A Novel Approach to Isolation and Screening of Calcifying Bacteria for Biotechnological Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling, Biolog EcoPlate Assay, and Physico–Chemical Analyses
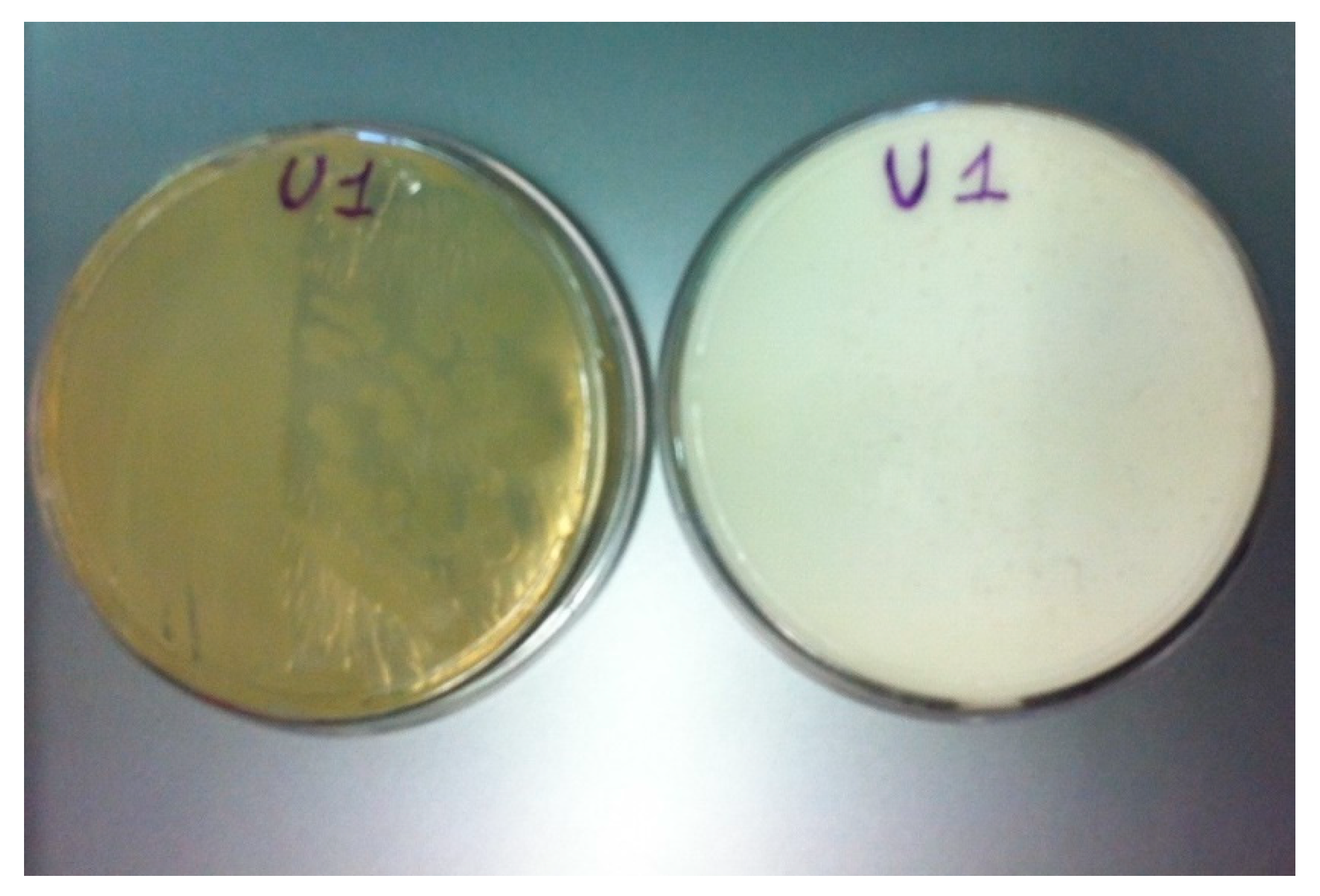
2.2. Isolation of CaCO3 Producing Bacteria
2.3. Preliminary Characterization and Biolog Identification
2.4. Calcification and CaCO3 Solubilization Activities
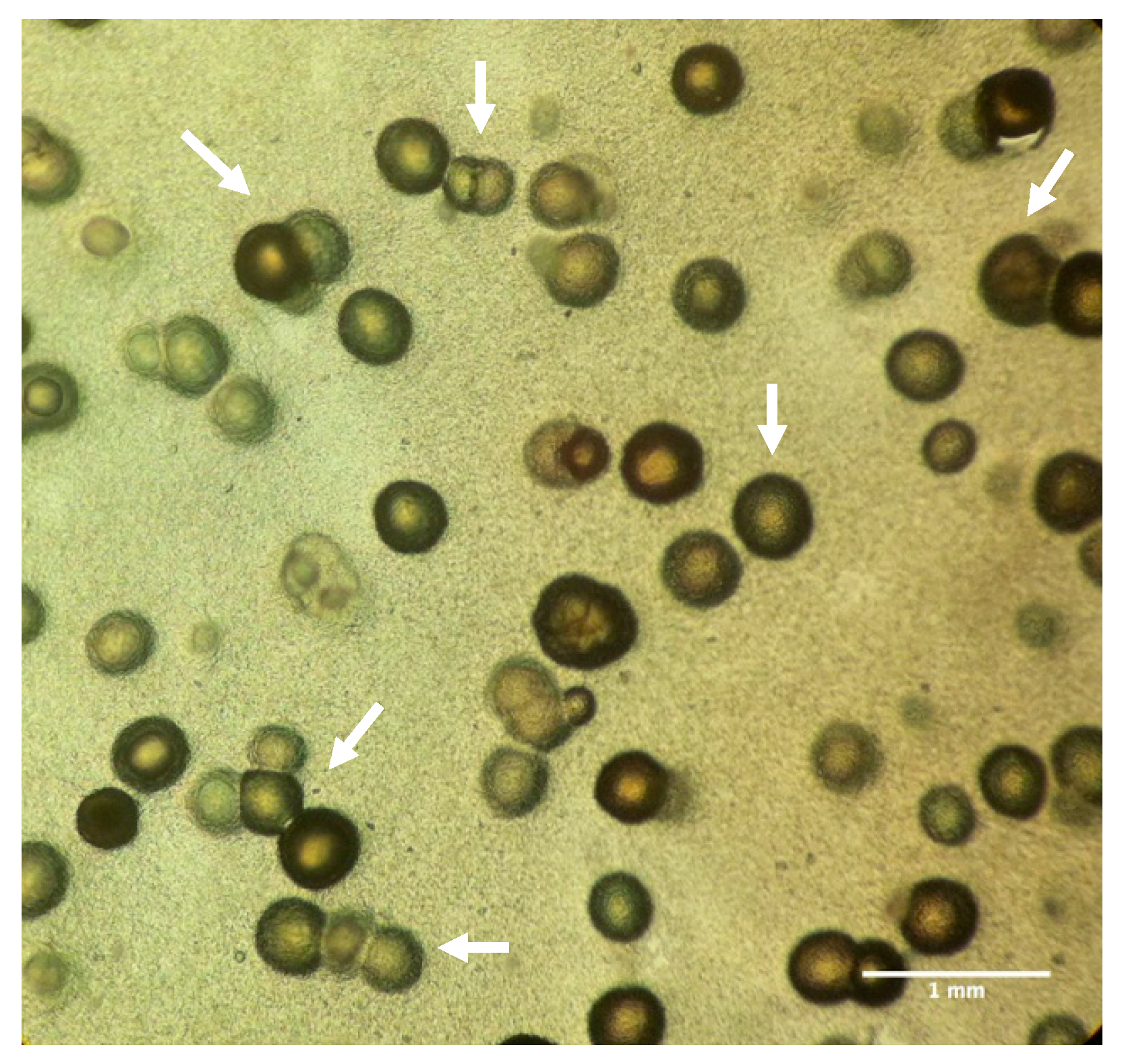
2.5. ImageJ Analysis
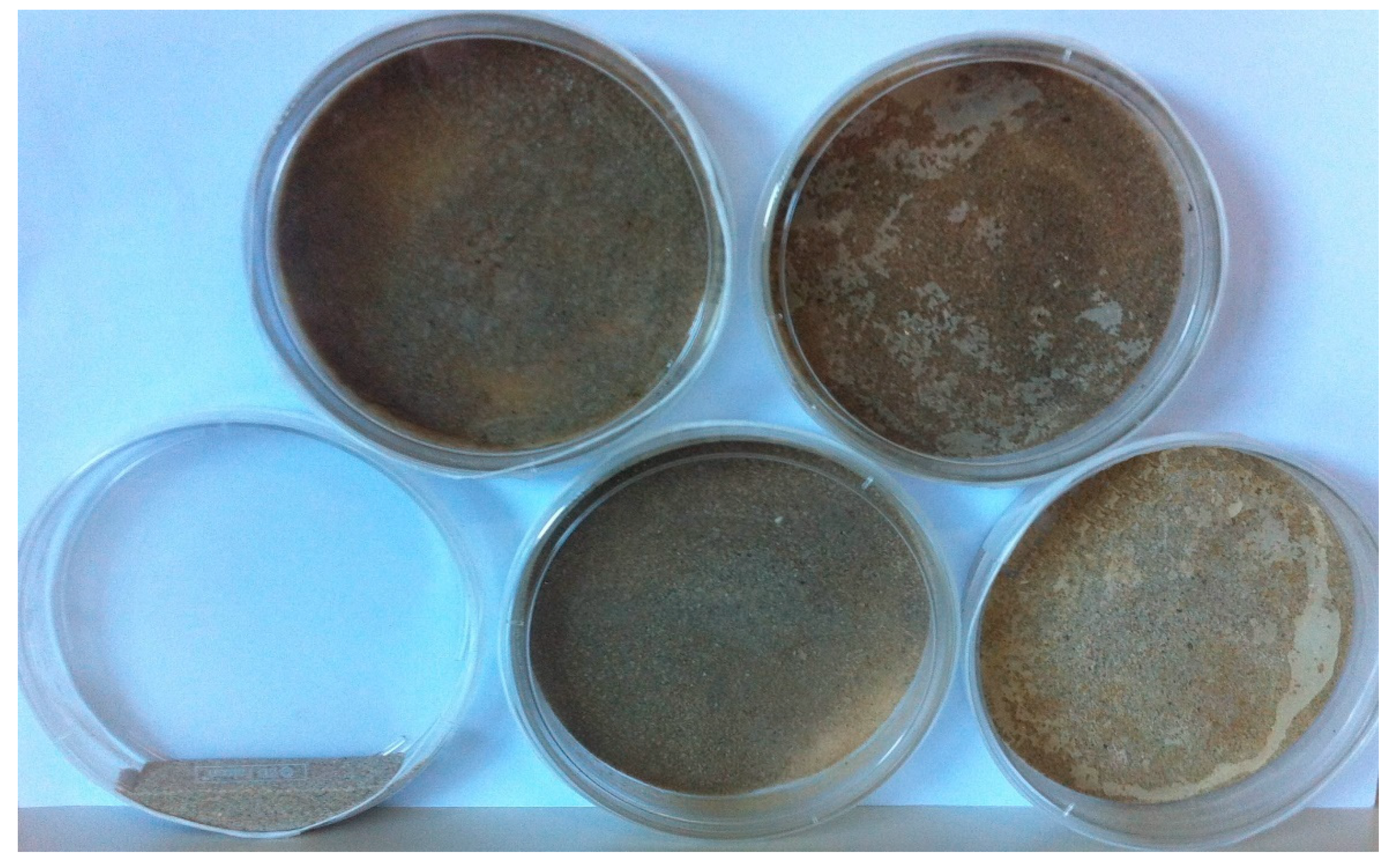
2.6. Sand Biocementation Test
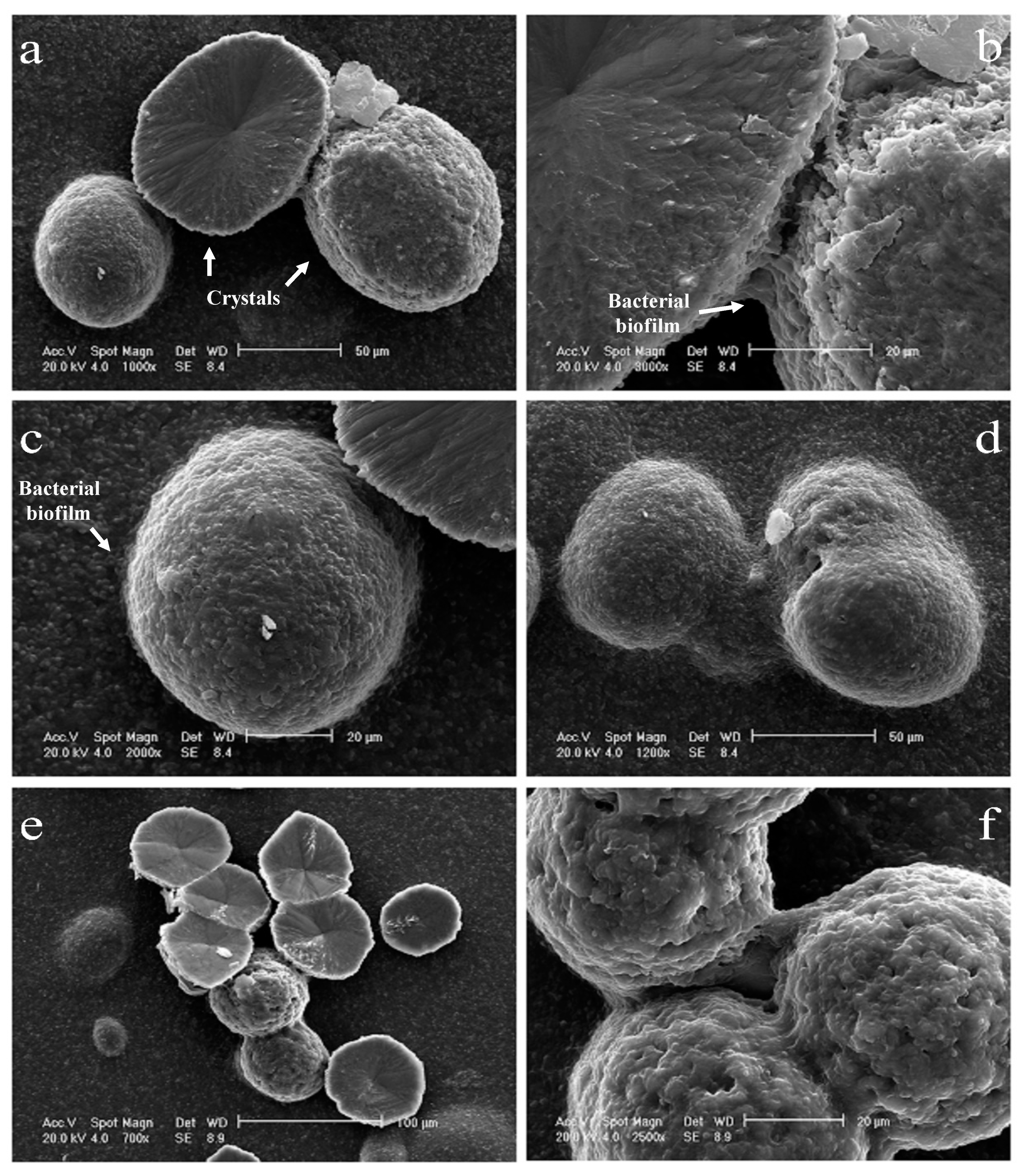
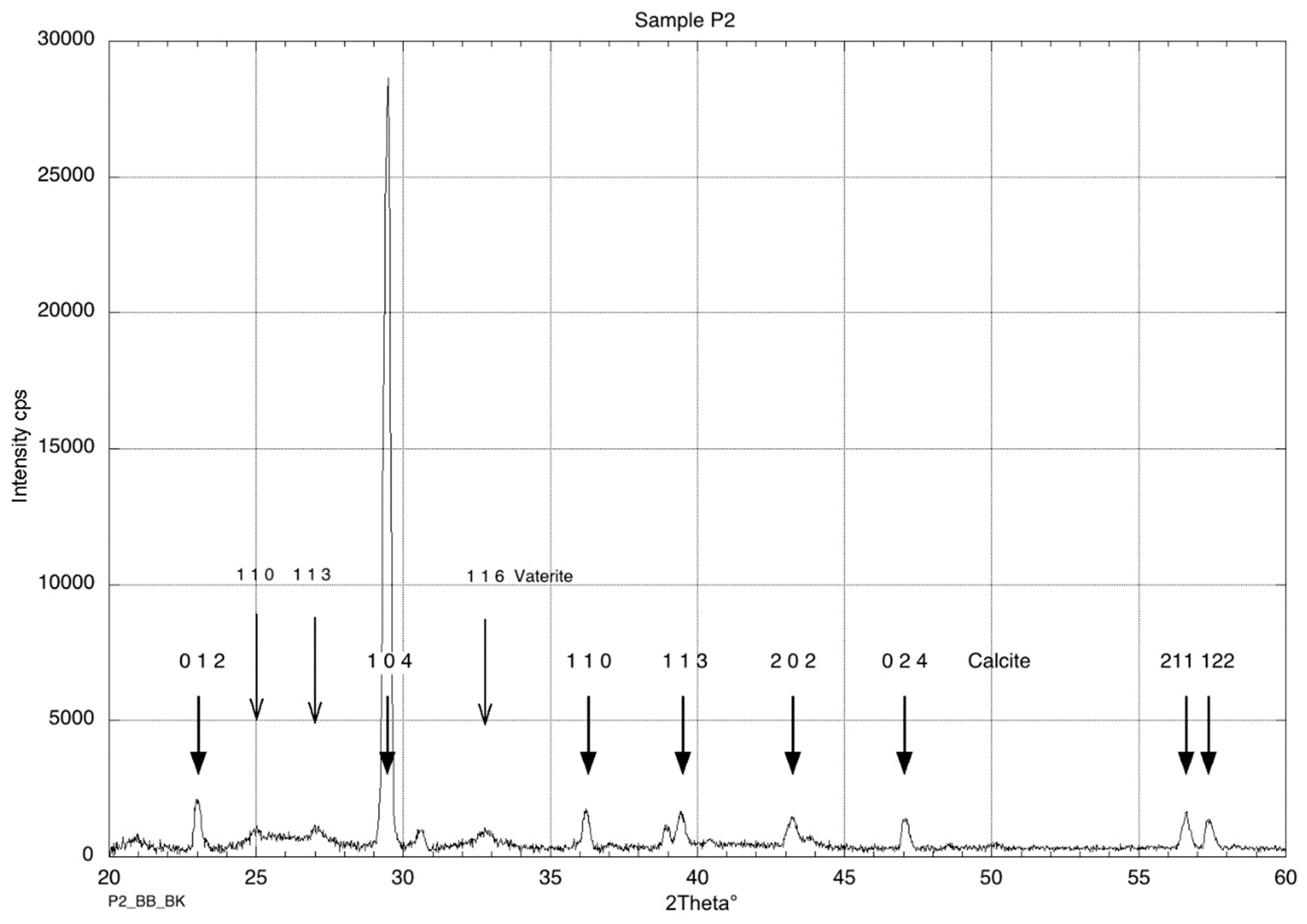
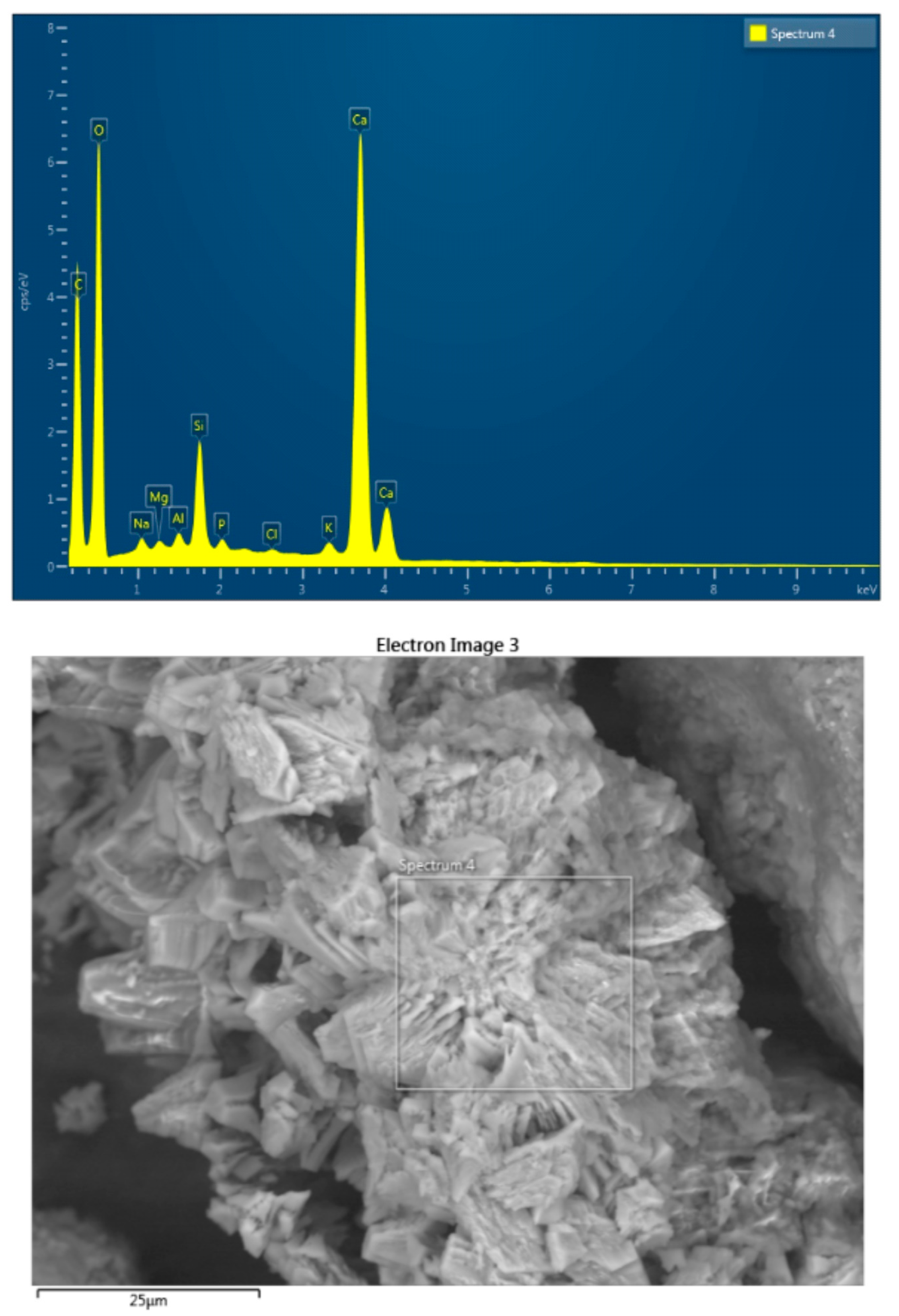
2.7. SEM, XRD, and EDS Analyses
3. Results and Discussion
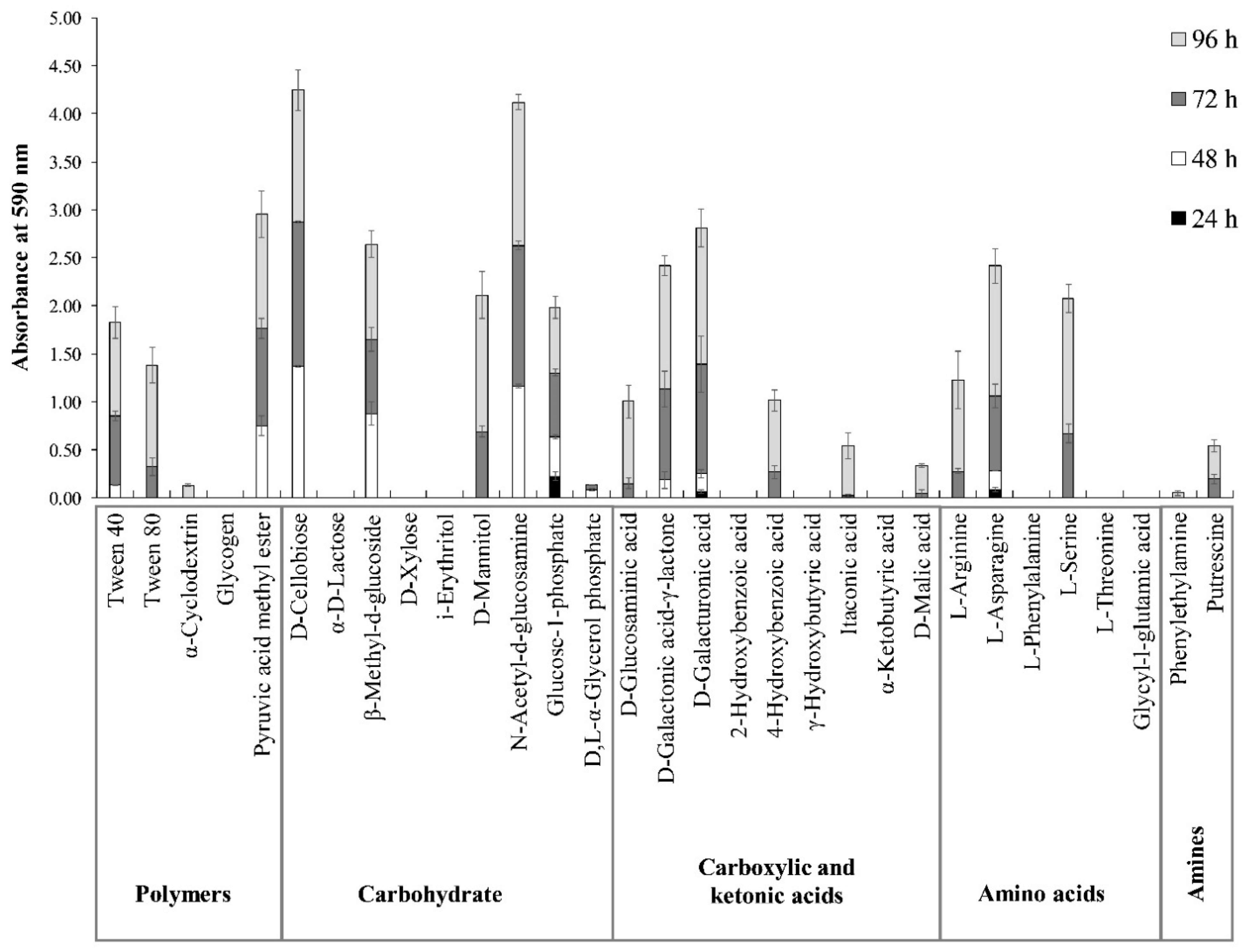
3.1. Biolog EcoPlates Assay
3.2. Isolation and Preliminary Characterization of Heterotrophic Calcifying Bacterial Strains
3.3. Identification of the Calcifying Bacterial Strains
3.4. Calcification and CaCO3 Solubilization Activities
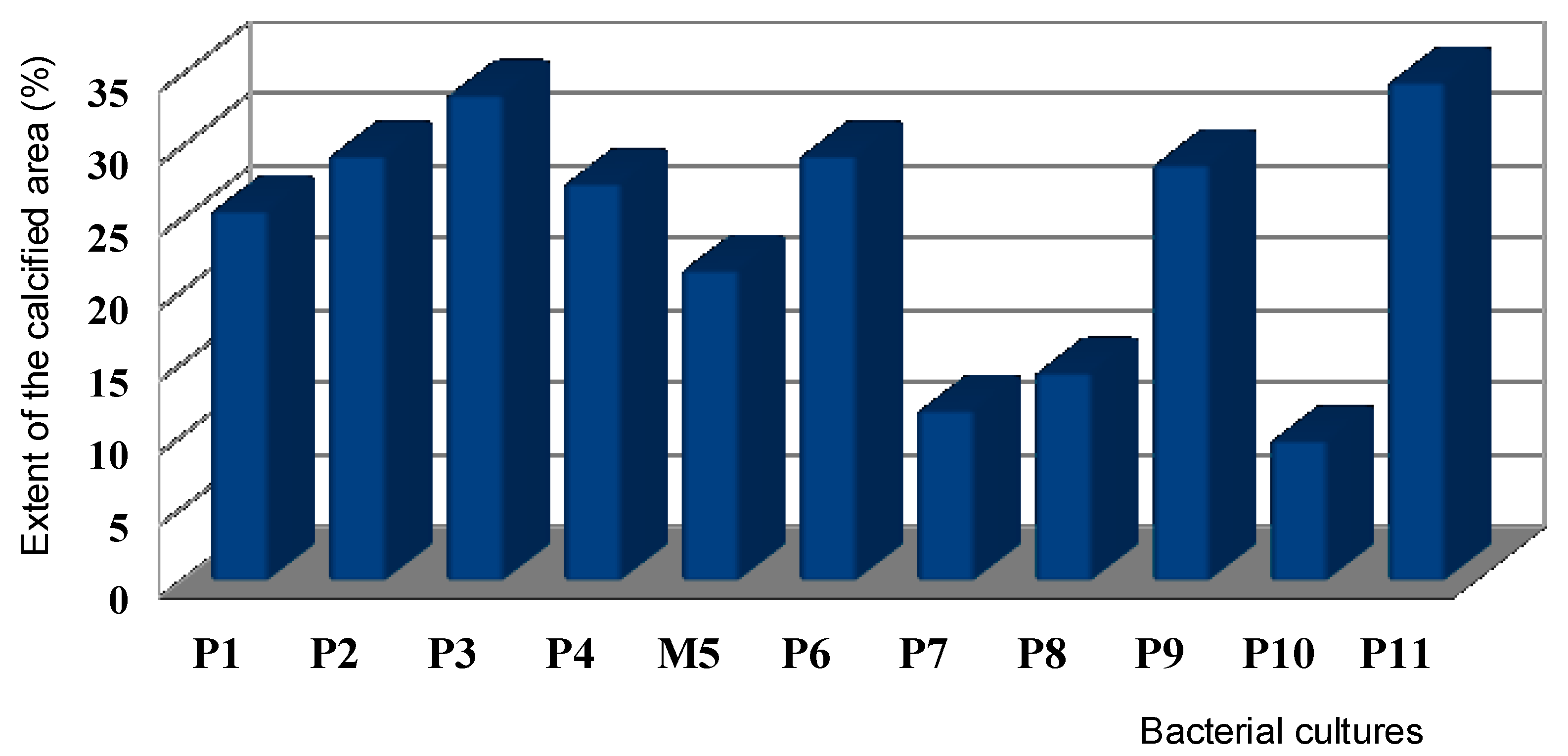
3.5. Bacterial Calcification Activity by the Imagej Software
3.6. Calcifying Strain Selection for Sand Biocementation
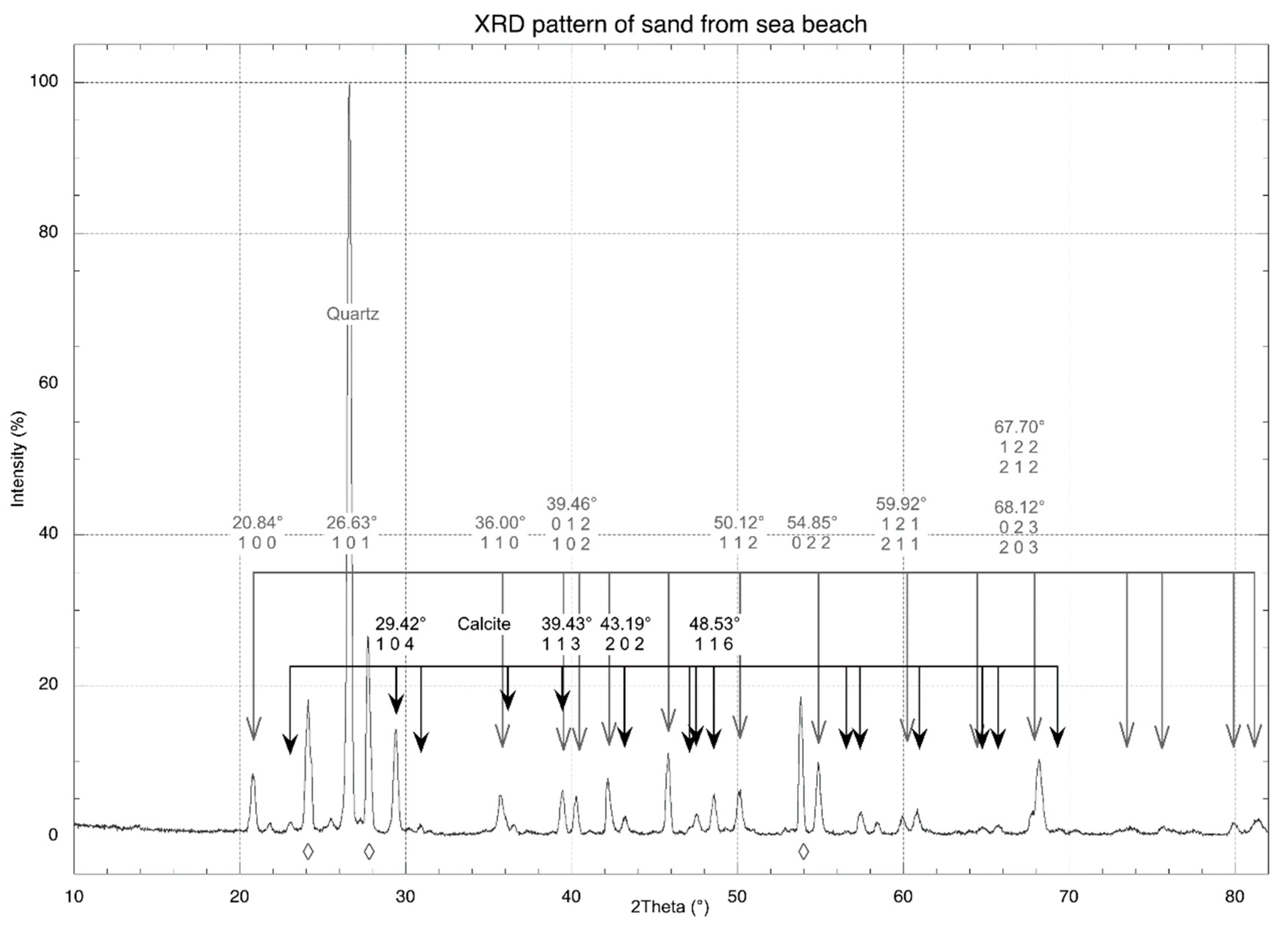
3.7. SEM, XRD, and EDS Analyses
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Banks, E.D.; Taylor, N.M.; Gulley, J.; Lubbers, B.R.; Giarrizo, J.G.; Bullen, H.A.; Hoehler, T.M.; Barton, H.A. Bacterial calcium carbonate precipitation in cave environments: A function of calcium homeostasis. Geomicrobiol. J. 2010, 27, 444–454. [Google Scholar] [CrossRef]
- Baskar, S.; Baskar, R.; Mauclaire, L.; Mckenzie, J.A. Role of microbial community in stalactite formation, Sahastradhara caves, Dehradun, India. Curr. Sci. 2005, 88, 1305–1308. [Google Scholar]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Cacchio, P.; Ercole, C.; Lepidi, A. Calcium carbonate deposition in limestone caves: Microbiological aspects. Subterr. Biol. 2003, 1, 57–63. [Google Scholar]
- Cacchio, P.; Ercole, C.; Cappuccio, G.; Lepidi, A. Calcium carbonate precipitation by bacterial strains isolated from a limestone cave and from a loamy soil. Geomicrobiol. J. 2003, 20, 85–98. [Google Scholar] [CrossRef]
- Cacchio, P.; Contento, R.; Ercole, C.; Cappuccio, G.; Preite Martinez, M.; Lepidi, A. Involvement of microorganisms in the formation of carbonate speleothems in the Cervo Cave (L’Aquila-Italy). Geomicrobiol. J. 2004, 21, 497–509. [Google Scholar] [CrossRef]
- Cacchio, P.; Ercole, C.; Lepidi, A. Evidences for bioprecipitation of pedogenic calcite by calcifying bacteria from three different soils of L’Aquila Basin (Abruzzi, Central Italy). Geomicrobiol. J. 2015, 32, 701–711. [Google Scholar] [CrossRef]
- Danielli, H.M.C.; Edington, M.A. Bacterial calcification in limestone caves. Geomicrobiology 1983, 3, 1–16. [Google Scholar] [CrossRef]
- Lipman, C.B. Further studies on marine bacteria with special reference to the drew hypothesis on CaCO3 precipitation on sea. Carnegie Inst. 1929, 39, 231–248. [Google Scholar]
- Morita, R.Y. Calcite precipitation by marine bacteria. Geomicrobiology 1980, 2, 63–82. [Google Scholar] [CrossRef]
- Northup, D.E.; Reysenbach, A.L.; Pace, N.R. Microorganisms and speleonthems. In Cave Minerals of the World, 2nd ed.; Hill, C., Forti, P., Eds.; National Speleological Society: Huntsville, AL, USA, 1997; pp. 261–266. ISBN 1-879961-07-5. [Google Scholar]
- Novitsky, J.A. Calcium carbonate precipitation by marine bacteria. Geomicrobiology 1981, 2, 375–388. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Zhuang, D.; Yan, H.; Tuckerd, M.E.; Zhao, H.; Han, Z.; Zhao, Y.; Sun, B.; Li, D.; Pan, J.; Zhao, Y.; et al. Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2+ concentrations: Further insights into biotic and abiotic calcite. Chem. Geol. 2018, 500, 64–87. [Google Scholar] [CrossRef]
- Castanier, S.; Le Metayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Castanier, S.; Métayer-Levrel, G.L.; Perthuisot, J.-P. Bacterial Roles in the Precipitation of Carbonate Minerals. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Heidelberg/Berlin, Germany, 2000; pp. 32–39. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Fortin, D.; Davis, B.S.; Beveridge, T.J. Mineralisation of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–287. [Google Scholar] [CrossRef]
- Southam, G. Bacterial Surface-Mediated Mineral Formation. In Environmental Microbe-Metal Interactions; Lovley, D., Ed.; ASM Press: Washington, DC, USA, 2000; pp. 257–276. [Google Scholar]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Decho, A.W. Overview of biopolymer-induced mineralization: What goes on in biofilms? Ecol. Eng. 2009, 30, 1–8. [Google Scholar] [CrossRef]
- Ercole, C.; Cacchio, P.; Botta, A.L.; Centi, V.; Lepidi, A. Bacterially induced mineralization of calcium carbonate: The role of exopolysaccharides and capsular polysaccharides. Microsc. Microanal. 2007, 13, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Verrecchia, E.P.; Aragno, M. Is the contribution of bacteria to terrestrial carbon budget greatly underestimated? Naturwissenschaften 2002, 89, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Arp, G.; Volker, T.; Reimer, A.; Michaelis, W.; Reitner, J. Biofilm exopolymers control microbialite formation at thermal springs discharging into the alkaline Pyramid Lake, Nevada, USA. Sediment. Geol. 1999, 126, 159–176. [Google Scholar] [CrossRef]
- McConnaughey, T.A.; Whelan, J.F. Calcification generates protons for nutrient and bicarbonate uptake. Earth Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906. [Google Scholar] [CrossRef]
- Wright, D.T. The role of sulphate-reducing bacteria and cyanobacteria in dolomite formation in distal ephemeral lakes of Coorong region, South Australia. Sed. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-induced biofilm calcification and calcium concentrations in Phanerozoic oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef]
- Dittrich, M.; Obst, M. Are picoplankton responsible for calcite precipitation in lakes? Ambio 2004, 33, 559–564. [Google Scholar] [CrossRef]
- Plée, K.; Pacton, M.; Ariztegui, D. Discriminating the role of photosynthetic and heterotrophic microbes triggering low-Mg calcite precipitation in freshwater biofilms (Lake Geneva, Switzerland). Geomicrobiol. J. 2010, 27, 391–399. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Small, D.P.; Dipple, G.M.; Wan, W.K.; Southam, G. Microbially mediated mineral carbonation: Roles of phototrophy and heterotrophy. Environ. Sci. Technol. 2011, 45, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth-Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Pires, I.; Duarte, S.O.D.; Monteiro, G.A. Effects of clay’s chemical interactions on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Morales, L.; Garzón, E.; Sánchez-Soto, S.P.J.; Romero, E. Microbiological induced carbonate (CaCO3) precipitation using clay phyllites to replace chemical stabilizers (cement or lime). Appl. Clay Sci. 2019, 174, 15–28. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.H.; Shin, Y.J.; So, J.S. Formation of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef]
- Vekariya, M.S.; Pitroda, J. Concrete: New Era for Construction Industry. Intern. J. Eng. Tre. Tech. 2013, 4, 4128–4137. [Google Scholar]
- Chahal, N.; Rajor, A.; Siddique, R. Calcium carbonate precipitation by different bacterial strains. Afr. J. Biotechnol. 2011, 10, 8359–8372. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Inter. 2004, 11, 36–42. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 213–222. [Google Scholar] [CrossRef]
- Frąc, M.; Oszust, K.; Lipiec, J. Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 2012, 12, 3253–3268. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V. Extracellular synthesis of Calcium Precipitating Bacteria Isolated from Rock Environment Region and its Characterization Studies. Adv. Bioequiv. Bioavailab. 2018, 1. [Google Scholar] [CrossRef]
- Otlewska, A.; Gutarowska, B. Environmental parameters conditioning microbially induced mineralization under the experimental model conditions. Acta Biochim. Pol. 2016, 63, 343–351. [Google Scholar] [CrossRef]
- Marvasi, M.; Visscher, P.T.; Perito, B.; Mastromei, G.; Martinez, L. Physiological requirements for carbonate precipitation during biofilm development of Bacillus subtilis etfA mutant. FEMS Microbiol. Ecol. 2010, 71, 341–350. [Google Scholar] [CrossRef]
- Urzì, C.; Garcia-Valles, M.; Vendrell, M.; Pernice, A. Biomineralization process on rock and monument surfaces observed in field and in laboratory conditions. Geomicrobiol. J. 1999, 16, 39–54. [Google Scholar]
- Baskar, S.; Baskar, R.; Mauclaire, L.; McKenzie, J.A. Microbially induced calcite precipitation in culture experiments: Possible origin for stalactites in Sahastradhara caves, Dehradun, India. Curr. Sci. 2006, 90, 58–64. [Google Scholar]
- Smibert, R.M.; Krieg, N.R. General characterization. In Manual Methods for General Bacteriology; Gerhardt, P., Ed.; American Society for Microbiology: Washington, DC, USA, 1981; pp. 409–443. [Google Scholar]
- Christensen, W.B. Urea Decomposition as a Means of Differentiating Proteus and Paracolon Cultures from Each Other and from Salmonella and Shigella Types. J. Bacteriol. 1946, 52, 461–466. [Google Scholar] [PubMed]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Williams & Wilkins: Philadelphia, PA, USA, 2000; p. 298. ISBN 0683053183. [Google Scholar]
- NORMAL Commission. NORMAL 9/88 recommendations. In Autotrophic and Heterotrophic Microflora: Culture Methods for Isolation; Consiglio Nazionale delle Ricerche/Istituto Centrale per il Restauro: Rome, Italy, 1990. [Google Scholar]
- Martino, T.; Salamone, P.; Zagari, M.; Urzì, C. Adesione a substrati solidi e solubilizzazione del CaCO3 quale misura della capacità deteriorante di batteri isolati dal marmo Pentelico. In Proceedings of the XI Meeting of the Italian Society of General Microbiology and Microbial Biotechnology (SIMGBM), Gubbio, Italy, 25–28 September 1992; pp. 249–250. [Google Scholar]
- Andrei, A.-S.; Păuşan, M.R.; Tămaş, T.; Har, N.; Barbu-Tudoran, L.; Leopold, N.; Banciu, H.L. Diversity and biomineralization potential of the epilithic bacterial communities inhabiting the oldest public stone monument of Cluj-Napoca (Transylvania, Romania). Front. Microbiol. 2017, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Florenzano, G. Fondamenti di Microbiologia del Terreno; REDA: Rome, Italy, 1983; p. 123. [Google Scholar]
- Marilley, J.; Aragno, M. Phylogenetic diversity of bacterial communities differing in degree of proximity of Lolium perenne and Trifolium repens roots. Appl. Soil Ecol. 1999, 13, 127–136. [Google Scholar] [CrossRef]
- Atlas, R.M.; Bartha, R. Microbial Ecology: Fundamentals and Applications, 4th ed.; The Benjamin/Cummings Publishing Company: San Francisco, CA, USA, 1998; ISBN 0805306552. [Google Scholar]
- Kourtev, P.S.; Ehrenfeld, J.G.; Haggblom, M. Exotic plant species alter the microbial community structure and function in the soil. Ecology 2002, 83, 3152–3166. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Williams, B.C.; Crawford, R.L. Urease activity of ureolytic bacteria isolated from six soils in which calcite was precipitated by indigenous bacteria. Geomicrobiol. J. 2012, 29, 389–395. [Google Scholar] [CrossRef]
- Davis, M.J.; Gillaspie, A.G.; Vidaver, A.K.; Harris, R.W. Clavibacter: A new genus containing some phytopathogenic coryneform bacteria, including, Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stunting disease of sugarcane and bermudagrass stunting disease. Int. J. Bacteriol. 1984, 34, 107–117. [Google Scholar]
- Riley, I.T. Serological relationships between strains of coryneform bacteria responsible for annual ryegrass toxicity and other plant pathogenic corynebacteria. Int. J. Syst. Bacteriol. 1987, 37, 153–159. [Google Scholar] [CrossRef]
- Riley, I.T.; Reardon, T.B.; McKay, A.C. Genetic Analysis of plant pathogenic bacteria in the genus clavibacter using allozyme electrophoresis. J. Gen. Microbiol. 1988, 134, 3025–3030. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Garayzabal, J.F.; Dominguez, L.; Pascual, C.; Jones, D.; Collins, M. Phenotypic and phylogenetic characterization of some unknown coryneform bacteria isolated from bovine blood and milk: Description of Sanguibacter gen. nov. Lett. Appl. Microbiol. 1995, 20, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of Vibrios. J. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Tubiash, H.S.; Chanley, P.E.; Leifson, E. Bacillary necrosis, a disease of larval and juvenile bivalve mollusks. I. Etiology and epizootiology. J. Bacteriol. 1965, 90, 1036–1044. [Google Scholar] [PubMed]
- Okada, K.; Iida, T.; Kita-Tsukamoto, K.; Takeshi, H. Vibrios commonly possess two chromosomes. J. Bacteriol. 2005, 187, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, M.A.; Delgado, R.; del Moral, A.; Ferrer, M.R.; Ramos-Cormenzana, A. Precipitation of calcium carbonate by Vibrio spp. from an inland saltern. FEMS Microbiol. Ecol. 1994, 13, 197–204. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Complex biomineralized vaterite structures encapsulating bacterial cells. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]



| Skel. | Sand (%) | Silt (%) | Clays (%) | Text. | O.M. (%) | pH | E.C. (mS) | Tot. CaCO3 (%) | Act. CaCO3 (%) | N (%) | Ca (ppm) | Mg (ppm) | K (ppm) | Na (ppm) | P (ppm) | Fe (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | C.E.C. (meq) | Ca (meq) | Mg (meq) | K (meq) | Na (meq) | B. S. (%) | Mg/K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con. | 48 | 15 | 37 | C.S. | 1.06 | 7.6 | 0.403 | 16.4 | 3.0 | 0.07 | 4100 | 244 | 203 | 66 | 6 | 9.6 | 13.0 | 1.9 | 1.2 | 23.35 | 20.50 | 2.04 | 0.52 | 0.29 | 100 | 3.9 |
| Skel. | Sand (%) | Silt (%) | Clay (%) | Text. | O.M. (%) | pH | E.C. (mS) | Tot. CaCO3 (%) | Act. CaCO3 (%) | N (%) | Ca (ppm) | Mg (ppm) | K (ppm) | Na (ppm) | P (ppm) | Fe (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | C.E.C. (meq) | Ca (meq) | Mg (meq) | K (meq) | Na (meq) | B. S. (%) | Mg/K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRA. | 94 | 2 | 4 | S. | 0.04 | 7.9 | 0.57 | 33.9 | 0.9 | 0.009 | 2940 | 77 | 54 | 119 | 6 | 3.6 | 4.6 | 0.4 | 0.8 | 16.01 | 14.7 | 0.65 | 0.14 | 0.52 | 100 | 4.6 |
| Strain | Cell | Gram | 4 °C | 20 °C | 28 °C | Anaerobic Growth | Urease |
|---|---|---|---|---|---|---|---|
| P1 | Short rod | + | - | + | + | - | + + |
| P2 | Short rod | - | - | + | + | - | + + |
| P3 | Short/irregular rod | + | - | + | + | - | + + + |
| P4 | Short/irregular rod | + | - | + | + | - | + + + |
| P5 | Rod | + | - | + | + | + | + |
| P6 | Short rod | + | - | + | + | + | + + |
| P7 | Rod | - | - | + | + | - | + + + |
| P8 | Short/irregular rod | + | + | + | + | + | + + + |
| P9 | Short/irregular rod | + | - | + | + | + | + + |
| P10 | Short/irregular rod | + | - | + | + | - | + + |
| P11 | Short/branched rod | + | - | + | + | - | + |
| P12 * | Rod | - | n.d. | + | + | + | + |
| P13 * | Rod | - | n.d. | + | + | + | n.d. |
| P14 * | Rod | - | n.d. | + | + | + | - |
| Strain | Identified Species | % ID |
|---|---|---|
| P1 | Not identified | |
| P2 | Vibrio tubiashii | 94 |
| P3 | Clavibacter agropyri | 93 |
| P4 | Clavibacter agropyri | 73 |
| P6 | Corynebacterium urealyticum | 78 |
| P7 | Not identified | |
| P8 | Clavibacter agropyri | 87 |
| P9 | Clavibacter agropyri | 73 |
| P10 | Clavibacter agropyri | 87 |
| P11 | Sanguibacter suarezii | 98 |
| P12 | Sphingomonas sanguinis | 98 |
| P13 | Not identified | |
| P14 | Pseudomonas syringae pv persicae | 92 |
| Timing a | Position b | Shape | Size | Amount c | Aggregates | Liquid Media | |||
|---|---|---|---|---|---|---|---|---|---|
| Strain | 4 °C | 20 °C | 28 °C | 28 °C | 28 °C | 28 °C | 28 °C | 28 °C | 28 °C |
| P1 | - | 30 | 14 | In/Out | G/O | M/L | + + + | + | + |
| P2 | - | 13 | 8 | In/Out | P/O | S/M | + + + + | + + + | + |
| P3 | - | 20 | 8 | In/Out | G | M/L | + + + | + + | + + |
| P4 | - | 23 | 10 | In/Out | G | L | + + + | + | - |
| P5 | n.d. | n.d. | n.d. | In/Out | G/O | S | + | - | n.d. |
| M5 d | - | 16 | 14 | In/Out | G/O | S | + + + + | ++ | - |
| P6 | - | 11 | 8 | In | G/P | M/L | + + + + | + + | +++ |
| P7 | - | 8 | 4 | In/Out | G | L | ++ | + + | - |
| P8 | 20 | 8 | 8 | In/Out | G/P | S/L | ++ | + | ++ |
| P9 | - | 22 | 10 | In/Out | G | M/L | + + + + | ++/+ + + | - |
| P10 | - | 13 | 8 | In | G/P | S/M/L | ++ | - | + |
| P11 | - | 12 | 8 | In/Out | G/P | S | + + + | +/+ + | + |
| Strains | Identified Species | Solubilization | |
|---|---|---|---|
| CaCO3 (0.14%) | CaCO3 (0.5%) | ||
| P1 | Not identified | + + + | +/- a |
| P2 | Vibrio tubiashii | + + + | +/- |
| P3 | Clavibacter agropyri | + + + | +/+ + |
| P4 | Clavibacter agropyri | + + | + + |
| M5 | Sphingomonas sanguinis (P12), Not identified (P13), Pseudomonas syringae (P14) | + + | + |
| P6 | Corynebacterium urealyticum | + + | + + |
| P7 | Not identified | + + + | +/+ + |
| P8 | Clavibacter agropyri | + + | +/- |
| P9 | Clavibacter agropyri | + + | - |
| P10 | Clavibacter agropyri | + + + | + |
| P11 | Sanguibacter suarezii | + + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacchio, P.; Del Gallo, M. A Novel Approach to Isolation and Screening of Calcifying Bacteria for Biotechnological Applications. Geosciences 2019, 9, 479. https://doi.org/10.3390/geosciences9110479
Cacchio P, Del Gallo M. A Novel Approach to Isolation and Screening of Calcifying Bacteria for Biotechnological Applications. Geosciences. 2019; 9(11):479. https://doi.org/10.3390/geosciences9110479
Chicago/Turabian StyleCacchio, Paola, and Maddalena Del Gallo. 2019. "A Novel Approach to Isolation and Screening of Calcifying Bacteria for Biotechnological Applications" Geosciences 9, no. 11: 479. https://doi.org/10.3390/geosciences9110479
APA StyleCacchio, P., & Del Gallo, M. (2019). A Novel Approach to Isolation and Screening of Calcifying Bacteria for Biotechnological Applications. Geosciences, 9(11), 479. https://doi.org/10.3390/geosciences9110479





