δ13C and δ18O Stable Isotope Analysis Applied to Detect Technological Variations and Weathering Processes of Ancient Lime and Hydraulic Mortars
Abstract
1. Introduction
2. Methods and Materials
2.1. Methods
2.2. Materials
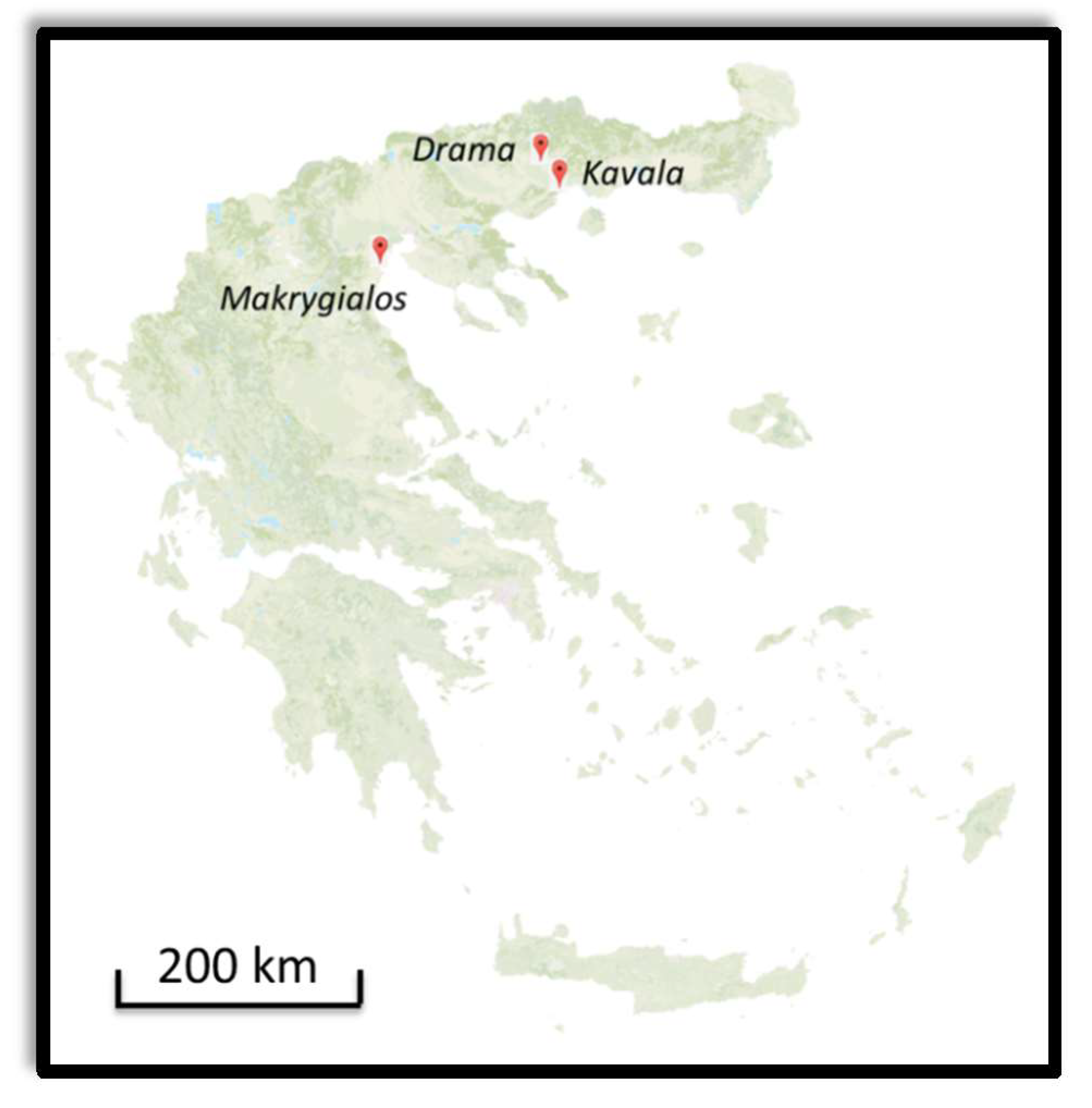
2.2.1. Anaktoroupoli and Land Walls in Kavala
2.2.2. Marmarion Tower in Kavala
2.2.3. Fortification Walls in Drama
2.2.4. Funerary Monuments in Makrygialos
3. Results
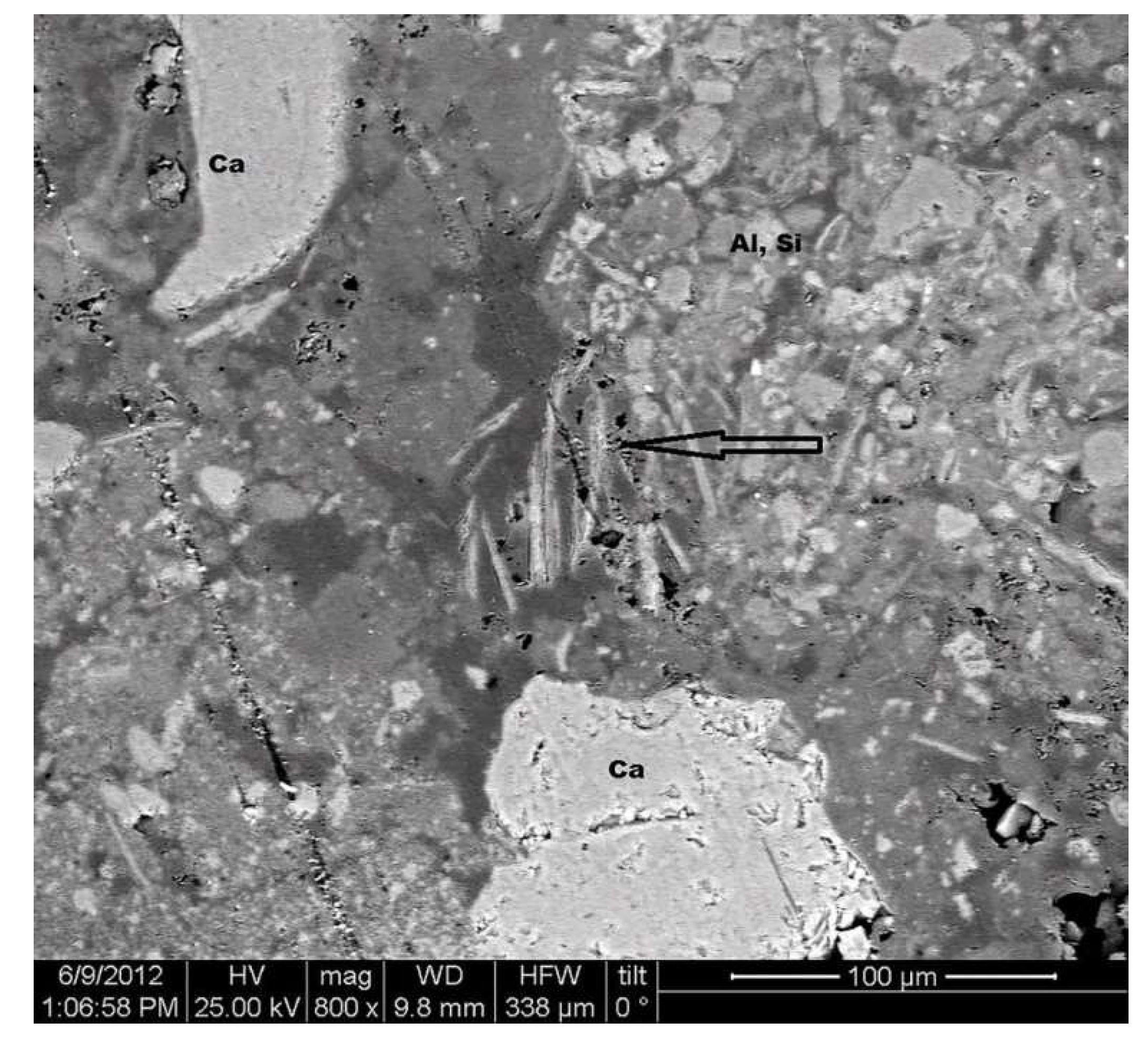
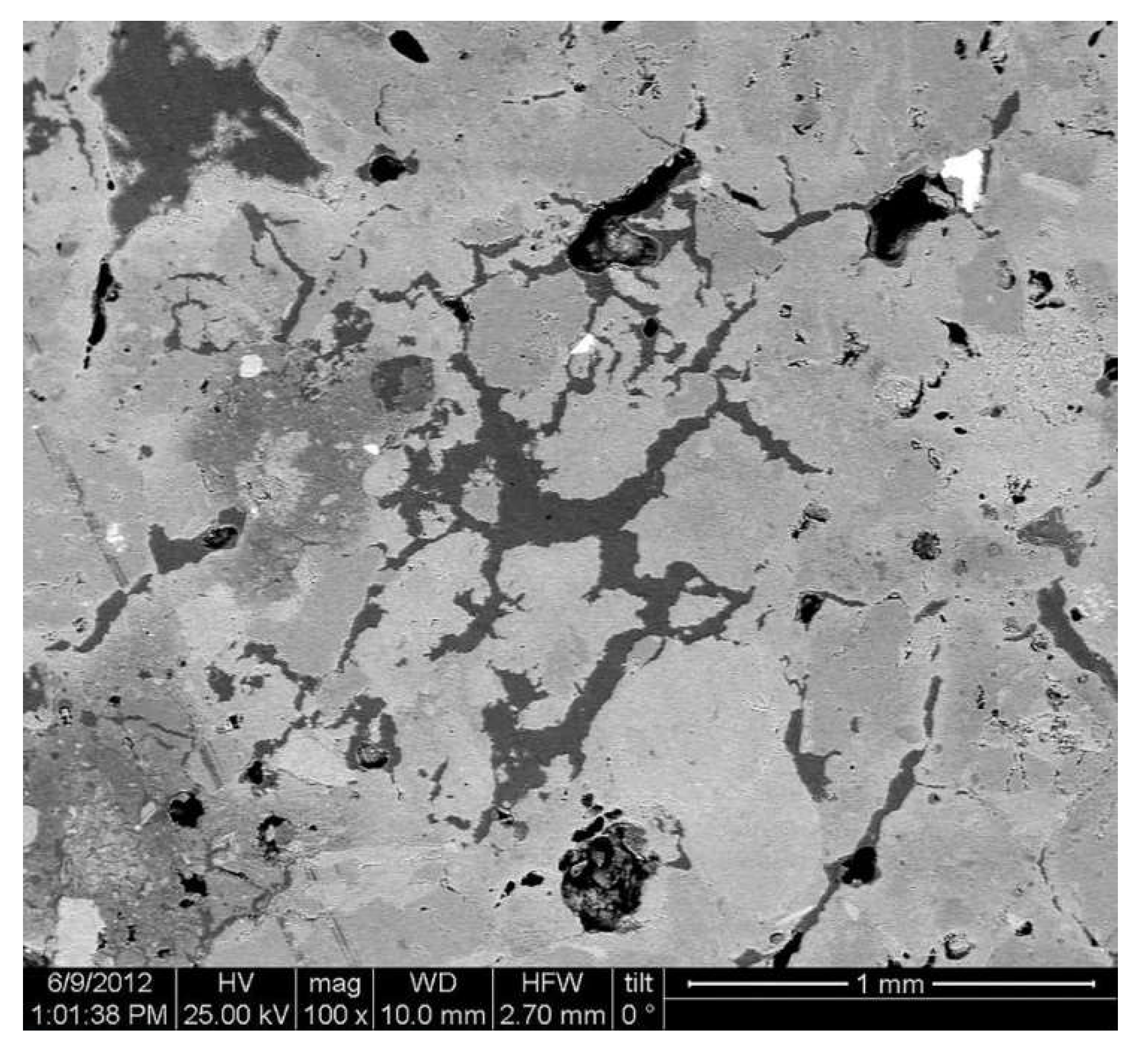
3.1. Micromorphological and Elemental Analysis
3.1.1. Anaktoroupoli-Land Walls Kavala
3.1.2. Fortification Walls in Drama
3.1.3. Marmarion Tower—Kavala
3.1.4. Makrygialos
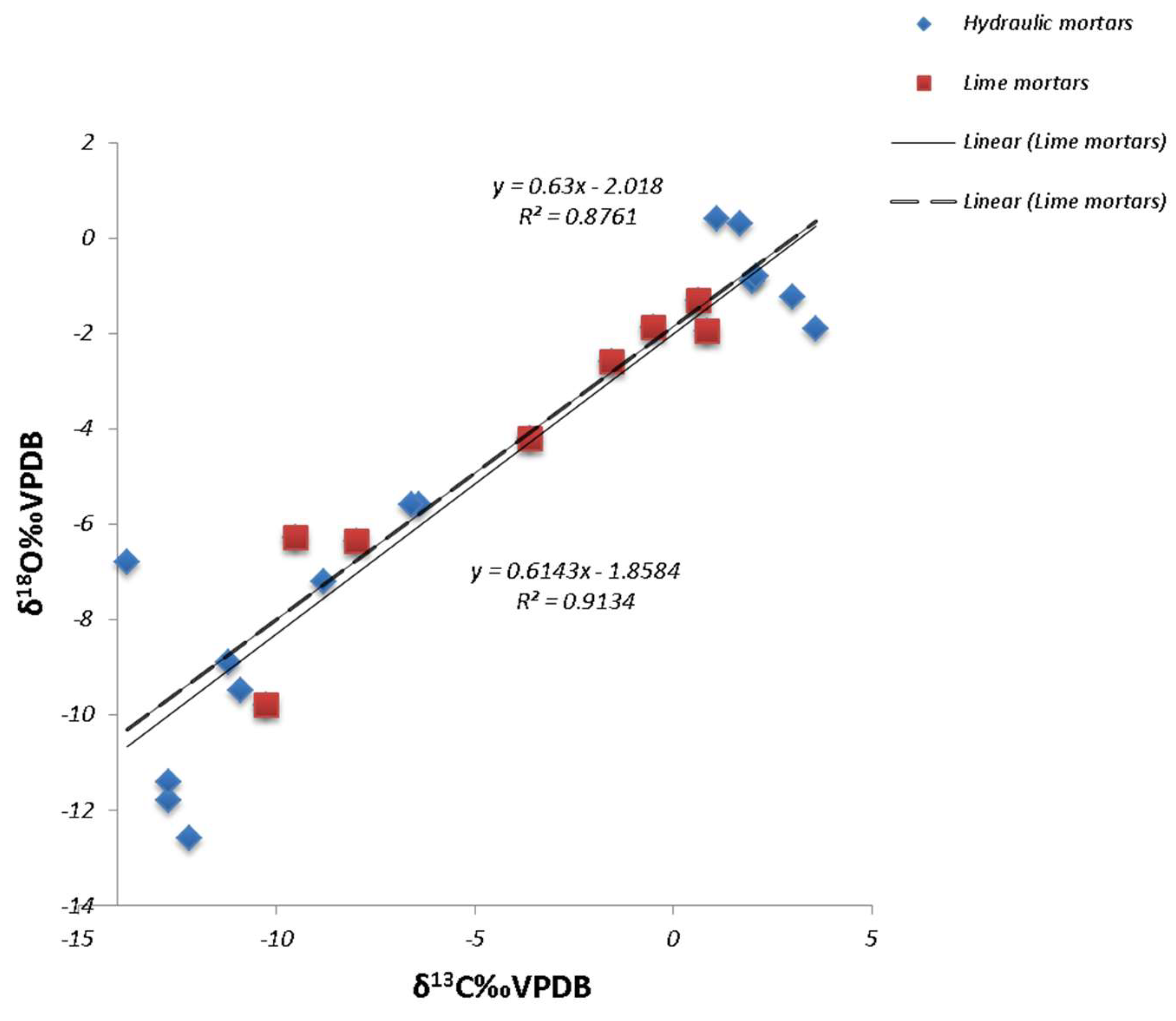
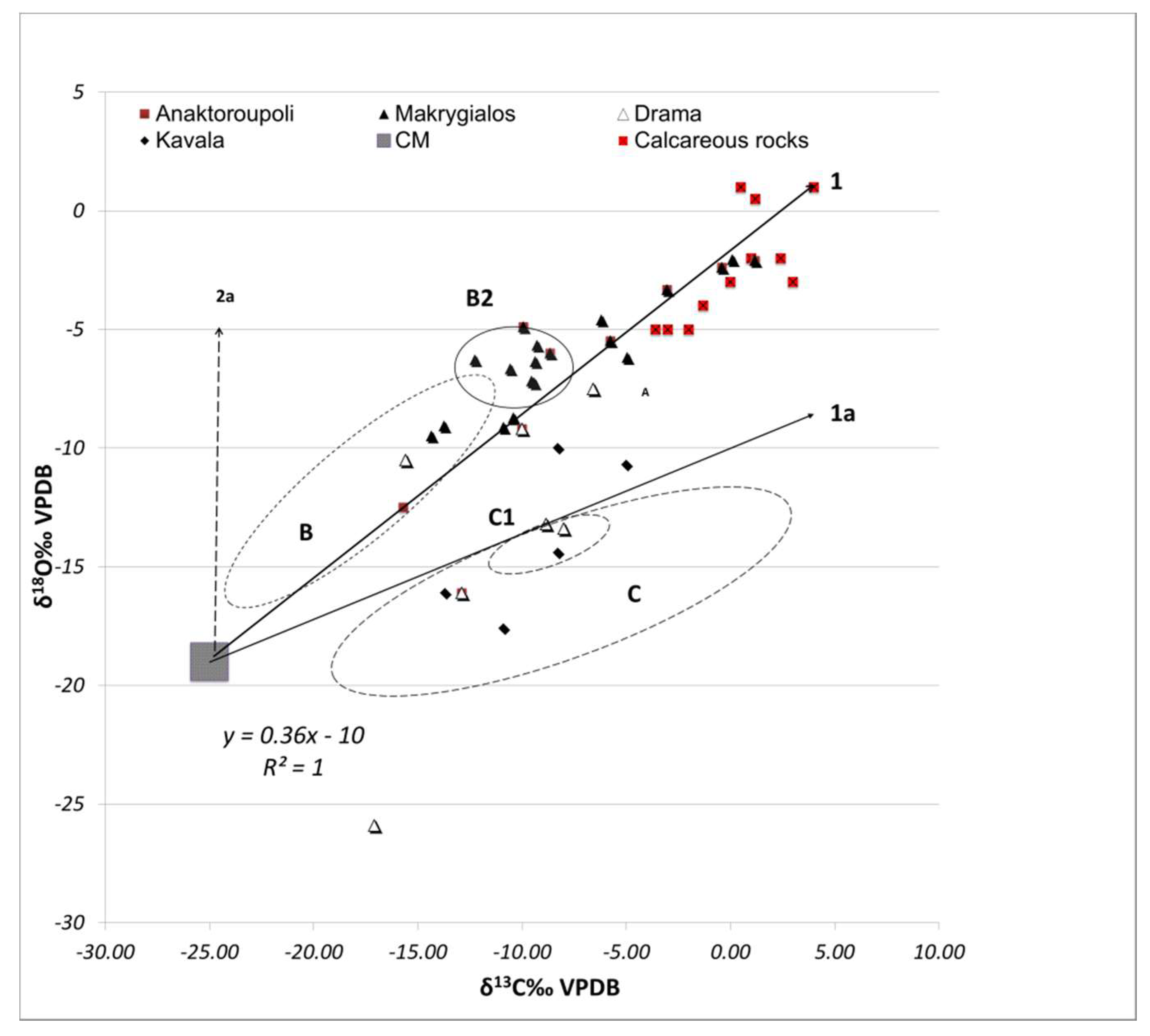
3.2. Stable Isotope Analysis
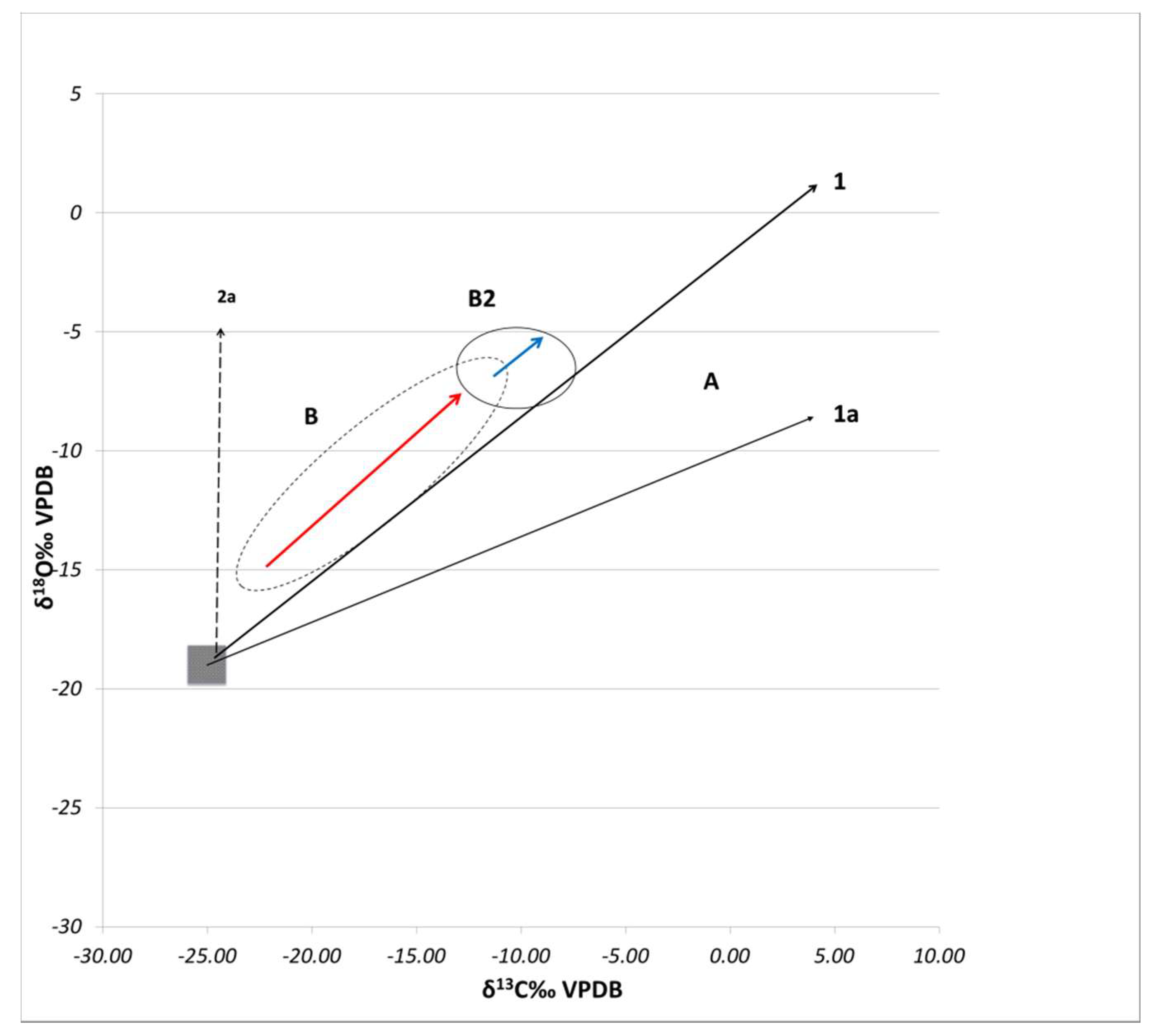
4. Discussion
4.1. Technological Variations and Degradation Mechanisms
4.2. Evaluation of Setting Environments and Processes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabbioni, C.; Zappia, G.; Riontino, C.; Blanco-Varela, M.T.; Aguilera, J.; Puertas, F.; Van Balen, K.; Toumbakari, E.E. Atmospheric deterioration of ancient and modern hydraulic mortars. Atmos. Environ. 2001, 35, 539–548. [Google Scholar] [CrossRef]
- van Balen, K.; Toumbakari, E.E.; Blanco-Varela, M.T.; Aguilera, J.; Puertas, F.; Palomo, A. Environmental deterioration of ancient and modern hydraulic mortars. WIT Trans. Built Environ. 1970, 42, 10. [Google Scholar]
- Yates, T. The Effects of Air Pollution on the Built Environment. Air Pollution Reviews; Brimblecombe, P., Ed.; Imperial College Press: London, UK, 2003; pp. 107–132. [Google Scholar]
- Zappia, G.; Sabbioni, C.; Pauri, M.; Gobbi, G. Mortar damage due to airborne sulfur compounds. Mater. Struct. 1994, 27, 469–473. [Google Scholar] [CrossRef]
- Stefanidou, M.; Papayianni, I. Salt accumulation in historic and repair mortars. In Proceedings of the Heritage, Weathering and Conservation Conference, Consejo Superior de Investigaciones Cientificas, Madrid, Spain, 21–24 June 2006; Taylor & Francis: London, UK, 2006; pp. 269–272. [Google Scholar]
- Stefanidou, M. A contribution to salt crystallization into the structure of traditional repair mortars through capillarity. In Proceedings of the 11th euroseminar on microscopy applied to building materials, Porto, Portugal, 5–9 June 2007. [Google Scholar]
- Theoulakis, P.; Moropoulou, A. Microstructural and mechanical parameters determining the susceptibility of porous building stones to salt decay. Constr. Build. Mater. 1997, 11, 65–71. [Google Scholar] [CrossRef]
- Rossi-Manaresi, R.; Tucci, A. Pore structure and the disruptive or cementing effect of salt crystallization in various types of stone. Stud. Conserv. 1991, 36, 56–58. [Google Scholar]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Papayianni, I.; Stefanidou, M.; Pachta, V.; Konopisi, S. Content and topography of salts in historic mortars. In Proceedings of the 3rd Historic mortars conference, Glasgow, Scotland, 11–14 September 2013. [Google Scholar]
- Gomides, M.d.J.; Molin, D.C.C.D.; Rêgo, J.H.d.S. Effect of the incorporation of aggregates with high sulfide content on the mechanical and microstructural properties of concrete with slag cement. Matéria (Rio de Janeiro) 2017, 22. [Google Scholar] [CrossRef]
- Chinchón, J.; Ayora, C.; Aguado, A.; Guirado, F. Influence of weathering of iron sulfides contained in aggregates on concrete durability. Cem. Concr. Res 1995, 25, 1264–1272. [Google Scholar] [CrossRef]
- Clark, I.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Maravelaki-Kalaitzaki, P.; Bakolas, A.; Moropoulou, A. Physico-chemical study of Cretan ancient mortars. Cement and Concrete Research 2003, 33, 651–661. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Investigation of the technology of historic mortars. J. Cult. Her. 2000, 1, 45–58. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Characterization of ancient, byzantine and later historic mortars by thermal and X-ray diffraction techniques. Thermochim. Act. 1995, 269, 779–795. [Google Scholar] [CrossRef]
- Bakolas, A.; Biscontin, G.; Moropoulou, A.; Zendri, E. Characterization of the lumps in the mortars of historic masonry. Thermochim. Acta 1995, 269, 809–816. [Google Scholar] [CrossRef]
- Dotsika, E.; Psomiadis, D.; Raco, B.; Poutoukis, D.; Gamaletsos, P. Isotopic analysis for degradation diagnosis of calcite matrix in mortar and plaster. Anal. Bioanal. Chem. 2009, 395, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Kosednar-Legenstein, B.; Dietzel, M.; Leis, A.; Stingl, K. Stable carbon and oxygen isotope investigation in historical lime mortar and plaster–Results from field and experimental study. Appl. Geochem. 2008, 23, 2425–2437. [Google Scholar] [CrossRef]
- Larbi, J. Microscopy applied to the diagnosis of the deterioration of brick masonry. Constr. Build. Mater. 2004, 18, 299–307. [Google Scholar] [CrossRef]
- Hughes, J.J.; Cuthbert, S.J. The petrography and microstructure of medieval lime mortars from the west of Scotland: Implications for the formulation of repair and replacement mortars. Mater. Struct. 2000, 33, 594–600. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Forming Minerals, 2nd ed.; Longman Scientific & Technical: London, UK, 1992; p. 696. [Google Scholar]
- Tucker, M.E. Sedimentary Petrology, 2nd ed.; Blackwell Scientific Publications: Hoboken, NW, USA, 1991. [Google Scholar]
- Adams, A.E.; MacKenzie, W.S.; Guildford, C. Atlas of Sedimentary Rocks Under the Microscope; Routledge: Abington, UK, 2017. [Google Scholar]
- Whitbread, I.K. The characterisation of argillaceous inclusions in ceramic thin sections. Archaeometry 1986, 28, 79–88. [Google Scholar] [CrossRef]
- Freestone, I.C. Ceramic Petrography. Am. J. Archaeol. 1995, 99, 111–115. [Google Scholar]
- Coplen, T.B; Kendall, C.; Hopple, J. Comparison of stable isotope reference samples. Nature 1983, 302, 236–238. [Google Scholar] [CrossRef]
- Coplen, T.B. Reporting of stable hydrogen, carbon and oxygen isotopic abundances (technical report). Int. Union Pure Appl. Chemi. 1994, 66, 273–276. [Google Scholar] [CrossRef]
- Coplen, T.B. Discontinuance of Smow and PDB. Nature 1995, 375, 285. [Google Scholar] [CrossRef]
- Chabas, A.; Jeannette, D. Weathering of marbles and granites in marine environment: petrophysical properties and special role of atmospheric salts. Environ. Geol. 2001, 40, 359–368. [Google Scholar] [CrossRef]
- Liu, P. Damage to concrete structures in a marine environment. Mater. Struct. 1991, 24, 302–307. [Google Scholar] [CrossRef]
- Tsouris, E.M.A. Byzantine fortifications in Evros. Byzantina 2006, 26, 153–209. [Google Scholar]
- Dadaki, S. (Greek Ministry of Culture, Athens, Greece). Historical overview of the antiquities of 12th EBA, 12th Ephoreia of Byzantine antiquities, Archaeological report. Unpublished work. 2010. [Google Scholar]
- Mpesios, M. Pieridon Stephanos: Pydna, Methone Kai Hoi Archaiotetes Tes Voreias Pierias; Anthropon Physeos Erga: Katerine, Greece, 2010. [Google Scholar]
- van Strydonck, M.J.; Dupas, M.; Keppens, E. Isotopic fractionation of oxygen and carbon in lime mortar under natural environmental-conditions. Radiocarbon 1989, 31, 610–618. [Google Scholar] [CrossRef]
- Papayianni, I.; Stefanidou, M. Strength–porosity relationships in lime–pozzolan mortars. Constr. Build. Mater. 2006, 20, 700–705. [Google Scholar] [CrossRef]
- Walker, R.; Pavía, S. Behaviour and Properties of Lime-Pozzolan Pastes. In Proceedings of the 8th International Masonry Conference 2010, Dresden, Germany, 4–7 July 2010; Jäger, W., Haseltine, B., Fried, A., Eds.; International Masonry Society: Shermanbury, UK, 2010; pp. 353–362. [Google Scholar]
- Walker, R.; Pavía, S. Physical properties and reactivity of pozzolans, and their influence on the properties of lime-pozzolan pastes. Mater. Struct. 2011, 44, 1139–1150. [Google Scholar] [CrossRef]
- Matias, G.; Faria, P.; Torres, I. Lime mortars with heat treated clays and ceramic waste: a review. Constr. Build. Mater. 2014, 73, 125–136. [Google Scholar] [CrossRef]
- O’Neil, J.R.; Barnes, I. 13C and 18O compositions in some fresh-water carbonates associated with ultramafic rocks and serpentinites: Western United States. Geochim. Cosmichim. Acta. 1971, 35, 687–697. [Google Scholar] [CrossRef]
- Pachiaudi, C.; Marechal, J.; van Strydonck, M.; Dupas, M.; Dauchot- Dehon, M. Isotopic fractionation of carbon during CO2 absorption by mortar. Radiocarbon 1986, 28, 691–697. [Google Scholar] [CrossRef]
- Rafai, N.; Letolle, R.; Blanc, P.; Gegout, P.; Revertegat, E. Carbonation–decarbonation of concretes studied by the way of carbon and oxygen stable isotopes. Cem. Concr. Res. 1992, 22, 882–890. [Google Scholar] [CrossRef]
- Dietzel, M.; Usdowski, E.; Hoefs, J. Chemical and 13C/12Cand 18O/16O-isotope evolution of alkaline drainage waters and the precipitation of calcite. Appl. Geochem. 1992, 7, 177–184. [Google Scholar] [CrossRef]
- Clark, I.D.; Fontes, J.C.; Fritz, P. Stable isotope disequilibria in travertine from high pH waters: Laboratory investigations and field observations from Oman. Geochem. Cosmchim. Acta 1992, 56, 2041–2050. [Google Scholar] [CrossRef]
- Dotsika, E.; Lykoudis, S.; Poutoukis, D. Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Glob. Planet. Chang. 2010, 71, 141–149. [Google Scholar] [CrossRef]
- Taylor, R.M. Roman Builders: A Study in Architectural Process; Cambridge University Press: Cambridge, UK, 2003; pp. 175–178. [Google Scholar]
- Usdowski, E.; Hoefs, J. Oxygen isotope exchange between carbonic acid, bicarbonate, carbonate, and water: A re-examination of the data of McCrea (1950) and an expression for the overall partitioning of oxygen isotopes between the carbonate species and water. Geochim. Cosmochim. Acta 1993, 57, 3815–3818. [Google Scholar] [CrossRef]




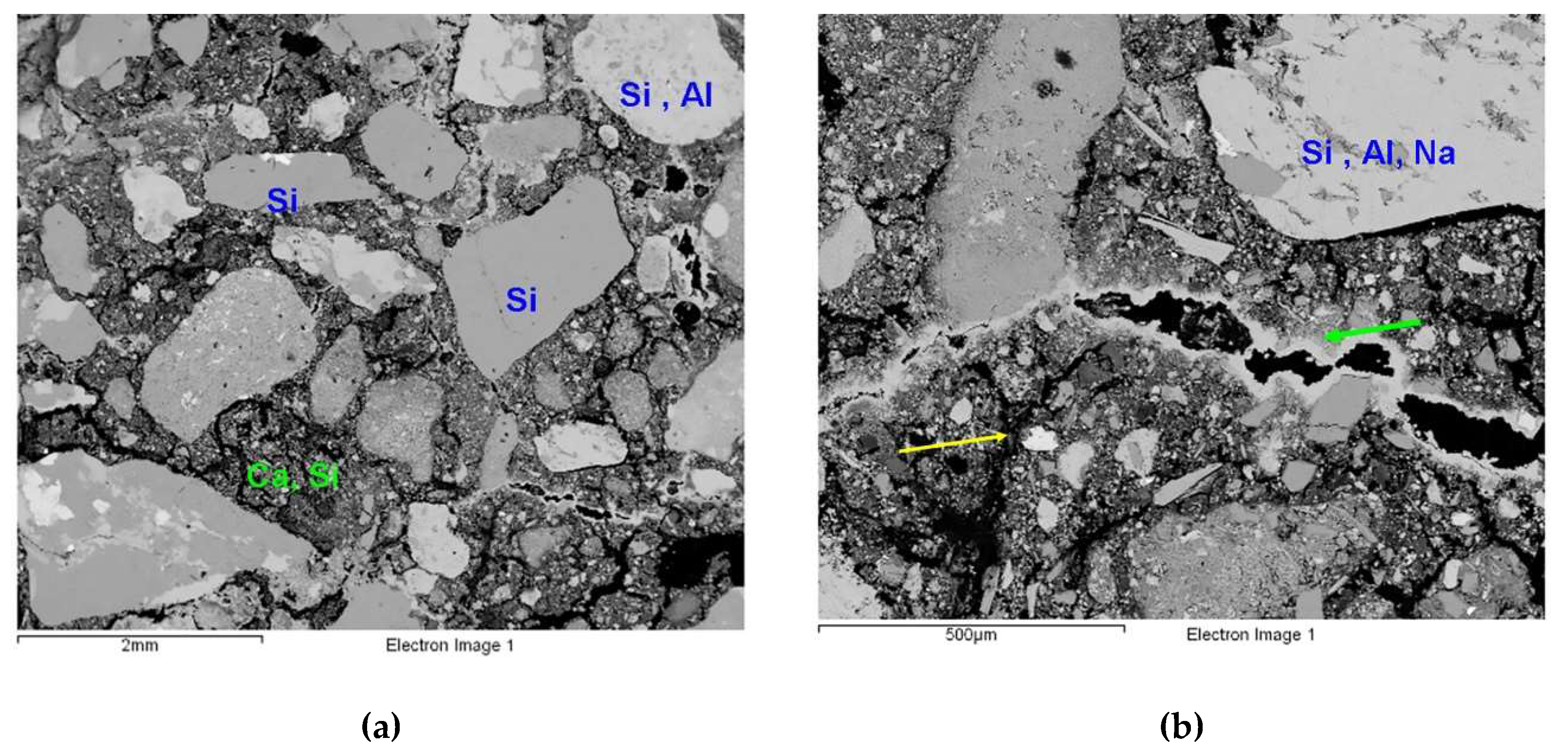
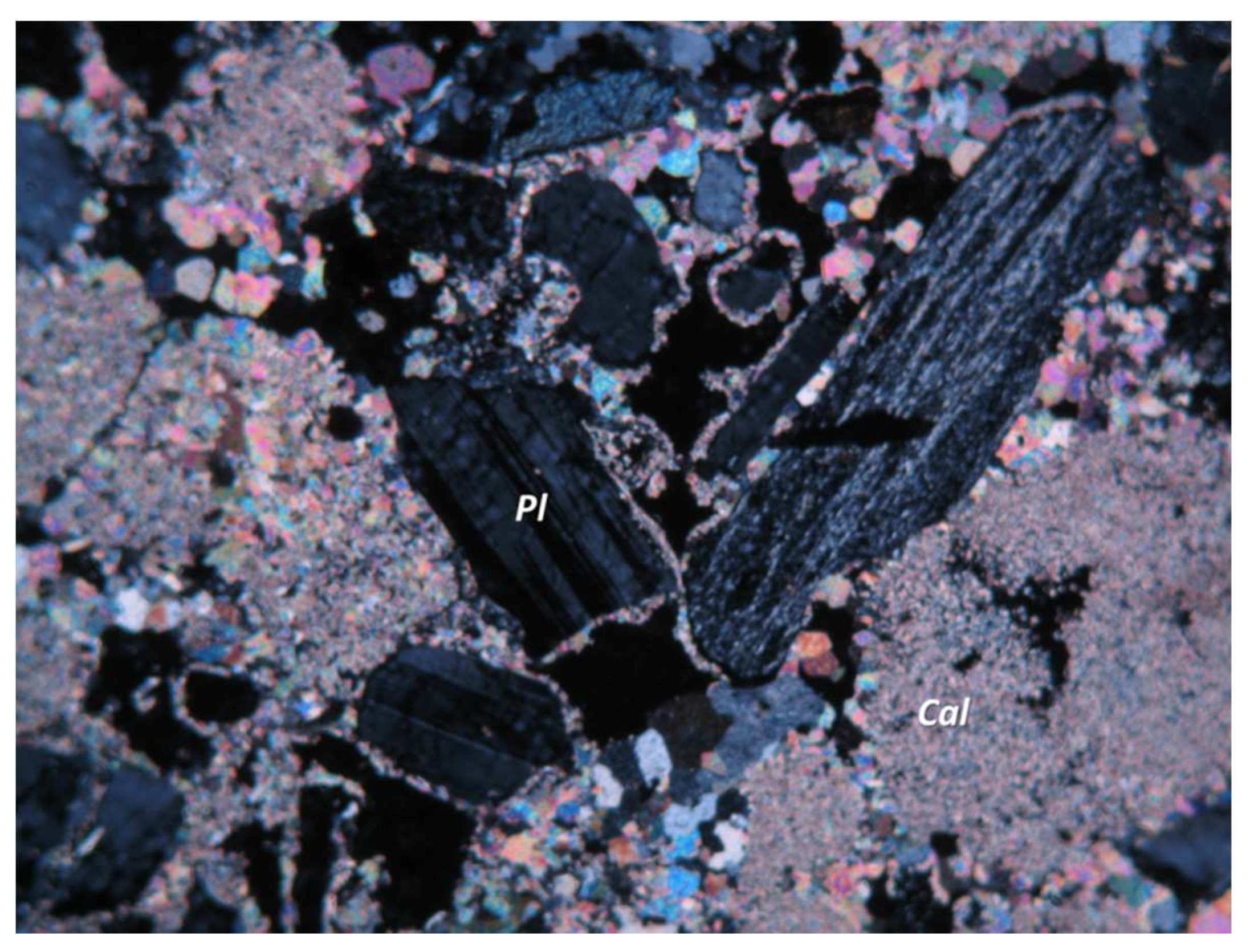
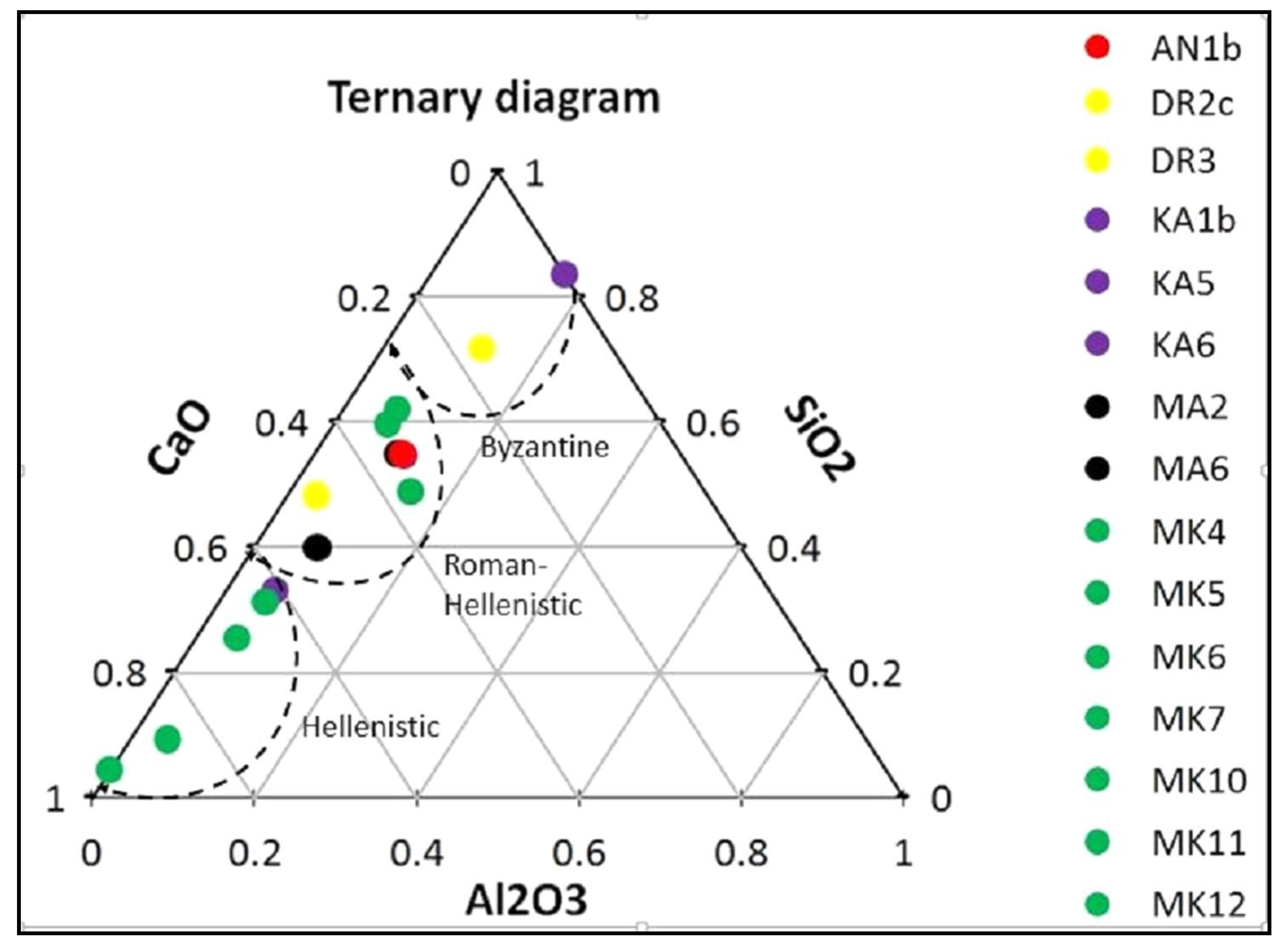
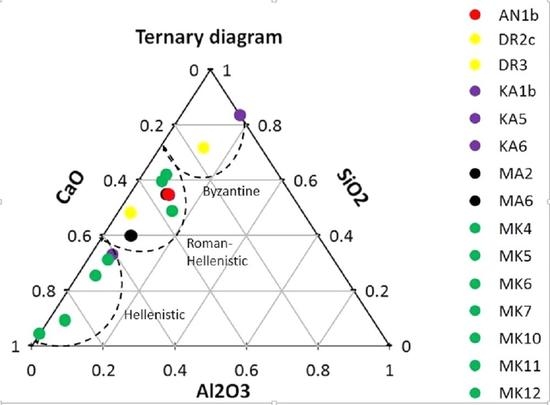
| Sample | Location | Al2O3 | SiO2 | CaO | MgO | Cl2O | K2O | Na2O | BaO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| AN1b | Anaktoroupoli, Kavala | 10.4 | 50.7 | 31.7 | na | 0.6 | 3.2 | 2.4 | na | na |
| DR2c | Drama | 9.8 | 57.6 | 13.0 | 13.2 | 1.4 | na | na | na | 5.1 |
| Dr3 | Drama | 3.5 | 49.0 | 49.0 | 1.3 | 1.1 | 1.1 | na | na | na |
| KA1b | Land walls, Kavala | 10.4 | 50.7 | 31.7 | na | 0.6 | na | 2.4 | na | na |
| KA5 | Land walls, Kavala | 5.3 | 29.8 | 54.5 | na | 2.4 | na | 3.8 | na | na |
| KA6 | Land walls, Kavala | 7.6 | 38.7 | na | 0.5 | 22.2 | 3.3 | 26.3 | 1.5 | na |
| MA2 | Marmarion Tower, Kavala | 9.8 | 52.9 | 33.4 | na | na | na | na | na | na |
| MA6 | Marmarion Tower, Kavala | 7 | 36.5 | 47.7 | 1.7 | 1.5 | na | na | na | 5.4 |
| MK4 | Makrygialos | 6.2 | 58.7 | 29.7 | 1.2 | na | 0.7 | 0.5 | na | 1.5 |
| MK5 | Makrygialos | 13.2 | 43.4 | 32.0 | 2.3 | na | 2.2 | na | na | 4.5 |
| MK6 | Makrygialos | 4.8 | 23.9 | 65.0 | 1.2 | na | 2.3 | na | na | 1.1 |
| MK7 | Makrygialos | 4.6 | 9.4 | 86.0 | na | na | na | na | na | na |
| MK10 | Makrygialos | na | 4.4 | 95.6 | na | na | na | na | na | na |
| MK11 | Makrygialos | 6.2 | 56.0 | 31.7 | 0.7 | na | 1.5 | na | na | 1 |
| MK12 | Makrygialos | 5.3 | 30.0 | 60.2 | 1.0 | na | 1.0 | na | na | 1.6 |
| SampleID | Observation | δ13C (‰VPDB) | δ18O (‰VPDB) | Location |
|---|---|---|---|---|
| AN1a-1 | Ext.Joint | −12.9 | −16.1 | Anaktoroupoli, Kavala |
| AN2a-1 | Ext.Joint | −10.0 | −9.2 | Anaktoroupoli, Kavala |
| AN2a-2 | Ext.Joint | −5.8 | −5.5 | Anaktoroupoli, Kavala |
| AN3a-1 | Ext.Joint | −0.4 | −2.4 | Anaktoroupoli, Kavala |
| AN1b-1 | Ext.Joint | −15.7 | −12.5 | Anaktoroupoli, Kavala |
| AN9-1 | Ext.Joint | −8.7 | −6 | Anaktoroupoli, Kavala |
| Anp-1 | Ext.Joint | −9.9 | −4.9 | Anaktoroupoli, Kavala |
| DRa-1 | Ext.Joint | −10.0 | −9.2 | Drama |
| DRa-2 | Ext.Joint | −17.1 | −25.9 | Drama |
| DR1b-1 | Ext.Joint | −8 | −13.4 | Drama |
| DR2a-1 | Ext.Joint | −8.9 | −13.2 | Drama |
| DR2c-1 | Ext.Joint | −13.7 | −16.1 | Drama |
| DR5a-1 | Ext.Joint | −12.9 | −16.1 | Drama |
| DR5b-1 | Ext.Joint | −15.6 | −10.5 | Drama |
| DR2g-1 | Ext.Joint | −6.6 | −7.5 | Drama |
| MA1-3 | Ext.Joint | −10.9 | −17.6 | Marmarion Tower, Kavala |
| MA3-1 | Ext.Joint | −8.3 | −14.4 | Marmarion Tower, Kavala |
| MA5-1 | Ext.Joint | −5 | −10.7 | Marmarion Tower, Kavala |
| MA5-2 | Int.joint | −8.3 | −10 | Marmarion Tower, Kavala |
| MK1-1 | Rend.Ext. | −12.3 | −6.3 | Makrygialos |
| MK1-2 | Rend.Int | −13.8 | −9.1 | Makrygialos |
| MK1-3 | Rend.Int | −14.4 | −9.5 | Makrygialos |
| MK2-1 | Rend.Ext. | −6.2 | −4.6 | Makrygialos |
| MK2-3 | Rend.Int | −9.4 | −7.3 | Makrygialos |
| MK3-1 | Rend.Ext. | −10.4 | −8.7 | Makrygialos |
| MK4-1 | Rend.Ext. | −9.4 | −6.4 | Makrygialos |
| MK4-2 | Rend.Int | −10.9 | −9.1 | Makrygialos |
| MK5-1 | Rend.Ext. | −9.3 | −5.7 | Makrygialos |
| MK5-2 | Rend.Int | −9.6 | −7.1 | Makrygialos |
| MK6-1 | Rend.Ext. | 0.1 | −2.0 | Makrygialos |
| MK6-2 | Rend.Int | −9.9 | −4.9 | Makrygialos |
| MK7-1 | Rend.Ext. | −5.0 | −6.2 | Makrygialos |
| MK7-2 | Rend.Int | −8.7 | −6.0 | Makrygialos |
| MK8-1 | Rend.Ext. | 1.2 | −2.1 | Makrygialos |
| M8-2 | Rend.Int | −3.0 | −3.3 | Makrygialos |
| MK9-1 | Rend.Ext. | −0.4 | −2.4 | Makrygialos |
| MK9-2 | Rend.Int | −10.6 | −6.7 | Makrygialos |
| MK10-1 | Rend.Ext. | −5.8 | −5.5 | Makrygialos |
| MK10-2 | Rend.Int | −3.0 | −3.4 | Makrygialos |
| Sample ID | Depth (cm) | δ13C (‰VPDB) | δ18O (‰VPDB) |
|---|---|---|---|
| Hydraulic. (lime-pozzolan) mortars. (Kavala. Drama) | −6 | 3.6 | −1.9 |
| −6 | 3 | −1.3 | |
| −6 | 2 | −0.9 | |
| −6 | 1.7 | 0.3 | |
| −6 | 1.1 | 0.4 | |
| −3 | −6.4 | −5.6 | |
| −3 | −6.6 | −5.6 | |
| −3 | −8.8 | −7.2 | |
| +0 | −10.9 | −9.5 | |
| +0 | −12.2 | −12.6 | |
| +0 | −11.2 | −8.9 | |
| +0 | −12.7 | −11.8 | |
| +0 | −12.7 | −11.4 | |
| +0 | −13.6 | −6.8 | |
| Lime mortars (Hellenistic mortars. Makrygialos) | −6 | 0.7 | −1.3 |
| −6 | 0.9 | −2 | |
| −6 | −0.5 | −1.9 | |
| −3 | −1.6 | −2.6 | |
| −3 | −3.6 | −4.2 | |
| +0 | −8 | −6.4 | |
| +0 | −9.5 | −6.3 | |
| +0 | −10.3 | −9.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsika, E.; Kyropoulou, D.; Christaras, V.; Diamantopoulos, G. δ13C and δ18O Stable Isotope Analysis Applied to Detect Technological Variations and Weathering Processes of Ancient Lime and Hydraulic Mortars. Geosciences 2018, 8, 339. https://doi.org/10.3390/geosciences8090339
Dotsika E, Kyropoulou D, Christaras V, Diamantopoulos G. δ13C and δ18O Stable Isotope Analysis Applied to Detect Technological Variations and Weathering Processes of Ancient Lime and Hydraulic Mortars. Geosciences. 2018; 8(9):339. https://doi.org/10.3390/geosciences8090339
Chicago/Turabian StyleDotsika, Elissavet, Dafni Kyropoulou, Vassilios Christaras, and Georgios Diamantopoulos. 2018. "δ13C and δ18O Stable Isotope Analysis Applied to Detect Technological Variations and Weathering Processes of Ancient Lime and Hydraulic Mortars" Geosciences 8, no. 9: 339. https://doi.org/10.3390/geosciences8090339
APA StyleDotsika, E., Kyropoulou, D., Christaras, V., & Diamantopoulos, G. (2018). δ13C and δ18O Stable Isotope Analysis Applied to Detect Technological Variations and Weathering Processes of Ancient Lime and Hydraulic Mortars. Geosciences, 8(9), 339. https://doi.org/10.3390/geosciences8090339