Abstract
We review the stable isotopic data of recovered Greek bones from the Early Neolithic to the Late Bronze period in order to examine dietary changes over time. As an isotopic baseline we use the published fauna data of the periods. The analysis revealed a diet that included a significant proportion of foods based on C3 plants, and the bulk of the animal protein must have been provided by terrestrial mammals with a small but detectable proportion of marine protein for coastal and island populations. A more significant contribution of marine protein is observed for Bronze Age populations while the enrichment in both C and N isotopes is connected, for some areas, to the introduction of millet during the Bronze Age, and to freshwater consumption. An extensive database of Greek food sources is presented and compared to the fauna from the prehistoric periods (Early Neolithic to Late Bronze Age) of the literature. We propose that this database can be used in palaeodiet reconstruction studies.
Keywords:
stable isotopes; palaeodiet; carbon; nitrogen; bone collagen; Bronze Age; Neolithic; Ancient Greece 1. Introduction
Stable isotopic analysis has been used to reconstruct the diet of past populations. The stable isotopic values 13C and 15N are used: to identify the introduction and spread of maize agriculture in the Americas [1] and millet cultivation in eastern Europe [2]; to distinguish between marine and terrestrial nutriments in the diet [3,4,5,6,7]; to recognize the proportion of legumes vs. non-legumes alimentation [8]; to distinguish between nitrogen-fixing and non-fixing plants; to examine dietary differences between contemporaneous populations [9]; to determine dietary differences (e.g. age, sex, and status differences) within populations [10,11,12,13,14,15]; to investigate infant feeding practices [16,17,18], and to observe dietary changes over time [19,20,21].
Although human remains are abundant, especially in Greece where there are numerous Holocene archaeological sites, very little is known regarding where and how people lived and what constituted their diet and habits.
The objective of this study is to reconstruct the diet of the populations living in Greece from the Early Neolithic to the Late Bronze period in order to examine a) the relative contribution of marine vs. terrestrial nutriments in the diet and b) explore possible chronological variations in the diet. To achieve the objectives of this study, we review published stable isotopic results of Greek human bones from the Early Neolithic to the Late Bronze period in relation to fauna data of the same periods as food sources. Measurements of 15N and 13C in recent Greek human samples (hair) and measurements of contemporary Greek food sources are also presented. These contemporary food sources are compared with the available fauna data of the studied periods in order to examine if they can be used as baseline indicators. This is especially important for marine food sources, where very few samples from the above periods are available.
1.1. Background on Stable Isotopes
The collagen of fossil bone and dentine should preserve C and N isotopic signatures, reflecting the dietary preferences of the individual. In particular, a stable carbon and nitrogen isotope ratio of bone and tooth collagen provides direct evidence of diet, and reflects the average isotopic composition of an individual’s intake of dietary protein as well as other nutrients [7,8,22] over a period of 10 years or more [23].
The stable carbon isotope analysis of collagen has been an effective tool for reconstructing the vegetable dietary patterns since the plants that follow different photosynthetic pathways exhibit different δ13C values. The C3 plants (temperate region plants, some subtropical grasses, including wheat, rice, barley, all trees, shrubs, nuts and fruits) have δ13C values ranging from −20‰ to −35‰ [24]. Due to fractionation, the δ13C value of the consumer’s bones collagen is approximately 5‰ [25,26] higher than that of their diet, and populations that consume only C3 plants have δ13C values from −20‰ to −21‰ [3,5]. The C4 plants (tropical grasses, maize, sugar cane, millet, sorghum, some amaranths and some chenopods) have δ13C values between −9‰ to −14‰ [24] and populations with diet rich in C4 plants have δ13C values as high as −10‰ [27]. Furthermore, the isotopic composition of bone collagen is mainly controlled by protein consumption, while the bone apatite reflects the whole carbon isotope ratios. A combination of collagen and apatite 13C measurements may introduce additional information [22] for the reconstruction of dietary habits, especially in mixed diets, as the consumption of fat from large marine animals and the consumption of carbohydrates from underground plants [26,28,29]. The isotopic preservation of bone apatite remains a serious concern but careful screening of the bone samples for alteration should reveal situations where bones retain useful information [30]. Another possible factor that must be considered when analyzing the isotopic values of 13C and 15N of bone collagen is the case when dietary protein is insufficient for tissue building and collagen amino acids might partially originate from other sources, such as carbohydrates [31,32]. That might be the case in regions of the Mediterranean where the reported high human δ15N values indicate consumption of marine foods, yet the low δ13C values indicate a terrestrial diet [31,32,33,34]. A possible explanation is that while nitrogen from marine foods was adequate for amino acid synthesis, marine carbon was insufficient to synthesize all the necessary amino acids in collagen, causing δ13C to be supplied from low 13C terrestrial sources (lipids and carbohydrates) [29].
Dietary reconstruction using stable nitrogen isotope analysis of bone collagen reproduces the trophic level, or the position of an individual in the food chain. So, the δ15N values of herbivores are approximately 3‰ higher than the plants they consume [4,35] while carnivores have δ15N values that are approximately 3‰ higher than the herbivores that they consume [4,35].
Carbon and nitrogen isotope ratios can also be used to investigate the proportion of terrestrial and marine nutriments in environments since marine animals and fish are systematically enriched by about 7‰ with respect to terrestrial consumers [5]. Most marine carnivores (fish, seals) have δ15N values greater than 12‰, while terrestrial plants and animals have values ranging from 0‰ to 10‰ [36]. Freshwater animals have more positive δ15N values than their terrestrial counterparts, whereas their δ13C values are relatively similar, and freshwater resources present in small rivers are readily distinguishable from fully terrestrial resources [12]. The δ15N value of human bone collagen is consequently about 3‰ higher than the δ15N value of the protein that the human has consumed [4,35,37]. Humans who obtain the majority of their dietary protein from marine species have δ15N values ranging from about 12‰ to 22‰ [38,39] while those who consume only terrestrial protein sources have δ15N between 5‰ and 12‰ [39].
In coastal areas where populations may have been consuming both marine resources and C4 plants [20,40], stable nitrogen and carbon isotope analysis is fundamental in order to distinguish between dietary proteins derived from marine resources vs. terrestrial foods [38]. Stable nitrogen isotope analysis can also be used to identify vegetable vs. non−vegetable eating, as vegetables have lower δ15N approaching 0‰. Climate may also affect the δ13C and δ15N values of terrestrial animals through its effect on the δ13C of plants [41,42] and on the δ15N of soil [43,44,45], at the base of the food chain.
1.2. Background on Ancient Greek Diet
Dietary habitudes of the Greek population were based on palaeobotanical and zooarchaeological analyses. Palaeobotanical study has been conducted to examine legume consumption [46] and the identification of plant phytoliths in order to distinguish between olive oil and wine [47]. In contrast zooarchaeological studies indicate that sheep and goats were the main source of meat in the Mediterranean region [48].
An indirect but important source of information on the diet of the ancient Greeks is the archaeological evidence such as plant remains, animal bones, food preparation utensils, and storage vessels. Food and drink offerings are also another source of information on the diet of the ancient Greeks. In the Neolithic period, the main source of nutrition was cereals, most notably wheat, fresh fruits, nuts and meat [18]. This is presumed to be so, because this period is characterized by the appearance of organized agricultural groups and the beginning of the domestication of plants and animals. The selection of food in the Neolithic settlements is connected to factors such as climate, geographical location, the cultural background and the local resources and landscape. So, it is assumed that Greek Neolithic populations’ diet was primarily terrestrial, based on C3 plants (wheat, barley, legumes). Meat and dairy products (from domestic animals) as well as wild resources (ex. Cervus elaphus, Sus scrofa, [49,50,51,52]) were, also incorporated into the diet, but to a lesser extent. This in accordance with the zooarchaeological observations, which also indicate that livestock was subsidiary to crop growing [53,54]. Furthermore, only occasional or periodic exploitation of near-shore marine or freshwater protein resources is assumed.
During the Bronze period the diet became more variable with the introduction of more cereal crops, wheat, barley and millet that constituted the primary source of protein. Also in this period vegetables, fish and meat are becoming more easily available and milk, wine, olive, are introduced in their alimentation [18]. The main source of meat in the Mediterranean region was sheep and goats although it is unknown to what extent these animals were also used for their milk. Meat was considered a luxury and was eaten only infrequently. Wild and domesticated birds were consumed only occasionally [55] although eggs may have been more commonly consumed. Fish were eaten less frequently than other foods [56]. According to literary sources significant dietary differences between the upper and lower classes and between males and females existed among the Greeks [57].
In Table 1 we present the key conclusions on the diet for all the sites according to the bibliography.

Table 1.
Key conclusions for the sites reviewed and relevant bibliography (E. Neolithic: Early Neolithic, L. Neolithic: Late Neolithic, EBA: Early Bronze Age, MBA: Middle Bronze Age, LBA: Late Bronze Age).
2. Materials
A total of 363 human bone samples from 22 archaeological sites across Greece were examined in this study obtained from the literature [17,49,50,51,58,59,60,61,62,63,64,65,66,67]. Figure 1 shows the spatial distribution of the sites and the number of samples for each one of them (solid circles). Apart from the human bones, some data on faunal samples were also available from the same literature sources and are indicated in Figure 1 (open circles).
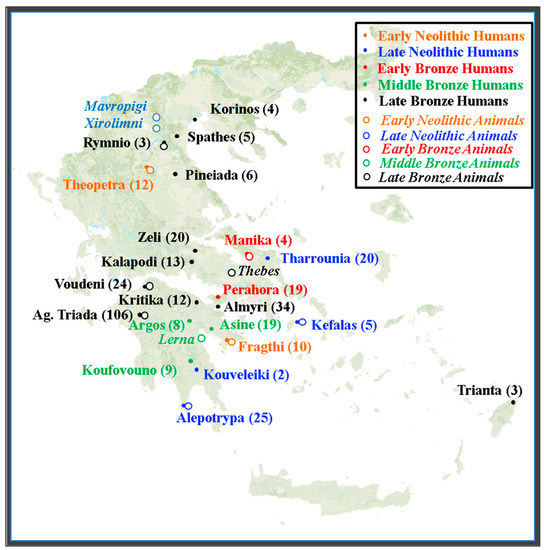
Figure 1.
Spatial distribution of human and fauna sites. Solid circles and the sites names in regular are human sites while hollow circles and the sites names in italics are fauna sites of the same or nearby areas of the respective time periods. The numbers in parenthesis denotes the number of human samples in each site.
Analysis of 23 hairs samples (δ13C and δ15N) from the recent Greek population were conducted in the Stable Isotope Unit of NCSR “Demokritos”, Greece (Table 2). These hair samples are from a selected population (elderly people of both genders, having not traveled for at least five years prior to the sampling, constantly consuming local products as diet source and drinking only tap water).

Table 2.
Isotopic values of contemporary humans from Greece.
Collagen samples from herbivore (n = 54), carnivore (n = 8), and flesh samples from marine low trophic level (n = 10), marine high trophic level (n = 22), freshwater fish (n = 8), birds (n = 5) and plant samples of C3 (n = 124) and C4 (n = 13), were studied isotopicaly (δ13C and δ15N) from Greek sources in the Stable Isotope Unit of NCSR “Demokritos” (Table 3 and Table 4). The classification of marine samples to low/high trophic level was conducted according to Stergiou and Karpouzi (2002) [68].

Table 3.
Isotopic values of terrestrial and marine animals from Greece.

Table 4.
Isotopic values of Greek plants.
3. Methods
For the contemporary Greek human samples, hairs were clipped from each subject, rinsed twice in distilled water for about 20 min each time. These samples were then dried overnight at 65 °C and ground to a fine powder (to be homogenized) before analysis.
The contemporary plant samples (C3 (n = 124) and C4 (n = 13)) were ground to a fine homogeneous powder (<250 μm size) under liquid nitrogen.
The contemporary fauna samples (marine low trophic level (n = 10), marine high trophic level (n = 2), freshwater fish (n = 8) and birds (n = 5)) muscle tissue was taken from each specimen and immediately frozen.
For the Herbivore (n = 54) and Carnivore (n = 8) samples, extraction of collagen from bone was based on those of Ambrose (1990) [69], which can be summarized as follows (see also Tykot (2004) [70]). Solid bone samples were first placed in 0.1 M NaOH to remove contaminants, followed by demineralization with 2% HCl, a second treatment with 0.1 M NaOH, and finally a 2:1:0.8 defatting mixture of CH3OH, CHCl3, and water. The dried and weighed samples were then analyzed with a FlashEA/IRMS for δ13C and δ15N.
The isotopic ratios [(R = 15N/14N or 13C/12C reported as δ15N or δ13C, where δ = ((Rsample − Rstandard)/Rstandard) × 1000] were measured versus atmospheric N2(AIR) and PDB (a marine carbonate) for nitrogen and carbon respectively. The reported values are the means of two or more consistent measurements of each sample. The standard deviation of the measurements ranges on average between ±0.1 and ±0.2‰ (2σ), for both 15N and 13C isotopes.
Collagen yields over 1 wt% are considered acceptable for carbon and nitrogen values [71], while the C:N ratio should range between 2.9 and 3.6 [72]. All our collagen samples were within these ranges as indicated in Table 2.
In order to compare contemporary 13C values of herbivores, carnivores and humans with the literature values of the Neolithic to Late Bronze age we applied a Suess effect correction [73] (we subtracted 1.5‰ from the values of Table 2 and Figure 2e). The Suess effect refers to the change in the ratios of carbon isotopes 13 C:12 C caused by the release of carbon (in the form of CO2) from the burning of fossil−fuels (burning fuels produces carbon dioxide, whose carbon consists almost entirely of the 12C isotope, and thus dilutes the ratios in all carbon reservoirs) and from land clearing (anthropogenic activities).
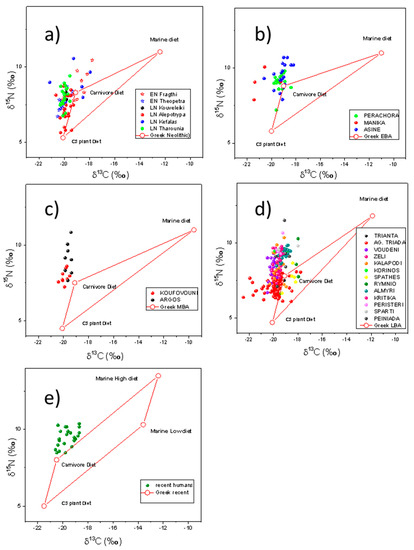
Figure 2.
(a–d) δ15N and δ13C values of human bone collagen as published in the literature covering the Neolithic (EN: Early Neolithic, LN: Late Neolithic), Early Bronze, Middle Bronze and Late Bronze age, respectively. (e) δ15N and δ13C values of human hair samples of contemporary Greek population. The red rectangles indicate the theoretical isotopic values of human bone of a subject under 100% herbivore (lower left), 100% carnivore (upper left) and 100% marine diet (right low/high for low marine and high marine respectively). The values were deducted from fauna of the respectively periods and archaeological sites, as published in the literature and for (e) from measurements of contemporary Greek food sources (Table 3 and Table 4).
In order to compare the contemporary marine flesh samples with the ancient fish bone samples of the studied periods a mean fractionation value (4‰) [26] was added to the flesh 13C values of Table 3 to reflect the expected bone values.
In order to compare the contemporary human hair samples with the human bone samples of the studied periods a fractionation to 13C and 15N equal to 1.41‰ and 0.86‰ was added to the hair isotopic values respectively [74], to reflect the expected bone values.
In this study, we considered four diet sources, i.e., marine high, marine low, terrestrial plants and terrestrial animals and two stable isotope systems (δ13C and δ15N).
4. Results and Discussion
In Table 2 we present the isotopic ratios (δ13C and δ15N) of contemporary Greek humans (hair samples). In Table 3 we present the isotopic ratios (δ13C and δ15N) of contemporary animals from Greece (bones samples for the terrestrial animals and flesh samples for the marine animals). In Table 4 we present the isotopic ratios (δ13C and δ15N) of Greek plants (C3 and C4).
Palaeodiet
The δ13C and δ15N values of human bone collagen as published in the literature covering the Early Neolithic, Late Neolithic, Early Bronze, Middle Bronze and Late Bronze period are presented in Figure 2. In the same Figure 2e we also present δ15N and δ13C values of human hair from contemporary Greek population (Table 2). These hair ceratin values were converted to bone collagen values according to O’ Connell (2001) [74]. The red rectangles in all cases represent the isotopic values for human bones consuming extreme diets (marine high, marine low, herbivore, carnivore) as indicated in Richards and Hedges 1999 [75]. Specifically, for Figure 2a–d the values are deducted from fauna samples from the archaeological sites [17,49,50,51,59,61,63,67] for Early/Late Neolithic, Early Bronze, Middle Bronze and Late Bronze periods, respectively. For Figure 2e the red rectangle are values as deducted from Table 3 and Table 4. The values used in Figure 2 for the red rectangles are summarized in Table 5 where all the conversions are indicated analytically. The “C3 plant diet” is the mean value of all herbivore isotopic data of each period, the “carnivore diet” is the value of the mean herbivore adjusted by one trophic level for each period and the “marine diet” is the value of the mean marine isotopic data of each period adjusted by one trophic level. For Figure 2e the “low/high marine diet” are the mean values of the low/high marine isotopic data of Table 3, after converted to bone and after adjusted by one trophic level.

Table 5.
Theoretical Isotopic values of human bones under extreme diets.
Human isotope values of δ13C range for Early Neolithic from −20.5‰ to −17.8‰ (Figure 2a). For Late Neolithic from −20.7‰ to −17.9‰ (Figure 2a), for EBA (Figure 2b) from −21.4‰ to −18.4‰, for MBA (Figure 2c) from −20.6‰ to −18.4‰, for LBA (Figure 2d) from −21.7‰ to −17.8‰ and for the recent Greek population (Figure 2e), after applying the conversion from ceratin hair to bone collagen [74], from −20.4‰ to −18.6‰. The δ15N human isotope values range from 6.7‰ to 10.4‰ (Early Neolithic), from 5.6‰ to 10.6‰ (Late Neolithic), 7.2‰ to 10.0‰ (EBA), 7.2‰ to 10.8‰ (MBA), 6.1‰ to 11.5‰ (LBA) and 8.5‰ to 10.4‰ (Recent).
According to the results presented in Figure 2, the diet of the residents of ancient Greece is dominated by terrestrial plant foods and meat with the addition of variable minor amounts of fish. Furthermore, we can observe a possible contribution of marine protein in Neolithic and Late Bronze age, in specific sites (Fracthi and Kefalas for Neolithic and Almyri, Kritika, Pineiada, and Rymnio for Late Bronze age), as their isotopic values are located to the right upper side of the red rectangle in Figure 2a,d respectively. For the EBA and MBA the marine protein consumption is not likely or inconclusive (for the EBA−Asine site [63]). Another general observation from Figure 2 is that the vast majority of the human isotopic values are outside the red rectangles, especially for the EBA (Figure 2b). This is a strong indication that the model of the four extreme diet sources [75] is not complex enough in order to accurately explain the isotopic values of the human samples. This is especially evident in the case of recent humans (2e) where the red rectangle was derived from contemporary Greek food sources. In Figure 3 we present the mean values of the human collagen bones for all the periods (the values for the recent human samples were corrected by adding 1.5‰ in the 13C value in order to account the Suess effect) along with all the archaeological fauna samples of the periods, (Figure 3a) as extracted from the literature and all recent Greek food sources (Figure 3b). All the values of the terrestrial Greek food sources were corrected to the Suess effect and the flesh samples were converted to bone collagen (see methodology section). The scales of Figure 3a,b are kept the same to facilitate comparison. There are not major variations in the absolute values of foods in Figure 3a,b indicating that both can be used as food database for palaeodiet reconstructions. Thus, if we include more food sources (like freshwater fish and birds) the interpretation of the isotopic values of Figure 2 (data from literature) or Figure 3b (mean values of the literature data) is more pleasing.
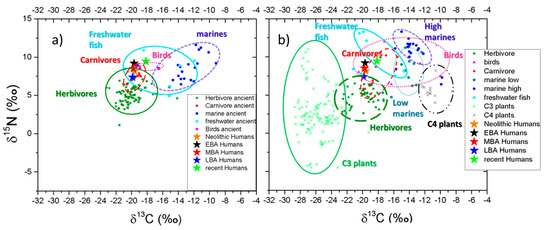
The human collagen samples are, in general, enriched in both 13C and 15N in comparison to the herbivores for all studied periods (Figure 3a). The mean human δ13C (−19.4‰ for Early Neolithic, −19.7‰ for Late Neolithic, −19.7‰ for EBA, −19.8‰ for MBA and −19.9‰ for LBA) are higher than the mean faunal herbivores values by 0.9‰ for Early Neolithic, 0.4‰ for Late Neolithic, 0.3‰ for EBA, 0.4‰ for MBA and 0.2‰ for LBA). The mean human δ15N (8.2‰ for Early Neolithic, 8.1‰ for Late Neolithic, 9.2‰ for EBA, 8.5‰ for MBA and 7.4‰ for LBA) are higher than the mean herbivores fauna values by 2.9‰ for Early Neolithic, 2.8‰ for Late Neolithic 3.4‰ for EBA, 4.0‰ for MBA and 2.7‰ for LBA. This is consistent with a trophic level and demonstrates the importance of terrestrial meat sources in the diet while one must retain in mind the possible influences to these values by the consumption of dairy products or manuring practices [76].
Humans living in the Greek mainland during the Neolithic are slightly enriched in δ13C over human Neolithic bones from the continental Europe (Bocherens et al., 2007] [76], which are usually between −20‰ and −21‰. This difference in δ13C values is attributable to both climatic variations and diet. Studies of wood, charcoal, and bone samples from European archaeological sites have shown that δ13C tends to become enriched in a north to south direction, following the climatic/temperature gradient toward the Mediterranean [77]. The δ15N values of the Neolithic Greek human bones are in general lower than the values of human Neolithic bones from the continental Europe [76] which are usually between 9.0‰ and 10.4‰ [76]. Only the δ15N bones found at coastal and island Neolithic sites (Fragthi and Kefala) have values similar to the ones of the continental Europe. This suggests that these humans were obtaining a detectable amount [67,78] of their dietary protein from marine sources (more than 20% in Fragthi and Kefala) and these fish consumption depends on the location of the individual.
Previous isotopic analyses of European coastal populations [79,80] have revealed a linear correlation between δ15N and δ 13C values within an area defined by terrestrial and marine (δ 13C, δ15N) values suggesting that individuals were eating varying proportions of terrestrial and marine foods [79]. In Figure 4 we present the isotopic values of human bones collagen from the referred archaeological sites versus the fauna baselines of the sites both terrestrial and marine (rectangles T and M respectively). The ranges of the fauna baselines are given in Table 6.
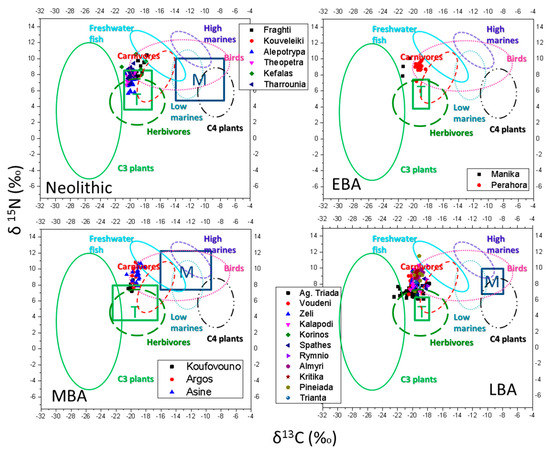
Figure 4.
Isotopic values of human bones collagen from the referred archaeological sites versus the fauna baselines of the periods, both terrestrial and marine (rectangles T and M respectively). The circles of the food database of Figure 3b are also presented.

Table 6.
Ranges of the archeological fauna for the studied periods as reported in the literature.
In Figure 4 we also overlap the food database (circles) of Figure 3b. In the MBA period of Figure 4 we can see that the rectangles (T and M) have almost the same boundaries with the corresponding circles of the food database (the herbivore and low/high marine circles). Contrary, in the LBA period of Figure 4, the rectangles (T and M) have considerably shorter boundaries than the corresponding food database circles as well as to the rectangles of the other periods (MBA and Neolithic). This may relate to the lack of adequate number of ancient fauna samples of the period (marine samples) and could lead to erroneous interpretations of the palaeodiet. By considering the food database circles (especially the extent of the herbivore circle) a more accurate interpretation may be visualized. For the EBA period there is no ancient marine sample available (no M rectangle in Figure 4) to establish the isotopic marine baseline and we have to solely rely on the food database circles in order to determine possible contribution of marine protein in the diet for that period.
The data from the Neolithic sites of Fragthi and Kefala (Figure 4) are consistent with the consumption of at least some marine protein challenging the general idea that during the Neolithic there was no signal of marine sources in the human diet [49,50,51] and agrees with the finding of modest numbers of fish in the deposits of Neolithic settlements of N. Greece [49,50,51].
In the rest of the Neolithic sites the isotopic analysis of human remains revealed a diet that included a significant proportion of foods based on C3 plants and the bulk of the dietary protein must have been provided by terrestrial mammals, either hunted wild mammals or husbanded domestic mammals [18]. The lower δ15N which was found in some human bones (Alepotrypa) suggest consumption of legumes that have extremely low δ15N [18].
A detectable [67,78] (≥20%) contribution of marine diet is also observed for the LBA sites of Almyri, Pineiada, Rymnio and Kritika (their mean δ15N are 9.3‰, 9.1‰, 8.9‰ and 9.3‰, respectively). It is not possible to determine the marine contribution with less than ±10 % accuracy. For example, if we assume a mean δ15N value equal to 8‰ for terrestrial diet and 11‰ for marine diet, the mass equation for the cases of (i.) 80% terrestrial + 20% marine diet (ii.) 70% terrestrial + 30% marine diet and (iii.) 60% terrestrial + 40% marine diet, yield δ15N values equal to 8.6‰, 8.9‰ and 9.2‰ respectively. These values are close to the reported mean values for the above four regions but seem unrealistic, underlying that the model of the four basic food sources (C3 plants, herbivores, marine high/low) [75] is not complex enough to explain the human diet. In addition, even though it is easy to justify the contribution of marine foods in the population of Almyri and Kritika that are coastal or near coastal areas, it is difficult to imagine fish consumption in Rymnio and Pinaiada, since these are inland sites. For these two areas, the enrichment in N isotope may be connected to consumption of dairy products or birds or even foraging of nearby large rivers (e.g., river Aliakmon, near Rymnio) in search of freshwater animals that have more positive δ15N values than their terrestrial counterparts.
For the EBA sites (Manika and Perachora) our analysis does not detect marine protein contrary to the bibliography that suggests a mixed diet of C3 with marine resources/few fish.
For the MBA sites, while our analysis agrees with the literature findings for Koufovouno (C3 consumption with legumes), for the sites of Argos and especially Asine, the fish contribution to diet seems detectable.
5. Conclusions
The results of the Neolithic populations revealed a diet that included a significant proportion of foods based on C3 plants and the bulk of the dietary protein must have been provided by terrestrial mammals with a small but detectable proportion of marine protein for coastal (Fragthi—Argolida) and Island (Kefala—Kea) populations. A detectable contribution of marine diet is observed in Almyri, Perahora, Rymnio and Kritika (Late Bronze age) populations. Further, the enrichment in both N isotopes maybe connected, for some areas, to freshwater consumption. In the rest of the sites the isotopic analysis of human remains revealed a diet that included a significant proportion of foods based on C3 plants and the bulk of the dietary protein must have been provided by terrestrial mammals. The isotopic values of the MBA sites of Argos and Asine seem to justify a detectable consumption of fish.
A database consisting of foods from the general territory of Greece was presented. The isotopic values of this database were compared to the available fauna bone collagen data from the archaeological sites reviewed in this study. No significant differences are observed and this database, in our opinion, may be used as a baseline for palaeodiet studies for the Neolithic and Bronze Age in Greece.
Author Contributions
Conceptualization, E.D. and G.D.; Data curation, P.K.; Formal analysis, E.D., G.D., S.L. and P.K.; Funding acquisition, E.D.; Methodology, E.D., G.D., S.L., S.G., E.K. and E.P.; Resources, E.K., D.M. and E.S.; Writing—original draft, E.D.; Writing—review & editing, G.D.
Funding
This research received no external funding.
Acknowledgments
We wish to express our gratitude to the 23 individuals that donated hair samples and gave their consent to use these samples for this study.
Conflicts of Interest
The authors declare no conflict of interest
References
- Katzenberg, M.A.; Schwarcz, H.P.; Knyf, M.; Melbye, F.J. Stable isotope evidence for maize horticulture and paleodiet in southern Ontario, Canada. Am. Antiq. 1995, 60, 335–350. [Google Scholar] [CrossRef]
- Murray, M.L.; Schoeninger, M.J. Diet, status, and complex social structure in Iron Age Central Europe: Some contributions of bone chemistry. In Tribe and Polity in Late Prehistoric Europe; Springer: Berlin/Heidelberg, Germany, 1988; pp. 155–176. [Google Scholar]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Chisholm, B.S.; Nelson, D.E.; Schwarcz, H.P. Stable-carbon isotope ratios as a measure of marine versus terrestrial protein in ancient diets. Science 1982, 216, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.L.; DeNiro, M.J. Stable nitrogen and carbon isotope ratios in bone collagen as indices of prehistoric dietary dependence on marine and terrestrial resources in southern California. Am. J. Phys. Anthropol. 1986, 71, 51–61. [Google Scholar] [CrossRef]
- Schwarcz, H.P. Some biochemical aspects of carbon isotopic paleodiet studies. In Biogeochemical Approaches to Paleodietary Analysis; Springer: Berlin/Heidelberg, Germany, 2002; pp. 189–209. [Google Scholar]
- DeNiro, M.J. Stable isotopy and archaeology. Am. Sci. 1987, 75, 182–191. [Google Scholar]
- White, C.D.; Healy, P.F.; Schwarcz, H.P. Intensive agriculture, social status, and Maya diet at Pacbitun, Belize. J. Anthropol. Res. 1993, 49, 347–375. [Google Scholar] [CrossRef]
- Katzenberg, M.A.; Saunders, S.R.; Fitzgerald, W.R. Age differences in stable carbon and nitrogen isotope ratios in a population of prehistoric maize horticulturists. Am. J. Phys. Anthropol. 1993, 90, 267–281. [Google Scholar] [CrossRef]
- Privat, K.L.; O’connell, T.C.; Richards, M.P. Stable isotope analysis of human and faunal remains from the Anglo-Saxon cemetery at Berinsfield, Oxfordshire: Dietary and social implications. J. Archaeol. Sci. 2002, 29, 779–790. [Google Scholar] [CrossRef]
- Richards, M.; Hedges, R.E.; Molleson, T.; Vogel, J. Stable isotope analysis reveals variations in human diet at the Poundbury Camp cemetery site. J. Archaeol. Sci. 1998, 25, 1247–1252. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; Katzenberg, M.A.; Doyon, L.G. Status and diet in precontact highland Ecuador. Am. J. Phys. Anthropol. 1995, 97, 403–411. [Google Scholar]
- Dotsika, E.; Michael, D.E. Using stable isotope technique in order to assess the dietary habits of a Roman population in Greece. J. Archaeol. Sci. Rep. 2018, 22, 470–481. [Google Scholar]
- Dotsika, E.; Michael, D.E.; Iliadis, E.; Karalis, P.; Diamantopoulos, G. Isotopic reconstruction of diet in Medieval Thebes (Greece). J. Archaeol. Sci. Rep. 2018, 22, 482–491. [Google Scholar] [CrossRef]
- Herring, D.; Saunders, S.R.; Katzenberg, M.A. Investigating the weaning process in past populations. Am. J. Phys. Anthropol. 1998, 105, 425–439. [Google Scholar] [CrossRef]
- Petroutsa, E.I.; Manolis, S.K. Reconstructing Late Bronze Age diet in mainland Greece using stable isotope analysis. J. Archaeol. Sci. 2010, 37, 614–620. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Richards, M.P.; Fox, S.C. Archaeodiet in the Greek World: Dietary Reconstruction from Stable Isotope Analysis; American School of Classical Studies: Athens, Greece, 2015; Volume 49. [Google Scholar]
- Katzenberg, M.A.; Weber, A. Stable isotope ecology and palaeodiet in the Lake Baikal region of Siberia. J. Archaeol. Sci. 1999, 26, 651–659. [Google Scholar]
- Larsen, C.S.; Schoeninger, M.J.; Van der Merwe, N.J.; Moore, K.M.; Lee-Thorp, J.A. Carbon and nitrogen stable isotopic signatures of human dietary change in the Georgia Bight. Am. J. Phys. Anthropol. 1992, 89, 197–214. [Google Scholar] [CrossRef]
- Lillie, M.C.; Richards, M. Stable isotope analysis and dental evidence of diet at the Mesolithic–Neolithic transition in Ukraine. J. Archaeol. Sci. 2000, 27, 965–972. [Google Scholar] [CrossRef]
- Krueger, H.W.; Sullivan, C.H. Models for Carbon Isotope Fractionation between Diet and Bone; ACS Publications: Washington, DC, USA, 1984. [Google Scholar]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef]
- Smith, B.N.; Epstein, S. Two categories of 13C/12C ratios for higher plants. Plant Physiol. 1971, 47, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, N.J. Carbon Isotopes, Photosynthesis, and Archaeology: Different pathways of photosynthesis cause characteristic changes in carbon isotope ratios that make possible the study of prehistoric human diets. Am. Sci. 1982, 70, 596–606. [Google Scholar]
- Lee-Thorp, J.A.; Sealy, J.C.; Van Der Merwe, N.J. Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. J. Archaeol. Sci. 1989, 16, 585–599. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Melbye, J.; Katzenberg, M.A.; Knyf, M. Stable isotopes in human skeletons of southern Ontario: Reconstructing palaeodiet. J. Archaeol. Sci. 1985, 12, 187–206. [Google Scholar] [CrossRef]
- Ambrose, S.H.; Norr, L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In Prehistoric Human Bone; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–37. [Google Scholar]
- Reitsema, L.J. Beyond diet reconstruction: Stable isotope applications to human physiology, health, and nutrition. Am. J. Hum. Biol. 2013, 25, 445–456. [Google Scholar]
- Clementz, M.T.; Fox-Dobbs, K.; Wheatley, P.V.; Koch, P.L.; Doak, D.F. Revisiting old bones: Coupled carbon isotope analysis of bioapatite and collagen as an ecological and palaeoecological tool. Geol. J. 2009, 44, 605–620. [Google Scholar] [CrossRef]
- Keenleyside, A.; Schwarcz, H.; Panayotova, K. Stable isotopic evidence of diet in a Greek colonial population from the Black Sea. J. Archaeol. Sci. 2006, 33, 1205–1215. [Google Scholar]
- Prowse, T.; Schwarcz, H.P.; Saunders, S.; Macchiarelli, R.; Bondioli, L. Isotopic paleodiet studies of skeletons from the Imperial Roman-age cemetery of Isola Sacra, Rome, Italy. J. Archaeol. Sci. 2004, 31, 259–272. [Google Scholar] [CrossRef]
- Craig, O.E.; Biazzo, M.; O’Connell, T.C.; Garnsey, P.; Martinez-Labarga, C.; Lelli, R.; Salvadei, L.; Tartaglia, G.; Nava, A.; Renò, L. Stable isotopic evidence for diet at the Imperial Roman coastal site of Velia (1st and 2nd Centuries AD) in Southern Italy. Am. J. Phys. Anthropol. 2009, 139, 572–583. [Google Scholar]
- Prowse, T.L.; Schwarcz, H.P.; Saunders, S.R.; Macchiarelli, R.; Bondioli, L. Isotopic evidence for age-related variation in diet from Isola Sacra, Italy. Am. J. Phys. Anthropol. 2005, 128, 2–13. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Schoeninger, M.J. Stable isotope analyses in human nutritional ecology. Am. J. Phys. Anthropol. 1991, 34, 283–321. [Google Scholar]
- Reed, D.M. Cuisine from Hun-Nal-Ye. In Reconstructing Ancient Maya Diet; University of Utah Press: Salt Lake, UT, USA, 1999; pp. 183–196. [Google Scholar]
- Ambrose, S.H. Isotopic analysis of palaeodiets: Methodological and interpretive consideration. In Investigation of Ancient Human Tissue; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Schoeninger, M.J.; DeNiro, M.J. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim. Cosmochim. Acta 1984, 48, 625–639. [Google Scholar] [CrossRef]
- Lubell, D.; Jackes, M.; Schwarcz, H.; Knyf, M.; Meiklejohn, C. The Mesolithic-Neolithic transition in Portugal: Isotopic and dental evidence of diet. J. Archaeol. Sci. 1994, 21, 201–216. [Google Scholar] [CrossRef]
- White, C.D. Dietary dental pathology and cultural change in the Maya. In Strength in Diversity; Worcester Historical Museum: Worcester, MA, USA, 1994; pp. 279–302. [Google Scholar]
- Bocherens, H.; Grupe, G.; Mariotti, A.; Turban-Just, S. Molecular preservation and isotopy of Mesolithic human finds from the Ofnet cave (Bavaria, Germany). Anthropol. Anz. 1997, 55, 121–129. [Google Scholar]
- Drucker, D.; Bocherens, H.; Bridault, A.; Billiou, D. Carbon and nitrogen isotopic composition of red deer (Cervus elaphus) collagen as a tool for tracking palaeoenvironmental change during the Late-Glacial and Early Holocene in the northern Jura (France). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 195, 375–388. [Google Scholar]
- Wada, E.; Kadonaga, T.; Matsuo, S. 15N abundance in nitrogen of naturally occurring substances and global assessment of denitrification from isotopic viewpoint. Geochem. J. 1975, 9, 139–148. [Google Scholar]
- Cheng, H.; Bremner, J.; Edwards, A. Variations of nitrogen-15 abundance in soils. Science 1964, 146, 1574–1575. [Google Scholar]
- Mariotti, A.; Pierre, D.; Vedy, J.; Bruckert, S.; Guillemot, J. The abundance of natural nitrogen 15 in the organic matter of soils along an altitudinal gradient (Chablais, Haute Savoie, France). Catena 1980, 7, 293–300. [Google Scholar] [CrossRef]
- Hansen, J. Palaeoethnobotany and palaeodiet in the Aegean region: Notes on legume toxicity and related pathologies. In Palaeodiet in the Aegean; Oxbow: Oxford, UK, 2000; pp. 13–27. [Google Scholar]
- Tyree, E.L. Using phytoliths to identify plant remains from archaeological sites: A phytolith analysis of modern olive oil and wine sediment. In Palaeodiet in the Aegean (Wiener Laboratory Monograph); Oxbow: Oxford, UK, 1999; Volume 1, pp. 29–36. [Google Scholar]
- Lev-Tov, J. The influences of religion, social structure and ethnicity on diet: An example from Frankish Corinth. In Palaeodiet in the Aegean; Oxbow: Oxford, UK, 1999; pp. 85–98. [Google Scholar]
- Triantaphyllou, S. A Bioarchaeological Approach to Prehistoric Cemetery Populations from Central and Western Greek Macedonia; British Archaeological Reports Limited: Oxford, UK, 2001; Volume 976. [Google Scholar]
- Triantaphyllou, S.; Richards, M.P.; Touchais, G.; Philippa-Touchais, A.; Voutsaki, S. Analyses of Middle Helladic Skeletal Material from Aspis, Argos, 2. Stable Isotope Analysis of Human Remains. Bull. Corresp. Hellénique 2006, 130, 627–637. [Google Scholar] [CrossRef]
- Triantaphyllou, S.; Richards, M.P.; Zerner, C.; Voutsaki, S. Isotopic dietary reconstruction of humans from Middle Bronze age Lerna, Argolid, Greece. J. Archaeol. Sci. 2008, 35, 3028–3034. [Google Scholar] [CrossRef]
- Papathanasiou, A. Health, diet and social implications in Neolithic Greece from the study of human osteological material. In Human Bioarchaeology of the Transition to Agriculture; Wiley-Blackwell: Chichester, UK, 2011; pp. 87–106. [Google Scholar]
- Halstead, P. Land use in postglacial Greece: Cultural causes and environmental effects. In Landscape and Land Use in Postglacial Greece; Sheffield Academic Press: Sheffield, UK, 2000; Volume 3, pp. 110–130. [Google Scholar]
- Halstead, P. Between a rock and a hard place: Coping with marginal colonisation in the later Neolithic and early Bronze Age of Crete and the Aegean. In Escaping the Labyrinth: The Cretan Neolithic in Context; Oxbow Books: Oxford, UK, 2008; pp. 229–257. [Google Scholar]
- Bonsall, C.; Lennon, R.; McSweeney, K.; Stewart, C.; Harkness, D.; Boroneanţ, V.; Bartosiewicz, L.; Payton, R.; Chapman, J. Mesolithic and Early Neolithic in the Iron Gates: A palaeodietary perspective. J. Eur. Archaeol. 1997, 5, 50–92. [Google Scholar] [CrossRef]
- Bocherens, H.; Tresset, A.; Wiedemann, F.; Giligny, F.; Lafage, F.; Lanchon, Y.; Mariotti, A. Diagenetic evolution of mammal bones in two French Neolithic sites. Bull. Soc. Geol. Fr. 1997, 168, 555–564. [Google Scholar]
- Garnsey, P. Food and Society in Classical Antiquity; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Papathanasiou, A. Stable isotope analysis in Neolithic Greece and possible implications on human health. Int. J. Osteoarchaeol. 2003, 13, 314–324. [Google Scholar]
- Papathanasiou, A. A Bioarchaeological Analysis of Neolithic Alepotrypa Cave, Greece; British Archaeological Reports Ltd.: Oxford, UK, 2001; Volume 961. [Google Scholar]
- Kontopoulos, I.; Sampson, A. Prehistoric diet on the island of Euboea, Greece: An isotopic investigation. Mediterr. Archaeol. Archaeom. 2015, 15, 97–111. [Google Scholar]
- Petroutsa, E.I. An Investigation in the Nuitrition of Bronze Age Poplulations in Greece. Ph.D. Thesis, Biology Department, National University of Athens, Athens, Greece, 2007. (In Greek). [Google Scholar]
- Petroutsa, E.I.; Richards, M.P.; Manolis, S.K. Stable isotope analysis of human remains from the Early Helladic site of Perachora, Korinth, Greece. In Cooking Up the Past; Oxbow: Oxford, UK, 2007; pp. 290–296. [Google Scholar]
- Ingvarsson-Sundström, A.; Richards, M.P.; Voutsaki, S. Stable isotope analysis of the Middle Helladic population from two cemeteries at Asine: Barbouna and the east cemetery. Mediterr. Archaeol. Archaeom. 2009, 9, 1–14. [Google Scholar]
- Lagia, A.; Petroutsa, E.; Manolis, S. Health and Diet During the MBA in the Peloponnese: The site of Kouphovouno. In Cooking Up the Past: Food and Culinary Practices in the Neolithic and Bronze Age Aegean; Renard, E.J., Ed.; Oxbow Books Limited: Oxford, UK, 2007; pp. 313–328. [Google Scholar]
- Vika, E. From Diet to Society: Stable Isotope Analysis and Its Cultural Context in Bronze Age Peloponnese, Greece. Ph.D. Dissertation, University of Bradford, Bradford, UK, 2002. [Google Scholar]
- Petroutsa, E.I.; Richards, M.P.; Kolonas, L.; Manolis, S.K. Isotope Paleodietary Analysis of Humans and Fauna from the Late Bronze Age Site of Voudeni. Hesperia Suppl. 2009, 43, 237–243. [Google Scholar]
- Vika, E.; Theodoropoulou, T. Re-investigating fish consumption in Greek antiquity: Results from δ13C and δ15N analysis from fish bone collagen. J. Archaeol. Sci. 2012, 39, 1618–1627. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- Ambrose, S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- Tykot, R.H. Stable isotopes and diet: You are what you eat. Available online: http://luna.cas.usf.edu/~rtykot/PR39%20-%20Enrico%20Fermi%20isotopes.pdf (accessed on 10 February 2019).
- Van Klinken, G.J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 1999, 26, 687–695. [Google Scholar] [CrossRef]
- DeNiro, M.J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 1985, 317, 806–809. [Google Scholar] [CrossRef]
- Suess, H.E. The radioactivity of the atmosphere and hydrosphere. Annu. Rev. Nucl. Sci. 1958, 8, 243–256. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Hedges, R.E.; Healey, M.; Simpson, A. Isotopic comparison of hair, nail and bone: Modern analyses. J. Archaeol. Sci. 2001, 28, 1247–1255. [Google Scholar] [CrossRef]
- Richards, M.P.; Hedges, R.E. Stable isotope evidence for similarities in the types of marine foods used by Late Mesolithic humans at sites along the Atlantic coast of Europe. J. Archaeol. Sci. 1999, 26, 717–722. [Google Scholar]
- Bocherens, H.; Polet, C.; Toussaint, M. Palaeodiet of Mesolithic and Neolithic populations of Meuse Basin (Belgium): Evidence from stable isotopes. J. Archaeol. Sci. 2007, 34, 10–27. [Google Scholar] [CrossRef]
- Goude, G.; Fontugne, M. Carbon and nitrogen isotopic variability in bone collagen during the Neolithic period: Influence of environmental factors and diet. J. Archaeol. Sci. 2016, 70, 117–131. [Google Scholar] [CrossRef]
- Milner, N.; Craig, O.E.; Bailey, G.N.; Pedersen, K.; Andersen, S.H. Something fishy in the Neolithic? A re-evaluation of stable isotope analysis of Mesolithic and Neolithic coastal populations. Antiquity 2004, 78, 9–22. [Google Scholar] [CrossRef]
- Polet, C.; Katzenberg, M.A. Reconstruction of the diet in a mediaeval monastic community from the coast of Belgium. J. Archaeol. Sci. 2003, 30, 525–533. [Google Scholar]
- Tauber, H. 13C evidence for dietary habits of prehistoric man in Denmark. Nature 1981, 292, 332–333. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).