Thermal Conductivity of Frozen Sediments Containing Self-Preserved Pore Gas Hydrates at Atmospheric Pressure: An Experimental Study
Abstract
1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
- Frozen samples of the same composition with and without pore hydrates differ markedly in thermal conductivity measured at non-equilibrium conditions (at P = 0.1 MPa and t = −6 ± 1 °C). The difference may reach tens of percent or more, depending on the composition and structure of sediments, due to about four-fold thermal conductivity difference between pore ice and hydrate. It is the highest in the sand with high hydrate saturation where cracked gas hydrate forms at the boundaries of soil particles and impedes heat transfer. For instance, the thermal conductivity of frozen hydrate-bearing fine sand-2 (W = 10%) is 1.28 W/(m·K) but 2.09 W/(m·K) in the absence of pore hydrate.
- As the fraction of pore water converted to hydrate (and hydrate saturation) increases, the thermal conductivity of frozen samples decreases and becomes ever more different from that of hydrate-barren sediments.
- After the pressure in the test cell drops to 0.1 MPa, the thermal conductivity of frozen hydrate-bearing sediments increases as a result of slow pore hydrate dissociation and additional ice formation. For instance, it increased from 0.5 W/(m·K) to 1.74 W/(m·K) in 55 hours in fine sand-1, at t = −6 ± 1 °C, while the hydrate saturation reduced 36 to 9%.
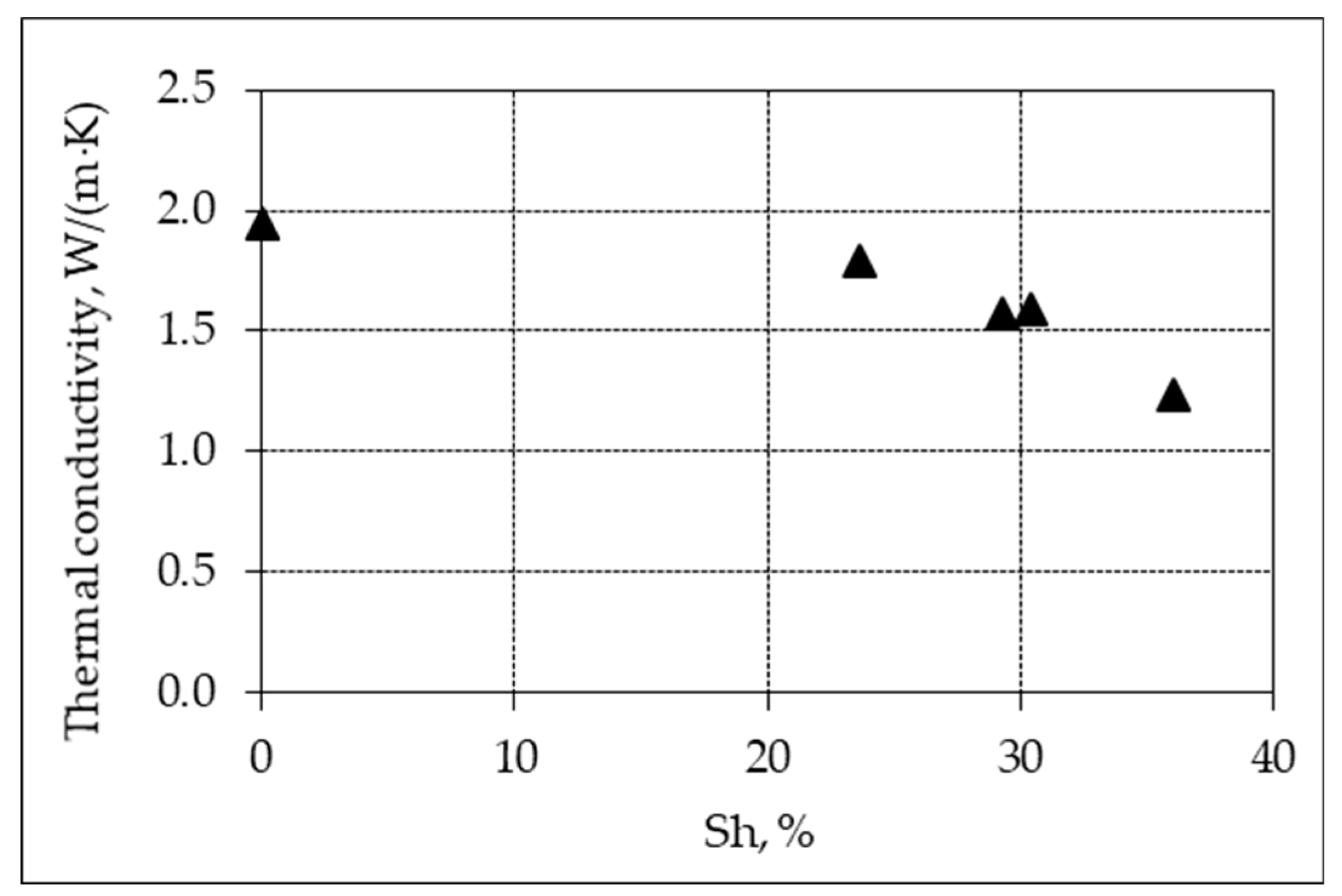
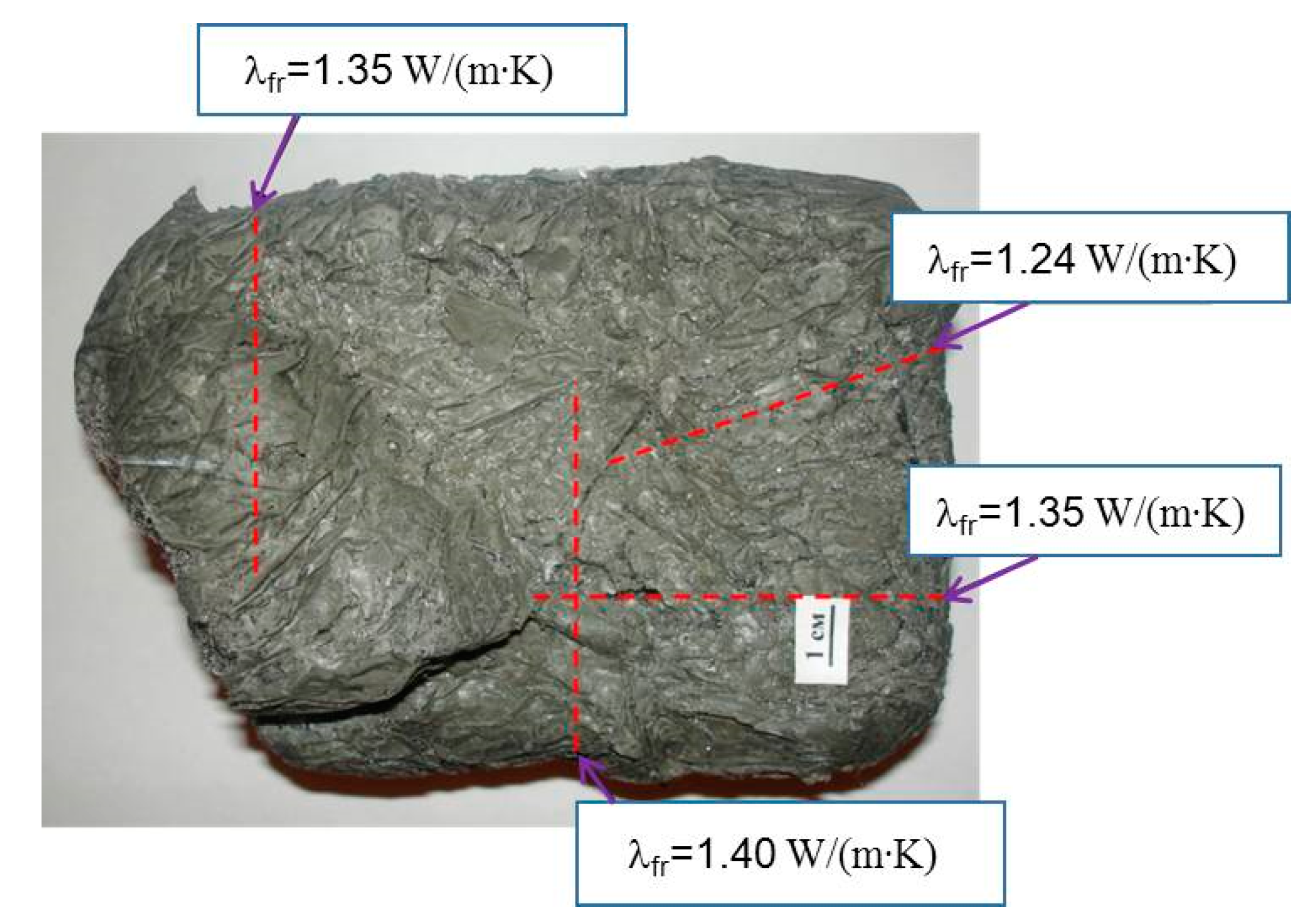
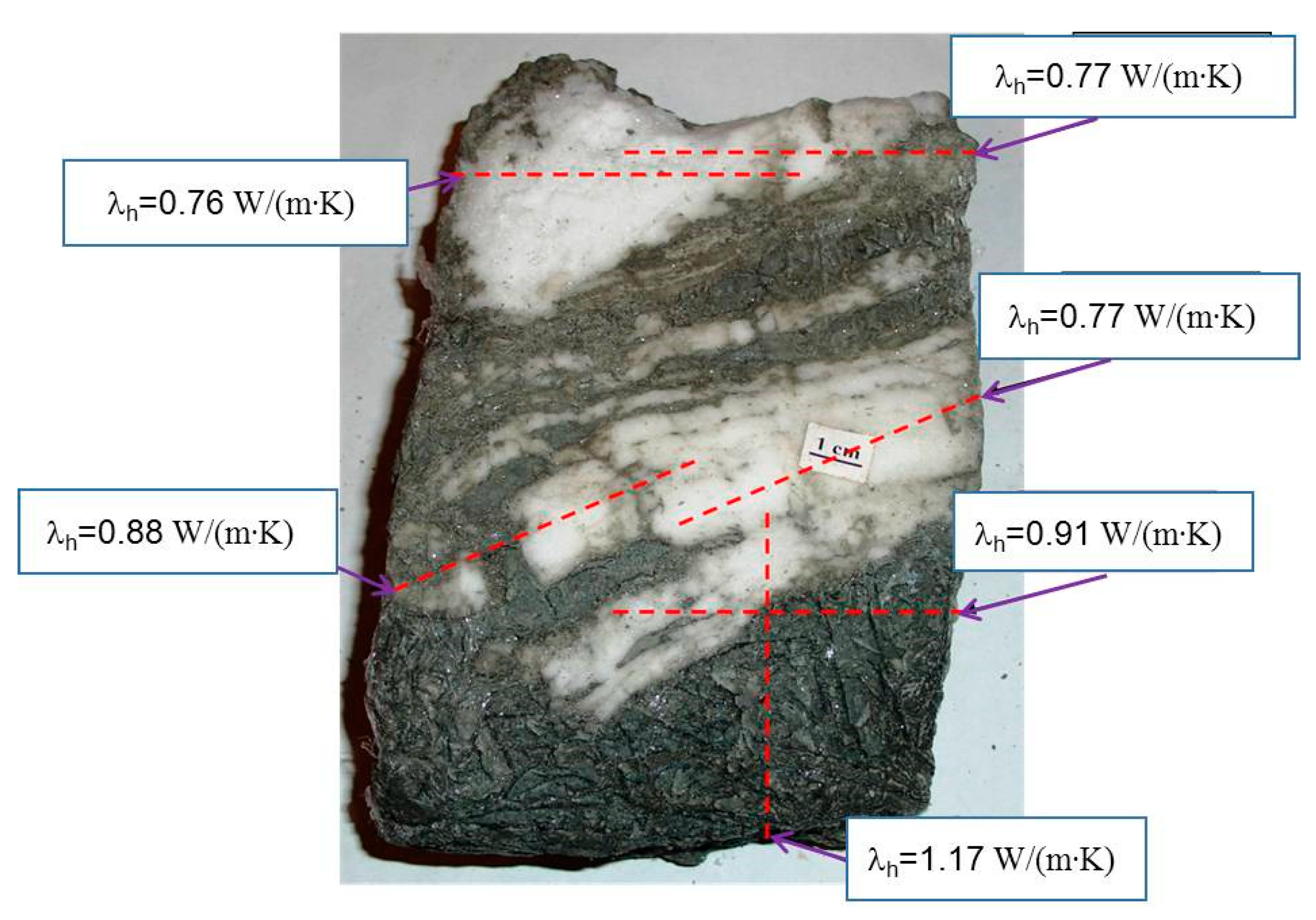
- The thermal conductivity of frozen natural hydrate-bearing core samples of Lake Baikal bottom sediments is anisotropic and varies with the direction of measurements with respect to ice-hydrate lenses: from 0.77 W/(m·K) along the lenses to 1.37 W/(m·K) in the orthogonal direction.
Author Contributions
Funding
Conflicts of Interest
References
- Makogon, Y.F. Hydrates of Natural Gases; NEDRA: Moscow, Russia, 1974; p. 208. ISBN 978-0878141654. (In Russian) [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 752. ISBN 978-0-8493-9078-4. [Google Scholar]
- Max, D.M.; Johnson, A.H.; Dillon, W.P. Natural Gas Hydrate—Arctic Ocean Deepwater Resource Potential; Springer: Dordrecht, The Netherlands, 2013; p. 113. ISBN 978-3-319-02508-7. [Google Scholar]
- Cherskiy, N.V.; Tsarev, V.P.; Nikitin, S.P. Investigation and prediction of conditions of accumulation of gas resources in gas-hydrate pools. Pet. Geol. 1985, 21, 65–89. (In Russian) [Google Scholar]
- Bird, K.J.; Magoon, L.B. Petroleum Geology of the Northern Part of the Arctic National Wildlife Refuge, Northeastern Alaska. U.S. Geological Survey, Bulletin 1778; US Government Printing Office: Washington, DC, USA, 1987; p. 329.
- Judge, A.S.; Majorowicz, J.A. Geothermal conditions for gas hydrate stability in the Beaufort-Mackenzie area: The global change aspect. Glob. Planet. Chang. 1992, 98, 251–263. [Google Scholar] [CrossRef]
- Judge, A.S.; Smith, S.L.; Majorowicz, J.A. The current distribution and thermal stability of gas hydrates in the Canadian Polar Region. In Proceedings of the Fourth Offshore and Polar Engineering Conference, Osaka, Japan, 10–15 April 1994; pp. 307–313. [Google Scholar]
- Dallimore, S.R.; Uchida, T.; Collett, T.S. Scientific Results from JAPEX/JNOC/GSC Mallik 2L-38 Gas Hydrate Research Well, Mackenzie Delta, Northwest Territories, Canada. Geological Survey of Canada, Bulletin 544; US Government Printing Office: Washington, DC, USA, 1999; p. 403. ISBN 0-660-17777-3.
- Yakushev, V.S.; Chuvilin, E.M. Natural gas and hydrate accumulation within permafrost in Russia. Cold Reg. Sci. Technol. 2000, 31, 189–197. [Google Scholar] [CrossRef]
- Liang, Y.P.; Li, X.S.; Li, B. Assessment of gas production potential from hydrate reservoir in Qilian mountain permafrost using five-spot horizontal well system. Energies 2015, 8, 10796–10817. [Google Scholar] [CrossRef]
- Ruppel, C. Permafrost-associated gas hydrate: Is it really approximately 1 % of the global system? J. Chem. Eng. Data 2015, 60, 429–436. [Google Scholar] [CrossRef]
- Henninges, J.; Schrötter, J.; Erbas, K.; Huenges, E. Temperature field of the Mallik gas hydrate occurrence-implications on phase changes and thermal properties. In Scientific Results from the Mallik 2002 Gas Hydrate Production Research WellPmgram, Mackenzie Delta, Northwest Territories, Canada. Geological Survey of Canada, Bulletin 585; Dallimore, S.R., Collett, T.S., Eds.; Ottawa, ON, Canada, 2005; p. 14. [Google Scholar]
- Collett, T.S.; Lee, M.W.; Agena, W.F.; Miller, J.J.; Lewis, K.A.; Zyrianova, M.V.; Boswell, R.; Inks, T.L. Permafrost associated natural gas hydrate occurrences on the Alaskan North Slope. Mar. Pet. Geol. 2011, 28, 279–294. [Google Scholar] [CrossRef]
- Fang, H.; Xu, M.; Lin, Z.; Zhong, Q.; Bai, D.; Liu, J.; Pei, F.; He, M. Geophysical characteristics of gas hydrate in the Muli area, Qinghai province. J. Nat. Gas Sci. Eng. 2017, 37, 539–550. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Collett, T.S. Intrapermafrost gas hydrates from a deep core hole in the Mackenzie Delta, Northwest Territories, Canada. Geology 1995, 23, 527–530. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Chuvilin, E.M.; Yakushev, V.S.; Grechischev, S.E.; Ponomarev, V.; Pavlov, A. Field and laboratory characterization of intrapermafrost gas hydrates, Mackenzie Delta, N.W.T., Canada. In Proceedings of the 2nd International Conference on Natural Gas Hydrates, Toulouse, France, 2–6 June 1996; pp. 525–531. [Google Scholar]
- Chuvilin, E.M.; Yakushev, V.S.; Perlova, E.V. Gas and possible gas hydrates in the permafrost of Bovanenkovo gas field, Yamal Peninsula, West Siberia. Polarforschung 2000, 68, 215–219. [Google Scholar]
- Chuvilin, E.; Bukhanov, B.; Davletshina, D.; Grebenkin, S.; Istomin, V. Dissociation and self-preservation of gas hydrates in permafrost. Geosciences 2018, 8, 431. [Google Scholar] [CrossRef]
- Yershov, E.D.; Lebedenko, Yu.P.; Chuvilin, E.M.; Istomin, V.A.; Yakushev, V.S. Features of gas hydrates in permafrost. Dokl. Akad. Nauk. 1991, 321, 788–791. (In Russian) [Google Scholar]
- Stern, L.; Circone, S.; Kirby, S.H.; Durham, W. Anomalous preservation of pure methane hydrates at 1 atm. J. Phys. Chem. 2001, 105, 537–542. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Bukhanov, B.A.; Grebenkin, S.I.; Doroshin, V.V.; Iospa, A.V. Shear strength of frozen sand with dissociating pore methane hydrate: An experimental study. Cold Reg. Sci. Technol. 2018, 153, 101–105. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Semenov, A.P.; Bogoyavlensky, V.I.; Medvedev, V.I.; Bogoyavlensky, I.V. Experimental modeling of methane release from intrapermafrost relic gas hydrates when sediment temperature change. Cold Reg. Sci. Technol. 2018, 31, 189–197. [Google Scholar] [CrossRef]
- Bukhanov, B.A.; Chuvilin, E.M.; Guryeva, O.M.; Kotov, P.I. Experimental study of the thermal conductivity of the frozen sediments containing gas hydrate. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, AK, USA, 23 June–3 July 2008; pp. 205–209. [Google Scholar]
- Li, D.; Liang, D.; Fan, S.; Peng, H. Estimation of ultra-stability of methane hydrate at 1 atm by thermal conductivity measurement. J. Nat. Gas Chem. 2010, 19, 229–233. [Google Scholar] [CrossRef]
- Stoll, R.D.; Bryan, G.M. Physical properties of sediments containing gas hydrates. J. Geophys. Res. 1979, 84, 1629–1634. [Google Scholar] [CrossRef]
- Groysman, A.G. Thermal Properties of Gas Hydrates; Nauka: Novosibirsk, Russia, 1985; p. 94. (In Russian) [Google Scholar]
- Huang, D.; Fan, S. Measuring and modeling thermal conductivity of gas hydrate-bearing sand. J. Geophys. Res. 2005, 110, B01311. [Google Scholar] [CrossRef]
- Rosenbaum, E.J.; English, N.J.; Johnson, J.K.; Shaw, D.W.; Warzinski, R.P. Thermal conductivity of methane hydrate from experiment and molecular simulation. J. Phys. Chem. B 2007, 111, 13193–13205. [Google Scholar] [CrossRef]
- Waite, W.F.; Stern, L.A.; Kirby, S.H.; Winters, W.J.; Mason, D.H. Simultaneous determination of thermal conductivity, thermal diffusivity and specific heat in sI methane hydrate. Geophys. J. Int. 2007, 169, 767–774. [Google Scholar] [CrossRef]
- Warzinski, R.P.; Gamwo, I.K.; Rosenbaum, E.J.; Myshakin, E.M.; Jiang, H.; Jordan, K.D.; English, N.J.; Shaw, D.W. Thermal properties of methane hydrate by experiment and modeling and impacts upon technology. In Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, BC, Canada, 6–10 July 2008; pp. 1–9. [Google Scholar]
- Asher, G.B. Development of a Computerized Thermal Conductivity Measurement System Utilizing the Transient Needle Probe Technique: An Application to Hydrates in Porous Media. Ph.D. Thesis, Dissertation T-3335. Colorado School of Mines, Golden, CO, USA, 1987; p. 179. [Google Scholar]
- Wright, J.F.; Nixon, F.M.; Dallimore, S.R.; Henninges, J.; Cote, M.M. Thermal conductivity of sediments within the gas-hydrate-bearing interval at the JAPEX/JNOC/GSC et al. Mallik 5L-38 gas hydrate production research well. In Mallik 5L-38 Gas Hydrate Production Research Well. Geological Survey of Canada, Bulletin 585; Dallimore, S.R., Collett, T.S., Eds.; US Government Printing Office: Washington, DC, USA, 2005; p. 10. [Google Scholar]
- Duchkov, A.D.; Manakov, A.Y.; Kazantsev, S.A.; Permyakov, M.E.; Ogienko, A.G. Experimental modeling and measurement of thermal conductivity of sediments containing methane hydrates. Dokl. Earth Sci. 2006, 409, 732–735. [Google Scholar] [CrossRef]
- Muraoka, M.; Ohtake, M.; Susuki, N.; Yamamoto, Y.; Suzuki, K.; Tsuji, T. Thermal properties of methane hydrate-bearing sediments and surrounding mud recovered from Nankai Trough wells. J. Geophys. Res. Solid Earth 2014, 119, 8021–8033. [Google Scholar] [CrossRef]
- Muraoka, M.; Susuki, N.; Yamaguchi, H.; Tsuji, T.; Yamamoto, Y. Thermal properties of a supercooled synthetic sand–water–gas–methane hydrate sample. Energy Fuels 2015, 29, 1345–1351. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Bukhanov, B.A. Effect of hydrate accumulation conditions on thermal conductivity of gas-saturated soils. Energy Fuels 2017, 31, 5246–5254. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Cheverev, V.; Motenko, R.; Grechishcheva, E. Effect of ice and hydrate formation on thermal conductivity of sediments. In Impact of Thermal Conductivity on Energy Technologies; Shahzad, A., Ed.; IntechOpen: London, UK, 2018; pp. 115–132. [Google Scholar] [CrossRef]
- De Batist, M.; Klerkx, J.; Van Rensbergen, P.; Vanneste, M.; Poort, J.; Golmshtok, A.Y.; Kremlev, A.A.; Khlystov, O.M.; Krinitsky, P. Active hydrate destabilization in Lake Baikal, Siberia. Terra Nova 2005, 14, 436–442. [Google Scholar] [CrossRef]
- Matveeva, T.V.; Mazurenko, L.L.; Soloviev, V.A.; Klerkx, J.; Kaulio, V.V.; Prasolov, E.M. Gas hydrate accumulation in the subsurface sediments of Lake Baikal (Eastern Siberia). Geo.-Mar. Lett. 2003, 23, 289–299. [Google Scholar] [CrossRef]
- Khlystov, O.; De Batist, M.; Shoji, H.; Hachikubo, A.; Nishio, S.; Naudts, L.; Poort, J.; Khabuev, A.; Belousov, O.; Manakov, A.; Kalmychkov, G. Gas hydrate of Lake Baikal: Discovery and varieties. J. Asian Earth Sci. 2013, 62, 162–166. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Lupachik, M.V.; Guryeva, O.M. Kinetics research of ice transition into gas hydrate in porous media. In Physics and Chemistry of Ice; Furukawa, Y., Sazaki, G., Uchida, T., Watanabe, N., Eds.; Hokkaido University Press: Sapporo, Japan, 2011; pp. 127–132. [Google Scholar]
- Chuvilin, E.M.; Ebinuma, T.; Kamata, Y.; Uchida, T.; Takeya, S.; Nagao, J.; Narita, H. Effects of temperature cycling on the phase transition of water in gas-saturated sediments. Can. J. Phys. 2003, 81, 343–350. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Istomin, V.A. Gas-hydrates self-preservation effect. In Physics and Chemistry of Ice; Maeno, N., Hondoh, T., Eds.; Hokkaido University Press: Sapporo, Japan, 1992; pp. 136–139. [Google Scholar]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Falenty, A.; Kuhs, W.F.; Glockzin, M.; Rehder, G. “Self-preservation” of CH4 hydrates for gas transport technology: Pressure-temperature dependence and ice microstructures. Energy Fuels 2014, 28, 6275–6283. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Kozlova, E.V. Experimental estimation of hydrate-bearing sediments stability. In Proceedings of the 5th International Conference on Gas Hydrate, Thermodynamic Aspects, Trondheim, Norway, 13–16 June 2005; Volume 3, pp. 1562–1567. [Google Scholar]
- Chuvilin, E.M.; Davletshina, D. Formation and accumulation of pore methane hydrates in permafrost: experimental modeling. Geosciences 2018, 8, 467. [Google Scholar] [CrossRef]
- Bristow, K.; White, R.D.; Kluitenberg, G.J. Comparison of single and dual probes for measuring soil thermal properties with transient heating. Aust. J. Soil Res. 1994, 32, 447–464. [Google Scholar] [CrossRef]
- Menditto, A.; Patriarca, M.; Magnusson, B. Understanding the meaning of accuracy, trueness and precision. Accred. Qual. Assur. 2007, 12, 45–47. [Google Scholar] [CrossRef]
- Côté, J.; Konrad, J.-M. Thermal conductivity of base-course materials. Can. Geotech. J. 2005, 42, 61–78. [Google Scholar] [CrossRef]
- Popov, Y.; Beardsmore, G.; Clauser, C.; Roy, S. ISRM suggested methods for determining thermal properties of rocks from laboratory tests at atmospheric pressure. Rock Mech. Rock Eng. 2016, 49, 4179–4207. [Google Scholar] [CrossRef]
- Hachikubo, A.; Takeya, S.; Chuvilin, E.; Istomin, V. Preservation phenomena of methane hydrate in pore spaces. Phys. Chem. Chem. Phys. 2011, 13, 17449–17452. [Google Scholar] [CrossRef]
- Takeya, S.; Fujihisa, H.; Gotoh, Y.; Istomin, V.; Chuvilin, E.; Sakagami, H.; Hachikubo, A. Methane clathrate hydrates formed within hydrophilic and hydrophobic porous media: Kinetics of dissociation and distortion of host structure. J. Phys. Chem. C 2013, 117, 7081–7085. [Google Scholar] [CrossRef]
- Yershov, E.D.; Lebedenko, Yu.P.; Chuvilin, E.M.; Yakushev, V.S. Microstructure of an ice–methane hydrate agglomerate: An experimental study. Eng. Geol. 1990, 3, 38–44. [Google Scholar]
- Sloan, E.D. Clathrate Hydrates of Natural Gases, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1998; p. 705. ISBN 0824799372. [Google Scholar]
- Chuvilin, E.M.; Guryeva, O.M. Experimental study of self-preservation effect of gas hydrates in frozen sediments. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, AK, USA, 23 June–3 July 2008; pp. 263–267. [Google Scholar]
- Shimada, W.; Takeya, S.; Kamata, Y.; Uchida, T.; Nagao, J.; Ebinuma, T.; Narita, H. Mechanism of self-preservation during dissociation of methane clathrate hydrate. In Proceedings of the 5th International Conference on Gas Hydrate, Trondheim, Norway, 13–16 June 2005; pp. 208–212. [Google Scholar]





| Type of Sediment | Sampling Site (Depth of Recovery) | Particle Size Distribution, % | Mineralogy, % | Salinity, % | ||
|---|---|---|---|---|---|---|
| 1–0.05 mm | 0.05–0.001 mm | <0.001 mm | ||||
| Fine sand-1 | ‒ | 94.8 | 3.1 | 2.1 | Quartz > 90 | 0.012 |
| Fine sand-2 | Yamburg GCF (64 m) | 83.2 | 14.4 | 2.4 | Quartz − 38 Microcline + albite − 45 Illite − 9 Kaolinite + chlorite − 5 | 0.09 |
| Silty sand | Vorkuta | 41.8 | 53.7 | 4.5 | Quartz − 64 Microcline − 9 Albite − 5 | 0.08 |
| Sandy clay | Zapolarnoe OGCF (199 m) | 87.6 | 4.1 | 8.3 | Quartz − 64 Microcline − 9 Albite − 5 | 0.2 |
| Bottom silt | Lake Baikal (water depth 1364 m) | 7.1 | 60.5 | 32.4 | Quartz − 66 Plagioclase − 12 Cristobalite − 10 Illite + smectite − 6 Kaolinite − 4 | 0.01 |
| Type of Material | Reference Value, W/(m·K) | Average Value, W/(m·K) | Number of Measurements | Random Error, W/(m·K) | Relative Error (precision), % | Systematic Error (trueness), % |
|---|---|---|---|---|---|---|
| Water | 0.60 (at +20 °C) | 0.56 (at +17 °C) | 21 | 0.03 | 5.7 | −7 |
| Glycerin | 0.28 (at +20 °C) | 0.30 (at +22 °C) | 21 | 0.00 | 0.0 | 7 |
| Marble | 2.64 | 2.15 | 15 | 0.05 | 2.3 | −23 |
| Limestone (Carbon Tan) | 1.77 | 1.47 | 25 | 0.06 | 4.3 | −20 |
| Quartz glass (TC) | 1.543 | 1.27 | 8 | 0.03 | 2.0 | −21 |
| Soil Type | W, % | ⍴, g/cm3 | Kh | λh, W/(m·K) | λfr, W/(m·K) |
|---|---|---|---|---|---|
| Fine sand-1 | 14 | 1.43 | 0.23 | 1.05 | 1.67 |
| Fine sand-1 | 16 | 1.53 | 0.28 | 1.11 | 2.07 |
| Fine sand-2 | 10 | 1.68 | 0.31 | 1.07 | 1.75 |
| Fine sand-2 | 21 | 1.68 | 0.60 | 0.51 | 2.32 |
| Silty sand | 15 | 1.77 | 0.29 | 1.04 | 1.61 |
| Silty sand | 23 | 1.94 | 0.19 | 1.86 | 2.15 |
| Sandy clay | 21 | 1.80 | 0.29 | 1.79 | 2.12 |
| W, % | Sh, % | Si, % | λh, W/(m·K) | λfr, W/(m·K) |
|---|---|---|---|---|
| 14 | 26 | 33 | 1.00 | 1.52 |
| 17 | 30 | 45 | 1.59 | 1.93 |
| 22 | 32 | 51 | 1.77 | 2,15 |
| Type of Sediment | Hydrate Forming Gas | Time after Gas Pressure Drop, hours | Sh, % | Si, % | λh, W/(m·K) |
|---|---|---|---|---|---|
| Fine sand-1 (W = 16%) | CH4 | 0.5 | 14 | 28 | 1.11 |
| 240 | 6 | 35 | 1.80 | ||
| Fine sand-1 (W = 14%) | CO2 | 0.5 | 32 | 35 | 1.17 |
| 135 | 4 | 55 | 1.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvilin, E.; Bukhanov, B. Thermal Conductivity of Frozen Sediments Containing Self-Preserved Pore Gas Hydrates at Atmospheric Pressure: An Experimental Study. Geosciences 2019, 9, 65. https://doi.org/10.3390/geosciences9020065
Chuvilin E, Bukhanov B. Thermal Conductivity of Frozen Sediments Containing Self-Preserved Pore Gas Hydrates at Atmospheric Pressure: An Experimental Study. Geosciences. 2019; 9(2):65. https://doi.org/10.3390/geosciences9020065
Chicago/Turabian StyleChuvilin, Evgeny, and Boris Bukhanov. 2019. "Thermal Conductivity of Frozen Sediments Containing Self-Preserved Pore Gas Hydrates at Atmospheric Pressure: An Experimental Study" Geosciences 9, no. 2: 65. https://doi.org/10.3390/geosciences9020065
APA StyleChuvilin, E., & Bukhanov, B. (2019). Thermal Conductivity of Frozen Sediments Containing Self-Preserved Pore Gas Hydrates at Atmospheric Pressure: An Experimental Study. Geosciences, 9(2), 65. https://doi.org/10.3390/geosciences9020065






