Trends in LVAD Placements and Outcomes: A Nationwide Analysis Using the National Inpatient Sample and National Readmissions Database
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, G.C.; Giertz, M.M. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation 2012, 125, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Liotta, D.; Hall, C.W.; Henly, W.S.; Cooley, D.A.; Crawford, E.S.; DeBakey, M.E. Prolonged assisted circulation during and after cardiac or aortic surgery: Prolonged partial left ventricular bypass by means of intracorporeal circulation. Am. J. Cardiol. 1963, 12, 399–405. [Google Scholar] [CrossRef] [PubMed]
- DeBakey, M.E. Left ventricular bypass pump for cardiac assistance: Clinical experience. Am. J. Cardiol. 1971, 27, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.C.; Stevenson, L.W. Keeping left ventricular assist device acceleration on track. Circulation 2011, 123, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W.; Guglin, M. Patient selection for ventricular assist devices: A moving target. J. Am. Coll. Cardiol. 2013, 61, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.S.; DeFilippis, E.M.; Cowger, J.A.; Netuka, I.; Pinney, S.P.; Givertz, M.M. Trends and Outcomes of Left Ventricular Assist Device Therapy: JACC Focus Seminar. J. Am. Coll. Cardiol. 2022, 79, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Moeller, C.M.; Valledor, A.F.; Oren, D.; Rubinstein, G.; Sayer, G.T.; Uriel, N. Evolution of Mechanical Circulatory Support for advanced heart failure. Prog. Cardiovasc. Dis. 2024, 82, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.M.; Nayak, A.; Wood, K.L.; Blumer, V.; Schettle, S.; Salerno, C.; Koehl, D.; Cantor, R.; Kirklin, J.K.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2024 Annual Report: Focus on Outcomes in Younger Patients. Ann. Thorac. Surg. 2025, 119, 34–58. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, K.; Lam, P.H.; Mehra, M.R. Evolving trends in mechanical circulatory support: Clinical development of a fully magnetically levitated durable ventricular assist device. Trends Cardiovasc. Med. 2020, 30, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hieda, M.; Sata, M.; Nakatani, T. The Importance of the Management of Infectious Complications for Patients with Left Ventricular Assist Device. Healthcare 2015, 3, 750–756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- International Classification of Diseases (ICD): International Classification of Diseases (ICD). Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 6 May 2025).
- Yuzefpolskaya, M.; Schroeder, S.E.; Houston, B.A.; Robinson, M.R.; Gosev, I.; Reyentovich, A.; Koehl, D.; Cantor, R.; Jorde, U.P.; Kirklin, J.K.; et al. The Society of Thoracic Surgeons Intermacs 2022 Annual Report: Focus on the 2018 Heart Transplant Allocation System. Ann. Thorac. Surg. 2023, 115, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Sanaiha, Y.; Downey, P.; Lyons, R.; Nsair, A.; Shemin, R.J.; Benharash, P. Trends in utilization, mortality, and resource use after implantation of left ventricular assist devices in the United States. J. Thorac. Cardiovasc. Surg. 2021, 161, 2083–2091.e4. [Google Scholar] [CrossRef] [PubMed]
- Mastoris, I.; Tonna, J.E.; Hu, J.; Sauer, A.J.; Haglund, N.A.; Rycus, P.; Wang, Y.; Wallisch, W.J.; Abicht, T.O.; Danter, M.R.; et al. Use of Extracorporeal Membrane Oxygenation as Bridge to Replacement Therapies in Cardiogenic Shock: Insights from the Extracorporeal Life Support Organization. Circ. Heart Fail. 2022, 15, e008777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acharya, D.; Manandhar-Shrestha, N.; Leacche, M.; Rajapreyar, I.; William, P.; Kazui, T.; Hooker, R.; Tonna, J.; Jovinge, S.; Loyaga-Rendon, R. Extracorporeal membrane oxygenation as a bridge to advanced heart failure therapies. J. Heart Lung Transplant. 2023, 42, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Schurr, J.W.; Ambrosi, L.; Fitzgerald, J.; Bermudez, C.; Genuardi, M.V.; Brahier, M.; Elliot, T.; McGowan, K.; Zaaqoq, A.; Laskar, S.; et al. Multicenter evaluation of left ventricular assist device implantation with or without ECMO bridge in cardiogenic shock. Artif. Organs. 2024, 48, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Chung, J.; Chen, C.W.; Gaffey, A.C.; Sotolongo, A.; Justice, C.; Ameer, A.E.; Rame, J.E.; Bermudez, C.; Acker, M.A.; et al. Different Clinical Course and Complications in Interagency Registry for Mechanically Assisted Circulatory Support 1 (INTERMACS) Patients Managed with or Without Extracorporeal Membrane Oxygenation. ASAIO J. 2018, 64, 318–322. [Google Scholar] [CrossRef] [PubMed]
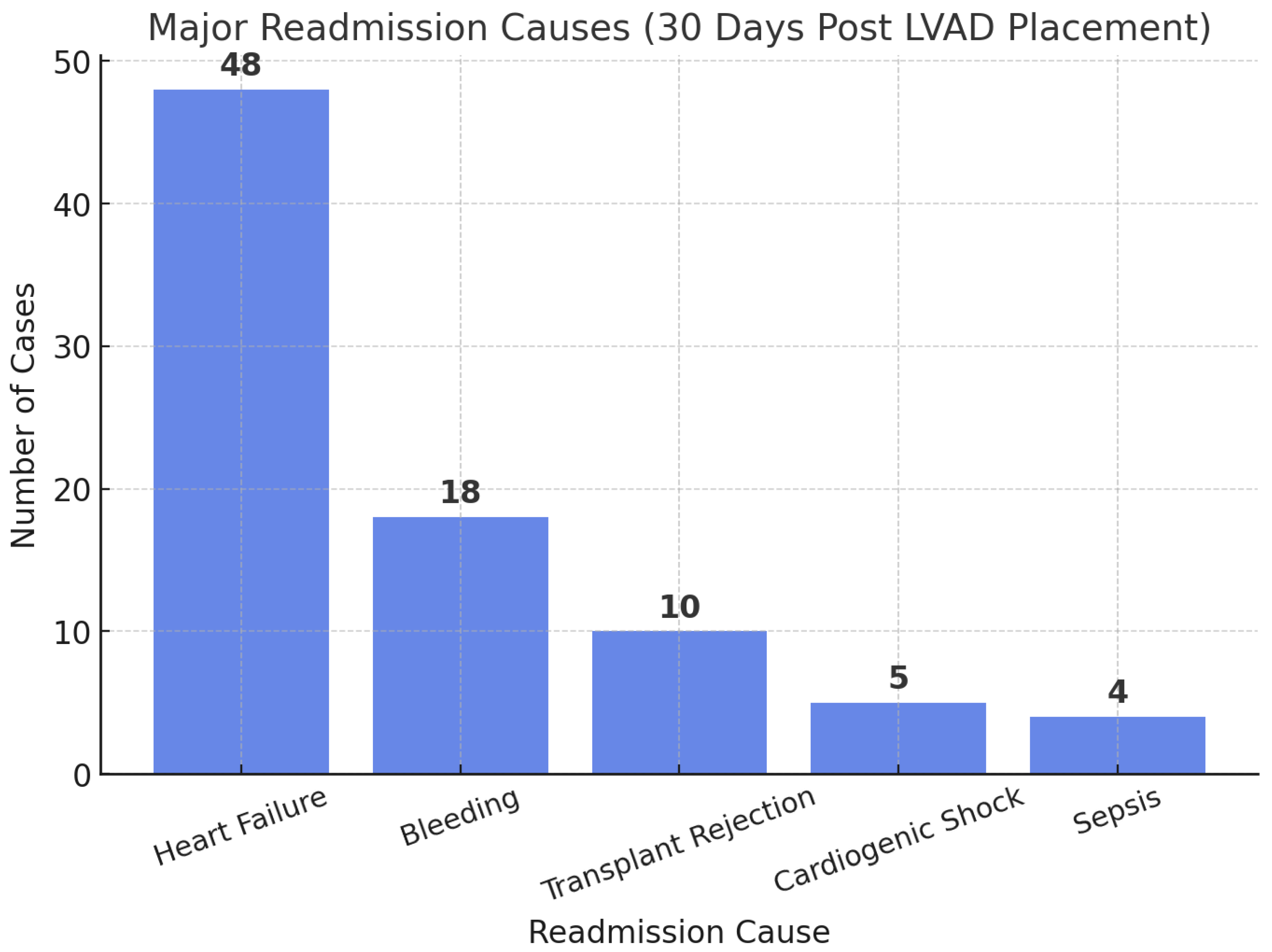
- Tripathi, B.; Arora, S.; Kumar, V.; Thakur, K.; Lahewala, S.; Patel, N.; Dave, M.; Shah, M.; Savani, S.; Sharma, P.; et al. Hospital Complications and Causes of 90-Day Readmissions After Implantation of Left Ventricular Assist Devices. Am. J. Cardiol. 2018, 122, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Mondellini, G.M.; Kurlansky, P.A.; Sun, J.; Ning, Y.; Feldman, V.R.; Tiburcio, M.; Maguire, C.W.; Ladanyi, A.; Clerkin, K.; et al. Unplanned hospital readmissions following HeartMate 3 implantation: Readmission rates, causes, and impact on survival. Artif. Organs. 2024, 48, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Cleveland, J.C., Jr.; Uriel, N.; Cowger, J.A.; Hall, S.; Horstmanshof, D.; Naka, Y.; Salerno, C.T.; Chuang, J.; Williams, C.; et al. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: A study of 2200 HeartMate 3 left ventricular assist device implants. Eur. J. Heart Fail. 2021, 23, 1392–1400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
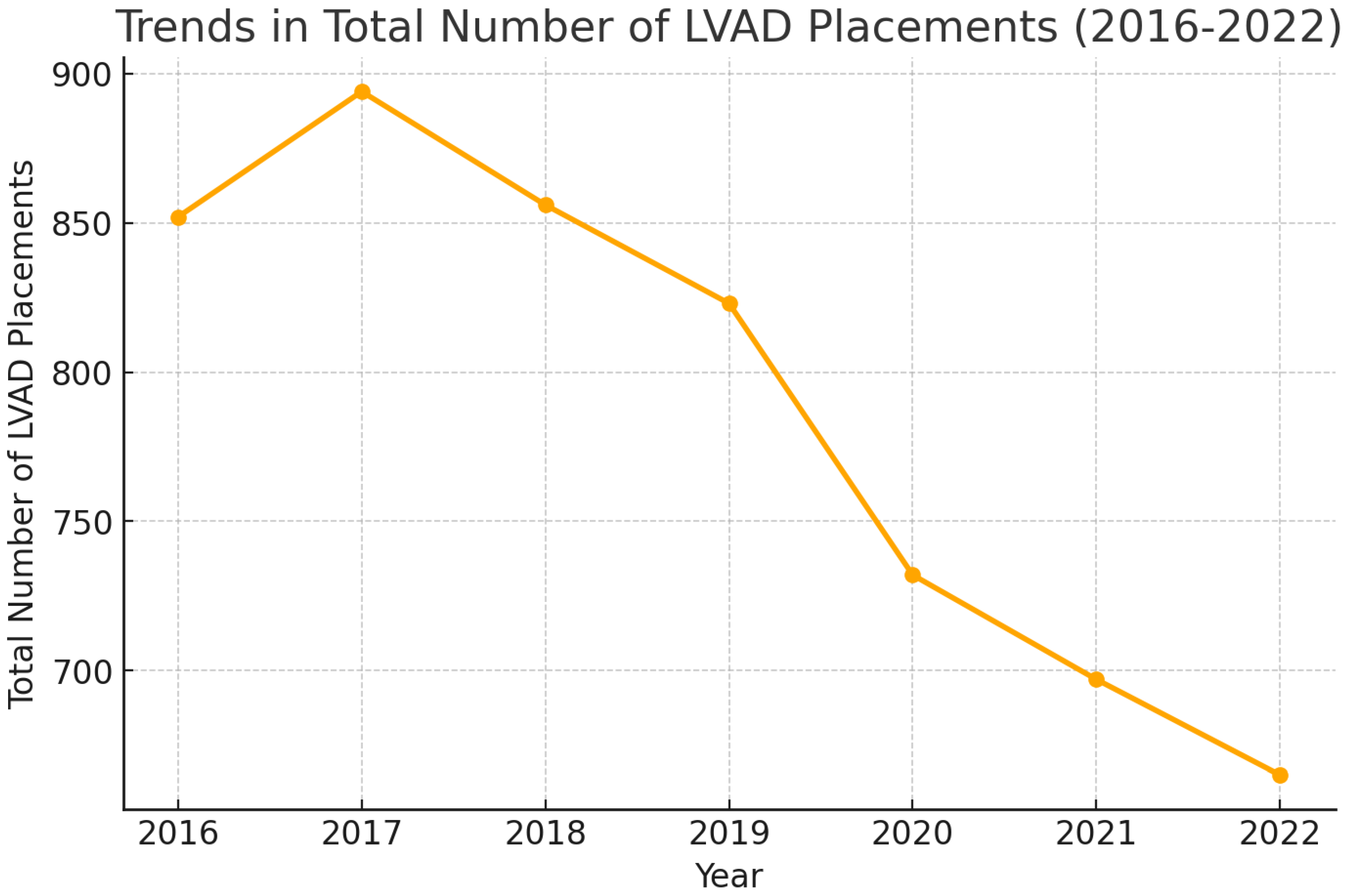
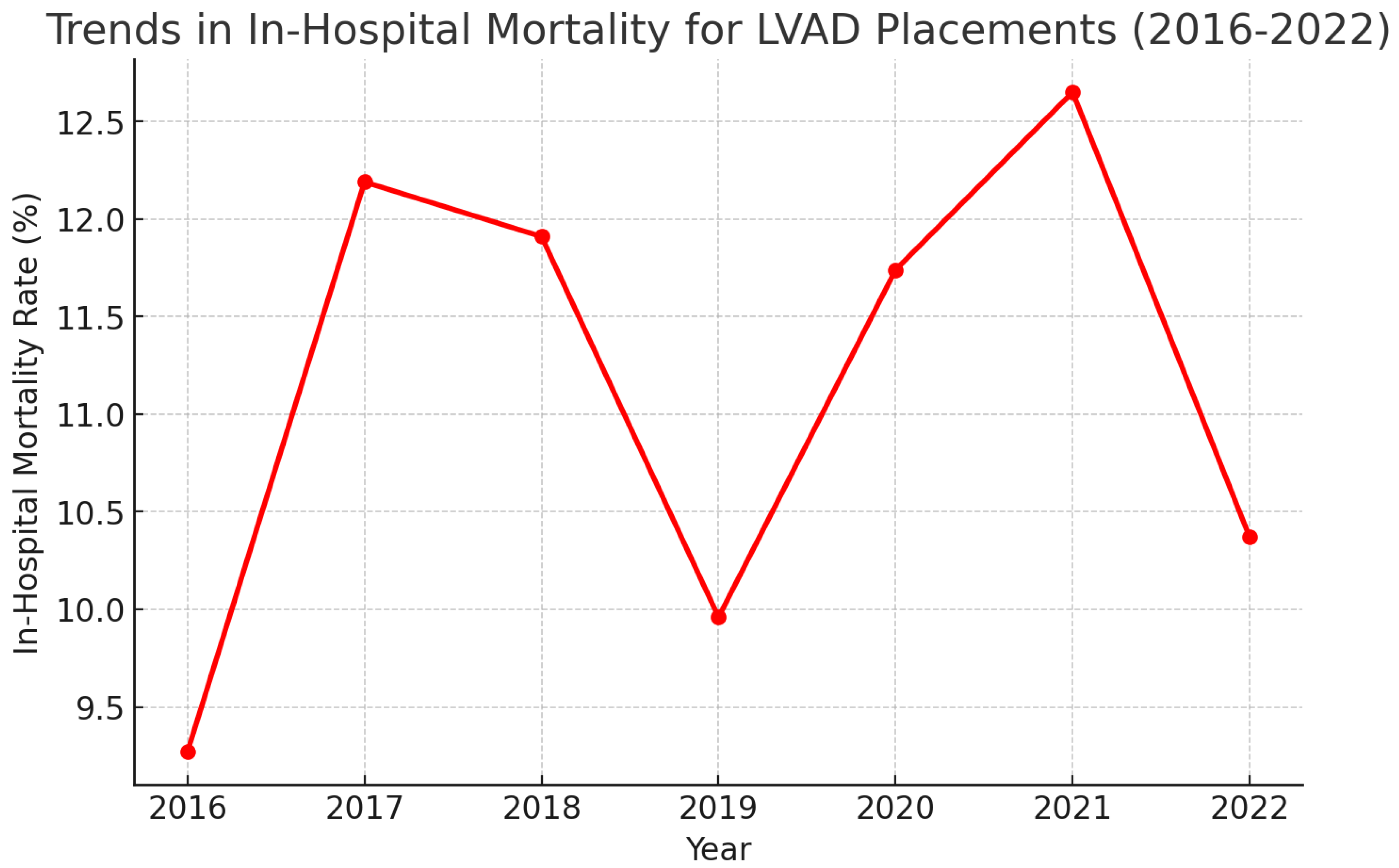
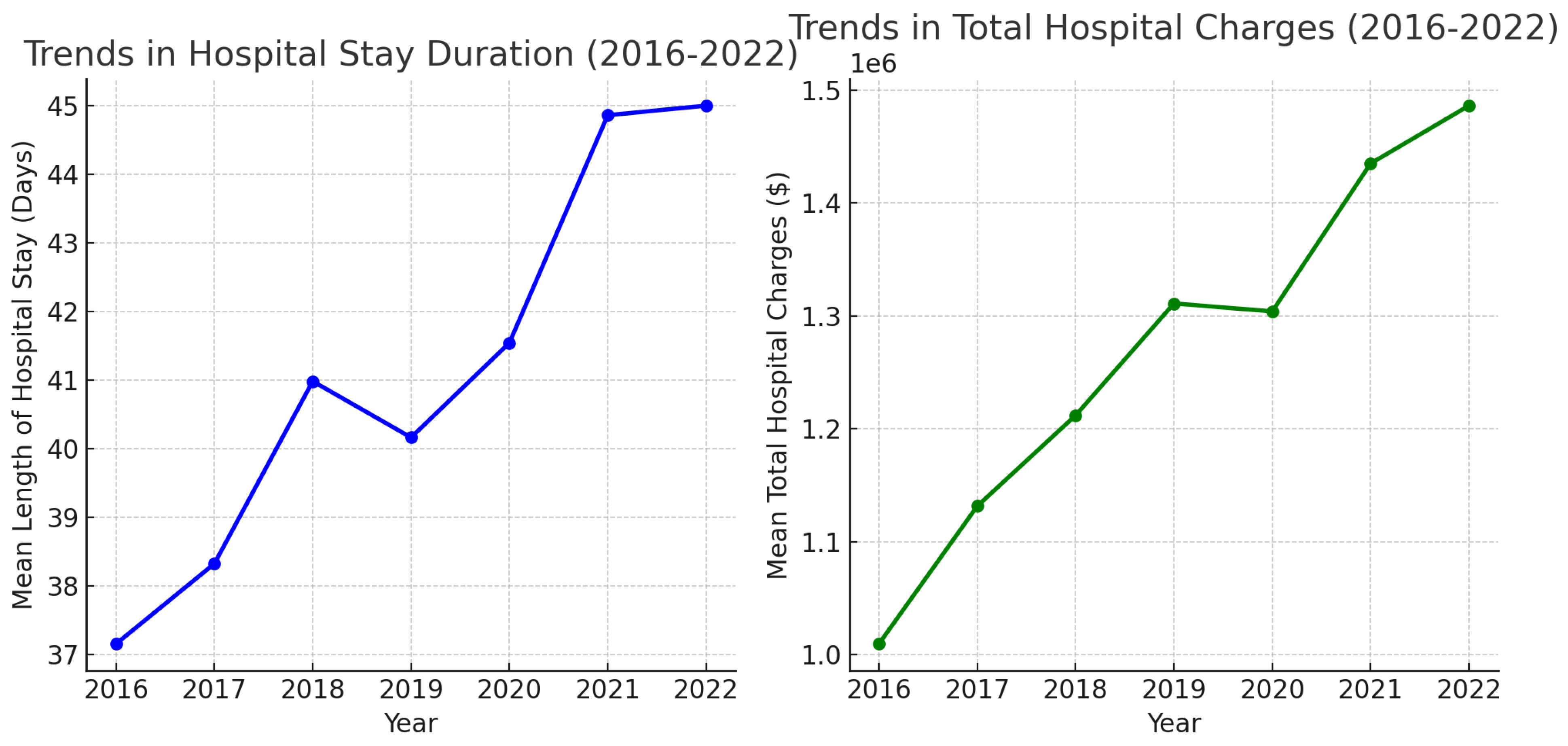
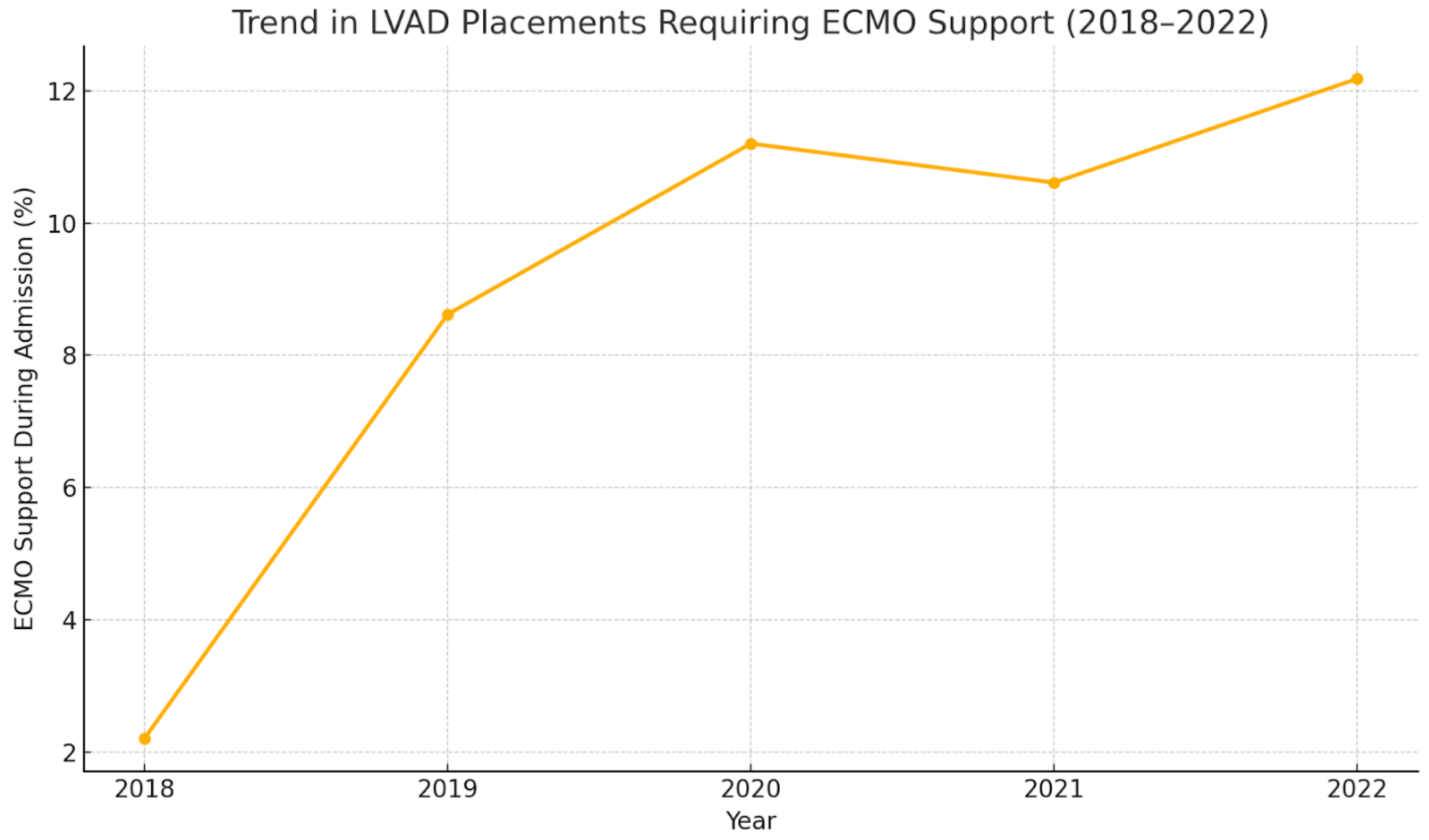
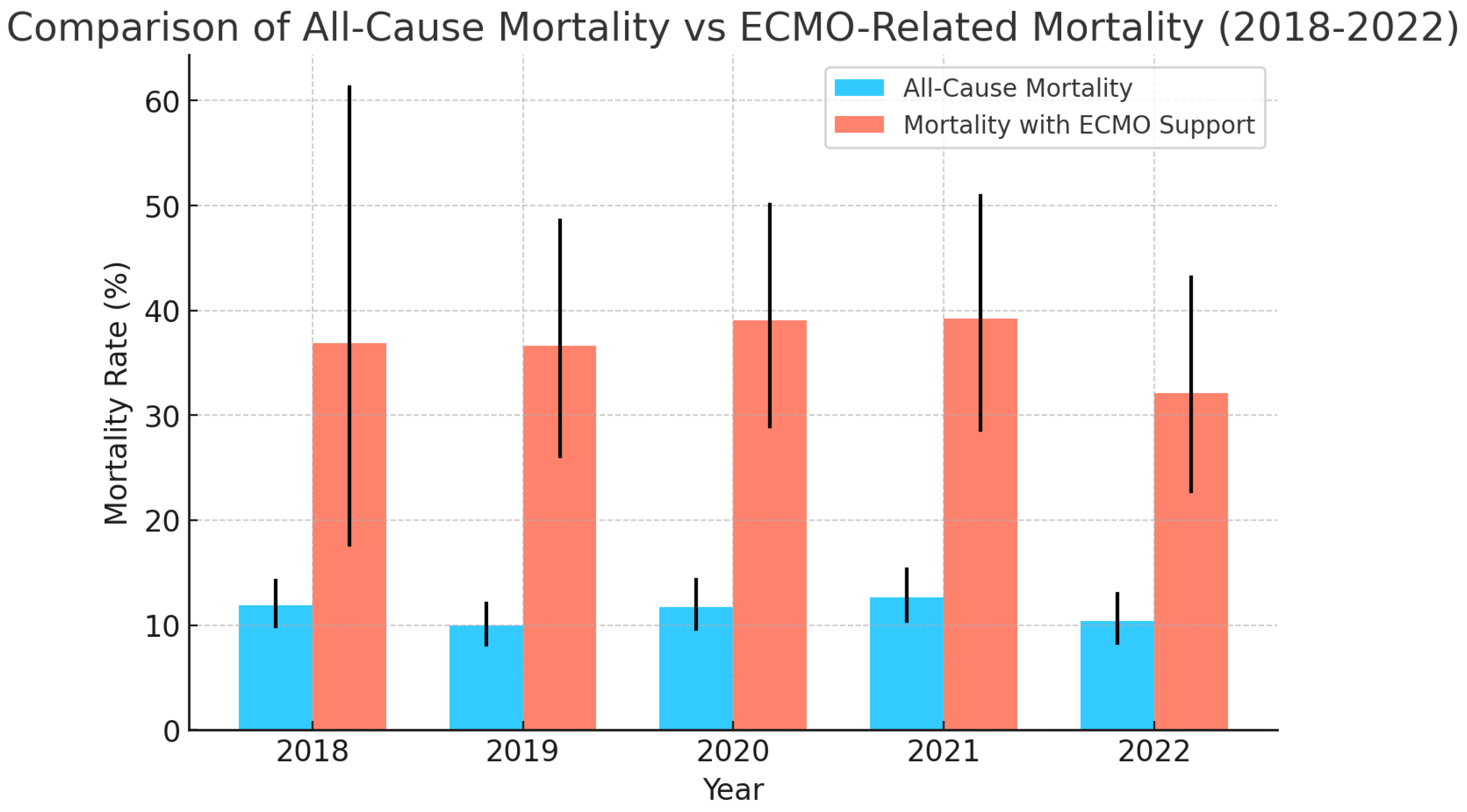
| Year | Total Number of LVAD Placements | Mean Age | Mean Length of Hospital Stay (Days) | Mean of Total Hospital Charges ($) | In-Hospital Mortality (All-Cause) % | Elective Procedures % | LVAD Placement Requiring ECMO Support During Admission % | In-Hospital Mortality for LVAD Placements Requiring ECMO Support % |
|---|---|---|---|---|---|---|---|---|
| 2016 | 852 | 55.45 | 37.15 | 1,009,442 | 9.27% (7.49–11.41%) | 25.55% (22.53–28.40%) | - | - |
| 2017 | 894 | 55.31 | 38.32 | 1,131,464 | 12.19% (10.20–14.50%) | 28.76% (25.88–31.83%) | - | - |
| 2018 | 856 | 54.84 | 40.98 | 1,211,810 | 11.91% (9.90–14.26%) | 24.12% (21.36–27.11%) | 2.21% (1.58–4.32%) | 36.84% (17.67–61.30%) |
| 2019 | 823 | 55.34 | 40.16 | 131,092 | 9.96% (8.09–12.08%) | 25.67% (22.43–28.78%) | 8.62% (7.93–12.47%) | 36.61% (26.11–48.56%) |
| 2020 | 732 | 54.1 | 41.54 | 1,303,891 | 11.74% (9.60–14.29%) | 25.03% (22.02–28.30%) | 11.20% (9.45–14.53%) | 39.02% (28.97–50.10%) |
| 2021 | 697 | 55.7 | 44.86 | 1,434,880 | 12.65% (10.35–15.30%) | 26.54% (23.39–29.95%) | 10.61% (9.84–15.67%) | 39.18% (28.61–50.88%) |
| 2022 | 665 | 54.67 | 45.0 | 1,486,108 | 10.37% (8.27–12.93%) | 24.73% (21.59–28.17%) | 12.18% (9.90–14.89%) | 32.09% (22.74–43.15%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varughese, V.J.; Nagesh, V.K.; Tran, H.H.-V.; Wadhwani, N.; Thu, A.; Weissman, S.; Atoot, A. Trends in LVAD Placements and Outcomes: A Nationwide Analysis Using the National Inpatient Sample and National Readmissions Database. Med. Sci. 2025, 13, 60. https://doi.org/10.3390/medsci13020060
Varughese VJ, Nagesh VK, Tran HH-V, Wadhwani N, Thu A, Weissman S, Atoot A. Trends in LVAD Placements and Outcomes: A Nationwide Analysis Using the National Inpatient Sample and National Readmissions Database. Medical Sciences. 2025; 13(2):60. https://doi.org/10.3390/medsci13020060
Chicago/Turabian StyleVarughese, Vivek Joseph, Vignesh Krishnan Nagesh, Hadrian Hoang-Vu Tran, Nikita Wadhwani, Audrey Thu, Simcha Weissman, and Adam Atoot. 2025. "Trends in LVAD Placements and Outcomes: A Nationwide Analysis Using the National Inpatient Sample and National Readmissions Database" Medical Sciences 13, no. 2: 60. https://doi.org/10.3390/medsci13020060
APA StyleVarughese, V. J., Nagesh, V. K., Tran, H. H.-V., Wadhwani, N., Thu, A., Weissman, S., & Atoot, A. (2025). Trends in LVAD Placements and Outcomes: A Nationwide Analysis Using the National Inpatient Sample and National Readmissions Database. Medical Sciences, 13(2), 60. https://doi.org/10.3390/medsci13020060





