphic adenomas of salivary gland origin: Intrasurgical rupture, histopathologic features, and pseudopodia. Cancer 1998, 82, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Cannavale, R.; Chiodini, P. Meta-analysis of surgical approaches to the treatment of parotid pleomorphic adenomas and recurrence rates. J. Craniomaxillofac. Surg. 2015, 43, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Natvig, K.; Søberg, R. Relationship of intraoperative rupture of pleomorphic adenomas to recurrence: An 11–25 year follow-up study. Head Neck 1994, 16, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.L. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope 2002, 112, 2141–2154. [Google Scholar] [CrossRef] [PubMed]

| Title, Year | Study Design | No. Patients | Mean Age | Mean Follow Up (Range) (ED/SP) | Mean Tumor Size (Range) (ED/SP) | Localization | ED | SP | Capsular Rupture (ED/SP) | Transient Facial Narve Palsy (ED/SP) | Permanent Facial Nerve Palsy (ED/SP) | Frey’s Syndrome (ED/SP) | Salivary Fistula (ED/SP) | Sialocele (ED/SP) | Seroma (ED/SP) | Hematoma (ED/SP) | Recurrence (ED/SP) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roh JL et al., 2024 [17] | Prospective | 36 | 54 | 44 mo (24–60) | 2.8 cm | Deep or superficial parotid lobe | 36 | 0 | 2 | 0 | 3 | 4 | 0 | ||||
| Chidambaram KS et al., 2023 [35] | Retrospective | 33 | 32.75 | (2–43) mo | (2–4) cm | Mainly the tail of the superficial lobe, with some having minimal superior or deep lobe extension | 33 | 0 | 0 | 1 | 0 | ||||||
| Barca I et al., 2020 [34] | Retrospective | 261 | 47 | 65 mo | 2.5/2.8 cm | Superficial parotid lobe with a diameter of 3.0 (0.5) cm | 210 | 51 | 7/3 | 7/9 | 0/2 | 0/0 | 1/1 | 6/3 | |||
| Qin H et al., 2020 [36] | Retrospective | 75 | 24 mo | Superficial parotid lobe | 37 | 2 | 3 | ||||||||||
| Bonavolontà P et al., 2019 [33] | Retrospective | 297 | 52 | 43 (25–168) mo * | ED: superficial parotid lobe SP: deep parotid lobe | 194 | 89 | 3/21 | 0/6 | 2/6 | 2/4 | 3/5 | 15/8 | 8/4 | |||
| Schaper M et al., 2019 [32] | Retrospective | 205 | 48 | 7.9 y | Deep or superficial parotid lobe | 77 | 2 | 0 | 4 | 2 | |||||||
| Espinosa CA et al., 2018 [31] | Retrospective | 198 | 46.18 | 56.7 (1–437) mo | 2.1 cm | 180: superficial parotid lobe 18: deep parotid lobe | 165 | 78 | 10 | ||||||||
| Infante-Cossio P et al., 2018 [18] | Prospective | 79 | 48 | 30.65 (12–45) mo | 2.44 (1–6) cm | Superficial parotid lobe | 79 | 41 | 0 | 9 | 0 | 0 | 0 | 10 | |||
| Ogreden S et al., 2016 [30] | Retrospective | 50 | 5 y | Superficial parotid lobe | 18 | 1 | 5 | 0 | |||||||||
| Cristofaro MG et al., 2014 [29] | Retrospective | 198 | 50.97 | 61.02/66.4 mo | 3.0/2.5 cm | Superficial parotid lobe | 153 | 45 | 6/1 | 7/9 | 0/1 | 1/1 | 5/1 | ||||
| Iro H et al., 2012 [28] | Retrospective | 76 | 51.35 | 7.38 (5.05–10.52) y | Superficial parotid lobe | 76 | 6 | 2 | 2 | 0 | |||||||
| Fukushima M et al., 2011 [27] | Retrospective | 31 | 43 | 61 (18–125) mo | 2.8 cm | Deep or superficial parotid lobe | 31 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| Riad MA et al., 2011 [20] | Prospective | 164 | 49.5 | 56.4 (34–93) mo | 141: superficial parotid lobe 19: deep parotid lobe 22: parapharyngeal space | 77 | 1 | 2 | |||||||||
| Uyar Y et a l., 2011 [19] | Prospective | 41 | 48.8 | 194 (117–264) mo | Superficial parotid lobe | 21 | 20 | 0/3 | 0/1 | 0/3 | 0/0 | ||||||
| Guntinas O et al., 2004 [13] | Retrospective | 295 | 253 | 8 y | Deep or superficial parotid lobe | 150 | 1 | 58 | 7 | 4 | 3 | 17 | 0 | ||||
| Piekarski J et al., 2004 [26] | Retrospective | 98 | 34.3 mo | 3.6 (1.5–10) mo | N/A | 98 | 7 | 8 | 8 | ||||||||
| Gosh S et al., 2003 [25] | Retrospective | 83 | 49.4 | 10.6 mo (0–10.5 y) | N/A | 22 | 49 | 1/3 | |||||||||
| Hancock BD et al., 1999 [24] | Retrospective | 101 | 43/46 | 10.3 (3–21)/8.3 (3–22) y | N/A | 28 | 73 | 0/0 | 2/6 | 0/0 | 0/18 | 0/0 | |||||
| Laskawi R et al., 1996 [23] | Retrospective | 475 | 63 mo | N/A | 139 | 2 | 20 | 1 | |||||||||
| Leverstein H et al., 1997 [22] | Retrospective | 245 | 95 mo | N/A | 61 | 0 | 8 | 0 | |||||||||
| McGurk M et al., 1996 [21] | Retrospective | 475 | 47 | 12.5 (1–34) y | N/A | 380 | 95 | 41/30 | 7/1 | 18/36 | 3/0 | 7/2 |
| Study | Selection | Comparison | Outcome |
|---|---|---|---|
| Roh JL et al., 2024 [17] | xxx | x | xxx |
| Chidambaram KS et al., 2023 [35] | xxx | x | xxx |
| Barca I et al., 2020 [34] | xx | xx | xx |
| Qin H et al., 2020 [36] | xxx | x | xx |
| Bonavolontà P et al., 2019 [33] | xxx | xx | xxx |
| Schaper M et al., 2019 [32] | xxx | x | xxx |
| Espinosa CA et al., 2018 [31] | xxx | xx | xxx |
| Infante-Cossio P et al., 2018 [18] | xxx | x | xx |
| Ogreden S et al., 2016 [30] | xxx | x | xx |
| Cristofaro MG et al., 2014 [29] | xxx | x | xxx |
| Iro H et al., 2012 [28] | xxx | x | xxx |
| Fukushima M et al., 2011 [27] | xxx | x | xxx |
| Riad MA et al., 2011 [20] | xxx | x | xx |
| Uyar Y et al., 2011 [19] | xxx | xx | xx |
| Guntinas O et al., 2004 [13] | xxx | x | xxx |
| Piekarski J et al., 2004 [26] | xxx | x | xxx |
| Gosh S et al., 2003 [25] | xxx | x | xxx |
| Hancock BD et al., 1999 [24] | xxx | x | xx |
| Laskawi R et al., 1996 [23] | xxx | x | xx |
| Leverstein H et al., 1997 [22] | xxx | x | xxx |
| McGurk M et al., 1996 [21] | xxx | x | xxx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, G.; Scocca, V.; Troise, S.; Abbate, V.; Bonavolontà, P.; Vaira, L.A.; Committeri, U.; Lechien, J.R.; Tramontano, S.; Canterino, V.; et al. Pleomorphic Adenoma: Extracapsular Dissection vs. Superficial Parotidectomy—An Updated Systematic Review and Meta-Analysis. Med. Sci. 2025, 13, 104. https://doi.org/10.3390/medsci13030104
Salzano G, Scocca V, Troise S, Abbate V, Bonavolontà P, Vaira LA, Committeri U, Lechien JR, Tramontano S, Canterino V, et al. Pleomorphic Adenoma: Extracapsular Dissection vs. Superficial Parotidectomy—An Updated Systematic Review and Meta-Analysis. Medical Sciences. 2025; 13(3):104. https://doi.org/10.3390/medsci13030104
Chicago/Turabian StyleSalzano, Giovanni, Veronica Scocca, Stefania Troise, Vincenzo Abbate, Paola Bonavolontà, Luigi Angelo Vaira, Umberto Committeri, Jerome R. Lechien, Sara Tramontano, Vitanna Canterino, and et al. 2025. "Pleomorphic Adenoma: Extracapsular Dissection vs. Superficial Parotidectomy—An Updated Systematic Review and Meta-Analysis" Medical Sciences 13, no. 3: 104. https://doi.org/10.3390/medsci13030104
APA StyleSalzano, G., Scocca, V., Troise, S., Abbate, V., Bonavolontà, P., Vaira, L. A., Committeri, U., Lechien, J. R., Tramontano, S., Canterino, V., & Dell’Aversana Orabona, G. (2025). Pleomorphic Adenoma: Extracapsular Dissection vs. Superficial Parotidectomy—An Updated Systematic Review and Meta-Analysis. Medical Sciences, 13(3), 104. https://doi.org/10.3390/medsci13030104











