Abstract
Background: Heart failure (HF) has become increasingly difficult to manage given its increasing incidence. Despite the availability of novel treatment target relieving inhibition and congestions for neurohormonal activation, heart failure is one of leading health conditions associated with high hospitalization and readmission rates, resulting in poor quality of life. In light of this, this article serves to demonstrate the effect of anakinra as one of the treatment paradigms for HF to explore the need for advanced novel interventions. Methods: We conducted a search in five electronic databases, including Embase, MEDLINE, Cochrane, Scopus, and PubMed, for RCTs (randomized controlled trials) evaluating the effects of anakinra against placebo in HF. Meta-analysis was performed using RevMan version 5.4. Results: Eight RCTs were obtained and included for analysis in this study. The results demonstrate that anakinra significantly reduces the levels of CRP (C-reactive protein), with significant difference between anakinra- and placebo-treated groups. Analyses also show that CRP failed to cause an improvement in peak oxygen consumption and ventilatory efficiency. Additionally, the treatment-related adverse events were insignificant. Some considerable limitations are that the same set of researchers were involved in most of the studies; hence, more independent studies need to be encouraged. Conclusion: Anakinra was associated with a reduction in CRP levels, indicating some anti-inflammatory effects but no effect on function, exercise capacity, and adverse effects.
1. Introduction
Heart failure (HF) incidence and prevalence increases year by year, which has caused an increased pressure and strain on healthcare systems around the world. Prior to the initial reports on patients experiencing symptoms of HF despite having normal left-ventricular ejection fraction (LVEF) and small hearts, the term “heart failure” (HF) was only used to refer to patients with diminished ejection fraction [1,2,3]. Furthermore, due to its distinct appearance from “systolic heart failure”, this condition was initially referred to as “diastolic heart failure”. As a result, this resulted in discussions among scientific researchers as a community since there is a distinct difference between systolic and diastolic dysfunction that is more fictitious than real [4]. In regard, it was even demonstrated that patients with systolic function impairment may experience more severe diastolic dysfunction than those without, and patients with maintained ejection fraction can nonetheless experience systolic dysfunction [5,6]. Earlier, a classification based on ejection fraction was proposed, and the heart failure classification included HFrEF (heart failure with reduced ejection fraction) and HFpEF (heart failure with preserved ejection fraction). Presently, there is an emerging nomenclature where a distinct subset of HFmEF (heart failure with mid-range ejection fraction) has been identified [7]. This has led to the move towards a classification that recognizes a continuous spectrum of heart failure [8,9]. The absolute cutoffs vary across different guidelines, but the European Society of Cardiology’s most recent classification advocates the following: HFrEF; LVEF < 40%, HFpEF; LVEF ≥ 50%, HFmrEF; LVEF 40–49% [9].
Particularly, managing AHF (acute heart failure) is a difficult problem that necessitates early detection of the HF clinically, confirmation by diagnostic testing (including echocardiography, biological, and X-ray), evaluation of severity, and appropriate urgent therapy. AHF is highly difficult to manage in an emergency environment due to the variety of clinical presentations, which range from mild congestion with just the symptoms of being breathless (dyspnea) to a severe shock-state syndrome [10]. Some observational research studies have associated the biomarkers of the HF-related systemic inflammation to the effects of impaired cardiac function, which in turn leads to poor prognosis in patients suffering from the HFrEF condition as well as in those with chronic inflammatory infections [11,12,13,14].
Exploring new biomarker-targeted treatments for heart failure have gained recent attention due to the recent success of canakinumab in the CANTOS trial [15]. Furthermore, since IL-1 plays a role in both systemic and localized inflammation, it is a potential target for anti-inflammatory therapy in heart failure [16]. Even a novel marker of isoform IL-1 receptor-like 1 has been found to be elevated in heart failure [17]. In experiments with animals, IL-1 has proven significant in altering systolic and diastolic function [18]. It is responsible for a myriad of inflammatory processes in the heart and hence is a potential target of treatment. Additionally, rheumatoid arthritis patients exhibit symptoms of decreased LV (left ventricular) diastolic function with therapy with anakinra, an IL-1 blocker, which proved to quickly restore normal LV diastolic function in these patients. Figure 1 demonstrates the possible effect of anakinra on cardiovascular functions of IL-1.
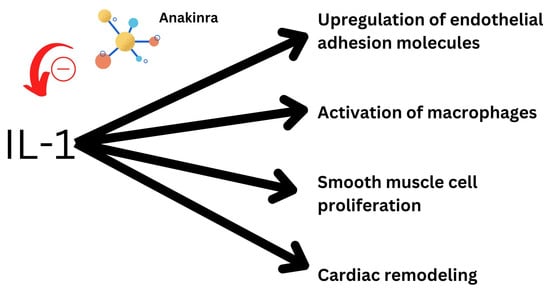
Figure 1.
Effect of anakinra and its possible inhibitory effect on IL-1’s functions.
There is limited evidence on the efficacy of anakinra in improving outcomes of heart failure with reduced ejection fraction. Anakinra is an IL-1 receptor antagonist that has recently been demonstrated to increase aerobic exercise capacity in individuals with HFpEF, minimize the incidence of HF following ST-segment elevation AMI (acute myocardial infarction), and treat chronic systemic inflammatory disorders [19,20,21]. In regard to this, this article seeks to demonstrate the effects of anakinra in HF. To achieve this, we performed a systematic review and meta-analysis to evaluate how the drug impacts outcomes in heart failure, mainly focusing on biomarkers, ventilatory efficiency, peak oxygen consumption, and adverse events.
2. Materials and Methods
2.1. Study Design and Data Sources
The article herein is a systematic review and meta-analysis of randomized controlled trials (RCTs) conducted based on the guidelines and standards as dictated by the PRISMA framework [22]. Five medical databases were systematically searched for appropriate primary studies pertinent to the topic under study. To find RCTs exploring the use of anakinra in HFrEF that were available in English, searches were conducted in the following databases: Embase, MEDLINE, Cochrane, Scopus, and PubMed.
2.2. Search Strategy
A thorough search strategy focusing on keywords and essential concepts pertinent to this article was used. Additionally, the search method included the Boolean expression, which mostly consists of “AND” and “OR”. For effectiveness, the following set of keywords were utilized: “Anakinra” AND “Heart Failure” OR “HF” OR “Acute Heart Failure” OR “AHF” OR “Heart failure with reduced ejection fraction” OR “HFrEF” OR “HF with preserved ejection fraction” OR “HFpEF”. The search was limited to published random controlled trials in the English language.
2.3. Eligibility Criteria
The following inclusion criteria were adopted for careful selection of primary articles to be analyzed in this study:
- Primary research articles evaluating the effects of anakinra in HF;
- Original articles comprising RCTs only;
- Articles published in English and dating from 2010 to 2022.
In contrast, studies were excluded based on the following criteria:
- Secondary sources of anakinra effects on HF, including newspapers, magazines, and other systematic reviews;
- Studies evaluating the use of anakinra other than in HF;
- Primary articles published in languages other than the preferred English language to avoid the loss of information and distortion during translations;
- Case reports and other study designs.
2.4. Data Extraction Quality Assessment
We tasked two independent investigators to conduct data selection and extraction, where information was obtained from the studies that met the inclusion criteria based on PICO framework [23]. The data obtained by these reviewers include information on authors, study protocol, patients’ characteristics (participants), anakinra dosage (intervention), placebo treatment (comparator), and key findings (outcomes). An additional reviewer was consulted to help resolve issues resulting from data extraction to harmonize and elucidate meaningful information for data and statical analysis. In addition, quality appraisal was performed using the Cochrane risk of bias tool; the six-criteria tool—reporting, blinding, selection, binding, attrition, and other biases—is used to categorize studies as having a low, high, or unknown risk of bias [24].
2.5. Statistical Analysis
Based on the requirements and the data obtained, the Cochrane Review Manager Software (RevMan version 5.4) was effectively put to use for data analysis. In order to perform meta-analysis, the Cochrane guidelines dictate that data must be in the same format and measure similar aspects. Regarding this, most of the data recorded in the included articles was presented numerically as median and interquartile range (IQR). As a result, to use RevMan to perform the meta-analysis, imputations were made for median as mean and IQR/4 as standard deviation to facilitate similar format of numerical data for the analysis. Due to approximations and imputations, mean difference (MD) and poled odds ratio were selected as the effect measure at a 95% level of confidence. Therefore, statistical significance was evaluated at p < 0.05, while the I-square test was employed to test for heterogeneity, where above 50% (I2 > 50%) was deemed significant heterogeneity, and low heterogeneity was determined otherwise [25].
3. Results
3.1. Search Results
The total citations identified through the various databases in addition to reviewing their reference lists included 823 articles. Of these, only eight studies were RCTs meeting the inclusion criteria, namely exploring the effect of anakinra on HFrEF, and were acquired for analysis. Duplicates were 342 in number, and 178 were excluded after title and abstract screening, and 186 studies were not retrieved due to the requirement of purchase and special licenses needed for access and also due to URL errors during searching for their full texts. Figure 2 below depicts the search process used to select the eight pertinent articles for this study. Table 1 depicts the outcomes of the use of anakinra in HF that were evaluated in various trials and those included in this systematic review.
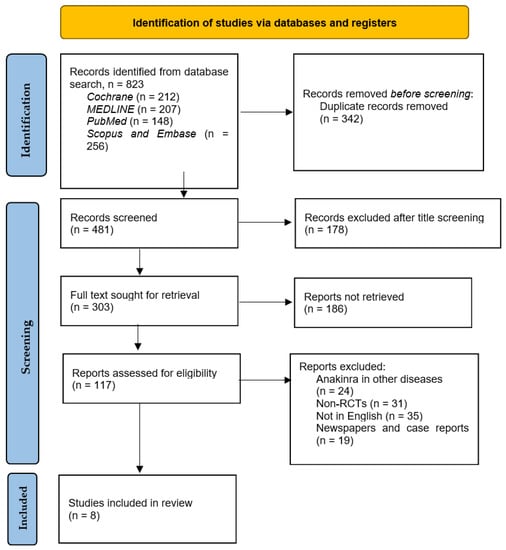
Figure 2.
PRISMA flowchart depicting the search process utilized to arrive at the eight RCTs.

Table 1.
Outcomes studied in the trials of use of anakinra in heart failure.
Table 2 describes the individual study characteristics and the outcomes that were studies in each of those studies related to anakinra.

Table 2.
Study characteristics. NOTE: RCT, randomized controlled trial; CRP-C, reactive protein; Vo2, peak oxygen consumption; VE/Vco2, minute ventilation–carbon dioxide production slope; NT, proBNP-N-terminal pro-B-type natriuretic peptide; AEs, adverse events; QoL, quality of life; CPX, cardiopulmonary exercise testing.
3.2. Risk of Bias Evaluation
Based on the protocols adopted by the Cochrane risk of bias tool of evaluation, all the eight RCTs were majorly of low bias across all the items as depicted in Figure 3 and Figure 4. The low risk was manifested by the green shading, and high risk corresponds to red, while yellow represents uncertain bias or unclear result. This was achieved by disregarding primary articles characterized by high risk of bias to avoid altering and inconveniencing the results of this study. The same protocol was applied for those with unclear bias to eliminate doubtful outcomes of our study.
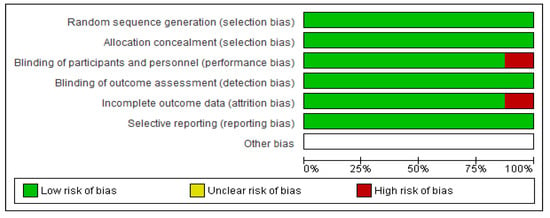
Figure 3.
Depicting the risk of bias graph judgments for the six items.
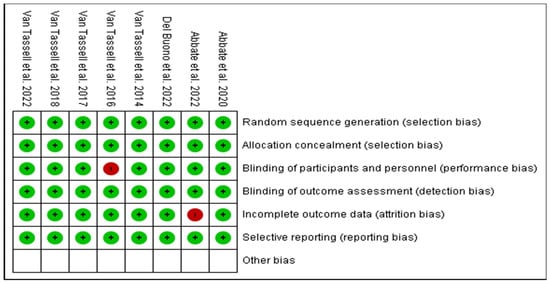
Figure 4.
Risk of bias summary of the eight RCTs against various item evaluations [19,26,27,28,29,30,31,32].
3.3. Effect of Anakinra on CRP Levels
Seven RCTs evaluated the impact on C-reactive protein (CRP) levels caused by anakinra treatment. The changes in the level of CRP from baseline were used to perform a meta-analysis of the seven RCTs as depicted in Figure 5. This analysis reveals a significant difference in CRP levels between the two groups, with anakinra treatment showing a significantly higher reduction from the baseline CRP level compared to placebo treatment (MD 2.17, 95% CI 1.81 to 2.53; I2 = 95%) at p = 0.0009. The overall heterogeneity across the seven studies was significantly high, with I2 = 95%.
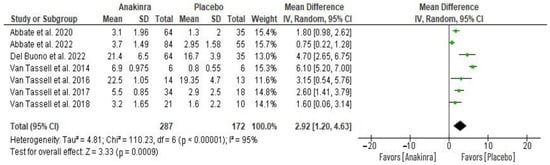
Figure 5.
Anakinra effect on CRP levels [19,26,27,28,29,30,31,32].
3.4. Effect of Anakinra on VO2
The analysis of the anakinra effect on peak oxygen consumption (VO2) in patients with HF was determined using four RCTs as in Figure 6. In comparison to placebo or standard treatment, anakinra was associated with greater change in peak VO2 from baseline VO2. However, the difference between anakinra and placebo in peak VO2 changes was not statistically significant (MD 0.30, 95% CI 0.00 to 0.59; I2 = 0%), with an overall test effect of Z = 0.96 at p = 0.05. The heterogeneity was insignificant (I2 = 0%).
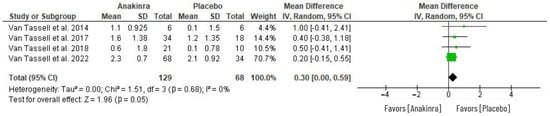
Figure 6.
Effect of anakinra on peak oxygen consumption (VO2) changes from baseline values [28,30,31,32].
3.5. Effect of Anakinra on VE/VCO2 Slope
The effect of anakinra on ventilatory efficiency in HF patients was determined by the VE/VCO2 slope as in Figure 7. Among the three RCTs that evaluated VE/VCO2 slope, no difference was depicted between anakinra treatment relative to placebo, and thus, the statistical difference was insignificant between the two groups (MD 0.89, 95% CI –0.23 to 2.01; I2 = 72%) at p = 0.12.
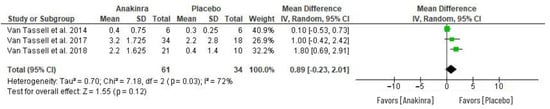
Figure 7.
Effect of anakinra on ventilatory efficiency [28,30,31].
3.6. Adverse Events (AEs)
An odds ratio effect measure was performed in five RCTs to evaluate the adverse events associated with the use of anakinra in HF treatment. The AEs evaluated includes the cases of nonserious infections, respiratory infections, cardiac and non-cardiac evets, and the occurrence of other clinical events. A random effect model of the analysis revealed insignificant difference between anakinra and placebo treatment although the individual studies indicated that there was considerably small number of patients with adverse events in the anakinra group compared to placebo (OR 0.67, 95% CI 0.38 to 1.20; I2 = 0%) at p = 0.18, while the heterogeneity was insignificant. See Figure 8 for the results of our analysis.
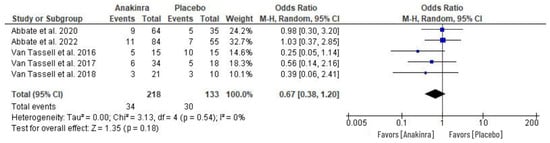
Figure 8.
Adverse events associated with the use of anakinra vs. placebo treatment in HF patients [26,27,29,30,31].
4. Discussion
Our study was conducted with the aim of providing insight on whether anakinra is a useful therapeutic intervention for HF that will be instrumental in alleviating associated symptoms and, most importantly, improve the patients’ cardiorespiratory functioning. The results of our analysis revealed that anakinra has significant effect on the CRP levels but minimal to no impact on ventilatory capacity, exercise capacity, and adverse events. Many of the studies evaluated the effect of anakinra administered once or twice a day through 100 mg subcutaneous injections for 2 to 24 weeks. However, two weeks of treatment were considered in our study for uniformity in the statistical analysis since most trials were evaluated during this period. This indicates that substantial evidence on the effect of the drug in longer duration of treatment is sparse. More granular analysis of the adverse event data could not be performed, as there were inconsistencies in the representation of individual adverse events across the studies, which could not be harmonized and used to provide meaningful statistical analysis. Van Tassell et al. (2022) is an ongoing trial that is measuring CRP as an outcome, but the current trial data only provided a theoretical description of the biomarker levels and could not provide numerical data (in terms of mean +/− SD) that could be used in our analysis, similar to other studies. Hence, it could not be included for analyzing the effect of anakinra on CRP [32].
Our study evaluated the effect of anakinra (IL-1 blocker), which is a significant recombinant of IL-1 receptor antagonist, administered once or twice a day through 100 mg subcutaneous injections for periods ranging from 2 to 24 weeks. Based on the results of our study, anakinra treatment bolstered a significant effect in the reduction of CRP levels relative to standard treatment (placebo). Since CRP production is directly related to IL-6 rather than IL-1, anakinra’s effect on the marker is indirect through a possible mechanism that alters persistent myocardial inflammation in HF [15]. This result implies that anakinra is significant as an inhibitor to the systemic inflammatory response, indicated by the statistically significant difference between patients treated with anakinra versus those in the placebo group. This finding seems coherent with the results of randomized placebo phase II trials, which observed that anakinra was associated with significant reduction of CRP from baseline levels than placebo at 12 weeks of treatment [28,30,31]. However, our findings diverge from previous research that employed alternative cytokines, including infliximab, in such a way that the levels of CRP only declined in 2 weeks of treatment although afterwards, it gradually returned back to the baseline values irrespective of changes, and treatment with infliximab progressed [33]. Similarly, etanercept could not cause significant changes to CRP levels despite having modest impact on IL-6 [34].
Despite its prowess as an inflammatory response inhibitor [27,29], anakinra, however, did not show a significant difference in peak oxygen consumption. In comparison to placebo treatment, anakinra failed to significantly improve the aerobic capacity as indicated by the associated modest effect on VO2 (see Figure 6). Similarly, ventilatory efficiency was not improved from the baseline values, with an insignificant difference between anakinra and placebo treatment (Figure 7). These analyses are consistent with the results of three RCTs that indicated that the ventilatory efficiency and peak oxygen consumption levels remained unchanged from baseline after 12 weeks of anakinra intervention [24,25,29]. However, this outcome contradicts the results of a D-HART study using anakinra as a robust impact on exercise capacity in patients with HF, revealing improved peak VO2 in 2 weeks of treatment [28]. Nonetheless, the observed improvement was relatively smaller in patients with HFrEF [21,28]. Despite the modest change due to anakinra, the peak VO2 was considered significant in their results. On the contrary, in a REDHART trail, anakinra treatment for 2 weeks had no significant difference but, with continued treatment at 4 and 12 weeks, was associated with an improved peak VO2 relative to the baseline values [30,32].
Furthermore, this study evaluated adverse events as treatment-related side effects of anakinra. Among the AEs were injection-site reactions [27], serious and non-serious sinus and respiratory infections [31], events of ischemic or hemorrhagic stroke [30], and worsened pulmonary congestion [26]. The high rate of readmissions and longer hospitalizations can be articulated along with these adverse events, which may result in deaths among HF patients if not well-managed [27]. Our study indicates that there was a smaller number of patients with adverse events in the anakinra-treated group than in the placebo group. However, the statistical difference between the two was insignificant (Figure 8). Generally, there were several cases in both anakinra and placebo groups within the included studies with high rates of hospital readmissions following AEs associated with HF across all the populations under considerations [30]. This correlates with one study that showed no infections in the anakinra group compared to 12 AEs among ten patients in the placebo group [29]. Furthermore, despite the occurrence of adverse events, data collected from the Duke Activity Status Index (DASI) and Minnesota Living with Heart Failure Questionnaire (MLWHFQ questionnaires) indicated a positive change in functional capacity restoration in the anakinra group compared to the placebo group [31,32]. Similarly, a significant improvement in DASI was also recorded in the anakinra group [28,30].
The existing interventional treatment paradigm for acute HF, including HFrEF and HFpEF, work by targeting to relieve the patients from acute decompensation and cardiac remodeling. Recent insights from the use of anti-inflammatory drugs such as canakinumab produced improvement in heart damage associated with the persistent inflammation seen in some types of heart failure. Furthermore, novel biomarkers related to the development and progression of heart failure are being studied. The cardio-inflammatory phenotype of heart failure is of particular interest since markers such as CRP, a soluble isoform of IL-1 receptor-like 1 (also known as sST2), have been seen to be increased in the phenotype, indicating a vital role in the disease process. Even in preclinical studies, TNF, IL-1β, IL-6, and IL-18 have been found to be increased in HF [35]. HFpEF has been difficult to study due to its heterogenous nature and lack of reliable pre-clinical models [36]. No study was particularly aimed at the cardio-inflammatory phenotype, and only one biomarker was repeatedly used in the studies (i.e., CRP). sST2 has been found to be useful as a prognostic marker with a better predictive value of fatal outcomes [37]. In the future, in more focused studies with broad range of biomarkers, HFpEF should be evaluated to better understand the effect of drugs such as anakinra on the inflammation associated with HF [38].
Limitations to the Study
The article is limited due to constrictions in using RCTs only in addition to insufficient literature exploring anakinra treatment for HF. The statistical analysis was conducted largely based on imputations since the depicted values in the studies were provided in median and IQR, which might be a source of errors and inaccuracies in the analysis. Moreover, there is the chance of unreliable results due to the varying small populations utilized in individual studies in addition to different treatment periods and frequency per day of anakinra injection from one study to another. All of RCTs included belong to the same group of researchers with similar procedures, locations, and patients’ characteristics. Therefore, this is also a source of bias. The time of treatment of anakinra ranged from 2 to 24 weeks, but our analysis only took 2-week treatment into consideration for uniformity in the data obtained. Hence, it is vital that future studies take into consideration the relationship of time with the outcomes of anakinra in heart failure if used for longer durations.
5. Conclusions
Our study sought to explore the effects of anakinra in heart failure as a means of providing insight on whether it can prove to be a novel treatment approach to tackling HF. Based on our analysis, anakinra, as a combinatory IL-1 blocker, was instrumental in the reduction of CRP levels among the HF patients. Consequently, this demonstrates that anakinra is a key inhibitor to inflammatory response, which is crucial in improving functional capacity in HF patients. Future trials studying the drug specifically in patients with a cardio-inflammatory phenotype maybe fruitful in analyzing its impact. Nevertheless, short-term treatment with anakinra was not effective in improving the ventilatory efficiency or changing the peak oxygen consumption in heart failure.
Author Contributions
K.M., conceptualization, methodology, investigation, data curation, writing—original draft, and writing—review and editing; A.R., literature review, data extraction, and writing—original draft; K.P., conceptualization, data extraction, and writing—original draft; C.P.—conceptualization, data extraction, and writing—original draft; A.K.S., conceptualization, data extraction, and writing—original draft; S.P.G., conceptualization, data extraction, writing—original draft; A.A., conceptualization, data extraction, and writing—original draft; R.A., conceptualization, data-analysis, and writing—original draft; A.V., conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing, visualization, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. Trials described in the study were publicly available.
Informed Consent Statement
Not applicable.
Data Availability Statement
Trials described in the study were publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borlaug, B.A.; Melenovsky, V.; Russell, S.D.; Kessler, K.; Pacak, K.; Becker, L.C.; Kass, D.A. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients with Heart Failure and a Preserved Ejection Fraction. Circulation 2006, 114, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, A.H.; Naccarelli, G.V.; Gray, E.L.; Hicks, C.H.; Goldstein, R.A. Congestive heart failure with normal systolic function. Am. J. Cardiol. 1984, 54, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J.; Traill, T.A.; Fortuin, N.J. Hypertensive Hypertrophic Cardiomyopathy of the Elderly. N. Engl. J. Med. 1985, 312, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Böhm, M.; Cleland, J.G.; Paulus, W.J.; Pieske, B.; Rapezzi, C.; Tavazzi, L. Heart failure with preserved ejection fraction: Uncertainties and dilemmas. Eur. J. Heart Fail. 2015, 17, 665–671. [Google Scholar] [CrossRef]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and Diastolic Heart Failure in the Community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired Systolic Function by Strain Imaging in Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2013, 63, 447–456. [Google Scholar] [CrossRef]
- Lam, C.S.; Teng, T.-H.K. Understanding Heart Failure With Mid-Range Ejection Fraction. JACC Heart Fail. 2016, 4, 473–476. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Butler, J.; Abboud, F.; Armstrong, P.; Adamopoulos, S.; Atherton, J.; Backs, J.; Bauersachs, J.; Burkhoff, D.; Bonow, R.; et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur. Heart J. 2019, 40, 2155–2163. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200, Erratum in: Eur. Heart J. 2018, 39, 860. [Google Scholar] [CrossRef]
- Malik, A.; Brito, D.; Vaqar, S.; Chhabra, L. Congestive Heart Failure; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430873/ (accessed on 29 October 2022).
- Michowitz, Y.; Arbel, Y.; Wexler, D.; Sheps, D.; Rogowski, O.; Shapira, I.; Berliner, S.; Keren, G.; George, J.; Roth, A. Predictive value of high sensitivity CRP in patients with diastolic heart failure. Int. J. Cardiol. 2008, 125, 347. [Google Scholar] [CrossRef]
- Shah, S.J.; Marcus, G.M.; Gerber, I.L.; Mckeown, B.H.; Vessey, J.C.; Jordan, M.V.; Huddleston, M.; Foster, E.; Chatterjee, K.; Michaels, A.D. High-Sensitivity C-Reactive Protein and Parameters of Left Ventricular Dysfunction. J. Card. Fail. 2006, 12, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.P.; Myasoedova, E.; Crowson, C.S.; Davis, J.; Roger, V.L.; Karon, B.L.; Borgeson, D.D.; Therneau, T.M.; Rodeheffer, R.J.; Gabriel, S.E. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T. Inhibition of Interleukin-1 by Anakinra Improves Vascular and Left Ventricular Function in Patients with Rheumatoid Arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy with Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Damonte, J.I.; Trankle, C.R.; Kadariya, D.; Carbone, S.; Thomas, G.; Turlington, J.; Markley, R.; Canada, J.M.; Biondi-Zoccai, G.G.; et al. Effect of interleukin-1 blockade with anakinra on leukocyte count in patients with ST-segment elevation acute myocardial infarction. Sci. Rep. 2022, 12, 1254. [Google Scholar] [CrossRef]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of Interleukin-1 Blockade with Anakinra on Adverse Cardiac Remodeling and Heart Failure After Acute Myocardial Infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) Pilot Study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Arena, R.A.; Toldo, S.; Mezzaroma, E.; Azam, T.; Seropian, I.M.; Shah, K.; Canada, J.; Voelkel, N.F.; Dinarello, C.A.; et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE 2012, 7, e33438. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://jhu.pure.elsevier.com/en/publications/cochrane-handbook-for-systematic-reviews-of-interventions (accessed on 30 October 2022).
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment–Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Abbate, A.; Wohlford, G.F.; Del Buono, M.G.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Kadariya, D.; Trankle, C.R.; Biondi-Zoccai, G.; Lipinski, M.J.; et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: Results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 8, 503–510. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Abouzaki, N.A.; Oddi Erdle, C.; Carbone, S.; Trankle, C.R.; Melchior, R.D.; Turlington, J.S.; Thurber, C.J.; Christopher, S.; Dixon, D.L.; et al. Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study. J. Cardiovasc. Pharmacol. 2016, 67, 544–551. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Oddi Erdle, C.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef]
- Van Tassell, B.; Mihalick, V.; Thomas, G.; Marawan, A.; Talasaz, A.H.; Lu, J.; Kang, L.; Ladd, A.; Damonte, J.I.; Dixon, D.L.; et al. Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): A randomized, double blind, placebo controlled, single center, phase 2 study. J. Transl. Med. 2022, 20, 270. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [PubMed]
- Deswal, A.; Bozkurt, B.; Seta, Y.; Parilti-Eiswirth, S.; Hayes, F.A.; Blosch, C.; Mann, D.L. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation 1999, 99, 3224–3226. [Google Scholar] [CrossRef] [PubMed]
- Gulick, T.; Chung, M.K.; Pieper, S.J.; Lange, L.G.; Schreiner, G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc. Natl. Acad. Sci. USA 1989, 86, 6753–6757. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Hill, J.A.; Singh, A.; Valero-Muñoz, M.; Sam, F. Heart Failure with Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models. Circ. Res. 2022, 130, 1906–1925. [Google Scholar] [CrossRef]
- Miftode, R.-S.; Constantinescu, D.; Cianga, C.M.; Petris, A.O.; Timpau, A.-S.; Crisan, A.; Costache, I.-I.; Mitu, O.; Anton-Paduraru, D.-T.; Miftode, I.-L.; et al. A Novel Paradigm Based on ST2 and Its Contribution towards a Multimarker Approach in the Diagnosis and Prognosis of Heart Failure: A Prospective Study during the Pandemic Storm. Life 2021, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Pistulli, R.; Lichtenauer, M.; Wernly, B.; Paar, V.; Motloch, L.J.; Rezar, R.; Jung, C.; Hoppe, U.C.; Schulze, P.C.; et al. Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared with ICM, DCM, and Controls. J. Clin. Med. 2020, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).