Peripheral Prosthetic Vascular Graft Infection: A 5-Year Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimmel, R.M.; Murphy, R.X.J.; Chowdary, R.P. Optimal management of inguinal vascular graft infections. Ann. Plast. Surg. 1994, 32, 623–629. [Google Scholar] [CrossRef]
- Zetrenne, E.; McIntosh, B.C.; McRae, M.H.; Gusberg, R.; Evans, G.R.; Narayan, D. Prosthetic vascular graft infection: A multi-center review of surgical management. Yale J. Biol. Med. 2007, 80, 113–121. [Google Scholar] [PubMed]
- Herrera, F.A.; Kohanzadeh, S.; Nasseri, Y.; Kansal, N.; Owens, E.L.; Bodor, R. Management of vascular graft infections with soft tissue flap coverage: Improving limb salvage rates—A veterans affairs experience. Am. Surg. 2009, 75, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.A.; Hamdan, A.D.; Pomposelli, F.B.; Wyers, M.C.; Siracuse, J.J.; Schermerhorn, M.L. Body mass index: Surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005–2007. Ann. Vasc. Surg. 2010, 24, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.Y.; Rajamanickam, V.; Mell, M.W. Predictors of surgical site infection after open lower extremity revascularization. J. Vasc. Surg. 2011, 54, 433–439. [Google Scholar] [CrossRef]
- Vogel, T.R.; Dombrovskiy, V.Y.; Carson, J.L.; Haser, P.B.; Lowry, S.F.; Graham, A.M. Infectious complications after elective vascular surgical procedures. J. Vasc. Surg. 2010, 51, 122–129. [Google Scholar] [CrossRef]
- Wilson, S.E. New alternatives in management of the infected vascular prosthesis. Surg. Infect. 2001, 2, 171–177. [Google Scholar] [CrossRef]
- Williams, M.; Milling, M.; Shandall, A. Vascularized muscular flaps and arterial graft infection in the groin. Eur. J. Vasc. Surg. 2003, 25, 390–395. [Google Scholar] [CrossRef]
- Mertens, R.A.; O’Hara, P.J.; Hertzer, N.R.; Krajewski, L.P.; Beven, E.G. Surgical management of infrainguinal arterial prosthetic graft infections: Review of a thirty-five-year experience. J. Vasc. Surg. 1995, 21, 782–791. [Google Scholar] [CrossRef]
- Henke, P.K.; Bergamini, T.M.; Rose, S.M.; Richardson, J.D. Current options in prosthetic vascular graft infection. Am. Surg. 1998, 64, 39–46. [Google Scholar]
- Morasch, M.D.; Sam, A.D., 2nd; Kibbe, M.R.; Hijjawi, J.; Dumanian, G.A. Early results with use of gracilis muscle flap coverage of infected groin wounds after vascular surgery. J. Vasc. Surg. 2004, 39, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, J.H.; Connolly, J.E. Successful management of prosthetic graft infection with continuous povidone-iodine irrigation. Arch. Surg. 1981, 116, 716–720. [Google Scholar] [CrossRef]
- Calligaro, K.D.; Veith, F.J.; Gupta, S.K.; Ascer, E.; Dietzek, A.M.; Franco, C.D.; Wengerter, K.R. A modified method for management of prosthetic graft infections involving an anastomosis to the common femoral artery. J. Vasc. Surg. 1990, 11, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Mixter, R.C.; Turnipseed, W.D.; Smith Jr, D.J.; Acher, C.W.; Rao, V.K.; Dibbell, D.G. Rotational muscle flaps: A new technique for covering infected vascular grafts. J. Vasc. Surg. 1989, 9, 472–478. [Google Scholar] [CrossRef]
- Meland, N.B.; Arnold, P.G.; Pairolero, P.C. Infected vascular prosthesis management with muscle transposition. Plast Reconstr. Surg. 1994, 93, 1005–1011. [Google Scholar] [CrossRef]
- Edwards, W.H., Jr.; Martin, R.S., III; Jenkins, J.M.; Edwards, W.H., Sr.; Mulherin, J.L., Jr. Primary graft infections. J. Vasc. Surg. 1987, 6, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Dosluoglu, H.H.; Loghmanee, C.; Lall, P.; Cherr, G.S.; Harris, L.M.; Dryjski, M.L. Management of early (<30 day) vascular groin infections using vacuum-assisted closure alone without muscle flap coverage in a consecutive patient series. J. Vasc. Surg. 2010, 51, 1160–1166. [Google Scholar]
- Landry, G.J.; Carlson, J.R.; Liem, T.K.; Mitchell, E.L.; Edwards, J.M.; Moneta, G.L. The sartorius muscle flap: An important adjunct for complicated femoral wounds involving vascular grafts. Am. J. Surg. 2009, 197, 655–659. [Google Scholar] [CrossRef]
- Mayer, D.; Hasse, B.; Koelliker, J.; Enzler, M.; Veith, F.J.; Rancic, Z.; Lachat, M. Long-term results of vascular graft and artery preserving treatment with negative pressure wound therapy in Szilagyi grade III infections justify a paradigm shift. Ann. Surg. 2011, 254, 754–759. [Google Scholar] [CrossRef]
- Monsen, C.; Wann-Hansson, C.; Wictorsson, C.; Acosta, S. Vacuum-assisted wound closure versus alginate for the treatment of deep perivascular wound infections in the groin after vascular surgery. J. Vasc. Surg. 2014, 59, 145–151. [Google Scholar] [CrossRef]
- Hisata, Y.; Hashizume, K.; Tanigawa, K.; Miura, T.; Odate, T.; Tasaki, Y.; Eishi, K. Vacuum-assisted closure therapy for salvaging a methicillin-resistant Staphylococcus aureus-infected prosthetic graft. Asian J. Surg. 2014, 37, 46–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massara, M.; Cascio, A.; Benedetto, F.; Caridi, G.; Lentini, S.; Spinelli, F. Septic skin lesions: An uncommon manifestation of peripheral prosthetic vascular graft infection. Acta Medica Mediterr 2014, 30, 651–654. [Google Scholar]
- Massara, M.; De Caridi, G.; Barberi, G.; Spinelli, F.; Cascio, A. Peripheral septic arterial embolism of non cardiac source: A systematic review of literature. Rev. Vasc. Med. Dec. 2014, 2, 127135. [Google Scholar] [CrossRef]
- Piano, G. Infections in lower extremity vascular grafts. Surg. Clin. N. Am. 1995, 74, 799–809. [Google Scholar] [CrossRef]
- Edwards, M.J.; Richardson, J.D.; Klamer, T.W. Management of aortic prosthetic infections. Am. J. Surg. 1988, 155, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.M.M.; Ehrenfeld, W.K.M.; Goldstone, J.M.; Stoney, R.J.M. Gastrointestinal tract involvement by prosthetic graft infection. The significance of gastrointestinal hemorrhage. Ann. Surg. 1985, 202, 342–348. [Google Scholar] [CrossRef]
- O’connor, S.; Andrew, P.; Batt, M.; Becquemin, J.P. A systematic review and meta-analysis of treatments for aortic graft infection. J. Vasc. Surg. 2006, 44, 38–45. [Google Scholar] [CrossRef]
- Neufang, A.; Doemland, M. Clinical results with the denaturated human umbilical vein (HUV) and the ovine collagen prothesis (Omniflow) as small caliber prosthetic vascular replacement. Gefäßchirurgie 2010, 15, 83–89. [Google Scholar] [CrossRef]
- Amann, W.; Tiesenhausen, K.; Fruhwirth, J.; Thalhammer, M.; Allmayer, T.; Tomka, M.; Koch, G. Aneurysms in biosynthetic grafts. Z. Für Herz Thorax-Und Gefäßchirurgie 2000, 14, 113–116. [Google Scholar] [CrossRef]
- Koch, G.; Gutschi, S.; Pascher, O.; Fruhwirth, H.; Glanzer, H. Analysis of 274 Omniflow Vascular Prosthesis implanted over an eight-year period. Aust. N. Z. J. Surg. 1997, 67, 637–639. [Google Scholar] [CrossRef]
- Töpel, I.; Betz, T.; Uhl, C.; Wiesner, M.; Bröckner, S.; Steinbauer, M. Use of biosynthetic prosthesis (Omniflow II®) to replace infected infrainguinal prosthetic grafts—First results. Vasa 2012, 41, 215–220. [Google Scholar] [CrossRef]
- Chaudhry, A.; Shetty, R.; Crimmin, S.; Singh-Ranger, R. Bio-synthetic Graft Repair of Mycotic Aneurysm of the Common Femoral Artery. EJVES Extra 2009, 18, 1–2. [Google Scholar] [CrossRef][Green Version]
- Ozelci, A.; Yetkin, U.; Akyuz, M. A clinical application of the Omniflow II collagen- polymer vascular prosthesis in a polytetrafluoroethylene graft infection case. Int. J. Thorac. Cardiovasc. Surg. 2008, 13, 2. [Google Scholar]
- De Caridi, G.; Serra, R.; Massara, M.; Barone, M.; Grande, R.; Butrico, L.; Mastroroberto, P.; de Franciscis, S.; Monaco, F. VAC therapy for the treatment of complex wounds after cardio-thoracic surgery. Int. Wound J. 2016, 13, 759–762. [Google Scholar] [CrossRef] [PubMed]
- De Caridi, G.; Massara, M.; Greco, M.; Pipitò, N.; Spinelli, F.; Grande, R.; Butrico, L.; de Franciscis, S.; Serra, R. VAC therapy to promote wound healing after surgical revascularisation for critical lower limb ischaemia. Int. Wound J. 2014, 13, 336–342. [Google Scholar] [CrossRef]
- Svensson, S.; Monsen, C.; Kölbel, T.; Acosta, S. Predictors for outcome after vacuum assisted closure therapy of peri-vascular surgical site infections in the groin. Eur. J. Vasc. Endovasc. 2008, 36, 84–89. [Google Scholar] [CrossRef][Green Version]
- Pinocy, J.; Albes, J.M.; Wicke, C.; Ruck, P.; Ziemer, G. Treatment of periprosthetic soft tissue infection of the groin following vascular surgical procedures by means of polyvinyl alcohol-vacuum sponge system. Wound Repair. Regen. 2003, 11, 104–109. [Google Scholar] [CrossRef]
- Dee, A. The successful management of a dehisced surgical wound with TNP following femoropopliteal bypass. J. Wound Care 2007, 16, 42–44. [Google Scholar] [CrossRef]
- Acosta, S.; Monsen, C. Outcome after VAC® therapy for infected bypass grafts in the lower limb. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 294–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siracuse, J.J.; Nandivada, P.; Giles, K.A.; Hamdan, A.D.; Wyers, M.C.; Chaikof, E.L.; Pomposelli, F.B.; Schermerhorn, M.L. Prosthetic graft infections involving the femoral artery. J. Vasc. Surg. 2013, 57, 700–705. [Google Scholar] [CrossRef]
- Massara, M.; De Caridi, G.; Serra, R.; Barillà, D.; Cutrupi, A.; Volpe, A.; Cutrupi, F.; Alberti, A.; Volpe, P. The role of procalcitonin as a marker of diabetic foot ulcer infection. Int. Wound J. 2015, 14, 31–34. [Google Scholar] [CrossRef]
- Cherry, K.J., Jr.; Roland, C.F.; Pairolero, P.C.; Hallett, J.W., Jr.; Meland, N.B.; Naessens, J.M.; Gloviczki, P.; Bower, T.C. Infected femorodistal bypass: Is graft removal mandatory? J. Vasc. Surg. 1992, 15, 295–303. [Google Scholar] [CrossRef][Green Version]


| Patient Sex, Age | Type of Bypass | Type of Graft | Time, Type and Location of Infection | Organism. Site of Bacteriological Sampling | Surgical Treatment | Antibiotic Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1, M 82 y | Right axillo-femoral bypass | Dacron | Late infection. Abscess along the abdominal wall | Staphylococcus epidermidis. Prosthesis | Bypass excision. New bypass in a clean field | Tigecycline, gentamicin | Limb salvage |
| 2, F 72 y | Right ilio-tibial bypass (redo-bypass) | PTFE | Early infection. Abscess and surgical wound dehiscence with pus discharge | MSSA. Prosthesis, dehiscent groin wound | Bypass excision. Impossible to revascularize | Ceftriaxone, amikacin | Amputation |
| 3, M 78 y | Left ilio-peroneal bypass (redo-bypass) | PTFE | Early infection. Leg abscess with surgical wound dehiscence | Pseudomonas aeruginosa. Prosthesis, dehiscent leg wound. Negative blood culture | Surgical debridement of the dehiscent wound. VAC therapy. Surgical closure | Piperacillin-tazobactam | Limb salvage |
| 4, F 81 y | Right femoro-tibial bypass (redo-bypass) | PTFE | Early infection. Graft occlusion | Pseudomonas aeruginosa. Prosthesis, dehiscent groin wound | Bypass excision. Impossible to revascularize | Amikacin | Amputation |
| 5, M 61 y | Left femoro-tibial bypass (redo-bypass) | PTFE | Early infection. Proximal false aneurysm | Sterile. Prosthesis | Proximal bypass excision and replacement with Omniflow II | Ceftazidime, gentamicin | Limb salvage |
| 6, F 79 y | Left femoro-tibial bypass (redo-bypass) | PTFE | Early infection. Bleeding | Sterile. Prosthesis. | Bypass excision. Impossible to revascularize | Ceftazidime | Amputation |
| 7, M 77 y | Right femoro-popliteal bypass | PTFE | Late infection. Groin suture dehiscence with persistent pus discharge | MRSA (dehiscent groin wound); Pseudomonas aeruginosa (prosthesis) | Bypass excision and replacement in GSV | Imipenem, ciprofloxacin | Limb salvage |
| 8, M 76 y | Left ilio-femoral bypass | PTFE | Early infection. Abscess | Sterile (periprosthetic material) | Surgical site debridement and pus evacuation. VAC therapy. Muscle flap transposition | Ceftriaxone | Limb salvage |
| 9,M 78 y | Right femoro-popliteal bypass | PTFE | Late infection. Acute ischemia | Staphylococcus lugdunensis. Prosthesis | Bypass excision and femoro-peroneal bypass in GSV | Amoxicillin clavulanic acid, Gentamicin | Limb Salvage |
| 10, M 78 y | Femoro-tibial bypass (redo-bypass) | PTFE | Early infection. Bleeding | MRSA. Prosthesis and dehiscent groin wound | Bypass excision. Impossible to revascularize | Teicoplanin, vancomycin | Amputation. Death during follow-up |
| 11, M 75 | Femoro-femoral crossover bypass | PTFE | Late infection. Graft occlusion | Sterile (prosthesis) | Bypass excision. Revascularization not required | Vancomycin, rifampicin | Limb salvage |
| 12, M 69 y | Right femoro-tibial bypass (redo- bypass) | PTFE | Early infection. Surgical wound dehiscence with persistent discharge | Sterile (prosthesis) | Bypass excision. Impossible to revascularize | Ceftazidime | Amputation |
| 13, F 78 y | Left femoro-popliteal bypass | PTFE | Early infection. Bleeding | Enterococcus faecalis. Prosthesis and dehiscent groin wound | Bypass excision. Femoro-peroneal bypass in GSV | Piperacillin-tazobactam, amikacin | Limb salvage |
| 14, M 67 y | Right femoro-popliteal bypass | PTFE | Late infection. Proximal false aneurysm | Pseudomonas aeruginosa. Prosthesis, dehiscent groin wound | Proximal bypass excision and replacement in Omniflow II with muscle flap transposition. | Ampicillin sulbactam | Limb salvage |
| 15, M 67 y | Left axillo-femoral bypass (Dacron) | Dacron | Late infection. Seroma | MRSA. Prosthesis | Bypass excision and femoral artery patch in GSV. Revascularization not required | Teicoplanin, vancomycin | Limb salvage |
| 16, M 82 y | Right axillo-femoral bypass (Dacron) | Dacron | Late infection. Seroma | Escherichia coli (periprosthetic fluid), Pseudomonas aeruginosa (prosthesis) | Bypass excision and replacement in a clean field | Amikacin, colistimethate sodium | Limb salvage. Death during follow-up |
| 17, F 68 y | Right femoro-popliteal bypass | PTFE | Early infection. Acute ischemia | MRSA (blood culture). Pseudomonas aeruginosa and Staphylococcus aureus (prosthesis) | Bypass excision. Impossible to revascularize | Meropenem, colistimethate sodium | Amputation. Death during hospital stay for sepsis |
| 18, F 65 y | Left femoro-popliteal bypass | PTFE | Late infection. Proximal false aneurysm | Proteus mirabilis (dehiscent groin wound). MSSA (dehiscent thigh wound) | Proximal bypass excision and replacement in Omniflow II. Muscle flap transposition. Graft occlusion | Ertapenem | Amputation after new bypass occlusion. Death during follow-up |
| 19,F 71 y | Right femoro-popliteal bypass | PTFE | Early infection. Acute ischemia and peripheral septic arterial embolism on the skin | Staphylococcus warneri (thrombus and prosthesis) | Bypass excision and femoro-peroneal bypass in GSV | Teicoplanin | Limb salvage |
| 20, M 65 y | Femoro-femoral crossover bypass | PTFE | Early infection. Proximal false aneurysm | MSSA. Prosthesis | Bypass excision and axillo-bifemoral bypass | Rifampicin, trimethoprim/sulfamethoxazole, daptomycin | Limb salvage |
| 21, F 48 y | Left Ilio-tibial bypass (redo-bypass) | PTFE | Early infection. Surgical wound dehiscence with persistent discharge | Pseudomonas aeruginosa. Prosthesis | Bypass excision and replacement in cephalic vein. Graft occlusion | Amikacin | Amputation after new bypass occlusion |
| 22, F 83 y | Right axillo-femoral bypass in Dacron | Dacron | Late infection. Groin surgical wound dehiscence with persistent discharge | MSSA. Dehiscent groin wound | Surgical debridement of the dehiscent groin wound. VAC therapy. Surgical closure of the wound | Meropenem | Limb salvage |
| 23, M 80 y | Right femoro-popliteal bypass | PTFE | Early infection. Groin surgical wound dehiscence with persistent discharge | Escherichia coli, Pseudomonas aeruginosa. Dehiscent groin wound | Surgical debridement of the dehiscent groin wound. VAC therapy. Surgical closure of the dehiscent wound | Amikacin, colistimethate sodium | Limb salvage |
| 24, F 77 y | Left ilio-femoral bypass | PTFE | Early infection. Surgical wound dehiscence with persistent discharge | MRSA | Surgical debridement, VAC therapy. Muscle flap transposition. | Teicoplanin, vancomycin | Limb salvage |
| 25, M 83 y | Left femoro-popliteal bypass | PTFE | Early infection. Surgical wound dehiscence with persistent discharge | MRSA | Surgical debridement of the dehiscent groin wound and VAC therapy. Surgical closure of the dehiscent wound | Teicoplanin, vancomycin | Limb salvage. Death during follow-up |
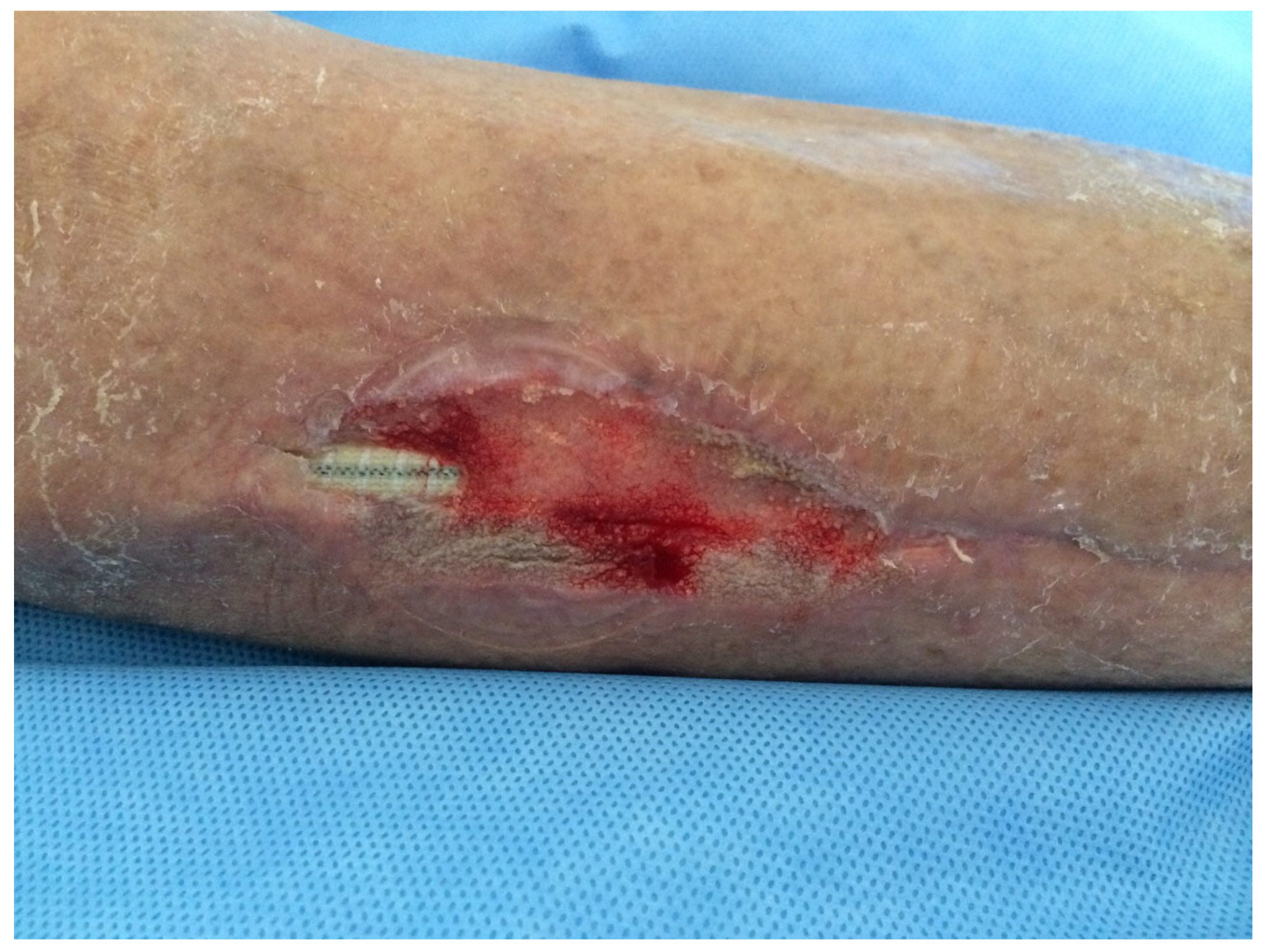
| 26, F 79 y (Figure 1, Figure 2 and Figure 3) | Right femoro- peroneal bypass (redo-bypass) | PTFE | Early infection. Dehiscence of the distal surgical wound with prosthesis exposure | Pseudomonas aeruginosa. Periprosthetic material | Surgical debridements and VAC therapy | Piperacillin-tazobactam | Limb salvage |
| 27, M 73 y | Femoro-femoral crossover bypass | PTFE | Late infection. Proximal false aneurysm | Sterile Periprosthetic material | Surgical repair | Ceftriaxone | Limb salvage |
| 28, M 76 y | Left femoro-tibial bypass (redo-bypass) | PTFE | Early infection. Dehiscence of the proximal surgical wound with prosthesis exposure | Pseudomonas aeruginosa. Periprosthetic fluid | Surgical debridements and VAC therapy | Piperacillin-tazobactam | Limb salvage |
| 29, M 74 y | Right femoro-popliteal bypass | PTFE | Late infection. Proxiaml false aneurysm | MSSA. Prosthesis | Bypass excision and replacement with a femoro-peroneal bypass in GSV | Rifampicin, trimethoprim/sulfamethoxazole, daptomycin | Limb salvage |
| Vascular Bypass and Type of Prosthesis Implanted | In our Institution (N, %) | In Other Institutions |
|---|---|---|
| Axillo-femoral (Dacron) | 2 (4.9) | 2 |
| Femoro-femoral crossover (PTFE) | 1 (2.3) | 2 |
| Ilio-femoral (PTFE) | 1 (3.7) | 1 |
| Femoro-popliteal (PTFE) | 2 (4.9) | 8 |
| Redo-bypass after failure of multiple attempts of revascularization (PTFE): -Ilio-tibial -Femoro-tibial | 10 (22.7) 3 7 | |
| TOT = 29 | 16 (8.2)/196 | 13 |
| Number | Type of Bypass | Surgical Treatment | Limb Outcome | Mortality |
|---|---|---|---|---|
| 4 | Axillo-femoral bypass | 2 Bypass excisions and new reconstruction 1 Bypass excision. Revascularization not required 1 Surgical debridement of the dehiscent groin wound.VAC therapy. Surgical closure of the wound | Limb salvage Limb salvage Limb salvage | |
| 2 | Ilio-tibial bypass | 1 Bypass excision. Impossible to revascularize 1 Bypass excision and replacement in cephalic vein. | Amputation Amputation after new bypass occlusion | |
| 1 | Ilio-peroneal bypass | Surgical debridement of the dehiscent wound. VAC therapy. Surgical closure | Limb salvage | |
| 6 | Femoro-tibial bypass | 4 Bypass excisions. Impossible to revascularize 1 Proximal bypass excision and replacemnt with Omniflow II 1 Debridements and VAC therapy | Amputation 1 Limb salvage—1 Amputation Limb salvage | 1 1 |
| 10 | Femoro-popliteal bypass | 5 Bypass excisions and replacement in GSV 1 Bypass excision. Impossible to revascularize 2 Proximal bypass excisions and replacement in Omniflow II with muscle flap transposition. 2 Surgical debridements of the dehiscent groin wound. VAC therapy. | Limb salvage Amputation Limb Salvage Amputation Limb salvage | 1 1 1 |
| 2 | Ilio-femoral bypass | Surgical site debridement. VAC therapy. Muscle flap transposition | Limb salvage | |
| 3 | Femoro-femoral crossover bypass | Bypass excision. Revascularization not required Bypass excision and axillo-bifemoral bypass Surgical repair | Limb salvage Limb salvage Limb salvage | |
| 1 | Femoro-peroneal bypass | Surgical debridements and VAC therapy | Limb salvage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caridi, G.; Massara, M.; Barilla, C.; Benedetto, F. Peripheral Prosthetic Vascular Graft Infection: A 5-Year Retrospective Study. Med. Sci. 2025, 13, 71. https://doi.org/10.3390/medsci13020071
De Caridi G, Massara M, Barilla C, Benedetto F. Peripheral Prosthetic Vascular Graft Infection: A 5-Year Retrospective Study. Medical Sciences. 2025; 13(2):71. https://doi.org/10.3390/medsci13020071
Chicago/Turabian StyleDe Caridi, Giovanni, Mafalda Massara, Chiara Barilla, and Filippo Benedetto. 2025. "Peripheral Prosthetic Vascular Graft Infection: A 5-Year Retrospective Study" Medical Sciences 13, no. 2: 71. https://doi.org/10.3390/medsci13020071
APA StyleDe Caridi, G., Massara, M., Barilla, C., & Benedetto, F. (2025). Peripheral Prosthetic Vascular Graft Infection: A 5-Year Retrospective Study. Medical Sciences, 13(2), 71. https://doi.org/10.3390/medsci13020071






