Microtopography Controls of Carbon and Related Elements Distribution in the West Siberian Frozen Bogs
Abstract
1. Introduction
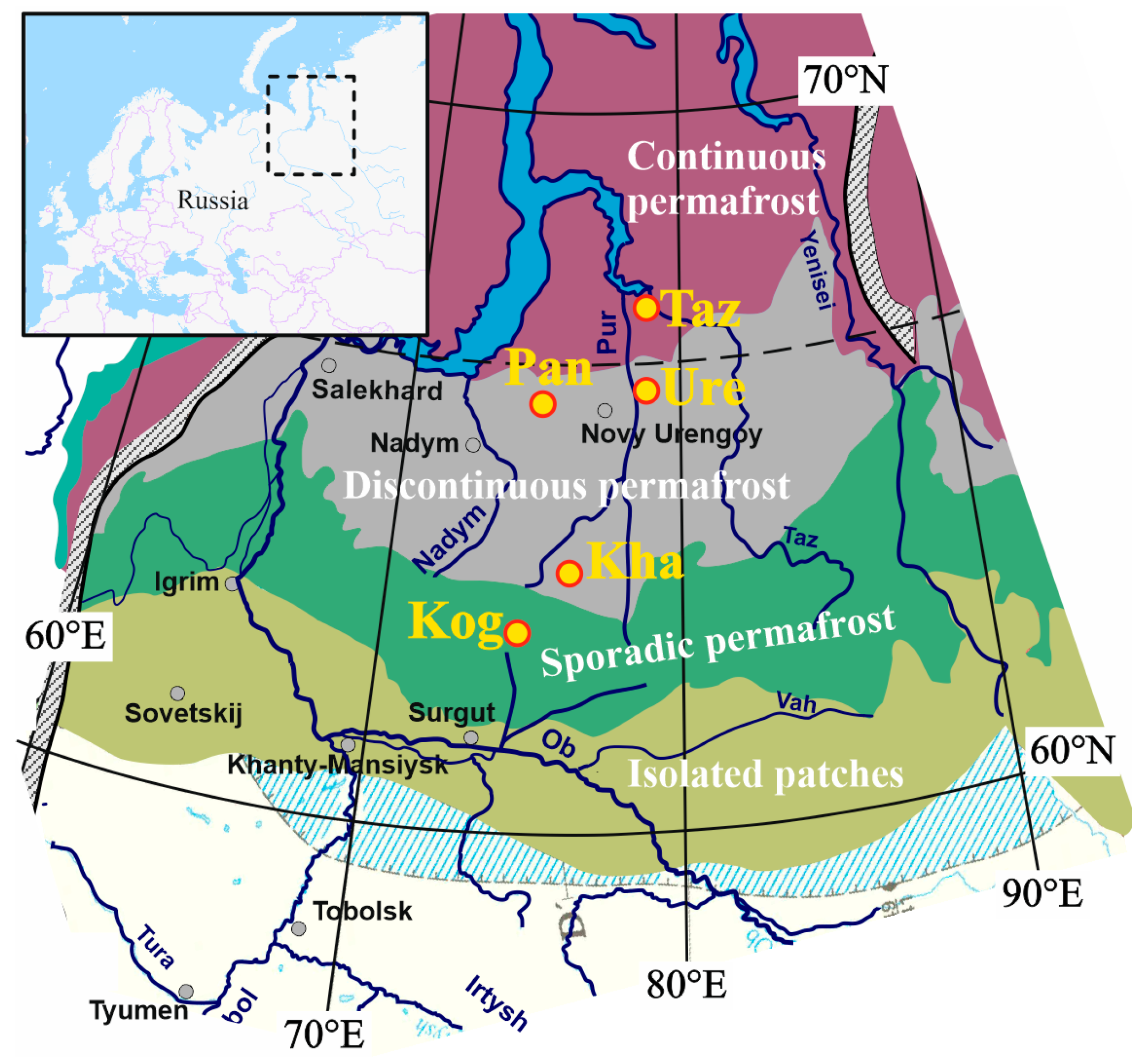
2. Materials and Methods
3. Results
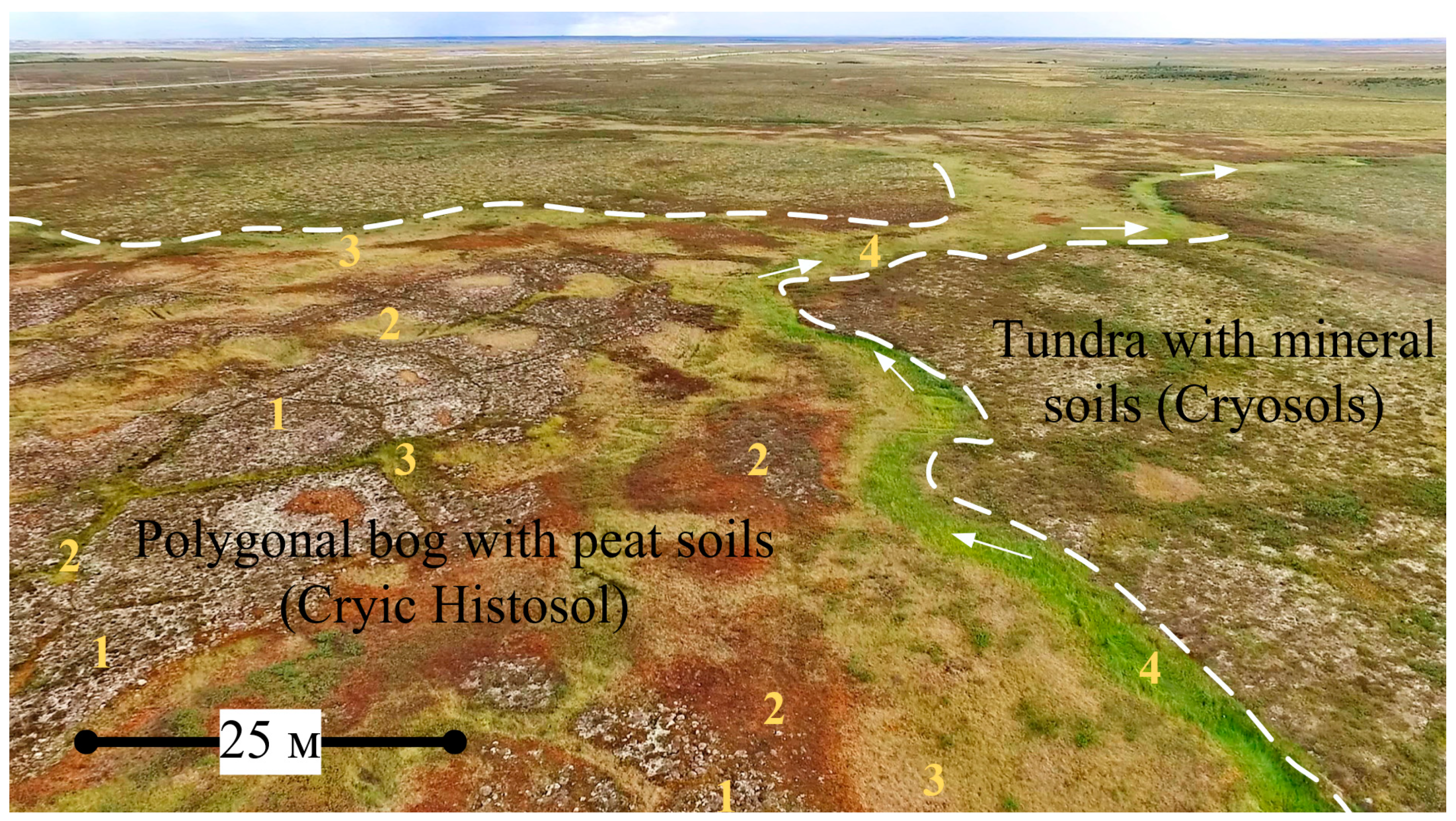
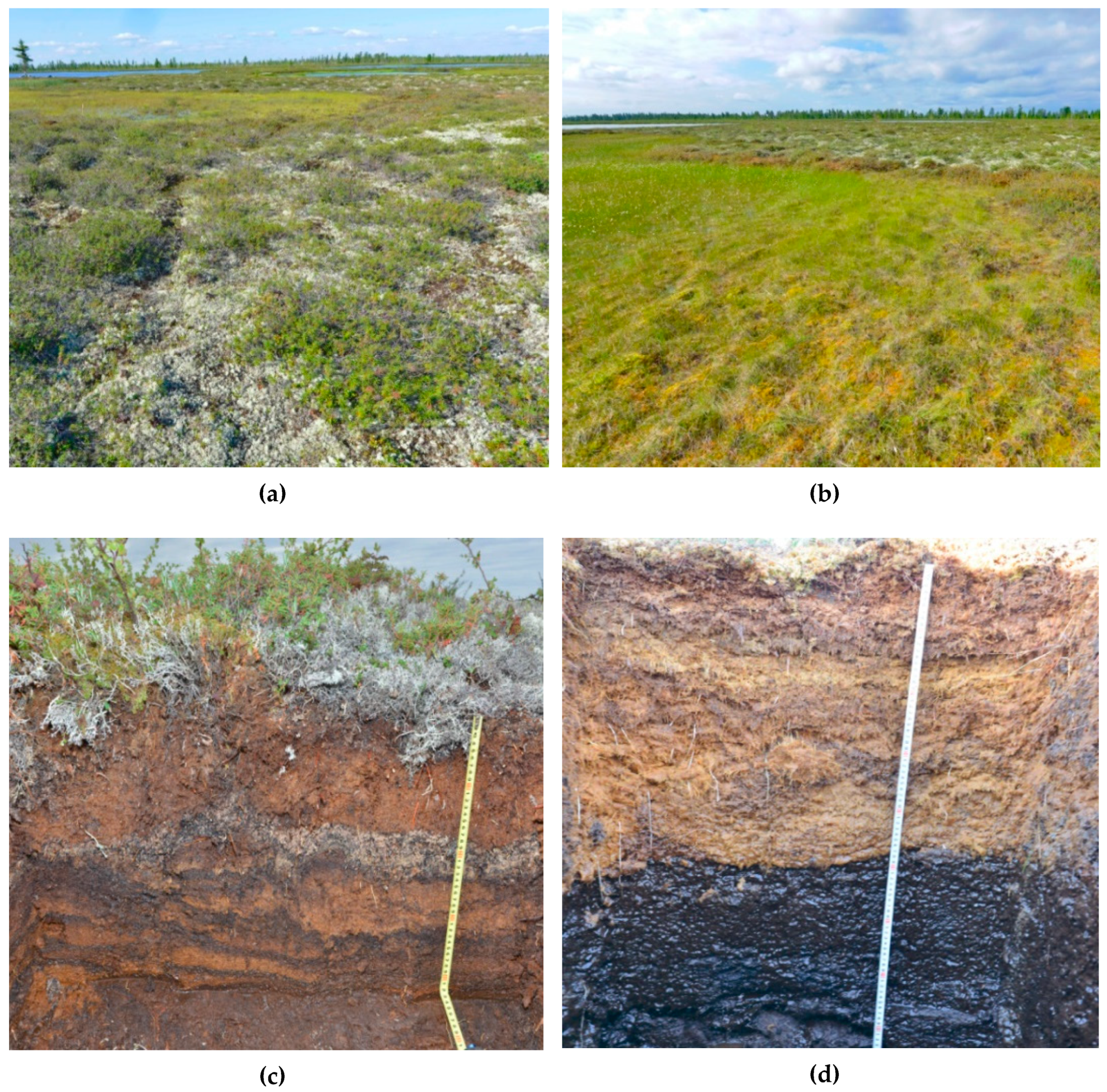
3.1. Features of Key Site Bogs and Soil Identification
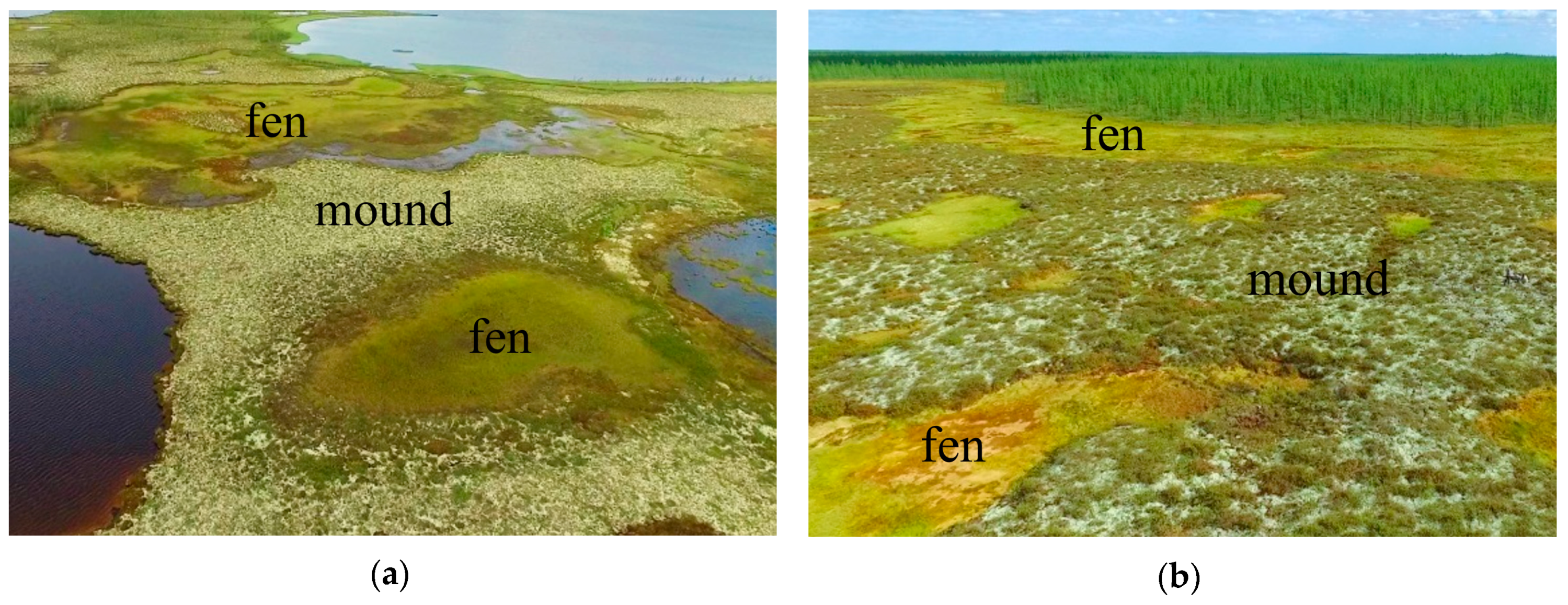
3.2. Properties of Histosols Formed on Various Bog Microtopes
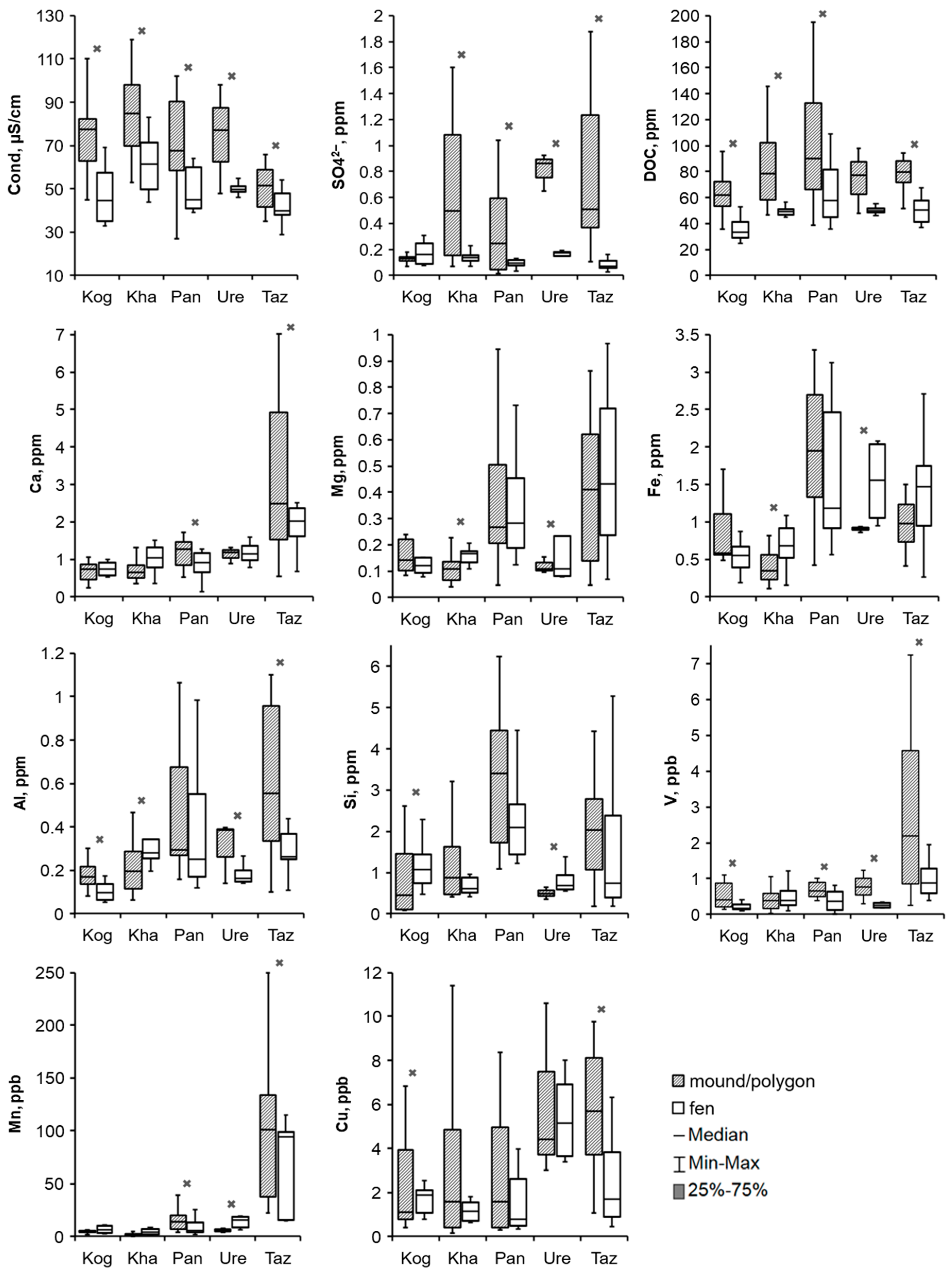
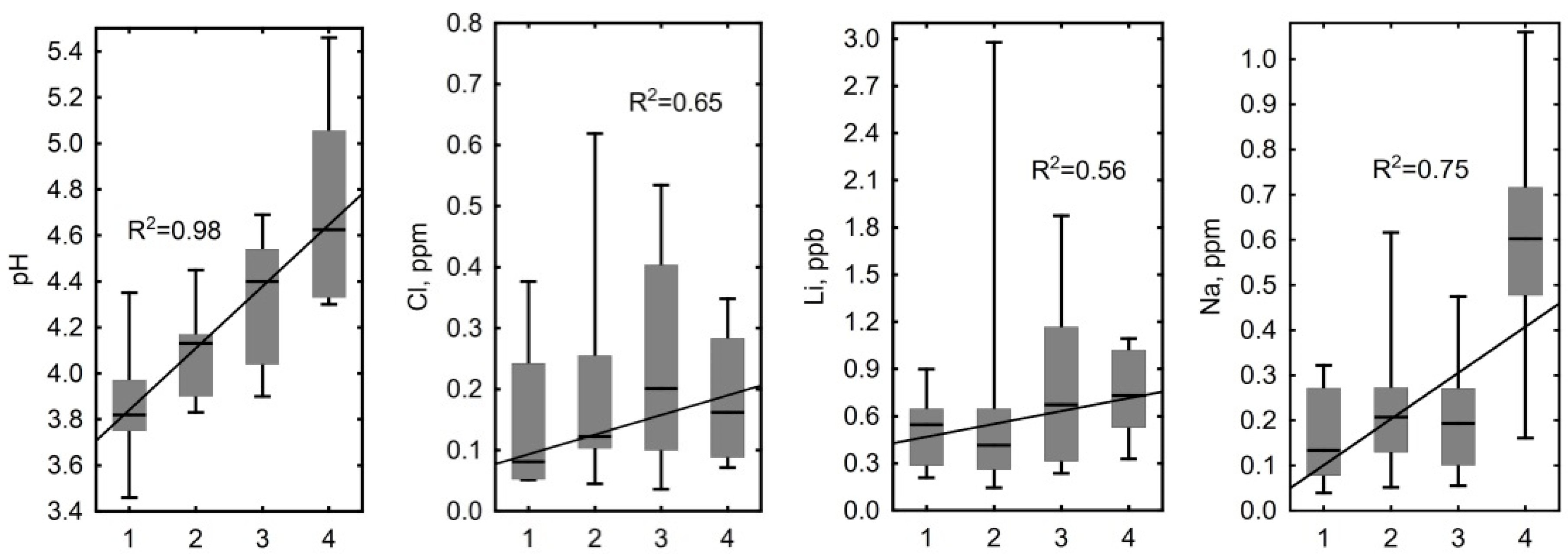
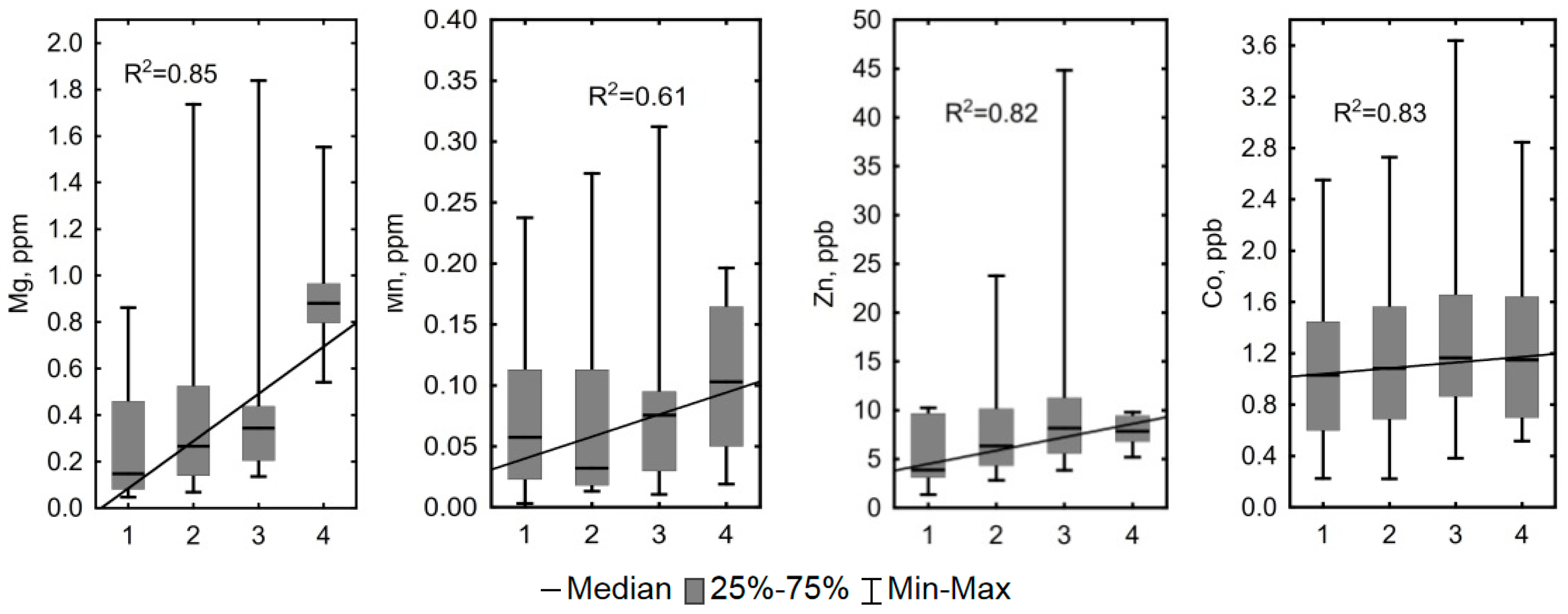
3.3. Hydrochemical Contrast of the Convex and Concave Microtopes
4. Discussion
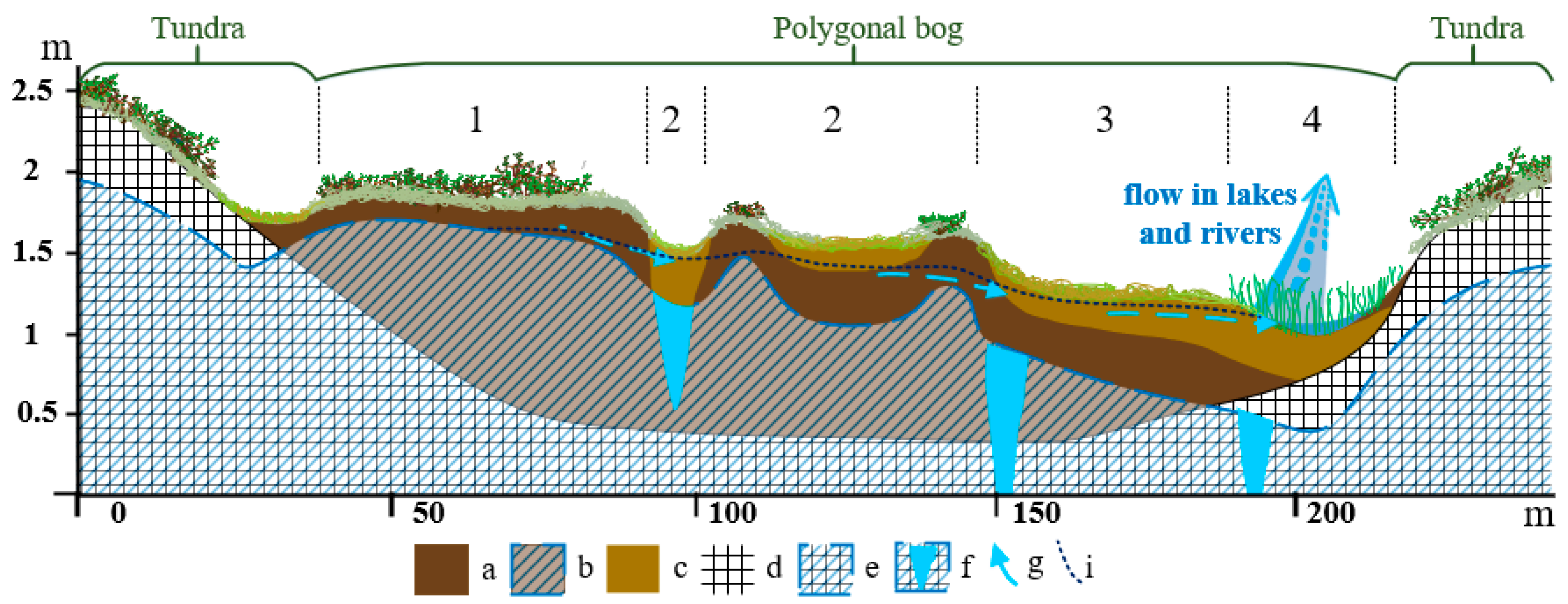
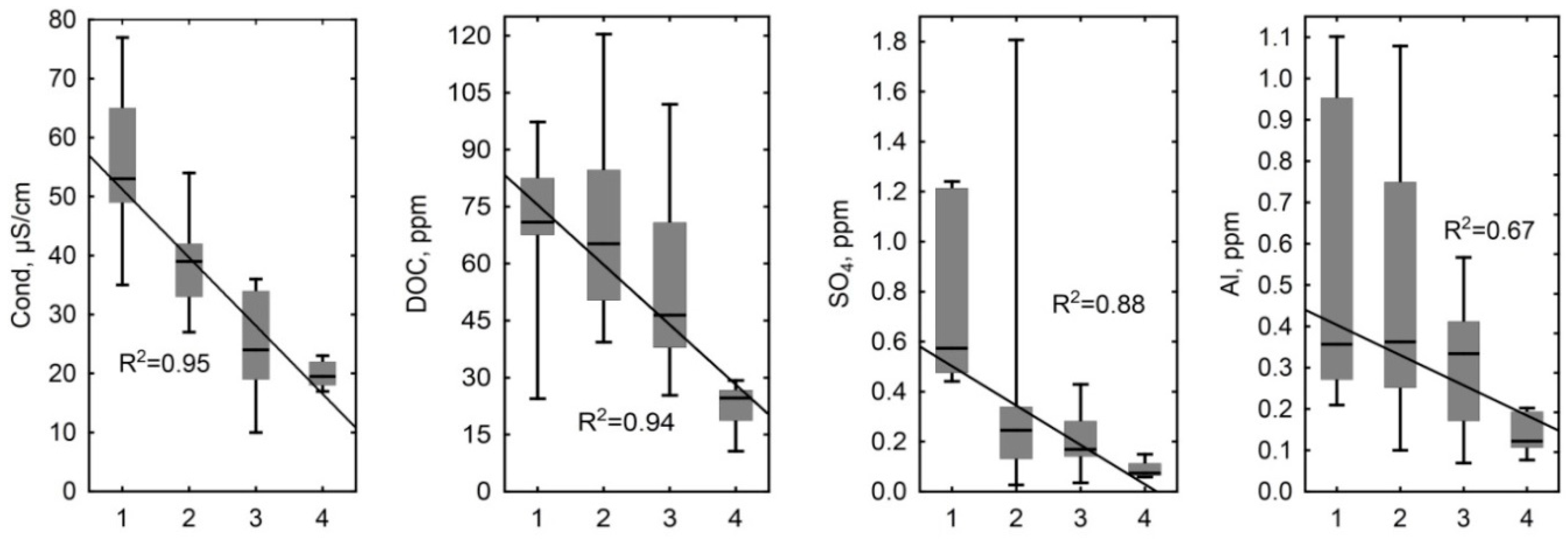
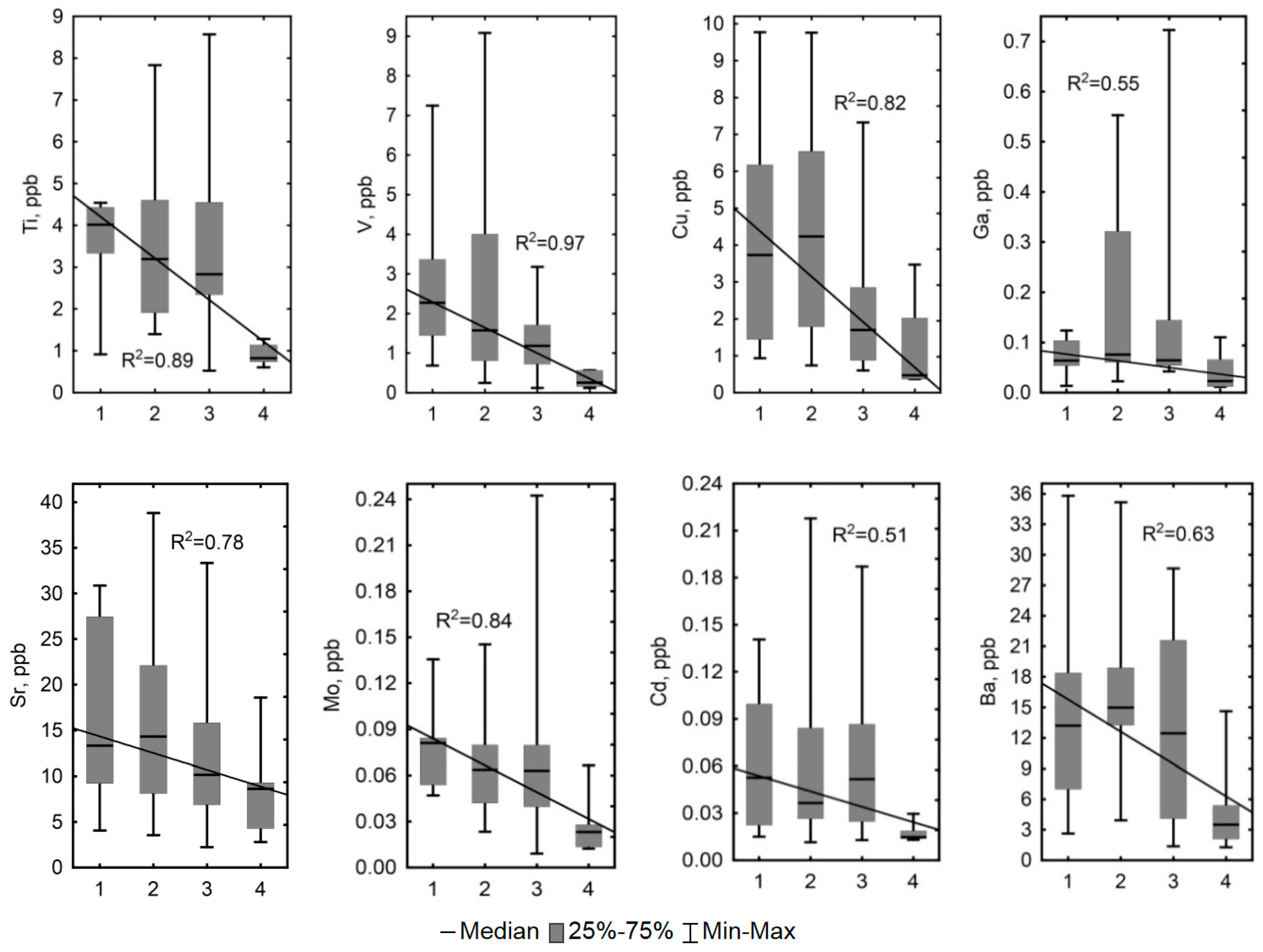
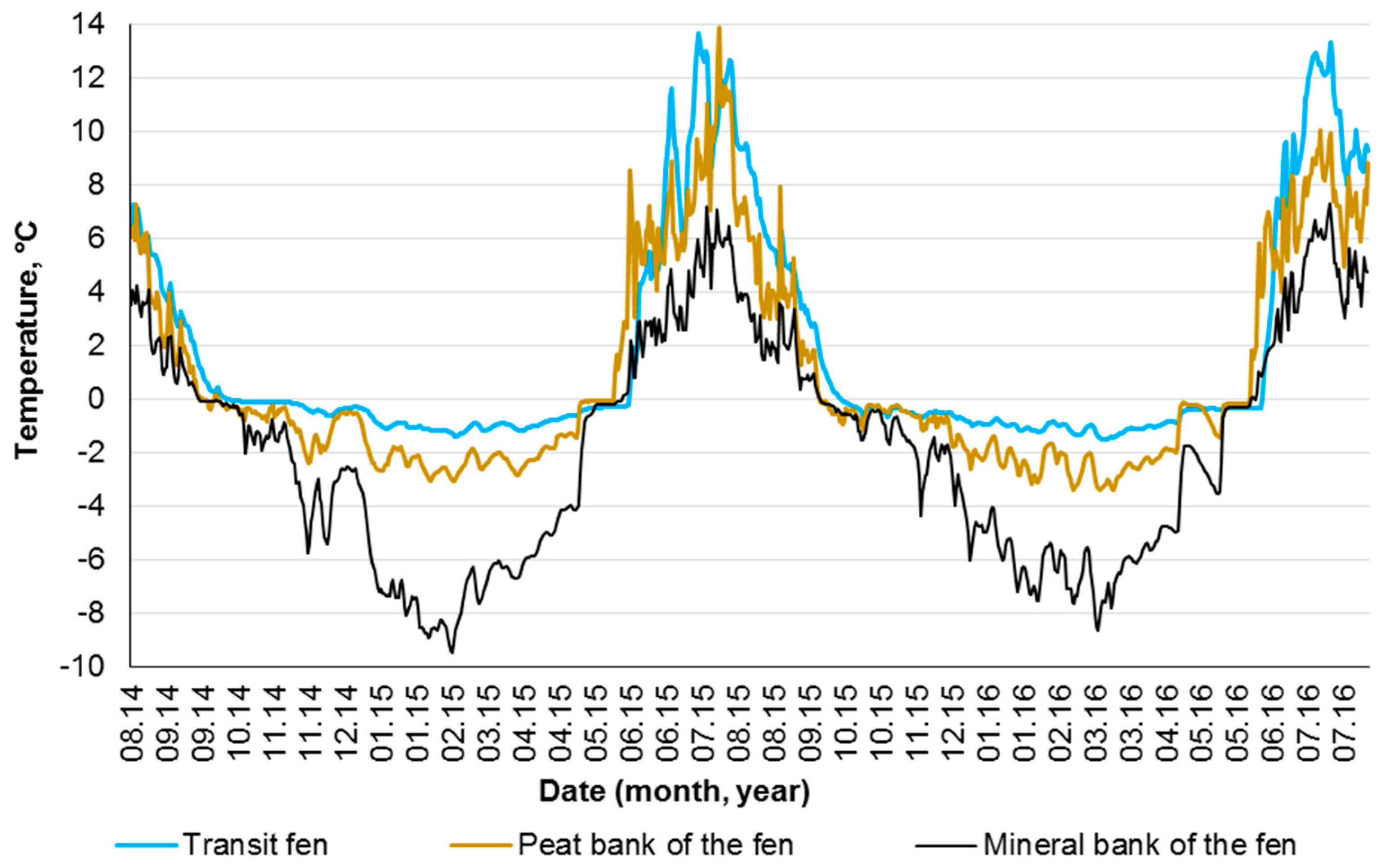
4.1. Influence of Hydrological and Thermal Regimes of Different Microtopes on the Bog Water Properties
4.2. Other Factors Determining Major and Trace Element Concentrations in the Bog Water of Different Microtopes
4.3. Prospective Climate Change in Western Siberia
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | KS «Kogalym» | KS «Khanymey» | KS «Pangody» | KS «Urengoy» | KS «Tazovsky» | All KS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mound (n = 8) | hollow /fen (n = 8) | z | p | mound (n = 34) | hollow /fen (n = 8) | z | p | mound (n = 34) | hollow /fen (n = 34) | z | p | mound (n = 3) | hollow /fen (n = 4) | z | p | polygon (n = 20) | hollow /fen (n = 17) | z | p | mound/ polygon | hollow /fen | p | |
| Cond | 73.8 ± 21 | 47.9 ± 14.5 | 2.46 | 0.01 | 87.1 ± 23 | 61.6 ± 14 | 2.95 | <0.001 | 72.5 ± 30.1 | 51.5 ± 17 | 2.17 | 0.03 | 45 ± 15 | 25 ± 3 | 2.12 | 0.03 | 50.3 ± 10.1 | 41.5 ± 7.7 | 2.24 | 0.03 | 73.4 ± 27 | 47.4 ± 16 | <0.001 |
| pH | 3.8 ± 0.3 | 3.9 ± 0.4 | −0.52 | 0.60 | 3.5 ± 0.3 | 3.8 ± 0.2 | −2.50 | 0.01 | 3.9 ± 0.2 | 3.9 ± 0.2 | −0.11 | 0.91 | 4.1 ± 0.2 | 4.6 ± 0.3 | −2.12 | 0.03 | 4.2 ± 0.3 | 4.2 ± 0.4 | 0.26 | 0.79 | 3.78 ± 0.4 | 4.06 ± 0.4 | <0.001 |
| Cl– | 0.6 ± 0.42 | 0.55 ± 0.30 | 0.000 | 1.00 | 0.43 ± 0.3 | 0.24 ± 0.1 | 1.84 | 0.07 | 0.35 ± 0.3 | 0.39 ± 0.4 | 0.22 | 0.83 | 0.47 ± 0.33 | 0.54 ± 0.41 | −0.35 | 0.72 | 0.31 ± 0.3 | 0.21 ± 0.1 | 0.16 | 0.87 | 0.41 ± 0.3 | 0.35 ± 0.3 | 0.17 |
| SO42– | 0.13 ± 0.04 | 0.21 ± 0.17 | −0.73 | 0.46 | 0.62 ± 0.5 | 0.13 ± 0.1 | 2.78 | 0.01 | 0.33 ± 0.3 | 0.12 ± 0.1 | 1.08 | 0.28 | 0.81 ± 0.14 | 0.16 ± 0.05 | 2.12 | 0.03 | 0.75 ± 0.5 | 0.1 ± 0.1 | 4.13 | <0.001 | 0.54 ± 0.5 | 0.13 ± 0.11 | <0.001 |
| DOC | 62.1 ± 19 | 36.1 ± 10.1 | 2.73 | 0.01 | 82.4 ± 26 | 50.3 ± 9.1 | 3.56 | <0.001 | 99 ± 45.9 | 68.8 ± 36 | 1.95 | 0.05 | 74.3 ± 25.2 | 50.2 ± 3.64 | 1.06 | 0.29 | 79.8 ± 14.6 | 50.1 ± 10.1 | 4.25 | <0.001 | 82.9 ± 30 | 53.3 ± 23 | <0.001 |
| DIC | 1.35 ± 0.2 | 1.43 ± 0.21 | −0.63 | 0.53 | 1.68 ± 0.3 | 1.77 ± 0.8 | 0.75 | 0.45 | 1.8 ± 0.3 | 1.78 ± 0.7 | 0.79 | 0.43 | 1.36 ± 0.17 | 1.58 ± 0.7 | 0.35 | 0.72 | 1.43 ± 0.2 | 1.74 ± 0.5 | −2.24 | 0.03 | 1.61 ± 0.3 | 1.69 ± 0.6 | 0.89 |
| Ca | 0.73 ± 0.4 | 0.8 ± 0.32 | −0.42 | 0.67 | 0.78 ± 0.4 | 1.01 ± 0.4 | −1.56 | 0.12 | 1.17 ± 0.4 | 0.84 ± 0.4 | 2.16 | 0.03 | 1.13 ± 0.22 | 1.17 ± 0.35 | −0.42 | 0.67 | 3.17 ± 2.2 | 2.26 ± 1.4 | 1.98 | 0.05 | 1.36 ± 1.4 | 1.26 ± 1 | 0.33 |
| Mg | 0.16 ± 0.06 | 0.13 ± 0.06 | 0.63 | 0.53 | 0.12 ± 0.1 | 0.18 ± 0.1 | −2.56 | 0.01 | 0.36 ± 0.3 | 0.32 ± 0.2 | 0.35 | 0.73 | 0.12 ± 0.03 | 0.19 ± 0.18 | 1.95 | 0.05 | 0.42 ± 0.3 | 0.48 ± 0.32 | −0.60 | 0.55 | 0.23 ± 0.2 | 0.29 ± 0.2 | 0.02 |
| K | 0.83 ± 0.46 | 0.62 ± 0.39 | 1.05 | 0.29 | 0.32 ± 0.1 | 0.48 ± 0.5 | −0.41 | 0.69 | 0.66 ± 0.5 | 0.46 ± 0.3 | 0.83 | 0.41 | 0.21 ± 0.06 | 0.16 ± 0.05 | −1.41 | 0.16 | 0.28 ± 0.2 | 0.21 ± 0.1 | 0.28 | 0.78 | 0.43 ± 0.4 | 0.4 ± 0.3 | 0.48 |
| P | 0.41 ± 0.16 | 0.23 ± 0.06 | 2.34 | 0.02 | 0.18 ± 0.1 | 0.15 ± 0.2 | 1.64 | 0.10 | 0.20 ± 0.1 | 0.17 ± 0.1 | 0.44 | 0.66 | – | – | – | – | 0.18 ± 0.1 | 0.14 ± 0.02 | −0.37 | 0.72 | 0.2 ± 0.1 | 0.17 ± 0.1 | 0.30 |
| Al | 0.18 ± 0.08 | 0.10 ± 0.05 | 2.1 | 0.04 | 0.22 ± 0.1 | 0.33 ± 0.1 | −2.19 | 0.03 | 0.57 ± 0.6 | 0.38 ± 0.3 | 1.50 | 0.13 | 0.31 ± 0.15 | 0.18 ± 0.05 | 2.10 | 0.04 | 0.61 ± 0.4 | 0.32 ± 0.2 | 2.04 | 0.04 | 0.37 ± 0.4 | 0.29 ± 0.2 | 0.56 |
| Fe | 0.86 ± 0.47 | 0.59 ± 0.33 | 1.26 | 0.21 | 0.44 ± 0.3 | 0.68 ± 0.3 | −2.19 | 0.03 | 2.08 ± 1.1 | 1.61 ± 0.9 | 1.41 | 0.16 | 0.90 ± 0.04 | 1.54 ± 0.6 | −2.12 | 0.03 | 1.08 ± 0.6 | 1.53 ± 0.9 | −1.53 | 0.13 | 0.97 ± 0.9 | 1.24 ± 0.8 | 0.02 |
| Si | 0.84 ± 1 | 1.17 ± 0.61 | 1.99 | 0.048 | 1.31 ± 1.2 | 0.78 ± 0.5 | 0.97 | 0.33 | 3.30 ± 1.9 | 2.4 ± 1.3 | 1.05 | 0.30 | 0.49 ± 0.14 | 0.82 ± 0.38 | 2.00 | 0.046 | 2 ± 1.3 | 1.64 ± 1.67 | 0.60 | 0.55 | 1.76 ± 1.6 | 1.57 ± 1.3 | 0.70 |
| Li | 0.44 ± 0.1 | 0.48 ± 0.16 | 0.11 | 0.92 | 0.48 ± 0.3 | 0.47 ± 0.1 | −0.87 | 0.38 | 1.12 ± 0.7 | 0.81 ± 0.4 | 1.41 | 0.16 | 0.17 ± 0.01 | 0.37 ± 0.36 | −1.41 | 0.16 | 0.5 ± 0.2 | 0.9 ± 0.7 | −0.98 | 0.33 | 0.6 ± 0.5 | 0.68 ± 0.5 | 0.28 |
| B | – | – | – | – | 3.85 ± 2.4 | 2.57 ± 1.1 | 1.38 | 0.17 | 2.06 ± 1.2 | 2.88 ± 2.7 | −0.37 | 0.71 | 0.63 ± 0.34 | – | – | – | 4.45 ± 2.7 | 1.44 ± 0.59 | 2.32 | 0.02 | 2.33 ± 5.7 | 1.07 ± 3.9 | 0.06 |
| Na | 0.39 ± 0.2 | 0.42 ± 0.27 | 0.19 | 0.85 | 0.29 ± 0.1 | 0.34 ± 0.1 | −1.19 | 0.24 | 0.37 ± 0.2 | 0.32 ± 0.2 | 0.53 | 0.60 | 0.23 ± 0.1 | 0.25 ± 0.22 | 1.06 | 0.29 | 0.21 ± 0.1 | 0.25 ± 0.15 | −0.70 | 0.49 | 0.3 ± 0.2 | 0.32 ± 0.2 | 0.75 |
| Ti | 1.92 ± 0.7 | 0.96 ± 0.41 | 2.62 | 0.01 | 2.91 ± 1.8 | 2.54 ± 1.2 | 0.23 | 0.82 | 4.20 ± 1.61 | 2.56 ± 1.7 | 2.95 | <0.001 | 1.72 ± 0.36 | 1.38 ± 0.32 | 1.77 | 0.08 | 3.85 ± 0.7 | 3.26 ± 1.7 | 1.30 | 0.19 | 3.23 ± 1.6 | 2.36 ± 1.6 | <0.001 |
| V | 0.54 ± 0.4 | 0.22 ± 0.11 | 1.97 | 0.05 | 0.45 ± 0.4 | 0.50 ± 0.4 | −0.22 | 0.83 | 0.73 ± 0.34 | 0.46 ± 0.5 | 2.29 | 0.02 | 0.77 ± 0.47 | 0.26 ± 0.08 | −2.08 | 0.04 | 3.5 ± 2.7 | 1 ± 0.52 | 2.55 | 0.01 | 1.16 ± 1.8 | 0.55 ± 0.5 | 0.02 |
| Cr | 0.87 ± 0.5 | 0.67 ± 0.31 | 0.73 | 0.46 | 1.10 ± 0.4 | 1.15 ± 0.4 | −0.78 | 0.44 | 1.40 ± 1.2 | 1.26 ± 0.5 | −0.50 | 0.62 | 0.27 ± 0.18 | 0.39 ± 0.2 | −0.35 | 0.72 | 1.3 ± 0.6 | 0.97 ± 0.4 | 1.49 | 0.14 | 1.15 ± 0.7 | 0.99 ± 0.5 | 0.33 |
| Mn | 5.30 ± 2.8 | 6.90 ± 3.47 | −0.94 | 0.35 | 2.68 ± 2.6 | 4.41 ± 2.8 | −1.72 | 0.09 | 15.7 ± 10.3 | 9.17 ± 7.7 | 2.00 | 0.05 | 6.05 ± 2.02 | 14.4 ± 5.54 | 2.10 | 0.04 | 116.5 ± 87 | 87.4 ± 81 | 1.98 | 0.05 | 29.1 ± 59 | 28.8 ± 54 | 0.06 |
| Co | 0.16 ± 0.04 | 0.12 ± 0.08 | 1.26 | 0.21 | 0.19 ± 0.1 | 0.28 ± 0.1 | −2.50 | 0.01 | 1.48 ± 0.7 | 1.05 ± 0.6 | 1.50 | 0.14 | 0.26 ± 0.09 | 0.34 ± 0.14 | −0.71 | 0.48 | 1.32 ± 0.8 | 1.23 ± 0.89 | 0.33 | 0.75 | 0.68 ± 0.8 | 0.74 ± 0.7 | 0.15 |
| Ga | 0.05 ± 0.03 | 0.024 ± 0.01 | 1.57 | 0.12 | 0.30 ± 0.4 | 0.06 ± 0.0 | 0.62 | 0.53 | 0.08 ± 0.05 | 0.08 ± 0.1 | 0.96 | 0.34 | 0.59 ± 0.22 | 0.42 ± 0.18 | 1.06 | 0.29 | 0.11 ± 0.1 | 0.21 ± 0.2 | −1.21 | 0.23 | 0.2 ± 0.3 | 0.13 ± 0.2 | 0.29 |
| As | 0.88 ± 0.4 | 0.75 ± 0.2 | 1.15 | 0.25 | 0.53 ± 0.2 | 0.83 ± 0.3 | −2.79 | 0.01 | 1.27 ± 1.3 | 0.83 ± 0.6 | 0.87 | 0.38 | 0.2 ± 0.06 | 0.17 ± 0.06 | 0.35 | 0.72 | 2.61 ± 3 | 1.4 ± 1 | 0.63 | 0.53 | 1.14 ± 1.7 | 0.88 ± 0.7 | 0.35 |
| Rb | 0.85 ± 0.4 | 0.51 ± 0.26 | 2.10 | 0.04 | 0.59 ± 0.4 | 0.58 ± 0.4 | −0.13 | 0.90 | 1.02 ± 0.8 | 0.59 ± 0.5 | 1.83 | 0.07 | 0.23 ± 0.22 | 0.27 ± 0.15 | −0.71 | 0.48 | 0.55 ± 0.3 | 0.68 ± 0.6 | −0.09 | 0.93 | 0.68 ± 0.5 | 0.57 ± 0.5 | 0.18 |
| Zr | 0.08 ± 0.1 | 0.05 ± 0.03 | 1.05 | 0.29 | 0.18 ± 0.2 | 0.16 ± 0.1 | −0.34 | 0.73 | 0.38 ± 0.2 | 0.39 ± 0.3 | 0.04 | 0.97 | 0.14 ± 0.06 | 0.19 ± 0.2 | 0.00 | 1.00 | 0.95 ± 0.6 | 0.39 ± 0.16 | 2.04 | 0.04 | 0.37 ± 0.4 | 0.27 ± 0.2 | 0.89 |
| Nb | 0.012 ± 0.01 | 0.01 ± 0.01 | 0.63 | 0.53 | 0.02 ± 0.0 | 0.03 ± 0.0 | −1.72 | 0.09 | 0.05 ± 0.04 | 0.04 ± 0.02 | 0.33 | 0.74 | 0.004 ± 0.00 | 0.004 ± 0.00 | 0.71 | 0.48 | 0.04 ± 0.03 | 0.02 ± 0.18 | 1.96 | 0.05 | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.56 |
| Mo | 0.03 ± 0.02 | 0.05 ± 0.06 | −0.32 | 0.75 | 0.07 ± 0.1 | 0.08 ± 0.1 | −0.38 | 0.71 | 0.067 ± 0.1 | 0.04 ± 0.04 | 1.46 | 0.15 | 0.028 ± 0.02 | 0.028 ± 0.01 | 0.35 | 0.72 | 0.08 ± 0.04 | 0.055 ± 0.23 | 1.76 | 0.08 | 0.06 ± 0.1 | 0.05 ± 0.05 | 0.13 |
| Cd | 0.11 ± 0.1 | 0.14 ± 0.18 | −0.11 | 0.92 | 0.21 ± 0.4 | 0.25 ± 0.3 | −1.78 | 0.08 | 0.161 ± 0.2 | 0.07 ± 0.04 | 0.75 | 0.45 | 0.040.019 | 0.025 ± 0.01 | 2.79 | 0.04 | 0.07 ± 0.05 | 0.055 ± 0.04 | 1.39 | 0.16 | 0.16 ± 0.3 | 0.11 ± 0.2 | 0.56 |
| Ni | 0.79 ± 0.57 | 0.7 ± 0.35 | 0.21 | 0.83 | 0.78 ± 0.4 | 1.37 ± 0.6 | −2.68 | 0.01 | 3.63 ± 1.7 | 2.65 ± 1.4 | 1.79 | 0.07 | 1.43 ± 0.7 | 1.25 ± 0.45 | 0.71 | 0.48 | 3.92 ± 2.1 | 2.5 ± 0.97 | 1.81 | 0.07 | 2.04 ± 1.9 | 1.90 ± 1.2 | 0.25 |
| Cu | 2.58 ± 2.6 | 1.66 ± 0.65 | 2.75 | 0.04 | 3.15 ± 3.7 | 1.16 ± 0.5 | 0.38 | 0.71 | 2.88 ± 3.4 | 2.25 ± 3.1 | 0.25 | 0.80 | 6.02 ± 4 | 5.41 ± 2.24 | 0.00 | 1.00 | 5.61 ± 2.8 | 2.75 ± 2.5 | 2.60 | 0.01 | 3.65 ± 3.5 | 2.37 ± 2.5 | 0.25 |
| Zn | 9.44 ± 5.1 | 11.7 ± 2.22 | −1.37 | 0.17 | 8.46 ± 4.5 | 10.95 ± 5.1 | −1.40 | 0.16 | 6.58 ± 4.3 | 6.8 ± 4.3 | 0.04 | 0.97 | 8 ± 5.38 | 6.34 ± 2.04 | 0.00 | 1.00 | 7.32 ± 5 | 8.59 ± 3.2 | −1.49 | 0.14 | 7.92 ± 4.6 | 8.80 ± 4.1 | 0.15 |
| Sr | 5.95 ± 2.2 | 4.5 ± 1.8 | 1.26 | 0.21 | 6.26 ± 3.7 | 7.18 ± 2.9 | −1.40 | 0.16 | 12.5 ± 3.7 | 9.77 ± 6.2 | 2.12 | 0.03 | 5.9 ± 2.3 | 6.5 ± 3.6 | 0.00 | 1.00 | 19 ± 11 | 11.15 ± 7.6 | 1.76 | 0.08 | 10.2 ± 7.6 | 8.51 ± 5.8 | 0.85 |
| Sb | 0.06 ± 0.03 | 0.07 ± 0.03 | −1.05 | 0.29 | 0.04 ± 0.0 | 0.06 ± 0.0 | −2.53 | 0.01 | 0.04 ± 0.02 | 0.05 ± 0.02 | −2.08 | 0.04 | 0.013 ± 0.01 | 0.013 ± 0.00 | −0.71 | 0.48 | 0.04 ± 0.01 | 0.035 ± 0.01 | 1.86 | 0.06 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.30 |
| Cs | 0.03 ± 0.02 | 0.02 ± 0.01 | 1.37 | 0.17 | 0.04 ± 0.0 | 0.03 ± 0.0 | 0.87 | 0.38 | 0.03 ± 0.02 | 0.02 ± 0.02 | 1.00 | 0.32 | 0.004 ± 0.00 | 0.006 ± 0.00 | −1.06 | 0.29 | 0.02 ± 0.01 | 0.01 ± 0.006 | 2.04 | 0.04 | 0.03 ± 0.03 | 0.02 ± 0.02 | <0.001 |
| Ba | 17.5 ± 8.1 | 17 ± 4.6 | −0.63 | 0.53 | 26.6 ± 18 | 24.5 ± 17 | 0.19 | 0.85 | 23.9 ± 12.1 | 24.2 ± 14 | −0.08 | 0.93 | 18.76 ± 6.89 | 13.83 ± 6.35 | 1.06 | 0.29 | 17.1 ± 6.4 | 15.4 ± 7.7 | 0.37 | 0.71 | 22.9 ± 14.3 | 19.8 ± 12 | 0.52 |
| La | 0.16 ± 0.1 | 0.07 ± 0.05 | 2.68 | 0.04 | 0.24 ± 0.3 | 0.22 ± 0.1 | −0.72 | 0.47 | 0.24 ± 0.18 | 0.22 ± 0.2 | 1.12 | 0.26 | 0.354 ± 0.26 | 0.14 ± 0.07 | 1.71 | 0.05 | 0.55 ± 0.33 | 0.22 ± 0.13 | 3.25 | <0.001 | 0.30 ± 0.3 | 0.19 ± 0.2 | 0.22 |
| Ce | 0.34 ± 0.4 | 0.13 ± 0.08 | 2.10 | 0.04 | 0.45 ± 0.5 | 0.48 ± 0.4 | −0.75 | 0.45 | 0.67 ± 0.5 | 0.49 ± 0.47 | 1.04 | 0.30 | 0.66 ± 0.53 | 0.29 ± 0.136 | 2.00 | 0.05 | 1.24 ± 0.8 | 0.51 ± 0.3 | 2.97 | <0.001 | 0.65 ± 0.6 | 0.41 ± 0.4 | 0.17 |
| Pr | 0.03 ± 0.01 | 0.012 ± 0.01 | 2.31 | 0.02 | 0.05 ± 0.1 | 0.05 ± 0.0 | −0.34 | 0.73 | 0.07 ± 0.04 | 0.05 ± 0.05 | 1.37 | 0.17 | 0.05 ± 0.034 | 0.028 ± 0.01 | 1.41 | 0.16 | 0.16 ± 0.1 | 0.06 ± 0.04 | 2.79 | 0.01 | 0.08 ± 0.1 | 0.05 ± 0.04 | 0.11 |
| Nd | 0.17 ± 0.16 | 0.05 ± 0.03 | 2.42 | 0.02 | 0.22 ± 0.2 | 0.22 ± 0.2 | −0.91 | 0.37 | 0.24 ± 0.2 | 0.21 ± 0.2 | 0.37 | 0.71 | 0.194 ± 0.13 | 0.115 ± 0.05 | 2.12 | 0.03 | 0.7 ± 0.5 | 0.26 ± 0.16 | 2.79 | 0.01 | 0.32 ± 0.3 | 0.19 ± 0.2 | 0.27 |
| Sm | 0.023 ± 0.01 | 0.01 ± 0.00 | 2.84 | 0.01 | 0.05 ± 0.0 | 0.04 ± 0.0 | −0.50 | 0.62 | 0.05 ± 0.04 | 0.04 ± 0.03 | 0.54 | 0.59 | 0.04 ± 0.027 | 0.025 ± 0.01 | 1.06 | 0.29 | 0.16 ± 0.1 | 0.06 ± 0.04 | 2.79 | 0.01 | 0.07 ± 0.1 | 0.04 ± 0.03 | 0.19 |
| Eu | 0.008 ± 0.01 | 0.004 ± 0.001 | 2.52 | 0.01 | 0.01 ± 0.0 | 0.01 ± 0.006 | −0.59 | 0.55 | 0.014 ± 0.01 | 0.01 ± 0.01 | 0.54 | 0.59 | 0.012 ± 0.006 | 0.008 ± 0.004 | 1.06 | 0.29 | 0.04 ± 0.03 | 0.014 ± 0.01 | 2.97 | <0.001 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.28 |
| Gd | 0.022 ± 0.01 | 0.013 ± 0.01 | 1.58 | 0.12 | 0.05 ± 0.0 | 0.045 ± 0.01 | −0.72 | 0.47 | 0.051 ± 0.04 | 0.041 ± 0.03 | 0.54 | 0.59 | 0.042 ± 0.027 | 0.025 ± 0.013 | 1.06 | 0.29 | 0.17 ± 0.11 | 0.06 ± 0.04 | 2.88 | <0.001 | 0.07 ± 0.1 | 0.04 ± 0.03 | 0.30 |
| Tb | 0.005 ± 0.01 | 0.002 ± 0.00 | 2.00 | 0.05 | 0.01 ± 0.01 | 0.01 ± 0.005 | 0.62 | 0.53 | 0.007 ± 0.01 | 0.007 ± 0.004 | 0.46 | 0.65 | 0.006 ± 0.004 | 0.003 ± 0.002 | 1.06 | 0.29 | 0.024 ± 0.02 | 0.008 ± 0.005 | 2.93 | <0.001 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.02 |
| Dy | 0.029 ± 0.03 | 0.01 ± 0.005 | 2.42 | 0.02 | 0.04 ± 0.04 | 0.04 ± 0.025 | −0.81 | 0.42 | 0.039 ± 0.03 | 0.038 ± 0.04 | 0.33 | 0.74 | 0.031 ± 0.02 | 0.018 ± 0.009 | 1.06 | 0.29 | 0.14 ± 0.1 | 0.05 ± 0.03 | 2.88 | <0.001 | 0.06 ± 0.1 | 0.03 ± 0.03 | 0.28 |
| Ho | 0.005 ± 0.01 | 0.002 ± 0.00 | 1.79 | 0.07 | 0.01 ± 0.01 | 0.01 ± 0.006 | −1.19 | 0.24 | 0.008 ± 0.01 | 0.006 ± 0.005 | 0.50 | 0.62 | 0.007 ± 0.004 | 0.004 ± 0.002 | 0.71 | 0.48 | 0.029 ± 0.02 | 0.01 ± 0.006 | 2.88 | <0.001 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.44 |
| Er | 0.013 ± 0.01 | 0.005 ± 0.00 | 2.00 | 0.05 | 0.02 ± 0.02 | 0.02 ± 0.015 | −0.91 | 0.37 | 0.026 ± 0.02 | 0.018 ± 0.01 | 0.96 | 0.34 | 0.017 ± 0.01 | 0.012 ± 0.008 | 0.71 | 0.48 | 0.086 ± 0.1 | 0.03 ± 0.017 | 2.60 | 0.01 | 0.03 ± 0.04 | 0.02 ± 0.02 | 0.24 |
| Tm | 0.002 ± 0.00 | 0.001 ± 0.00 | 0.84 | 0.40 | 0.003 ± 0.00 | 0.003 ± 0.00 | −0.84 | 0.40 | 0.004 ± 0.003 | 0.003 ± 0.002 | 0.37 | 0.71 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.71 | 0.48 | 0.013 ± 0.01 | 0.004 ± 0.003 | 2.65 | 0.01 | 0.005 ± 0.01 | 0.003 ± 0.002 | 0.48 |
| Yb | 0.011 ± 0.01 | 0.004 ± 0.00 | 1.99 | 0.05 | 0.015 ± 0.01 | 0.02 ± 0.013 | −0.69 | 0.49 | 0.022 ± 0.02 | 0.024 ± 0.02 | −0.67 | 0.51 | 0.014 ± 0.008 | 0.012 ± 0.01 | 0.35 | 0.72 | 0.083 ± 0.06 | 0.029 ± 0.018 | 2.51 | 0.01 | 0.03 ± 0.04 | 0.02 ± 0.018 | 0.94 |
| Lu | 0.002 ± 0.00 | 0.001 ± 0.00 | 0.74 | 0.46 | 0.002 ± 0.00 | 0.002 ± 0.00 | −0.97 | 0.33 | 0.003 ± 0.003 | 0.002 ± 0.002 | 0.71 | 0.48 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.35 | 0.72 | 0.013 ± 0.01 | 0.004 ± 0.003 | 2.60 | 0.01 | 0.005 ± 0.01 | 0.003 ± 0.002 | 0.52 |
| Hf | 0.005 ± 0.00 | 0.004 ± 0.00 | 0.63 | 0.53 | 0.008 ± 0.01 | 0.01 ± 0.003 | −1.69 | 0.09 | 0.021 ± 0.02 | 0.021 ± 0.02 | −0.33 | 0.74 | 0.006 ± 0.003 | 0.005 ± 0.005 | 0.35 | 0.72 | 0.049 ± 0.05 | 0.017 ± 0.015 | 1.67 | 0.10 | 0.02 ± 0.03 | 0.01 ± 0.01 | 0.35 |
| W | 0.021 ± 0.02 | 0.02 ± 0.01 | 0.95 | 0.35 | 0.024 ± 0.03 | 0.03 ± 0.02 | −0.55 | 0.59 | 0.018 ± 0.01 | 0.018 ± 0.01 | 0.00 | 1.00 | 0.008 ± 0.007 | 0.004 ± 0.006 | 0.69 | 0.49 | 0.012 ± 0.01 | 0.013 ± 0.006 | −0.64 | 0.52 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.75 |
| Tl | 0.006 ± 0.01 | 0.003 ± 0.00 | 2.05 | 0.04 | 0.01 ± 0.004 | 0.01 ± 0.004 | 0.87 | 0.39 | 0.007 ± 0.01 | 0.004 ± 0.005 | 1.79 | 0.07 | 0.001 ± 0.001 | 0.002 ± 0.00 | −1.41 | 0.16 | 0.006 ± 0.01 | 0.005 ± 0.004 | 1.49 | 0.14 | 0.01 ± 0.01 | 0.004 ± 0.003 | 0.01 |
| Pb | 0.82 ± 0.6 | 0.74 ± 0.2 | −0.32 | 0.75 | 0.86 ± 0.7 | 1.042 ± 0.49 | −1.19 | 0.24 | 0.54 ± 0.3 | 0.8 ± 0.5 | −1.66 | 0.10 | 0.49 ± 0.42 | 0.27 ± 0.13 | 0.71 | 0.48 | 0.516 ± 0.23 | 0.536 ± 0.34 | 0.09 | 0.93 | 0.70 ± 0.5 | 0.72 ± 0.4 | 0.56 |
| Th | 0.026 ± 0.03 | 0.01 ± 0.005 | 2.31 | 0.02 | 0.045 ± 0.05 | 0.044 ± 0.04 | 0.09 | 0.93 | 0.068 ± 0.05 | 0.047 ± 0.03 | 1.21 | 0.23 | 0.073 ± 0.05 | 0.032 ± 0.02 | 1.06 | 0.29 | 0.115 ± 0.06 | 0.048 ± 0.023 | 3.30 | <0.001 | 0.06 ± 0.1 | 0.04 ± 0.03 | 0.08 |
| U | 0.011 ± 0.02 | 0.01 ± 0.006 | 0.84 | 0.40 | 0.02 ± 0.02 | 0.02 ± 0.017 | −0.34 | 0.73 | 0.021 ± 0.01 | 0.024 ± 0.03 | 0.87 | 0.38 | 0.008 ± 0.006 | 0.015 ± 0.01 | −0.35 | 0.72 | 0.03 ± 0.02 | 0.019 ± 0.018 | 2.04 | 0.04 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.22 |
| Parameter | Kruskal-Wallis (H-test) 1–2–3–4 | Mann-Whitney (U-test) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | p-level | 1–2 | 1–3 | 1–4 | 2–3 | 2–4 | 3–4 | |||||||
| U | p | U | p | U | p | U | p | U | p | U | p | |||
| Cond | 11.9 | 0.007 | 81 | 0.57 | 56 | 0.38 | 6 | 0.008 | 133 | 0.29 | 13 | 0.002 | 18 | 0.012 |
| pH | 13.2 | 0.004 | 66 | 0.21 | 63 | 0.65 | 2 | 0.002 | 146 | 0.51 | 15 | 0.002 | 13 | 0.005 |
| Cl– | 1.76 | 0.62 | 22 | 0.3 | 13 | 0.2 | 6 | 0.66 | 27 | 0.56 | 32 | 0.89 | 38 | 0.46 |
| SO42– | 18.3 | 0.000 | 20 | 0.016 | 0 | <0.001 | 0 | 0.014 | 147 | 0.69 | 12 | 0.033 | 11 | 0.06 |
| DOC | 19.8 | 0.000 | 88 | 0.65 | 46 | 0.15 | 4 | 0.004 | 109 | 0.048 | 0 | <0.0001 | 3 | <0.001 |
| DIC | 11.4 | 0.01 | 26 | 0.001 | 40 | 0.07 | 25 | 0.53 | 151 | 0.47 | 31 | 0.02 | 36 | 0.19 |
| Ca | 1.57 | 0.67 | 84 | 0.65 | 71 | 0.97 | 29 | 0.83 | 68 | 0.24 | 51 | 0.24 | 88 | 0.41 |
| Mg | 7.57 | 0.05 | 72 | 0.32 | 50 | 0.22 | 9 | 0.05 | 93 | 0.96 | 33 | 0.04 | 40 | 0.044 |
| K | 8.46 | 0.038 | 66 | 0.27 | 24 | 0.015 | 19 | 0.2 | 125 | 0.27 | 66 | 0.85 | 42 | 0.37 |
| Al | 13.9 | 0.003 | 80 | 0.52 | 43 | 0.22 | 0 | 0.001 | 131 | 0.26 | 12 | 0.001 | 19 | 0.015 |
| Fe | 10.1 | 0.018 | 78 | 0.59 | 41 | 0.18 | 8 | 0.015 | 113 | 0.14 | 28 | 0.021 | 18 | 0.012 |
| Si | 0.78 | 0.85 | 90 | 0.18 | 63 | 0.63 | 25 | 0.52 | 63 | 0.16 | 60 | 0.49 | 94 | 0.56 |
| Li | 6.71 | 0.08 | 40 | 0.34 | 47 | 0.64 | 16 | 0.11 | 102 | 0.07 | 32 | 0.05 | 69 | 0.36 |
| B | 6.96 | 0.07 | 62 | 0.75 | 22 | 0.055 | 1 | 0.055 | 69 | 0.15 | 10 | 0.11 | 38 | 0.15 |
| Na | 10.8 | 0.013 | 56 | 0.3 | 48 | 0.46 | 3 | 0.24 | 133 | 0.75 | 14 | 0.002 | 33 | 0.09 |
| Ti | 16.3 | 0.001 | 85 | 0.68 | 56 | 0.68 | 2 | 0.002 | 165 | 0.93 | 0 | <0.0001 | 7 | 0.001 |
| V | 16.5 | 0.001 | 72 | 0.32 | 26 | 0.02 | 0 | 0.001 | 139 | 0.38 | 14 | 0.002 | 10 | 0.002 |
| Cr | 5.21 | 0.15 | 81 | 0.56 | 69 | 0.89 | 13 | 0.055 | 85 | 0.68 | 41 | 0.09 | 46 | 0.054 |
| Mn | 2.69 | 0.44 | 79 | 0.49 | 68 | 0.84 | 25 | 0.53 | 81 | 0.55 | 46 | 0.15 | 63 | 0.060 |
| Co | 0.57 | 0.9 | 84 | 0.65 | 62 | 0.59 | 27 | 0.67 | 90 | 0.85 | 68 | 0.79 | 108 | 0.983 |
| Ga | 5.69 | 0.13 | 70 | 0.28 | 57 | 0.41 | 17 | 0.14 | 67 | 0.22 | 26 | 0.055 | 34 | 0.056 |
| As | 8.04 | 0.045 | 77 | 0.69 | 56 | 0.68 | 12 | 0.044 | 129 | 0.65 | 19 | 0.007 | 35 | 0.11 |
| Rb | 7.01 | 0.07 | 85 | 0.68 | 45 | 0.13 | 17 | 0.138 | 24 | 0.19 | 36 | 0.68 | 36 | 0.054 |
| Zr | 6.99 | 0.07 | 72 | 0.31 | 58 | 0.45 | 5 | 0.056 | 25 | 0.22 | 20 | 0.11 | 19 | 0.054 |
| Nb | 11.6 | 0.008 | 80 | 0.53 | 65 | 0.71 | 2 | 0.002 | 162 | 0.87 | 9 | <0.001 | 12 | 0.004 |
| Mo | 11.8 | 0.008 | 71 | 0.3 | 54 | 0.32 | 0 | 0.001 | 166 | 0.96 | 14 | 0.002 | 18 | 0.012 |
| Cd | 9.66 | 0.022 | 91 | 0.89 | 68 | 0.84 | 7 | 0.011 | 158 | 0.77 | 14 | 0.002 | 11 | 0.003 |
| Ni | 3.63 | 0.3 | 93 | 0.96 | 69 | 0.89 | 18 | 0.17 | 79 | 0.49 | 44 | 0.12 | 49 | 0.053 |
| Cu | 13.8 | 0.003 | 85 | 0.68 | 34 | 0.07 | 9 | 0.02 | 77 | 0.005 | 15 | 0.002 | 29 | 0.08 |
| Zn | 3.23 | 0.36 | 58 | 0.21 | 28 | 0.052 | 20 | 0.38 | 90 | 0.85 | 59 | 0.46 | 104 | 0.88 |
| Sr | 7.65 | 0.06 | 92 | 0.93 | 59 | 0.48 | 16 | 0.11 | 56 | 0.08 | 43 | 0.11 | 81 | 0.26 |
| Sb | 3.21 | 0.36 | 94 | 1 | 67 | 0.79 | 17 | 0.14 | 84 | 0.65 | 41 | 0.09 | 53 | 0.04 |
| Cs | 10.6 | 0.014 | 83 | 0.61 | 63 | 0.63 | 4 | 0.004 | 126 | 0.2 | 17 | 0.003 | 12 | 0.004 |
| Ba | 11.1 | 0.011 | 77 | 0.44 | 70 | 0.93 | 8 | 0.015 | 135 | 0.32 | 10 | <0.0001 | 24 | 0.035 |
| La | 10.7 | 0.013 | 81 | 0.56 | 40 | 0.07 | 10 | 0.03 | 113 | 0.09 | 20 | 0.005 | 33 | 0.13 |
| Ce | 7.77 | 0.05 | 77 | 0.44 | 39 | 0.06 | 11 | 0.05 | 22 | 0.14 | 12 | 0.049 | 48 | 0.18 |
| Pr | 7.80 | 0.049 | 74 | 0.37 | 42 | 0.09 | 10 | 0.03 | 146 | 0.51 | 29 | 0.019 | 33 | 0.13 |
| Nd | 7.65 | 0.05 | 70 | 0.28 | 43 | 0.11 | 10 | 0.046 | 68 | 0.24 | 31 | 0.046 | 62 | 0.05 |
| Sm | 9.35 | 0.025 | 59 | 0.11 | 27 | 0.012 | 7 | 0.011 | 155 | 0.7 | 39 | 0.071 | 35 | 0.17 |
| Eu | 7.54 | 0.05 | 65 | 0.19 | 41 | 0.084 | 10 | 0.046 | 73 | 0.34 | 34 | 0.039 | 62 | 0.05 |
| Gd | 7.22 | 0.05 | 63 | 0.16 | 42 | 0.095 | 10 | 0.046 | 64 | 0.17 | 37 | 0.05 | 66 | 0.08 |
| Tb | 6.73 | 0.08 | 65 | 0.19 | 43 | 0.107 | 10 | 0.052 | 69 | 0.26 | 39 | 0.07 | 64 | 0.06 |
| Dy | 6.93 | 0.07 | 64 | 0.17 | 36 | 0.055 | 10 | 0.052 | 68 | 0.24 | 44 | 0.12 | 66 | 0.08 |
| Ho | 6.44 | 0.09 | 67 | 0.22 | 38 | 0.06 | 11 | 0.052 | 67 | 0.22 | 43 | 0.11 | 67 | 0.086 |
| Er | 5.18 | 0.16 | 68 | 0.24 | 43 | 0.11 | 11 | 0.052 | 68 | 0.23 | 51 | 0.24 | 68 | 0.09 |
| Tm | 4.26 | 0.235 | 70 | 0.28 | 48 | 0.18 | 11 | 0.052 | 66 | 0.2 | 56 | 0.37 | 70 | 0.11 |
| Yb | 4.37 | 0.224 | 72 | 0.32 | 46 | 0.15 | 11 | 0.052 | 70 | 0.27 | 54 | 0.31 | 70 | 0.11 |
| Lu | 3.70 | 0.296 | 76 | 0.42 | 48 | 0.18 | 11 | 0.052 | 71 | 0.29 | 57 | 0.39 | 71 | 0.12 |
| Hf | 4.94 | 0.176 | 76 | 0.42 | 70 | 0.93 | 8 | 0.052 | 79 | 0.49 | 52 | 0.26 | 42 | 0.056 |
| W | 10.5 | 0.015 | 91 | 0.89 | 57 | 0.73 | 4 | 0.016 | 104 | 0.26 | 5 | 0.002 | 5 | 0.006 |
| Tl | 8.79 | 0.032 | 76 | 0.52 | 46 | 0.29 | 14 | 0.14 | 117 | 0.28 | 20 | 0.016 | 5 | 0.014 |
| Pb | 8.95 | 0.030 | 66 | 0.21 | 29 | 0.034 | 25 | 0.53 | 111 | 0.38 | 33 | 0.034 | 37 | 0.146 |
| Th | 20.5 | 0.000 | 41 | 0.02 | 16 | 0.003 | 5 | 0.006 | 123 | 0.17 | 5 | 0.0003 | 18 | 0.012 |
| U | 15.7 | 0.001 | 84 | 0.65 | 39 | 0.14 | 2 | 0.002 | 120 | 0.15 | 5 | 0.0003 | 19 | 0.015 |
Appendix B
References
- Liss, O.L.; Abramova, L.I.; Avetov, N.A.; Berezina, N.A.; Inisheva, L.I.; Kurnishkova, T.V.; Sluka, Z.A.; Tolpysheva, T.Y.; Shvedchikova, N.K. Marsh Systems of Western Siberia and Their Conservation Value; Grief and K: Tula, Russia, 2001. (In Russian) [Google Scholar]
- Novikov, S.M.; Moskvin, Y.P.; Trofimov, S.A.; Usova, L.I.; Batuev, V.I.; Tumanovskaya, S.M.; Smirnova, V.P.; Markov, M.L.; Korotkevicth, A.E.; Potapova, T.M. Hydrology of Bog Territories of the Permafrost Zone of Western Siberia; BBM publ. House: St. Petersburg, Russia, 2009. (In Russian) [Google Scholar]
- Friborg, T.; Soegaard, H.; Christensen, T.R.; Lloyd, C.R.; Panikov, N.S. Siberian wetland: Where a sink is a source. Geophys. Res. Lett. 2003, 30, 2129–2132. [Google Scholar] [CrossRef]
- Zemtsov, A.A.; Mezentsev, A.V.; Inisheva, L.I. Marshes of Western Siberia: Their Role in the Biosphere; Publishing House of Tomsk TSNTI: Tomsk, Russia, 1998. (In Russian) [Google Scholar]
- Turunen, J.; Tahvanainen, T.; Tolonen, K. Carbon accumulation in West Siberian mires, Russia. Glob. Biogeochem. Cycles 2001, 72, 285–296. [Google Scholar] [CrossRef]
- Kirpotin, S.N.; Berezin, A.; Bazanov, V.; Polishchuk, Y.; Vorobiov, S.; Mironycheva-Tokoreva, N.; Kosykh, N.; Volkova, I.; Dupre, B.; Pokrovsky, O.; et al. Western Siberia wetlands as indicator and regulator of climate change on the global scale. Int. J. Environ. Stud. 2009, 66, 409–421. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.G.; Krickov, I.V.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Dissolved organic carbon and major and trace elements in peat porewater of sporadic, discontinuous, and continuous permafrost zones of western Siberia. Biogeosciences 2017, 14, 3561–3584. [Google Scholar] [CrossRef]
- Amon, R.M.W.; Meon, B. The biogeochemistry of dissolved organic matter and nutrients in two large Arctic estuaries and potential implications for our understanding of the Arctic Ocean system. Mar. Chem. 2004, 92, 311–330. [Google Scholar] [CrossRef]
- Sukhanova, I.N.; Flint, M.V.; Sergeyeva, V.M.; Kremenetskiy, V.V. Phytoplankton of the southwestern part of the Kara Sea. Oceanology 2011, 51, 1039–1053. (In Russian) [Google Scholar] [CrossRef]
- Vasilchuk, Y.K. Modern southern limit of permafrost in Western Siberia Lowland. Earth’s Cryosphere 2013, 17, 17–27. (In Russian) [Google Scholar]
- Bedritsky, A.I. Global Climate and Soil Cover of Russia: Risk Assessment and Environmental and Economic Impacts of Land Degradation. Adaptive Systems and Environmental Management Technologies (Agriculture and Forestry); GEOS publ.: Moscow, Russia, 2018. (In Russian) [Google Scholar]
- Biskaborn, B.K.; Smith, S.L.; Noetzli, J.; Matthes, H.; Vieira, G.; Streletskiy, D.A.; Schoeneich, P.; Romanovsky, V.E.; Lewkowicz, A.G.; Abramov, A.; et al. Permafrost is warming at a global scale. Nat. Commun. Vol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Paromov, V.V.; Zemtsov, V.A.; Kopysov, S.G. Climate of West Siberia during the slowing phase of warming (1986-2015) and prediction of hydro-climatic resources for 2021–2030. Bull. Tomsk Polytechnic Univ. Geo Assets Eng. 2017, 328, 62–74. [Google Scholar]
- Frey, K.E.; Smith, L.C. Amplified carbon release from vast West Siberian peatlands by 2100. Geophys. Res. Lett. 2005, 32, L09401. [Google Scholar] [CrossRef]
- Hugelius, G.; Virtanen, T.; Kaverin, D.; Pastukhov, A.; Rivkin, F.; Marchenko, S.; Romanovsky, V.; Kuhry, P. High-resolution mapping of ecosystem carbon storage and potential effects of permafrost thaw in periglacial terrain, European Russian Arctic. J. Geophys. Res. Biogeo. 2011, 116, G03024. [Google Scholar] [CrossRef]
- Kaverin, D.A.; Pastukhov, A.V.; Lapteva, E.M.; Biasi, C.; Marushchak, M.; Martikainen, P. Morphology and properties of the soils of permafrost peatlands in the southeast of the Bol’shezemel’skaya tundra. Eurasian Soil Sci. 2016, 49, 498–511. [Google Scholar] [CrossRef]
- Vasil’chuk, Y.K.; Vasil’chuk, A.C. Thick polygonal peatlands in continuous permafrost zone of West Siberia. Earth’s Cryosphere 2016, 20, 3–13. [Google Scholar]
- Kaverin, D.A.; Pastukhov, A.V. Temperature state of soils of peat plateaus in the sporadic permafrost area (European northeast of Russia). Earth’s Cryosphere 2018, 21, 42–50. [Google Scholar]
- Quinton, W.L.; Hayashi, M. The flow and storage of water in the wetland-dominated central Mackenzie River basin: Recent advances and future directions, in Prediction in Ungauged Basins: Approaches for Canada’s Cold Regions. In Prediction in Ungauged Basins: Approaches for Canada’s Cold Regions; Spence, C., Pomeroy, J.W., Pietroniro, A., Eds.; Canadian Water Resources Association: Toronto, ON, Canada, 2005; pp. 45–66. [Google Scholar]
- Sullivan, P.F.; Arens, S.J.; Chimner, R.A.; Welker, J.M. Temperature and microtopography interact to control carbon cycling in a high arctic fen. Ecosystems 2008, 11, 61–76. [Google Scholar] [CrossRef]
- Wright, N.; Hayashi, M.; Quinton, W.L. Spatial and temporal variations in active layer thawing and their implication on runoff generation in peat-covered permafrost terrain. Water Resour. Res. 2009, 45, W05414. [Google Scholar] [CrossRef]
- Verry, E.S.; Jansenns, J. Geology, vegetation, and hydrology of the S2 bog at the MEF: 12,000 years in northern Minnesota. In Peatland Biogeochemistry and Watershed Hydrology at the Marcell Experimental Forest; Kolka, R.K., Sebestyen, S.D., Verry, E.S., Brooks, K.N., Eds.; CRC Press: New York, NY, USA, 2011; pp. 93–134. [Google Scholar]
- Rhew, R.C.; Teh, Y.A.; Abel, T. Methyl halide and methane fluxes in the northern Alaskan coastal tundra. J. Geophys. Res. 2007, 112, G02009. [Google Scholar] [CrossRef]
- Zona, D.; Lipson, D.A.; Zulueta, R.C.; Oberbauer, S.F.; Oechel, W.C. Microtopographic controls on ecosystem functioning in the Arctic Coastal Plain. J. Geophys. Res. Biogeo. 2011, 116, G00I08. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.; Manasypov, R.M.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kylizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Permafrost thaw and climate warming may decrease the CO2, carbon, and metal concentration in peat soil waters of the Western Siberia Lowland. Sci. Total Environ. 2018, 634, 1004–1023. [Google Scholar] [CrossRef]
- Tang, J.; Yurova, A.Y.; Schurgers, G.; Miller, P.A.; Olin, S.; Smith, B.; Siewert, M.B.; Olefeldt, D.; Pilesjö, P.; Poska, A. Drivers of dissolved organic carbon export in a subarctic catchment: Importance of microbial decomposition, sorption-desorption, peatland and lateral flow. Sci. Total Environ. 2018, 622-623, 260–274. [Google Scholar] [CrossRef]
- Wright, N.; Quinton, W.L.; Hayashi, M. Hillslope runoff from an ice-cored peat plateau in a discontinuous permafrost basin, Northwest Territories, Canada. Hydrol. Processes 2008, 22, 2816–2828. [Google Scholar] [CrossRef]
- Trofimova, I.E.; Balybina, A.S. Classification of climates and climatic regionalization of the West-Siberian plain. Geogr. Nat Resour. 2014, 35, 114–122. [Google Scholar] [CrossRef]
- Brown, J.; Ferrians, O.J., Jr.; Heginbottom, J.A.; Melnikov, E.S. Circum-Arctic Map of Permafrost and Ground Ice Conditions; NSIDC: Boulder, CO, USA, 2002. [Google Scholar]
- Lindsay, R.; Charman, D.J.; Everingham, F.; O’reilly, R.M.; Palmer, M.A.; Rowell, T.A.; Stroud, D.A. The flow Country: The Peatlands of Caithness and Sutherland; Joint Nature Conservation Committee: Peterborough, UK, 1988. [Google Scholar]
- Couwenberg, J.; Joosten, H. Self-organization in raised bog patterning: The origin of microtope zonation and mesotope diversity. J. Ecol. 2005, 93, 1238–1248. [Google Scholar] [CrossRef]
- Pouliot, R.; Rochefort, L.; Karofeld, E. Initiation of microtopography in revegetated cutover peatlands. Appl. Veg. Sci. 2011, 14, 158–171. [Google Scholar] [CrossRef]
- Minayeva, T.Y.; Bragg, O.M.; Sirin, A.A. Towards ecosystem-based restoration of peatland biodiversity. Mires Peat 2017, 19, 1–36. [Google Scholar]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Parshina, E.K. Biological productivity of the forest-tundra mires of Western Siberia. Tomsk State Pedagogical Uni. Bull. 2008, 78, 53–57. (In Russian) [Google Scholar]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Mikhailova, E.V. Palsa mire. In Soils in the Biosphere, Proceedings of the Russian Scientific Conference with International Participation, Dedicated to the 50th Anniversary of the Institute of Soil Science and Agrochemistry SB RAS, Novosibirsk, Russia, 10–14 September 2018; Publishing House of Tomsk State University: Tomsk, Russia, 2018. [Google Scholar]
- Peregon, A.; Maksyutov, S.; Kosykh, N.P.; Mironycheva-Tokareva, N.P. Map-based inventory of wetland biomass and net primary production in western Siberia. J. Geophys. Res.: Biogeosciences 2008, 113, G01007. [Google Scholar] [CrossRef]
- Ilina, I.S.; Lapshina, E.I.; Lavrenko, N.N.; Meltzer, L.I.; Romanova, E.A.; Bogoyavlensky, B.A.; Makhno, V.D. Vegetation cover of the West Siberian Plain; Nauka: Novosibirsk, Russia, 1985. (In Russian) [Google Scholar]
- Peregon, A.; Uchida, M.; Shibata, Y. Sphagnum peatland development at their southern climatic range in West Siberia: trends and peat accumulation patterns. Environ. Res. Lett. 2007, 2, 045014. [Google Scholar] [CrossRef]
- Peregon, A.; Uchida, M.; Yamagata, Y. Lateral extension in Sphagnum mires along the southern margin of the boreal region, Western Siberia. Environ. Res. Lett. 2009, 4, 045028. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106; FAO: Rome, 2014.
- Raudina, T.V.; Loyko, S.V.; Kritskov, I.V.; Lim, A.G. Comparison of the composition of soil waters of frozen bogs of Western Siberia, obtained by various methods. Tomsk State Uni.J. Biol. 2016, 35, 26–42. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Prokushkin, A.S.; Pokrovsky, O.S.; Shirokova, L.S.; Korets, M.A.; Viers, J.; Prokushkin, S.G.; Amon, R.; Guggenberger, G.; McDowell, W.H. Sources and export fluxes of dissolved carbon in rivers draining larch-dominated basins of the Central Siberian Plateau. Environ. Res. Lett. 2011, 6, 045212. [Google Scholar] [CrossRef]
- Yeghicheyan, D.; Bossy, C.; Bouhnik Le Coz, M.; Douchet, C.; Granier, G.; Heimburger, A.; Lacan, F.; Lanzanova, A.; Rousseau, T.C.C.; Seidel, J.-L.; et al. A compilation of silicon, rare earth element and twenty-one other trace element concentrations in the natural river water reference material SLRS-5 (NRC-CNRC). Geostand. Geoanal. Res. 2013, 37, 449–467. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Loiko, S.; Krickov, I.A.; Kopysov, S.G.; Kolesnichenko, L.G.; Vorobyev, S.N.; Kirpotin, S.N. Trace element transport in western Siberian rivers across a permafrost gradient. Biogeosciences 2016, 13, 1877–1900. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Loiko, S.V.; Shirokova, L.S. Organic and organo-mineral colloids in discontinuous permafrost zone. Geochim. Cosmochim. Ac 2016, 188, 1–20. [Google Scholar] [CrossRef]
- Kremenetski, K.V.; Velichko, A.A.; Borisova, O.K.; MacDonald, G.M.; Smith, L.C.; Frey, K.E.; Orlova, L.A. Peatlands of the Western Siberian lowlands: current knowledge on zonation, carbon content and Late Quaternary history. Quaternary Sci. Rev. 2003, 22, 703–723. [Google Scholar] [CrossRef]
- Panova, N.K.; Trofimova, S.S.; Antipina, T.G.; Zinoviev, E.V.; Gilev, A.V.; Erokhin, N.G. Holocene dynamics of vegetation and ecological conditions in the Southern Yamal Peninsula according to the results of comprehensive analysis of a relict peat bog deposit. Russian J. Ecol. 2010, 41, 20–27. [Google Scholar] [CrossRef]
- Maksimova, L.N.; Ospennikov, E.N. Evolution of mire systems and permafrost of the Bolshezemelskaya tundra in the Holocene. Earth’s Cryosphere 2012, 16, 53–61. (In Russian) [Google Scholar]
- Ponomareva, O.E.; Gravis, A.G.; Berdnikov, N.M. Contemporary dynamics of frost mounds and flat peatlands in north taiga of West Siberia (on the example of Nadym site). Earth’s Cryosphere 2012, 16, 21–30. (In Russian) [Google Scholar]
- Lupachev, A.V.; Gubin, S.V.; Veremeeva, A.A.; Kaverin, D.A.; Pastukhov, A.V.; Yakimov, A.S. Microrelief of the permafrost table: structure and ecological functions. Earth’s Cryosphere 2016, 20, 3–14. [Google Scholar]
- Inisheva, L.I.; Tsybukova, T.N. Ecological and geochemical assessment of peat in the south-east of the West Siberian Plain. Geogr. Nat. Resour. 1999, 1, 45–51. (In Russian) [Google Scholar]
- Inisheva, L.I. Peat soils: Genesis and classification. Eurasian Soil Science 2006, 39, 699–704. [Google Scholar] [CrossRef]
- Moskovchenko, D.V. Biogeochemical properties of the high bogs in Western Siberia. Geogr. Nat. Resour. 2006, 1, 63–70. (In Russian) [Google Scholar]
- Moskovchenko, D.V.; Babushkin, A.G. Background level of mobile forms of metals in soils of northwest Siberia. UT Res. J. Nat. Resour. Use Ecol. 2015, 1, 163–174. (In Russian) [Google Scholar]
- Stepanova, V.A.; Pokrovsky, O.S.; Viers, J.; Mironycheva-Tokareva, N.P.; Kosykh, N.P.; Vishnyakova, E.K. Elemental composition of peat profiles in western Siberia: Effect of the micro-landscape, latitude position and permafrost coverage. Appl. Geochem. 2015, 53, 53–70. [Google Scholar] [CrossRef]
- Batuev, V.I. Runoff formation from frost mound bogs (data: West Siberia). TSPU Bull. 2012, 122, 146–152. [Google Scholar]
- Shi, X.; Thornton, P.E.; Ricciuto, D.M.; Hanson, P.J.; Mao, J.; Sebestyen, S.D.; Bisht, G. Representing northern peatland microtopography and hydrology within the Community Land Model. Biogeosciences 2015, 12, 6463–6477. [Google Scholar] [CrossRef]
- Ivanov, K.E.; Novikov, S.M. Bogs of Western Siberia, Their Composition and Hydrological Regime; Gidrometeoizdat: Leningrad, Russia, 1976. (In Russian) [Google Scholar]
- Olefeldt, D.; Roulet, N.T. Effects of permafrost and hydrology on the composition and transport of dissolved organic carbon in a subarctic peatland complex. J. Geophys. Res. 2012, 117, G01005. [Google Scholar] [CrossRef]
- Batuev, V.I. Classification of the primary hydrographic network of frost mound bogs. Tomsk State Pedagog. Uni. Bull. 2010, 93, 70–77. (In Russian) [Google Scholar]
- Kaverin, D.A.; Pastukhov, A.V.; Novakovskiy, A.B. Active layer thickness dynamics in the tundra permafrost-affected soils: a calm site case study, the European north of Russia. Earth’s Cryosphere 2017, 21, 30–38. [Google Scholar]
- Bobrik, A.A.; Goncharova, O.Y.; Matyshak, G.V.; Ryzhova, I.M.; Moskalenko, N.G.; Ponomareva, O.E.; Ogneva, O.A. Correlation of active layer thickness and landscape parameters of peatland in northern West Siberia (Nadym station). Earth’s Cryosphere 2015, 19, 29–35. [Google Scholar]
- Makhatkov, I.D.; Ermolov, Y.V. The thermal regime of active layer of pit-covered terrain in northern taiga. Internat. J. Appl. Basic Res. 2015, 11, 400–407. (In Russian) [Google Scholar]
- Mazhitova, G.G. Soil temperature regimes in the discontinuous permafrost zone in the East European Russian Arctic. Eurasian Soil Sci. 2008, 41, 48–62. [Google Scholar] [CrossRef]
- Koronatova, N.G.; Mironycheva-Tokareva, N.P.; Solomin, Y.R. Thermal regime of peat deposits of palsas and hollows of peat plateaus in Western Siberia. Earth’s Cryosphere 2018, 22, 16–25. [Google Scholar]
- Pokrovsky, O.S.; Manasypov, R.M.; Loiko, S.V.; Shirokova, L.S.; Krickov, I.A.; Pokrovsky, B.G.; Kolesnichenko, L.G.; Kopysov, S.G.; Zemtzov, V.A.; Kulizhsky, S.P.; et al. Permafrost coverage, watershed area and season control of dissolved carbon and major elements in western Siberian rivers. Biogeosciences 2015, 12, 6301–6320. [Google Scholar] [CrossRef]
- Kawahigashi, M.; Kaiser, K.; Kalbitz, K.; Rodionov, A.; Guggenberger, G. Dissolved organic matter in small streams along a gradient from discontinuous to continuous permafrost. Glob. Change Biol. 2013, 10, 1576–1586. [Google Scholar] [CrossRef]
- Keller, K.; Blum, J.D.; Kling, G.W. Stream geochemistry as an indicator of increasing permafrost thaw depth in an Arctic watershed. Chem. Geol. 2010, 273, 76–81. [Google Scholar] [CrossRef]
- Bagard, M.L.; Schmitt, A.D.; Chabaux, F.; Pokrovsky, O.S.; Viers, J.; Stille, P.; Labolle, F.; Prokushkin, A.S. Biogeochemistry of stable Ca and radiogenic Sr isotopes in larch-covered permafrost-dominated watersheds of Central Siberia. Geochim. Cosmochim. Ac. 2013, 114, 169–187. [Google Scholar] [CrossRef]
- Rudmin, M.; Ruban, A.; Savichev, O.; Mazurov, A.; Dauletova, A.; Savinova, O. Authigenic and detrital minerals in peat environment of Vasyugan swamp western Siberia. Minerals 2018, 8, 500. [Google Scholar] [CrossRef]
- Fiałkiewicz-Kozieł, B.; Smieja-Król, B.; Frontasyeva, M.; Słowiński, M.; Marcisz, K.; Lapshina, E.; Gilbert, D.; Buttler, A.; Jassey, V.E.J.; Kaliszan, K.; et al. Anthropogenic- and natural sources of dust in peatland during the Anthropocene. Sci. Rep. 2016, 6, 38731. [Google Scholar] [CrossRef]
- Lim, A.G.; Loyko, S.V.; Raudina, T.V.; Volkova, I.I.; Seredina, V.P. Element composition of peat deposits in flat frost mound bogs of the Pyakupur River (northern taiga of West Siberia). Ukrainian J.Ecol. 2018, 8, 79–87. [Google Scholar] [CrossRef]
- Shevchenko, V.P.; Pokrovsky, O.S.; Vorobyev, S.N.; Krickov, I.V.; Manasypov, R.M.; Politova, N.V.; Kopysov, S.G.; Dara, O.M.; Auda, Y.; Shirokova, L.S.; et al. Impact of snow deposition on major and trace element concentrations and elementary fluxes in surface waters of the Western Siberian Lowland across a 1700’km latitudinal gradient. Hydrol. Earth Syst. Sci. 2017, 21, 5725–5746. [Google Scholar] [CrossRef]
- Opekunova, M.G.; Opekunov, A.Y.; Kukushkin, S.Y.; Ganul, A.G. Background Contents of Heavy Metals in Soils and Bottom Sediments in the North of Western Siberia. Eurasian Soil Sc. 2019, 52, 380–395. [Google Scholar] [CrossRef]
- Judd, K.E.; Kling, G.W. Production and export of dissolved C in arctic tundra mesocosms: the roles of vegetation and water flow. Biogeochemistry 2002, 60, 213. [Google Scholar] [CrossRef]
- Ma, Q.; Jin, H.; Yu, C.; Bense, V.F. Dissolved organic carbon in permafrost regions: A review. Sci. China Earth Sci. 2019, 62, 349–364. [Google Scholar] [CrossRef]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Mikhailova, E.V.; Kolesnichenko, L.G. Vegetation and plant material of the flat palsa peatlands. J. Soils Environ. 2019, 2, e55, (In Russian with English Abstract). [Google Scholar]
- Harris, L.I.; Moore, T.R.; Roulet, N.T.; Pinsonneault, A.J. Lichens: A limit to peat growth? J. Ecol. 2018, 106, 2301–2319. [Google Scholar] [CrossRef]
- Nikonova, L.G.; Kurganova, I.N.; Lopes de Gerenyu, V.O.; Zhmurin, V.A.; Golovatskaya, E.A. Impact of abiotic factors on the decomposition of litter of peat-forming plants in the incubation experiment. Tomsk State Uni. J. Biol. 2019, 46, 148–170. (In Russian) [Google Scholar]
- Golovatskaya, E.A.; Nikonova, L.G. The influence of the bog water level on the transformation of sphagnum mosses in peat soils of oligotrophic bogs. Eurasian Soil Science 2017, 50, 580–588. [Google Scholar] [CrossRef]
- Vishnyakova, E.K.; Mironycheva-Tokareva, N.P. Moss decomposition in Western Siberian mires. In Mosses: Ecology, Life Cycle and Significance, 4th ed.; Pokrovsky, O., Volkova, I., Kosykh, N., Shevchenko, V., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 217–241. [Google Scholar]
- Edwards, K.R.; Kaštovská, E.; Borovec, J.; Šantrůčková, H.; Picek, T. Species effects and seasonal trends on plant efflux quantity and quality in a spruce swamp forest. Plant and Soil 2018, 426, 179–196. [Google Scholar] [CrossRef]
- Ritson, J.P.; Bell, M.; Brazier, R.E.; Grand-Clement, E.; Graham, N.J.D.; Freeman, C.; Smith, D.; Templeton, M.R.; Clark, J.M. Managing peatland vegetation for drinking water treatment. Sci. Rep. 2016, 6, 36751. [Google Scholar] [CrossRef]
- Zeh, L.; Limpens, J.; Erhagen, B.; Bragazza, L.; Kalbitz, K. Plant functional types and temperature control carbon input via roots in peatland soils. Plant and Soil 2019, 438, 19–38. [Google Scholar] [CrossRef]
- Corbett, J.E.; Tfaily, M.M.; Burdige, D.J.; Cooper, W.T.; Glaser, P.H.; Chanton, J.P. Partitioning pathways of CO2 production in peatlands with stable carbon isotopes. Biogeochemistry 2013, 114, 327–340. [Google Scholar] [CrossRef]
- Bobrik, A.A.; Ryzhova, I.M.; Goncharova, O.Y.; Matyshak, G.V.; Makarov, M.I.; Walker, D.A. CO2 Emission and Organic Carbon Pools in Soils of the Northern Taiga Ecosystems of Western Siberia under Different Geocryological Conditions. Eurasian Soil Sci. 2018, 51, 628–636. [Google Scholar] [CrossRef]
- Anisimov, O.A.; Shiklomanov, N.I.; Nelson, F.E. Variability of seasonal thaw depth in permafrost regions: A stochastic modeling approach. Ecol. Modelling 2002, 153, 217–227. [Google Scholar] [CrossRef]
- Anisimov, O.; Reneva, S. Permafrost and changing climate: the Russian perspective. AMBIO: A J. Hum Environ. 2006, 35, 169–176. [Google Scholar] [CrossRef]
- Anisimov, O.A.; Zhiltsova, E.L.; Reneva, S.A. Assessment of critical levels of climate change impacts on natural terrestrial ecosystems in Russia. Meteorol. Hydrol. 2011, 11, 31–41. (In Russian) [Google Scholar]
- Anisimov, O.; Kokorev, V.; Zhil’tsova, Y. Temporal and spatial patterns of modern climatic warming: Case study of Northern Eurasia. Clim. Change 2013, 118, 871–883. [Google Scholar] [CrossRef]
- Anisimov, O.A.; Sherstiukov, A.B. Evaluating the effect of climatic and environmental factors on permafrost in Russia. Earth’s Cryosphere 2016, 20, 78–86. [Google Scholar]
- Malkova, G.V.; Gubarkov, A.A.; Drozdov, D.S.; Leybman, M.O.; Khomutov, A.V.; Sherstyukov, A.B. The Second Assessment Report of Roshydromet on Climate Change and Its Consequences on the Territory of the Russian Federation; Roshydromet: Moscow, Russia, 2014. [Google Scholar]
- Walvoord, M.A.; Kurylyk, B.L. Hydrologic impacts of thawing permafrost—A review. Vadose Zone J. 2016, 15, 1–20. [Google Scholar] [CrossRef]
- Nadyozhina, E.D.; Shkolnik, I.M.; Pavlova, T.V.; Molkentin, E.K.; Semioshina, A.A. Permafrost response to the climate warming as simulated by the regional climate model of the main geophysical observatory. Earth’s Cryosphere 2008, 12, 3–11. (In Russian) [Google Scholar]
- Pavlov, A.V.; Malkova, G.V. Small-scale mapping of trends of the contemporary ground temperature changes in the Russian North. Earth’s Cryosphere 2009, 13, 32–39. (In Russian) [Google Scholar]
- Anisimov, O.A.; Anokhin, A.; Lavrov, S.A.; Malkova, G.V.; Pavlov, A.V.; Romanovskiy, V.E.; Streletskiy, D.A.; Kholodov, A.L.; Shiklomanov, N.I. Continental multiyear permafrost. In Methods of Study the Sequences of Climate Changes for Nature Systems, 1st ed.; Semenov, S.M., Ed.; VNIIGMI: Moscow, Russia, 2012; pp. 268–328. (In Russian) [Google Scholar]
- Shkolnik, I.M.; Nadyozhina, E.D.; Pavlova, T.V.; Khlebnikova, E.I.; Semioshina, A.A.; Molkentin, E.K.; Stafeev, E.N. Simulation of the regional features of the seasonal thawing layer in the Siberian permafrost zone. Earth’s Cryosphere 2012, 16, 52–59. (In Russian) [Google Scholar]
- Moskalenko, N.G. Permafrost and vegetation changes in the Nadym region of West Siberian northern taiga due to the climate change and technogenesis. Earth’s Cryosphere 2009, 8, 18–23. (In Russian) [Google Scholar]
- Malmer, N.; Johansson, T.; Olsrud, M.; Christensen, T.R. Vegetation, climatic changes and net carbon sequestration in a North-Scandinavian subarctic mire over 30 years. Glob. Change Biol. 2005, 11, 1895–1909. [Google Scholar] [CrossRef]
- Sherstyukov, A.B.; Sherstyukov, B.G. Spatial features and new trends in thermal conditions of soil and depth of its seasonal thawing in the permafrost zone. Russ. Meteorol. Hydrol. 2015, 40, 73–78. [Google Scholar] [CrossRef]
- Reshetko, M.V.; Moiseeva, Y. Climatic features and statistical evaluation of climate change in permafrost regions in the north of western Siberia. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2016, 327, 108–118. (In Russian) [Google Scholar]
- Guo, D.; Wang, H. Simulated historical (1901–2010) changes in the permafrost extent and active layer thickness in the Northern Hemisphere. J. Geophys. Res.: Atmospheres 2017, 122, 12,285–12,295. [Google Scholar] [CrossRef]
- Keuper, F.; Van Bodegom, P.M.; Dorrepaal, E.; Weedon, J.T.; Van Hal, J.; Van Logtestijn, R.S.; Aerts, R. A frozen feast: Thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob. Change Biol. 2012, 18, 1998–2007. [Google Scholar] [CrossRef]
- Jorgenson, M.T.; Harden, J.; Kanevskiy, M.; O’Donnell, J.; Wickland, K.; Ewing, S.; Koch, J. Reorganization of vegetation, hydrology and soil carbon after permafrost degradation across heterogeneous boreal landscapes. Environ. Res. Lett. 2013, 8, 035017. [Google Scholar] [CrossRef]
| Key Site | Latitude °N | Mineral substrate | Micro- topes | Peat Thickness, m | ALT, cm | Peat Accumu- lation rate | Biomass, g/m2 | Mortmass, g/m2 | Vegetation Types and Dominant Plant Species | Soil (WRB, 2014) |
|---|---|---|---|---|---|---|---|---|---|---|
| Taz | 67.4 | Clay loam and loam | polygons | 2.0–4.0 | 41 | 0.02 | – | – | Dwarf shrub-green mosses-lichen Betula nana, Ledum palustre, Vaccinium vitis-idaea, V. uliginosum, Arctous alpine, Rubus chamaemorus, Empetrum nigrum, Cladonia stellaris, Alectoria ochroleuca, Cetraria nivalis, Sphagnum balticum, S. fuscum, Drepanocladus ssp., Polytrichum ssp. | Hemic Epicryic Histosols (Hyperorganic) |
| fens | 0.2–1.5 | 65 | 0.22, 0.09, 0.21 | – | – | Sedge-Sphagnum Carex chordorrhiza, C. rotundata, C. limosa, Eriophorum polystachyon, Menyanthes trifoliata, Comarum palustre, Sphagnum balticum, S. jensenii, S. majus, S. lindbergii | Epifibric Endohemic Cryic Histosols | |||
| Ur | 66.1 | Loam and silt loam | mounds | 2.0–2.5 | 49 | – | – | – | Dwarf shrub-green mosses-lichen Ledum palustre, Vaccínium vítis-idaéa, Rubus chamaemorus, Empetrum nigrum, Betula nana, Cladonia stellaris, Cl. rangiferina, Cl. sylvatica, Cetraria cucullata, Sphagnum balticum, S. fuscum, | Hemic Epicryic Histosols (Hyperorganic) |
| hollows | 0.3–1.2 | 98 | – | – | – | Sedge-cotton grass-Sphagnum Carex rotundata, C. limosa, C, chordorrhiza, Eriophorum polystachyon, E. russeolum, Scheuchzeria palustris, Menyanthes trifoliata, Comarum palustre, Sphagnum majus, S. balticum | Histic Gleysols (Loamic); Epifibric Histosols | |||
| Pan | 65.9 | Loam | mounds | 0.2–1.4 | 49 | 0.05, 0.11 | 2200 [34] | 9000– 25000 [34] | Dwarf shrub-lichens, dwarf shrub-Sphagnum-lichen Ledum palustre, Betula nana, Andromeda polifolia, Rubus chamaemorus, Empetrum nigrum, Cladonia stellaris, Cl. rangiferina, Sphagnum fuscum, S. lenense | Hemic Epicryic Histosols; Histic Cryosols (Loamic) |
| fens | 0.3–1.0 | 82 | 0.23, 0.16 | 750 [34] | 13000–19000 [34] | Sedge-Sphagnum Carex rotundata, Eriophorum vaginatum, Scheuchzeria palustris, Sphagnum lindbergii, S. balticum | Epifibric Endocryic Histosols; Histic Turbic Cryosols (Loamic) | |||
| Kha | 63.8 | Sand | mounds | 0.1–1.4 | 90 | 0.15, 0.28 | 1200–2400 [35] | 14000–21000 [35] | Dwarf shrub-lichen, dwarf shrub-Sphagnum-lichen Ledum palustre, Betula nana, Vaccinium vitis-idaea, Andromeda polifolia, Cladonia stellaris, C. stygia, Rubus chamaemorus, Sphagnum fuscum, S. capillifolium | Hemic Endocryic Histosols; Epifibric Endocryic Histosols; Spodic Histic Turbic Cryosols (Albic, Arenic) |
| fens | 0.4–1.0 | >200 | 0.54, 0.96 | 800 [35] | 9000– 16000 [35] | Sedge-Sphagnum, cotton grass-Sphagnum Eriophorum russeolum, Eriophorum vaginatum, Carex rotundata, Scheuchzeria palustris, Sphagnum balticum, Sphagnum lindbergii, Warnstorfia fluitans | Epifibric Histosols; Spodic Histic Turbic Cryosols (Arenic) | |||
| Kog | 62.3 | Sand | ridges | 1.7–2.3 | not | 0.3 | 2064 [36] | 6658 [36] | Pine-dwarf shrub-Sphagnum Pinus sylvestris, Ledum palustre, Betula nana, Andromeda polifolia, Chamaedaphne calyculata, Vaccinium myrtillus, Vaccinium uliginosum, Sphagnum fuscum | Ombric Fibric Histosols (Hyperorganic) |
| fens | 1.0–1.5 | not | – | 769–1274 [36] | 8865– 10973 [36] | Sedge-Sphagnum Carex rostrata, C. limosa, Eriophorum polystachyon, Sphagnum majus, S. jensenii | Ombric Fibric Histosols |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiko, S.; Raudina, T.; Lim, A.; Kuzmina, D.; Kulizhskiy, S.; Pokrovsky, O. Microtopography Controls of Carbon and Related Elements Distribution in the West Siberian Frozen Bogs. Geosciences 2019, 9, 291. https://doi.org/10.3390/geosciences9070291
Loiko S, Raudina T, Lim A, Kuzmina D, Kulizhskiy S, Pokrovsky O. Microtopography Controls of Carbon and Related Elements Distribution in the West Siberian Frozen Bogs. Geosciences. 2019; 9(7):291. https://doi.org/10.3390/geosciences9070291
Chicago/Turabian StyleLoiko, Sergey, Tatiana Raudina, Artem Lim, Daria Kuzmina, Sergey Kulizhskiy, and Oleg Pokrovsky. 2019. "Microtopography Controls of Carbon and Related Elements Distribution in the West Siberian Frozen Bogs" Geosciences 9, no. 7: 291. https://doi.org/10.3390/geosciences9070291
APA StyleLoiko, S., Raudina, T., Lim, A., Kuzmina, D., Kulizhskiy, S., & Pokrovsky, O. (2019). Microtopography Controls of Carbon and Related Elements Distribution in the West Siberian Frozen Bogs. Geosciences, 9(7), 291. https://doi.org/10.3390/geosciences9070291









