Waste Concrete Valorization; Aggregates and Mineral Carbonation Feedstock Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concrete Sampling
2.2. Sample Preparation
2.3. Carbonation Procedure
2.4. Analytical
2.5. Economic Modelling
3. Results and Discussion
3.1. Waste Concrete Treatment Efficiency
3.2. MCF Use for Cement Plant CO2 Abatement
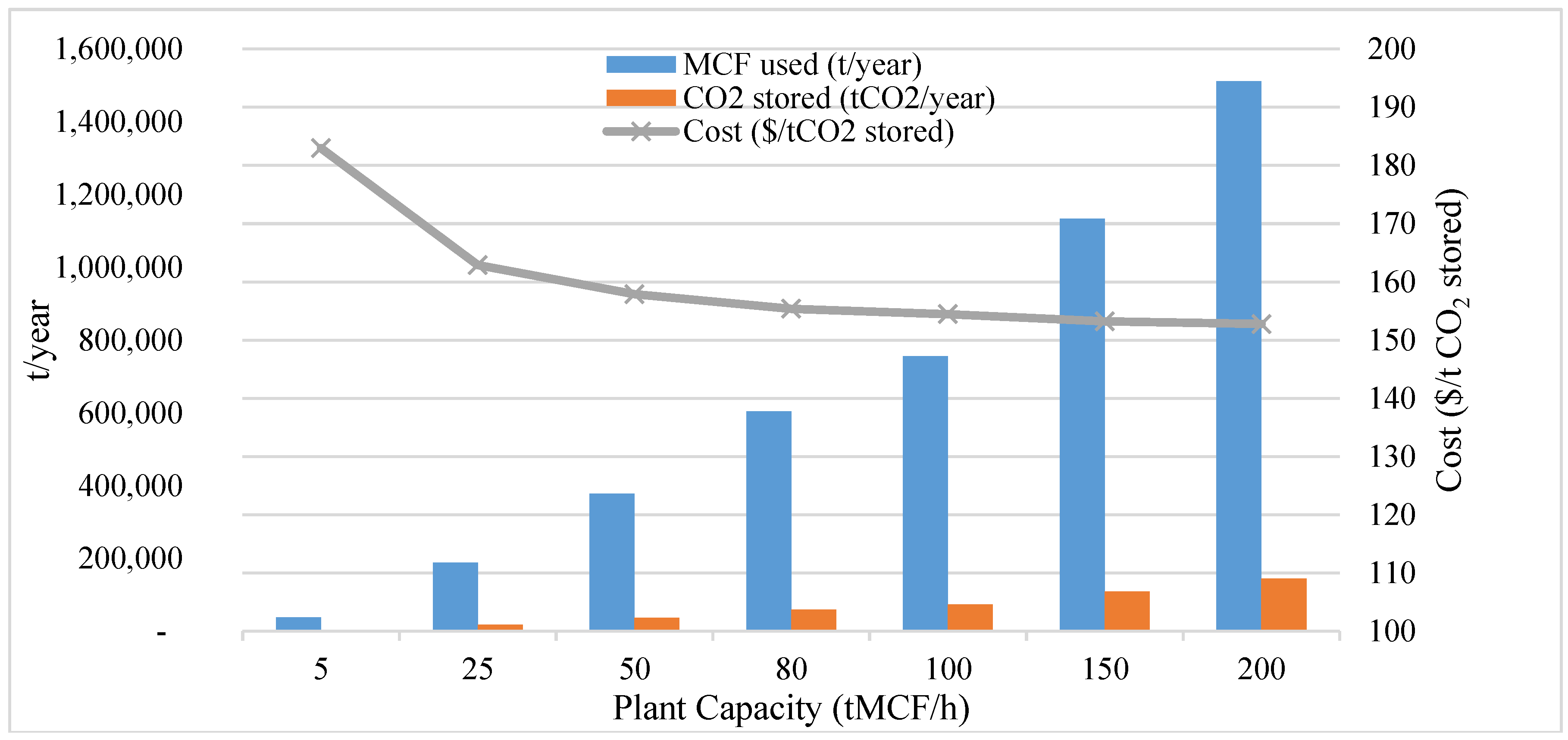
3.3. Economical Study
3.3.1. Aggregates Production
3.3.2. MCF Carbonation
4. Discussion
5. Conclusions
- The low carbonation potential requires a large amount of waste concrete to reach a significant amount of CO2 reduction.
- A carbon economy is important but should be completed by waste material legislation with an incentive effect to generate revenues from waste management fees.
- In its actual form, the process can be operated in areas where renewable energy is available. Operation at lower pressure is required for implementation in other areas.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P.; Houghton, R.; Marland, G.; Hackler, J.; Boden, T.A.; Conway, T.; Canadell, J.; Raupach, M.; Ciais, P.; Le Quere, C. Update on CO2 emissions. Nat. Geosci. 2010, 3, 811. [Google Scholar] [CrossRef]
- CSI. The Cement Sustainability Initiative. Recycling Concrete; World Business Council for Sustainable Development: Geneva, Switzerland, 2009. [Google Scholar]
- Robinson, G.R., Jr.; Menzie, W.D.; Hyun, H. Recycling of construction debris as aggregate in the mid-atlantic region, USA. Res. Conserv. Recycl. 2004, 42, 275–294. [Google Scholar] [CrossRef]
- ACI. Aci Design Handbook; American Concrete Institute: Farmington Hills, MN, USA, 2013. [Google Scholar]
- Nawy, E.G. Concrete Construction Engineering Handbook; CRC Press: Boca La ton, FL, USA, 2008. [Google Scholar]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geopolymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Rebeiz, K.; Fowler, D.W. Flexural strength of reinforced polymer concrete made with recycled plastic waste. ACI Struct. J. 1996, 93, 524–530. [Google Scholar]
- Buck, A.D. Recycled Concrete as a Source of Aggregates; US Army Engineer Waterways Experiement Station: Vicksburg, MS, USA, 1976; p. 23. [Google Scholar]
- Topcu, I.B.; Şengel, S. Properties of concretes produced with waste concrete aggregate. Cem. Concr. Res. 2004, 34, 1307–1312. [Google Scholar] [CrossRef]
- Tuyan, M.; Mardani-Aghabaglou, A.; Ramyar, K. Freeze–thaw resistance, mechanical and transport properties of self-consolidating concrete incorporating coarse recycled concrete aggregate. Mater. Des. 2014, 53, 983–991. [Google Scholar] [CrossRef]
- Richardson, A.; Coventry, K.; Bacon, J. Freeze/thaw durability of concrete with recycled demolition aggregate compared to virgin aggregate concrete. J. Clean. Prod. 2011, 19, 272–277. [Google Scholar] [CrossRef]
- Lotfi, S.; Eggimann, M.; Wagner, E.; Mróz, R.; Deja, J. Performance of recycled aggregate concrete based on a new concrete recycling technology. Constr. Build. Mater. 2015, 95, 243–256. [Google Scholar] [CrossRef]
- Rao, A.; Jha, K.N.; Misra, S. Use of aggregates from recycled construction and demolition waste in concrete. Resour. Conserv. Recycl. 2007, 50, 71–81. [Google Scholar] [CrossRef]
- Xiao, J. Recycled aggregate concrete. In Recycled Aggregate Concrete Structures; Springer: Beijing, China, 2018; pp. 65–98. [Google Scholar]
- Yoda, K.; Shintani, A. Building application of recycled aggregate concrete for upper-ground structural elements. Constr. Build. Mater. 2014, 67, 379–385. [Google Scholar] [CrossRef]
- IPCC. Mineral Carbonation and Industrial Uses of Carbon Dioxide; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; pp. 321–338. [Google Scholar]
- Thouvenot, P.; Bildstein, O.; Munier, I.; Cochepin, B.; Poyet, S.; Bourbon, X.; Treille, E. Modeling of Concrete Carbonation in Deep Geological Disposal of Intermediate Level Waste; EDP Science: Ellis, France, 2013. [Google Scholar]
- Katsuyama, Y.; Yamasaki, A.; Iizuka, A.; Fujii, M.; Kumagai, K.; Yanagisawa, Y. Development of a process for producing high purity calcium carbonate (CaCO3) from waste cement using pressurized CO2. Environ. Prog. Sustain. Energy 2005, 24, 162–170. [Google Scholar] [CrossRef]
- Vanderzee, S.; Zeman, F. Recovery and carbonation of 100% of calcium in waste concrete fines: Experimental results. J. Clean. Prod. 2018, 174, 718–727. [Google Scholar] [CrossRef]
- Ben Ghacham, A.; Cecchi, E.; Pasquier, L.-C.; Blais, J.-F.; Mercier, G. CO2 sequestration using waste concrete and anorthosite tailings by direct mineral carbonation in gas–solid–liquid and gas–solid routes. J. Environ. Manag. 2015, 163, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghacham, A.; Pasquier, L.-C.; Cecchi, E.; Blais, J.-F.; Mercier, G. Valorization of waste concrete through CO2 mineral carbonation: Optimizing parameters and improving reactivity using concrete separation. J. Clean. Prod. 2017, 166, 869–878. [Google Scholar] [CrossRef]
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Technical and economic evaluation of a mineral carbonation process using southern québec mining wastes for CO2 sequestration of raw flue gas with by-product recovery. Int. J. Greenh. Gas Control 2016, 50, 147–157. [Google Scholar] [CrossRef]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers, 4th ed.; McGraw-Hill: New York, NY, USA, 1991; p. 988. [Google Scholar]
- Ulrich, G.D. A Guide to Chemical Engineering Process Design and Economics; John Wiley & Sons: New York, NY, USA, 1984; p. 422. [Google Scholar]
- Canada Revenue Agency. Canadian Income Tax Rates for Individuals-Current and Previous Years. Available online: http://www.cra-arc.gc.ca/tx/ndvdls/fq/txrts-eng.html (accessed on 25 may 2018).
- Dumont, J. Le Marché du Carbone du Québec (Spede): Analyse et Enjeux; Univérsité de Sherbrooke: Sherbrooke, QC, Canada, 2013. [Google Scholar]
- Gouvernement du Québec. Règlement concernant le système de plafonnement et d’échange de droits d’émission de zaz à effet de serre. Éditeur officiel du Québec: Loi sur la qualité de l’environ 2013, 49, 524. [Google Scholar]
- MDDELCC. Vente Aux Enchères Conjointe n° 10 de Février 2017 Rapport Sommaire Des Résultats; California Environment Protection Agency-Air Resources Board: Sago Myanmar, CA, USA, 2017.
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; IGES: Tsukuba, Japan, 2006; p. 233. [Google Scholar]
- NYC DDC. Construction & Demolition Waste Manual; Prepared for NYC Department of Design & Construction by Gruzen Samton LLP with City Green Inc.: New York, NY, USA, 2003. [Google Scholar]
- Silva, R.; De Brito, J.; Dhir, R. Properties and composition of recycled aggregates from construction and demolition waste suitable for concrete production. Constr. Build. Mater. 2014, 65, 201–217. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.-C. Potential of CO2 sequestration through construction and demolition (c&d) waste—An overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar]
- Jeffrey, C. Construction and Demolition Waste Recycling: A Literature Review; Dalhousie University Office of Sustainabilit: Halifax, NS, Canada, 2011. [Google Scholar]
- Iizuka, A.; Fujii, M.; Yamasaki, A.; Yanagisawa, Y. Development of a new CO2 sequestration process utilizing the carbonation of waste cement. Ind. Eng. Chem. Res. 2004, 43, 7880–7887. [Google Scholar] [CrossRef]
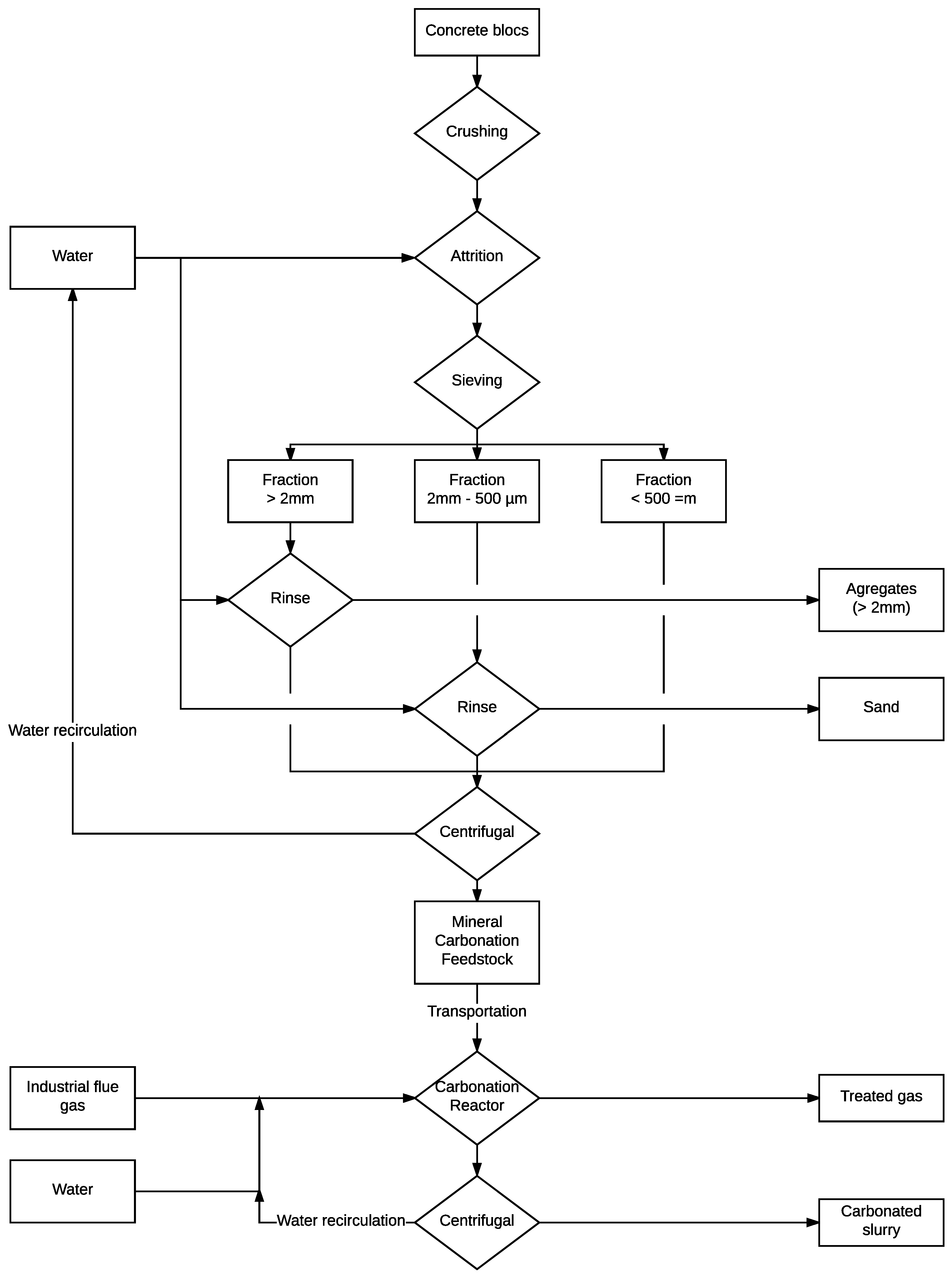
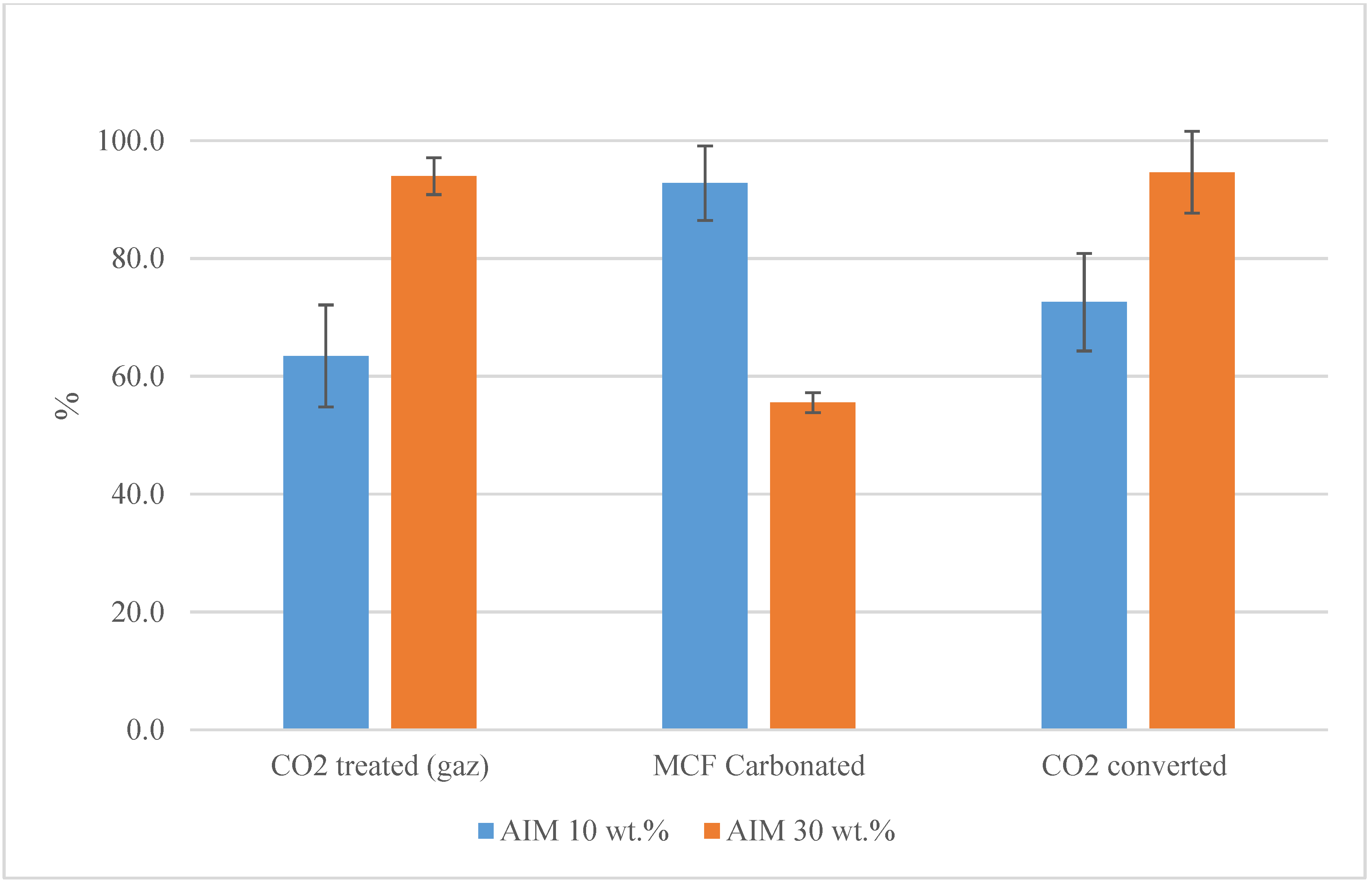
| Process Step | Volume (L) | Wet Mass (kg) | Dry Mass (kg) |
|---|---|---|---|
| Initial mass of concrete | 1000 | 1000 | |
| Water (attrition) | 2500 | 2500 | |
| Attrition | 3500 | 1000 | |
| Water (sieving) | 1600 | 1600 | |
| Sieving: | 5100 | 1000 | |
| >2 mm | 594 | 535 | |
| 2 mm 500 µm | 273 | 246 | |
| <500 µm | 270 | 216 | |
| Water for recirculation | 3962 | 3962 | |
| Mass balance | |||
| Inputs | 4100 | 5100 | 1000 |
| Outputs | 3962 | 5099 | 997 |
| Balance (%) | 96.6 | 99.9 | 99.7 |
| Parameter | MCF |
|---|---|
| Calcium concentration (mg/g) | 198.4 |
| Initial inorganic carbon content (%) | 2.6 |
| Initial CaO content (wt.% of total MCF) | 27.2 |
| Initial CaCO3 content (wt.% of CaO) | 44.3 |
| Rx (%) | 12 |
| Parameter | Unit | Value |
|---|---|---|
| Base case scenario parameters | ||
| Annual period operation | Days | 234 |
| Capacity | t of WC/hour | 75 |
| Daily operation | h/days | 8 |
| Annual capacity of WC | t/year | 131,220 |
| Sand (fine aggregates) | $/t | 6 |
| Coarse aggregates | $/t | 10 |
| Used concrete credit | $/t | 5 |
| Process cost & Revenues | ||
| CAPEX | $ | 1,159,510 |
| OPEX | $/t of WC | 4.31 |
| Sand | $/t of WC | 1.23 |
| Coarse aggregates | $/t of WC | 5.35 |
| Total revenues | $/t of WC | 11.58 |
| Balance | $/t of WC | 7.27 |
| Input Parameters | Unit | Base Case Value |
|---|---|---|
| Cost model parameters: | ||
| Plant treatment capacity | t rocks/h | 80 |
| Annual period of operation | Days | 350 |
| Daily period of operation | Hours | 24 |
| Annual Interest rate | % | 2 |
| Manpower rate | $/h | 25 |
| Distance | Km | 100 |
| Sequestration efficiency | kgCO2/t MCF | 110 |
| Energy unit costs (Electricity) | ¢/kWh | Hydroelectricity: 3.5 |
| Transportation unit cost | $/km | 0.12 |
| Profitability analysis parameters | ||
| Carbon credit price | $/tCO2 | 50 |
| Carbonated concrete value | $/t | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquier, L.-C.; Kemache, N.; Mocellin, J.; Blais, J.-F.; Mercier, G. Waste Concrete Valorization; Aggregates and Mineral Carbonation Feedstock Production. Geosciences 2018, 8, 342. https://doi.org/10.3390/geosciences8090342
Pasquier L-C, Kemache N, Mocellin J, Blais J-F, Mercier G. Waste Concrete Valorization; Aggregates and Mineral Carbonation Feedstock Production. Geosciences. 2018; 8(9):342. https://doi.org/10.3390/geosciences8090342
Chicago/Turabian StylePasquier, Louis-César, Nassima Kemache, Julien Mocellin, Jean-François Blais, and Guy Mercier. 2018. "Waste Concrete Valorization; Aggregates and Mineral Carbonation Feedstock Production" Geosciences 8, no. 9: 342. https://doi.org/10.3390/geosciences8090342
APA StylePasquier, L.-C., Kemache, N., Mocellin, J., Blais, J.-F., & Mercier, G. (2018). Waste Concrete Valorization; Aggregates and Mineral Carbonation Feedstock Production. Geosciences, 8(9), 342. https://doi.org/10.3390/geosciences8090342






