Influence of Climate on Stable Nitrogen Isotopic Values of Contemporary Greek Samples: Implications for Isotopic Studies of Human Remains from Neolithic to Late Bronze Age Greece
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Relation of δ15N versus Precipitation for Greece
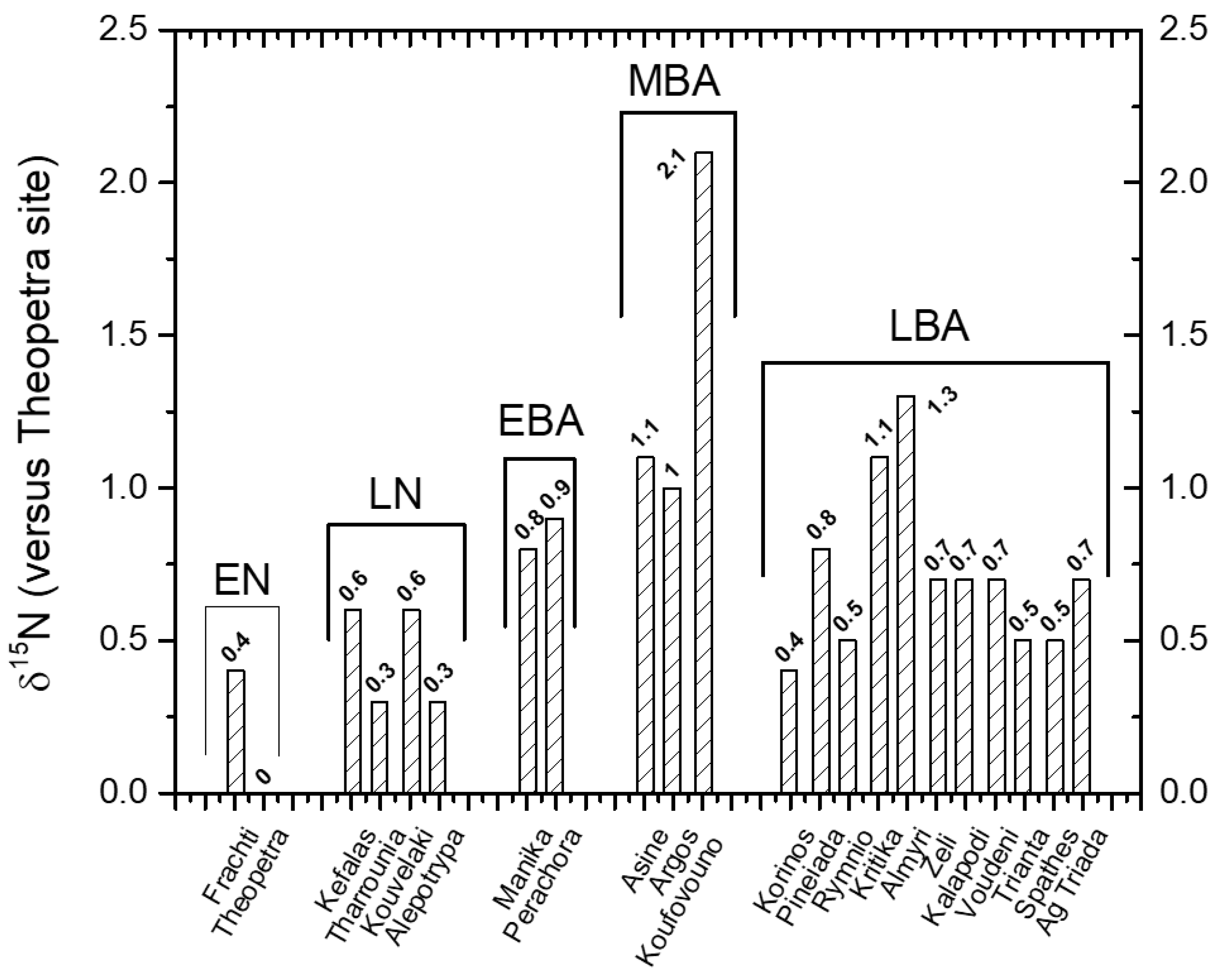
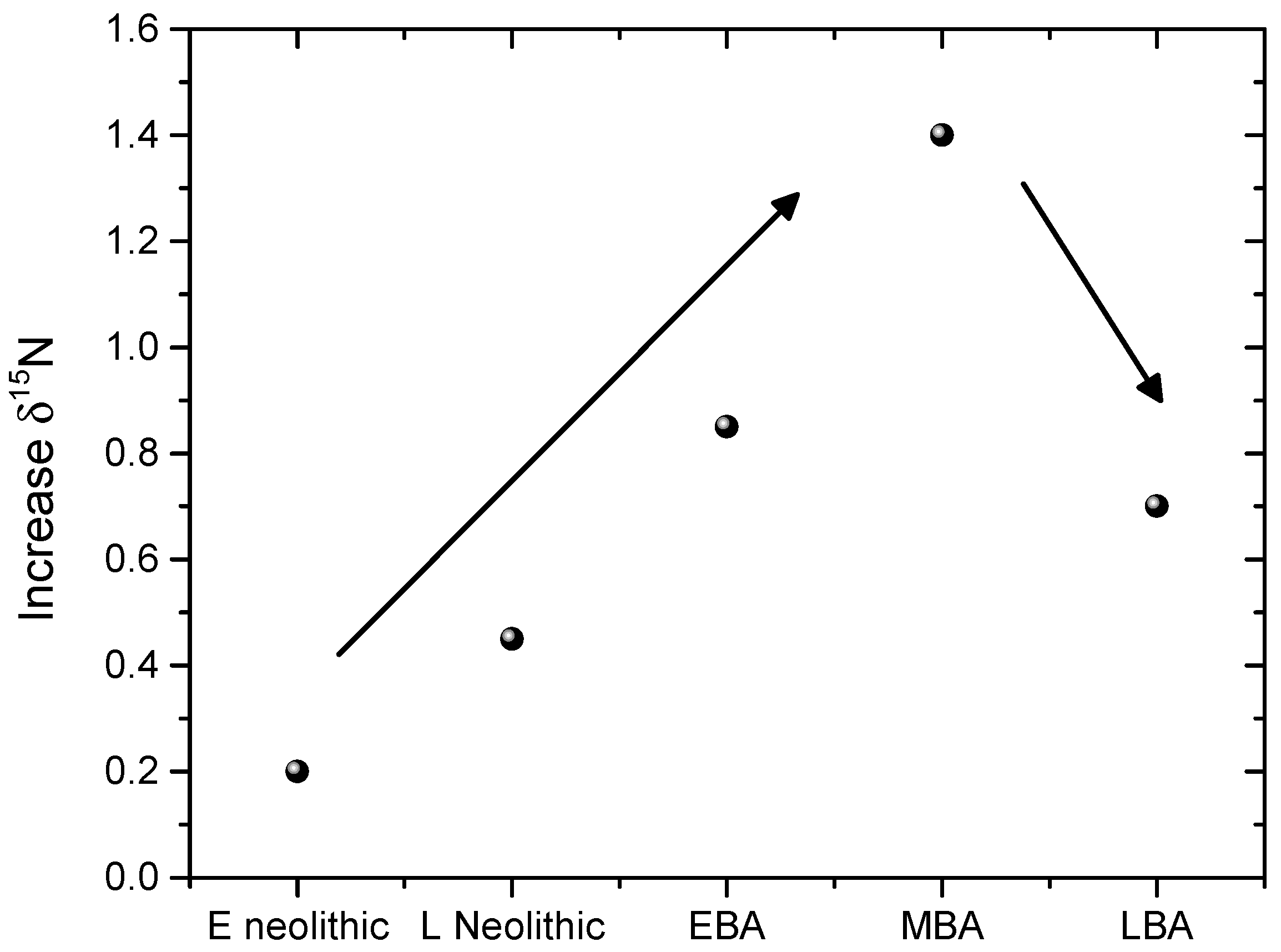
3.2. Case Study: Inter Comparison of δ15N Values of Human Bones of 22 Archeological Sites
3.3. Discussion
3.3.1. For the EN
3.3.2. For the LN
3.3.3. For the EBA
3.3.4. For the MBA
3.3.5. For the LBA
3.4. Intra-Site Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brand, W.A.; Coplen, T.B. Stable isotope deltas: Tiny, yet robust signatures in nature. Isotopes Environ. Health Stud. 2012, 48, 393–409. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Schoeninger, M.J. Stable isotope analyses in human nutritional ecology. Am. J. Phys. Anthropol. 1991, 34, 283–321. [Google Scholar] [CrossRef]
- Ambrose, S.H. Isotopic Analysis of Palaeodiets: Methodological and Interpretive Consideration. 1993. Available online: http://agris.fao.org/agris-search/search.do?recordID=US9514697 (accessed on 3 April 2019).
- Schoeninger, M.J.; DeNiro, M.J. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim. Cosmochim. Acta 1984, 48, 625–639. [Google Scholar] [CrossRef]
- Lubell, D.; Jackes, M.; Schwarcz, H.; Knyf, M.; Meiklejohn, C. The Mesolithic-Neolithic transition in Portugal: Isotopic and dental evidence of diet. J. Archaeol. Sci. 1994, 21, 201–216. [Google Scholar] [CrossRef]
- Iacumin, P.; Bocherens, H.; Delgado Huertas, A.; Mariottoi, A.; Longinelli, A. A stable isotope study of fossil mammal remains from the Paglicci cave, Southern Italy. N and C as palaeoenvironmental indicators. Earth Planet. Sci. Lett. 1997, 148, 349–357. [Google Scholar] [CrossRef]
- Wada, E.; Kadonaga, T.; Matsuo, S. 15N abundance in nitrogen of naturally occurring substances and global assessment of denitrification from isotopic viewpoint. Geochem. J. 1975, 9, 139–148. [Google Scholar] [CrossRef]
- Cheng, H.; Bremner, J.; Edwards, A. Variations of nitrogen-15 abundance in soils. Science 1964, 146, 1574–1575. [Google Scholar] [CrossRef]
- Mariotti, A.; Pierre, D.; Vedy, J.C.; Guillemot, J. The abundance of natural nitrogen 15 in the organic matter of soils along an altitudinal gradient (Chablais, Haute Savoie, France). CATENA 1980, 7, 293–300. [Google Scholar] [CrossRef]
- Heaton, T.H.; Vogel, J.C.; von la Chevallerie, G.; Collett, G. Climatic influence on the isotopic composition of bone nitrogen. Nature 1986, 322, 822. [Google Scholar] [CrossRef]
- Ambrose, S.H.; DeNiro, M.J. Climate and habitat reconstruction using stable carbon and nitrogen isotope ratios of collagen in prehistoric herbivore teeth from Kenya. Quat. Res. 1989, 31, 407–422. [Google Scholar] [CrossRef]
- Sealy, J.C.; van der Merwe, N.; Lee, J.A.; Lanham, J.L. Nitrogen isotopic ecology in southern Africa: Implications for environmental and dietary tracing. Geochim. Cosmochim. Acta 1987, 51, 2707–2717. [Google Scholar] [CrossRef]
- Drucker, D.; Bocherens, H.; Bridault, A.; Billiou, D. Carbon and nitrogen isotopic composition of red deer (Cervus elaphus) collagen as a tool for tracking palaeoenvironmental change during the Late-Glacial and Early Holocene in the northern Jura (France). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 195, 375–388. [Google Scholar] [CrossRef]
- Bogaard, A.; Heayon, T.H.E.; Poulton, P.; Merbach, I. The impact of manuring on nitrogen isotope ratios in cereals: Archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 2007, 34, 335–343. [Google Scholar] [CrossRef]
- Kreitler, C.W.; Jones, D.C. Natural soil nitrate: The cause of the nitrate contamination of ground water in Runnels County, Texas. Groundwater 1975, 13, 53–62. [Google Scholar] [CrossRef]
- Dotsika, E.; Diamantopoulos, G.; Lykoudis, S.; Gougoura, S.; Kranioti, E.; Karalis, P.; Michael, D.; Samartzidou, E.; Palaigeorgiou, E. Establishment of a Greek Food Database for Palaeodiet Reconstruction: Case Study of Human and Fauna Remains from Neolithic to Late Bronze Age from Greece. Geosciences 2019, 9, 165. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Hedges, R.E.M.; Healey, M.A.; Simpson, A.H.R.W. Isotopic comparison of hair, nail and bone: Modern analyses. J. Archaeol. Sci. 2001, 28, 1247–1255. [Google Scholar] [CrossRef]
- Ambrose, S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- Tykot, R.H. Stable Isotopes and Diet: You Are What You Eat. Available online: http://luna.cas.usf.edu/~rtykot/PR39%20-%20Enrico%20Fermi%20isotopes.pdf (accessed on 3 April 2019).
- Service, H.N.M. Climatic Atlas of Greece, 1971–2000. Available online: http://climatlas.hnms.gr/sdi/?lang=EN (accessed on 3 April 2019).
- Triantaphyllou, S. A Bioarchaeological Approach to Prehistoric Cemetery Populations from Central and Western Greek Macedonia. 2001. Available online: http://etheses.whiterose.ac.uk/14790/1/312328.pdf (accessed on 3 April 2019).
- Triantaphyllou, S.; Richards, M.P.; Zerner, C.; Voutsaki, S. Isotopic dietary reconstruction of humans from Middle Bronze age Lerna, Argolid, Greece. J. Archaeol. Sci. 2008, 35, 3028–3034. [Google Scholar] [CrossRef]
- Analyses of Middle Helladic Skeletal Material from Aspis, Argos, 2. Stable Isotope Analysis of Human Remains. Available online: https://www.persee.fr/doc/bch_0007-4217_2006_num_130_2_7430 (accessed on 3 April 2019).
- Papathanasiou, A. A Bioarchaeological Analysis of Neolithic Alepotrypa Cave, Greece; British Archaeological Reports Ltd.: Oxford, UK, 2001; Volume 961. [Google Scholar]
- Petroutsa, E.I.; Manolis, S.K. Reconstructing Late Bronze Age diet in mainland Greece using stable isotope analysis. J. Archaeol. Sci. 2010, 37, 614–620. [Google Scholar] [CrossRef]
- Petroutsa, E.I. An Investigation in the Nuitrition of Bronze Age Poplulations in Greece (in Greek); National University of Athens: Athens, Greece, 2007. [Google Scholar]
- Ingvarsson-Sundström, A.; Richards, M.P.; Voutsaki, S. Stable isotope analysis of the Middle Helladic population from two cemeteries at Asine: Barbouna and the east cemetery. Mediterr. Archaeol. Archaeometry 2009, 9, 1–14. [Google Scholar]
- Papathanasiou, A. Stable isotope analysis in Neolithic Greece and possible implications on human health. Int. J. Osteoarchaeol. 2003, 13, 314–324. [Google Scholar] [CrossRef]
- Kontopoulos, I.; Sampson, A. Prehistoric diet on the island of Euboea, Greece: An isotopic investigation. Mediterr. Archaeol. Archaeom. 2015, 15, 97–111. [Google Scholar]
- Lagia, A.; Petroutsa, E.; Manolis, S. Health and Diet During the MBA in the Peloponnese: The Site of Kouphovouno. In Cooking Up the Past: Food and Culinary Practices in the Neolithic and Bronze Age Aegean; Oxbow: Oxford, UK, 2007; pp. 313–328. [Google Scholar]
- Petroutsa, E.I.; Richards, M.P.; Manolis, S.K. Stable isotope analysis of human remains from the Early Helladic site of Perachora, Korinth, Greece. In Cooking Up the Past; Oxbow: Oxford, UK, 2007; pp. 290–296. [Google Scholar]
- Petroutsa, E.I.; Richards, M.P.; Kolonas, L.; Manolis, S.K. Isotope Paleodietary Analysis of Humans and Fauna from the Late Bronze Age Site of Voudeni; The American School of Classical Studies at Athens: Athina, Greece, 2009. [Google Scholar]
- Vika, E. From Diet to Society: Stable Isotope Analysis and Its Cultural Context in Bronze Age Peloponnese, Greece. Ph.D Thesis, University of Bradford, Bradford, UK, 2002. [Google Scholar]
- Heaton, T.H. The 15 N/14 N ratios of plants in South Africa and Namibia: Relationship to climate and coastal/saline environments. Oecologia 1987, 74, 236–246. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Dupras, T.L.; Fairgrieve, S.I. 15N enrichment in the Sahara: In search of a global relationship. J. Archaeol. Sci. 1999, 26, 629–636. [Google Scholar] [CrossRef]
- Papathanasopoulos, G.A.; Goulandre, H.N.P.; Technes, M.K. Neolithic Culture in Greece; Goulandris Foundation: Athens, Greece, 1996. [Google Scholar]
- Vika, E.; Theodoropoulou, T. Re-investigating fish consumption in Greek antiquity: Results from δ13C and δ15N analysis from fish bone collagen. J. Archaeol. Sci. 2012, 39, 1618–1627. [Google Scholar] [CrossRef]
- Garvie-Lok, S.J. Loaves and Fishes: A Stable Isotope Reconstruction of Diet in Medieval Greece; University of Calgary: Alberta, Canada, 2001. [Google Scholar]
- Moundrea-Agrafioti, A. Mesolithic Fish Hooks from the Cave of Cyclope, Youra; British School at Athens Studies: Athina, Greece, 2003; pp. 131–141. [Google Scholar]
- Farrand, W.R. Depositional Environments and Site Formation During the Mesolithic Occupations of Franchthi Cave, Peloponnesos, Greece; British School at Athens Studies: Athina, Greece, 2003; pp. 69–78. [Google Scholar]
- Psomiadis, D.; Dotsika, E.; Albanakis, K.; Ghaleb, B.; Hillaire-Marcel, C. Speleothem record of climatic changes in the northern Aegean region (Greece) from the Bronze Age to the collapse of the Roman Empire. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 489, 272–283. [Google Scholar] [CrossRef]
- Geraga, M.; Ioakim, C.; Lykousis, V.; Tsaila-Monopolis, S.; Mylona, G. The high-resolution palaeoclimatic and palaeoceanographic history of the last 24,000 years in the central Aegean Sea, Greece. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 287, 101–115. [Google Scholar] [CrossRef]
- Frogley, M.R.; Griffiths, H.I.; Heaton, T.H. Historical biogeography and Late Quaternary environmental change of Lake Pamvotis, Ioannina (north-western Greece): Evidence from ostracods. J. Biogeogr. 2001, 28, 745–756. [Google Scholar] [CrossRef]
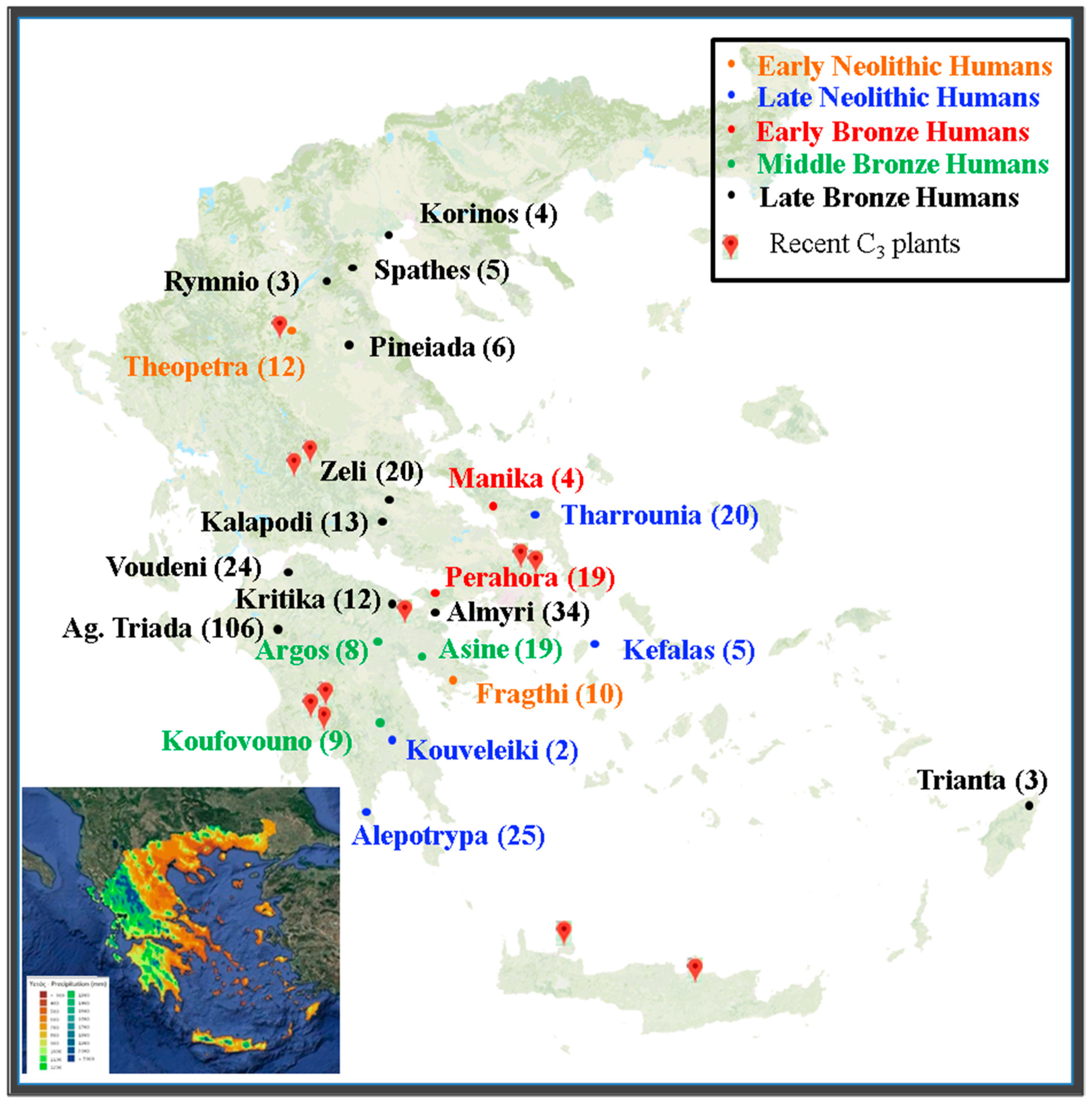
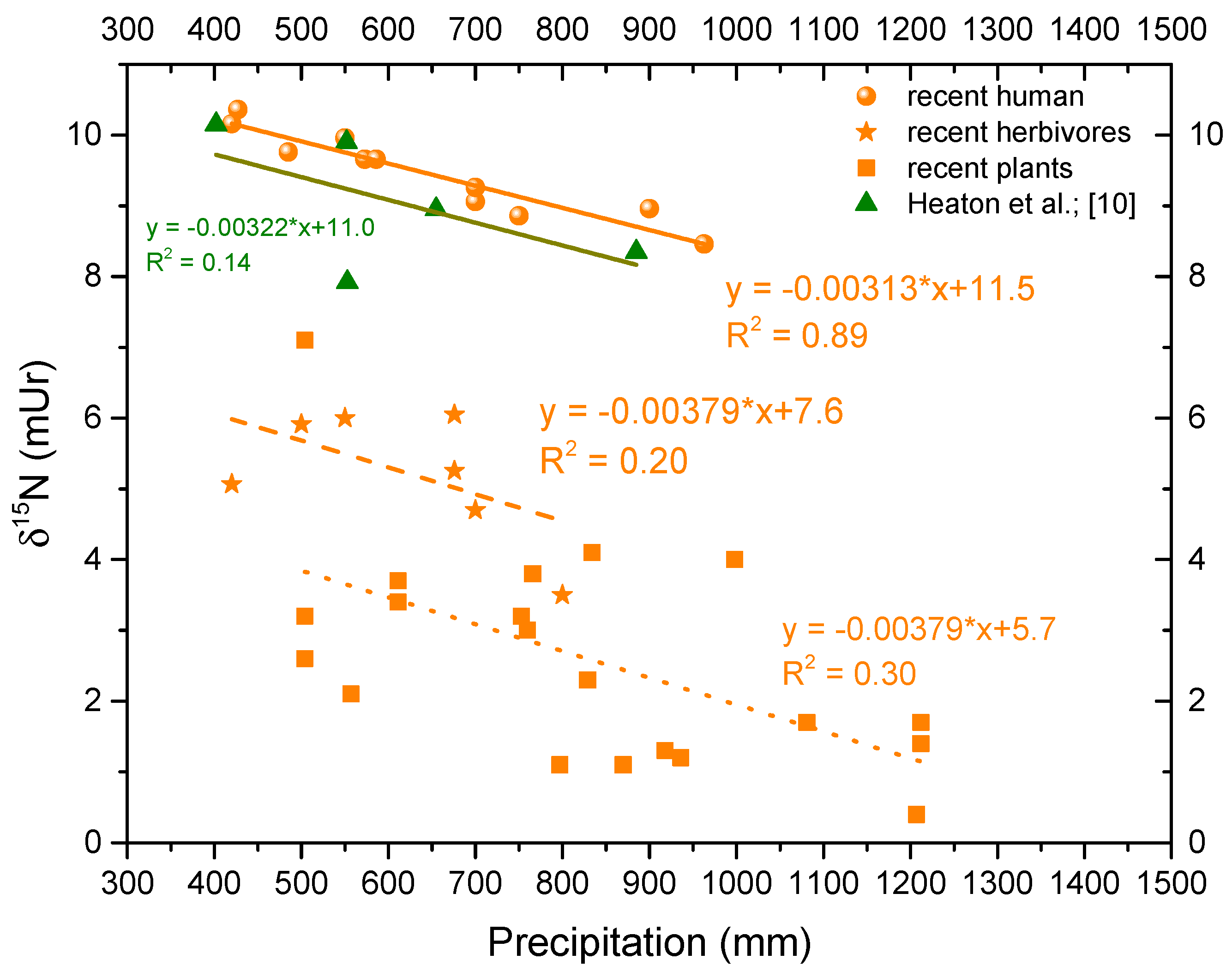

| Species | n | Location | Coordinates | Mean Annual Precipitation (mm) | δ13C | δ15N |
|---|---|---|---|---|---|---|
| Humans | ||||||
| 4 | Naxos-Damarionas | 37.05, 25.47 | 550 | −21.1 | 10.0 | |
| 2 | Imathia-Alexandria | 40.62, 22.44 | 485 | −20.3 | 9.8 | |
| 2 | Corinthia-Manna | 37.98, 22.51 | 963 | −21.4 | 8.5 | |
| 1 | Corinthia-Kamari | 38.09, 22.57 | 573 | −21.1 | 9.7 | |
| 1 | Xios | 38.38, 26.04 | 427 | −20.1 | 10.4 | |
| 4 | Messenia-Kopanaki | 37.28, 21.81 | 700 | −21.8 | 9.1 | |
| 1 | Messenia-Manesis | 37.08, 21.89 | 750 | −20.7 | 8.9 | |
| 1 | Messenia-Avramiou | 37.67, 21.46 | 700 | −20.5 | 9.3 | |
| 2 | Halkidiki-Polygyros | 40.37, 23.44 | 586 | −20.5 | 9.7 | |
| 1 | Aetolia-Acarnania Chrisovitsa | 38.57, 21.70 | 900 | −21.8 | 9.0 | |
| 1 | Attiki-Athens | 37.98, 23.73 | 420 | −21.2 | 10.2 | |
| Herbivores | ||||||
| Sheep, Ovis aries | 7 | Crete, Heraklion | 35.34, 25.14 | 500 | −20.6 | 5.9 |
| Sheep, Ovis aries | 5 | Sparta | 37.08, 22.43 | 700 | −23.5 | 4.7 |
| Sheep, Ovis aries | 6 | Chalkidiki | 40.51, 23.63 | 550 | −21.4 | 6.0 |
| Sheep, Ovis aries | 4 | Karditsa | 39.37, 21.93 | 676 | −20.7 | 6.1 |
| Cow, Bos taurus | 3 | Attiki | 37.92, 23.86 | 420 | −21.2 | 5.1 |
| Cow, Bos taurus | 7 | Karditsa | 39.37, 21.93 | 676 | −21.4 | 5.3 |
| Cow, Bos taurus | 3 | Sparta mountain | 37.08, 22.33 | 800 | −23.0 | 3.5 |
| C3 plants | ||||||
| Cercis | 6 | Parnitha | 38.13, 23.81 | 504 | −27.4 | 3.2 |
| Platanus orientalis | 7 | Parnitha | 38.13, 23.81 | 504 | −28.5 | 2.6 |
| Quercus sp. | 6 | Domnista | 38.58, 21.85 | 1207 | −28.4 | 0.4 |
| Olea europaea | 3 | Parnitha | 38.13, 23.81 | 504 | −26.2 | 7.1 |
| Juglans regia | 2 | Karpenisi | 38.92, 21.78 | 1212 | −28.2 | 1.7 |
| Corylus avellana | 2 | Karpenisi | 38.92, 21.78 | 1212 | −27.9 | 1.4 |
| Picea abies | 1 | Attiki | 37.98, 23.73 | 611 | −30.2 | 3.4 |
| Pinus Pinea | 1 | Attiki | 37.98, 23.73 | 611 | −27.1 | 3.7 |
| Olea europaea | 2 | Criti, Chania | 35.52, 24.02 | 557 | −28.3 | 2.1 |
| Olea europaea | 2 | Analipsi, Messinia | 37.02, 21.97 | 766 | −29.5 | 3.8 |
| Olea europaea | 1 | Vasilada, Messinia | 37.09, 21.94 | 936 | −30.1 | 1.2 |
| Olea europaea | 1 | Velika, Messinia | 37.01, 21.93 | 870 | −29.2 | 1.1 |
| Olea europaea | 1 | Diodia, Messinia | 37.08, 21.86 | 1081 | −28.9 | 1.7 |
| Olea europaea | 2 | Lykotrafos, Messinia | 37.05, 21.95 | 829 | −29 | 2.3 |
| Olea europaea | 1 | Madena, Messinia | 37.04, 21.96 | 797 | −29.4 | 1.1 |
| Olea europaea | 2 | Neochori, Messinia | 37.03, 21.92 | 918 | −29.6 | 1.3 |
| Olea europaea | 1 | Pilalistra, Messinia | 37.07, 21.97 | 834 | −28.6 | 4.1 |
| Olea europaea | 1 | Polylofos, Messinia | 37.09, 21.91 | 998 | −29.8 | 4 |
| Olea europaea | 3 | Avramio, Messinia | 37.03, 22.03 | 760 | −28.9 | 3 |
| Olea europaea | 1 | Messini, Messinia | 37.05, 22.01 | 753 | −29.6 | 3.2 |
| Site | Period | E (m) | P (mm) | δ15N | Δ P (mm) | Climatic Correction | Δ (δ15N) | Δ (δ15N) with Climatic Correction |
|---|---|---|---|---|---|---|---|---|
| Frachti | EN | 29 | 428 | 8.7 | −176 | −0.7 | 1.1 | 0.4 |
| Theopetra | EN | 236 | 604 | 7.6 | 0 | 0.0 | 0.0 | 0.0 |
| Kefalas | LN | 63 | 350 | 9.2 | −254 | −1.0 | 1.5 | 0.6 |
| Tharrounia | LN | 399 | 592 | 8.0 | −12 | 0.0 | 0.4 | 0.3 |
| Kouvelaki | LN | 350 | 660 | 8.1 | 56 | 0.2 | 0.4 | 0.6 |
| Alepotrypa | LN | 25 | 791 | 7.3 | 187 | 0.7 | −0.4 | 0.3 |
| Manika | EBA | 4 | 451 | 9.0 | −153 | −0.6 | 1.4 | 0.8 |
| Perachora | EBA | 255 | 460 | 9.0 | −144 | −0.5 | 1.4 | 0.9 |
| Asine | MBA | 110 | 411 | 9.5 | −193 | −0.7 | 1.8 | 1.1 |
| Argos | MBA | 49 | 486 | 9.1 | −118 | −0.4 | 1.5 | 1.0 |
| Koufovouno | MBA | 271 | 1091 | 7.9 | 487 | 1.8 | 0.2 | 2.1 |
| Korinos | LBA | 18 | 408 | 8.7 | −196 | −0.7 | 1.1 | 0.4 |
| Pineiada | LBA | 90 | 423 | 9.1 | −181 | −0.7 | 1.4 | 0.8 |
| Rymnio | LBA | 330 | 426 | 8.9 | −178 | −0.7 | 1.2 | 0.5 |
| Kritika | LBA | 118 | 444 | 9.3 | −160 | −0.6 | 1.7 | 1.1 |
| Almyri | LBA | 18 | 510 | 9.3 | −94 | −0.4 | 1.7 | 1.3 |
| Zeli | LBA | 179 | 573 | 8.4 | −31 | −0.1 | 0.8 | 0.7 |
| Kalapodi | LBA | 360 | 573 | 8.5 | −31 | −0.1 | 0.8 | 0.7 |
| Voudeni | LBA | 213 | 616 | 8.3 | 12 | 0.0 | 0.7 | 0.7 |
| Trianta | LBA | 14 | 640 | 8.0 | 36 | 0.1 | 0.4 | 0.5 |
| Spathes | LBA | 1080 | 733 | 7.6 | 129 | 0.5 | 0.0 | 0.5 |
| Ag Triada | LBA | 491 | 904 | 7.2 | 300 | 1.1 | −0.4 | 0.7 |
| Site | Period | δ15N | Number of Samples | % of Samples outside the ±0.4 δ Range |
|---|---|---|---|---|
| Frachti | EN | 8.7 | 10 | 70.0 |
| Theopetra | EN | 7.6 | 12 | 33.3 |
| Tharrounia | LN | 8.0 | 20 | 50.0 |
| Alepotrypa | LN | 7.3 | 25 | 60.0 |
| Perachora | EBA | 9.0 | 19 | 26.3 |
| Asine | MBA | 9.5 | 19 | 57.9 |
| Kritika | LBA | 9.3 | 12 | 25.0 |
| Almyri | LBA | 9.3 | 34 | 17.6 |
| Zeli | LBA | 8.4 | 20 | 40.0 |
| Kalapodi | LBA | 8.5 | 13 | 69.2 |
| Voudeni | LBA | 8.3 | 24 | 45.8 |
| Ag Triada | LBA | 7.2 | 106 | 32.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsika, E.; Diamantopoulos, G. Influence of Climate on Stable Nitrogen Isotopic Values of Contemporary Greek Samples: Implications for Isotopic Studies of Human Remains from Neolithic to Late Bronze Age Greece. Geosciences 2019, 9, 217. https://doi.org/10.3390/geosciences9050217
Dotsika E, Diamantopoulos G. Influence of Climate on Stable Nitrogen Isotopic Values of Contemporary Greek Samples: Implications for Isotopic Studies of Human Remains from Neolithic to Late Bronze Age Greece. Geosciences. 2019; 9(5):217. https://doi.org/10.3390/geosciences9050217
Chicago/Turabian StyleDotsika, Elissavet, and Georgios Diamantopoulos. 2019. "Influence of Climate on Stable Nitrogen Isotopic Values of Contemporary Greek Samples: Implications for Isotopic Studies of Human Remains from Neolithic to Late Bronze Age Greece" Geosciences 9, no. 5: 217. https://doi.org/10.3390/geosciences9050217
APA StyleDotsika, E., & Diamantopoulos, G. (2019). Influence of Climate on Stable Nitrogen Isotopic Values of Contemporary Greek Samples: Implications for Isotopic Studies of Human Remains from Neolithic to Late Bronze Age Greece. Geosciences, 9(5), 217. https://doi.org/10.3390/geosciences9050217





