Survivability of Soil and Permafrost Microbial Communities after Irradiation with Accelerated Electrons under Simulated Martian and Open Space Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects of Study
2.2. Preparation of Samples for Irradiation with Accelerated Electrons
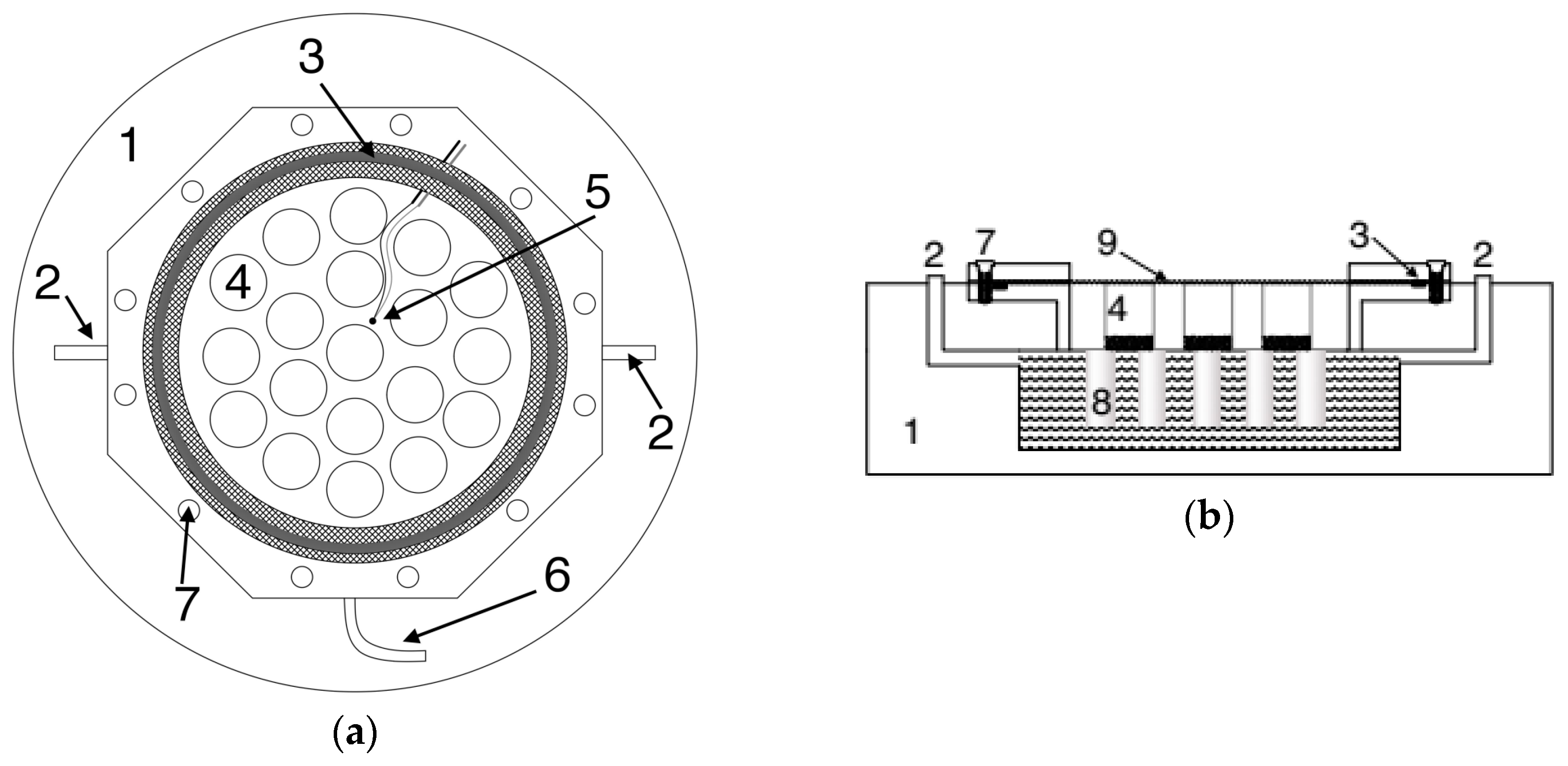
2.3. Description of the Climatic Chamber
2.4. Irradiation of Samples with Accelerated Electrons
2.5. Culturing of Microbial Communities
2.6. Accounting for the Total Numbers of Prokaryotes
2.7. Multisubstrate Testing of Integral Metabolic Activity and Functional Diversity of Microbial Communities (MST)
2.8. Identification of Bacterial Isolates
2.9. Investigation of the Physiological Characteristics of Isolates
2.10. Irradiation of Pure Bacterial Cultures with Gamma Radiation
3. Results and Discussion
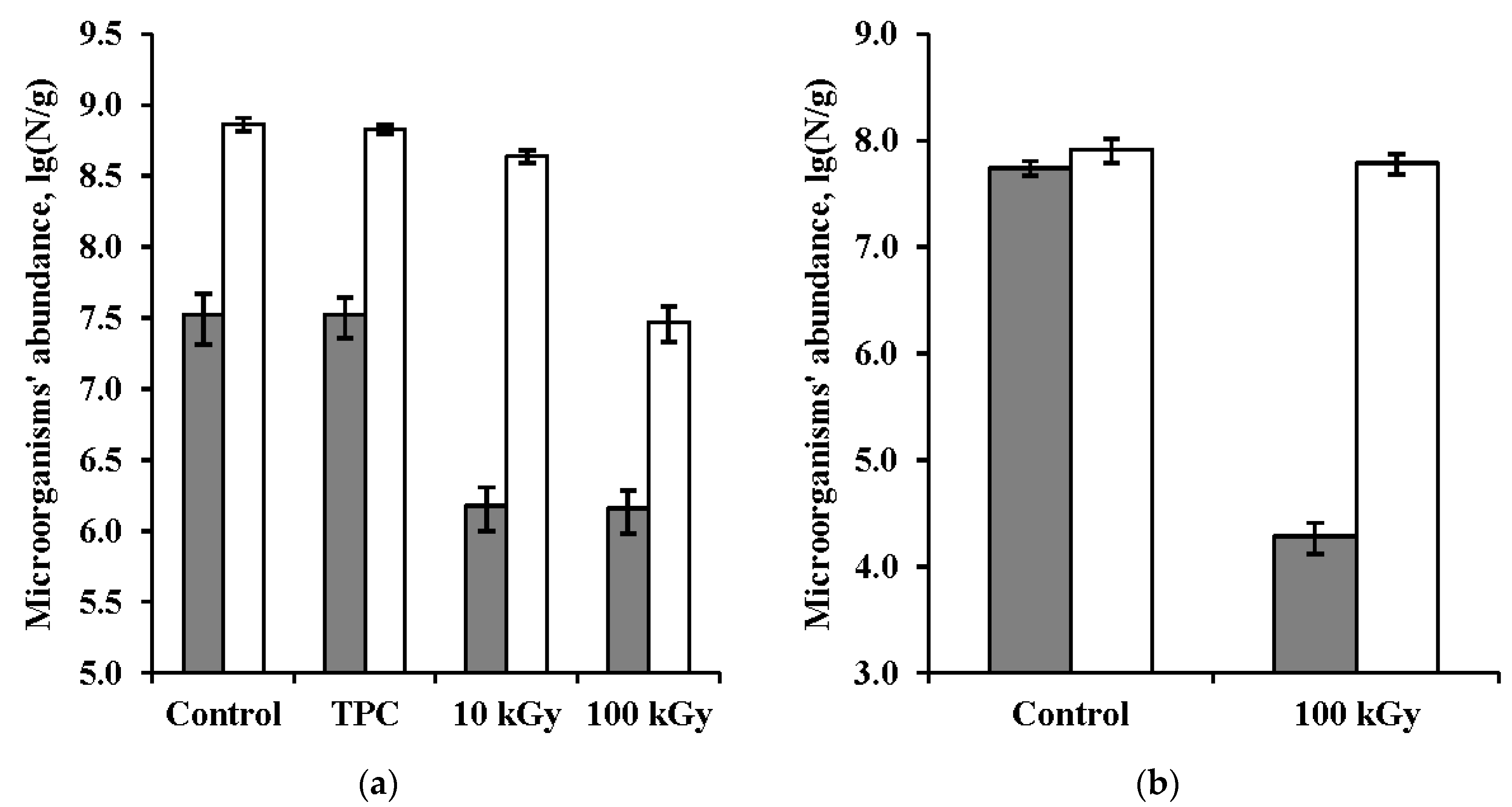
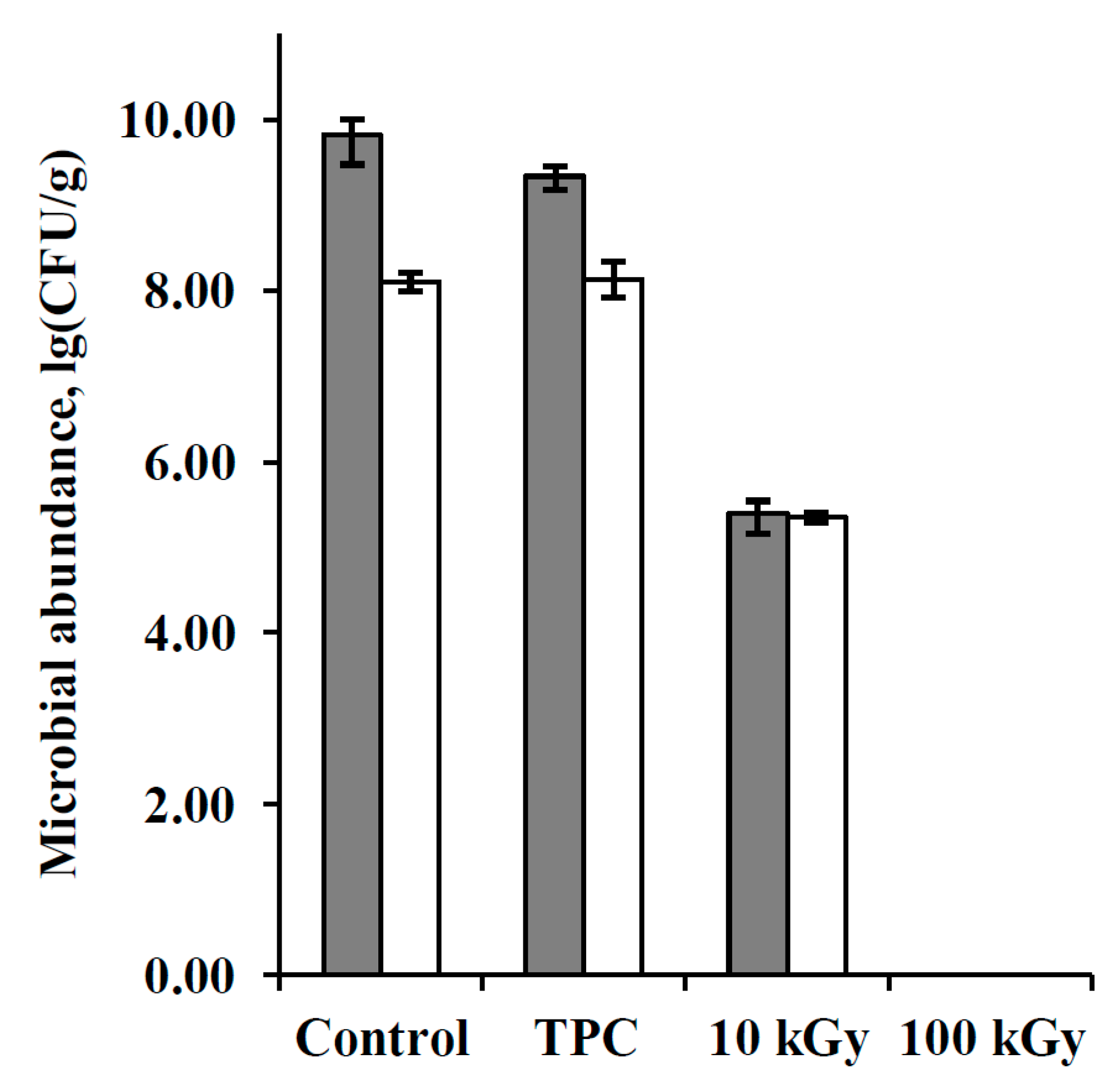
3.1. Bacteria Abundance in the Samples
3.2. Metabolic Activity of Microbial Communities
3.3. Taxonomic Affiliation and Physiological Characteristics of Bacterial Isolates
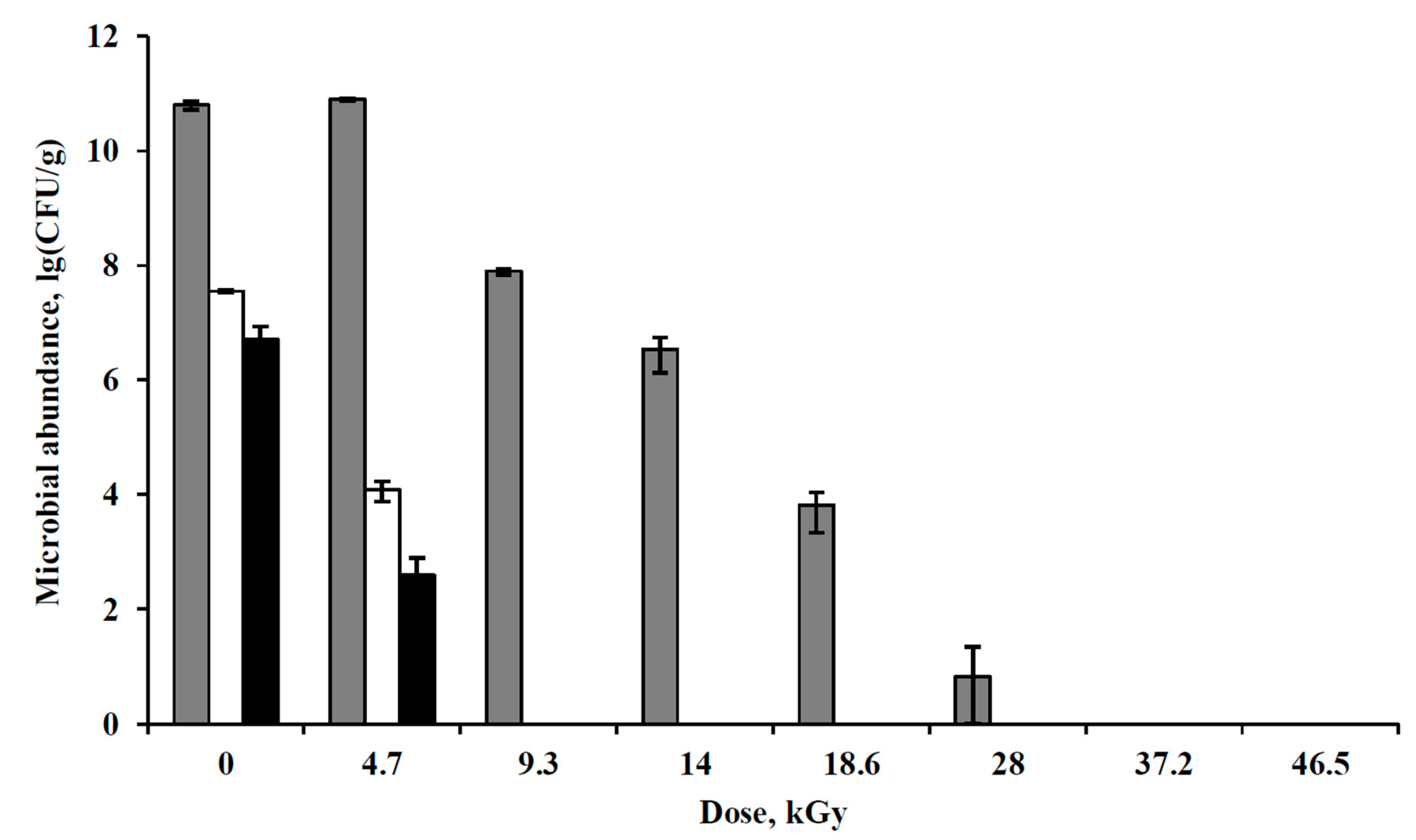
3.4. Irradiation of Pure Bacterial Cultures with Accelerated Electrons under Simulated Extraterrestrial Conditions
3.5. Survivability of Pure Bacterial Cultures under Gamma Irradiation
3.6. Implications for Habitability Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Strain—GenBank Accession Number | Primers Used for the Amplification | Primers Used for the Sequencing |
|---|---|---|
| KBP.AS.531—MH050938 | 63F+1387R | 1100R |
| KBP.AS.532—MH050939 | 27F+537R | 537R |
| KBP.AS.340—MH050940 | 63F+1387R | 1100R |
| KBP.AS.341—MH050941 | 63F+1387R | 1100R |
| KBP.AS.342—MH050942 | 63F+1387R | 1100R |
| KBP.AS.343—MH050943 | 63F+1387R | 1100R |
| KBP.AS.344—MH050944 | 63F+1387R | 1100R |
| KBP.AS.345—MH050945 | 63F+1387R | 1100R; 537R |
| KBP.AS.346—MH050946 | 63F+1387R | 1100R |
| KBP.AS.347—MH050947 | 63F+1387R | 1100R |
| KBP.AS.298—MH050948 | 63F+1387R | 1100R |
| KBP.AS.299—MH050949 | 63F+1387R | 1100R |
| KBP.AS.300—MH050950 | 63F+1387R | 1100R |
| KBP.AS.533—MH050951 | 63F+1387R | 1100R |
| KBP.AS.301—MH050952 | 63F+1387R | 1100R |
| KBP.AS.302—MH050953 | 63F+1387R | 1100R |
| KBP.AS.303—MH050954 | 63F+1387R | 1100R |
| KBP.AS.534—MH050955 | 27F+Un1492R | 1100R |
| KBP.AS.297—MH050956 | 63F+1387R | 1100R |
| KBP.AS.321—MH050957 | 63F+1387R | 1100R; 537R |
| KBP.AS.322—MH050958 | 63F+1387R | 1100R |
| KBP.AS.323—MH050959 | 63F+1387R | 1100R |
| KBP.AS.324—MH050960 | 63F+1387R | 1100R |
| KBP.AS.325—MH050961 | 63F+1387R | 1100R |
| KBP.AS.535—MH050962 | 63F+1387R | 1100R |
| KBP.AS.536—MH050963 | 63F+1387R | 1100R |
| KBP.AS.326—MH050964 | 63F+1387R | 1100R; 537R |
| KBP.AS.317—MH050965 | 63F+1387R | 1100R |
| KBP.AS.319—MH050966 | 63F+1387R | 1100R |
| KBP.AS.320—MH050967 | 63F+1387R | 1100R |
| KBP.AS.537—MH050968 | 63F+1387R | 1100R |
Appendix B
| SN2, TPC | SN2, 10 kGy | SN2, 100 kGy |
|---|---|---|
| Arthrobacter | Arthrobacter | Arthrobacter |
| Saccharothrix | ||
| Phenylobacterium | ||
| Cryobacterium | ||
| Streptomyces | ||
| Microbacterium | ||
| Micrococcus | Micrococcus | |
| Sphingoaurantiacus | ||
| Bacillus | ||
| Spirosoma | Spirosoma | |
| Rufibacter | Rufibacter | |
| Planomicrobium | ||
| Massilia | ||
| Microvirga | ||
| Pontibacter |
References
- Cockell, C.S.; Schwendner, P.; Perras, A.; Rettberg, P.; Beblo-Vranesevic, K.; Bohmeier, M.; Rabbow, E.; Moissl-Eichinger, C.; Wink, L.; Marteinsson, V.; et al. Anaerobic microorganisms in astrobiological analogue environments: From field site to culture collection. Int. J. Astrobiol. 2017, 1–15. [Google Scholar] [CrossRef]
- Horneck, G.; Walter, N.; Westall, F.; Grenfell, J.L.; Martin, W.F.; Gomez, F.; Leuko, S.; Lee, N.; Onofri, S.; Tsiganis, K.; et al. AstRoMap: European astrobiology roadmap. Astrobiology 2016, 16, 201–243. [Google Scholar] [CrossRef] [PubMed]
- Domagal-Goldman, S.D.; Wright, K.E.; Adamala, K.; Arina de la Rubia, L.; Bond, J.; Dartnell, L.R.; Goldman, A.D.; Lynch, K.; Naud, M.-E.; Paulino-Lima, I.G.; et al. The astrobiology primer v2.0. Astrobiology 2016, 16, 561–653. [Google Scholar] [CrossRef] [PubMed]
- Moeller, R.; Raguse, M.; Leuko, S.; Berger, T.; Hellweg, C.E.; Fujimori, A.; Okayasu, R.; Horneck, G. STARLIFE—An international campaign to study the role of galactic cosmic radiation in astrobiological model systems. Astrobiology 2017, 17, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Baumstark-Khan, C.; Facius, R. Life under conditions of ionizing radiation. In Astrobiology: The Quest for the Conditions of Life; Horneck, G., Baumstark-Khan, C., Eds.; Springer: Berlin, Germany, 2002; pp. 261–284. ISBN 978-3-642-59381-9. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Manucharova, N.A.; Gorlenko, M.V.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Bulat, S.A. 100 kGy gamma-affected microbial communities within the ancient Arctic permafrost under simulated Martian conditions. Extremophiles 2017, 21, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Cheptsov, V.S.; Vorobyova, E.A.; Gorlenko, M.V.; Manucharova, N.A.; Pavlov, A.K.; Lomasov, V.N. Effect of gamma radiation on viability of a soil microbial community under conditions of Mars. Paleontol. J. 2018, 52, 118–124. [Google Scholar] [CrossRef]
- Vorobyova, E.A.; Cheptsov, V.S.; Osipov, G.A.; Kotsyurbenko, O.R.; Soina, V.S. Gamma-IR resistance of bacteria in soil and permafrost. Paleontol. J. 2018, in press. [Google Scholar]
- Cheptsov, V.S.; Vorobyova, E.A.; Osipov, G.A.; Manucharova, N.A.; Polyanskaya, L.M.; Gorlenko, M.V.; Pavlov, A.K.; Rosanova, M.S.; Lomasov, V.N. Microbial activity in Martian analog soils after ionizing radiation: Implications for the preservation of subsurface life on Mars. AIMS Microbiol. 2018, 4, 541–562. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Hunter, S.J.; Lovell, K.V.; Coates, A.J.; Ward, J.M. Low-temperature ionizing radiation resistance of Deinococcus radiodurans and Antarctic Dry Valley bacteria. Astrobiology 2010, 10, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; p. 896. ISBN 9780198717485. [Google Scholar]
- Gaziev, A.; Shaikhaev, G. Limited repair of critical DNA damage in cells exposed to low dose radiation. In Current Topics in Ionizing Radiation Research; Nenoi, M., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0196-3. [Google Scholar] [CrossRef]
- Benhamda, C.; Benkahla, A.; Miled, S.B.; Ouled-Haddar, H.; del Carmen Montero-Calasanz, M.; Gtari, M.; Cherif, A.; Hofner, B.; Ghedira, K.; Sghaier, H. The RadioP1–An integrative web resource for radioresistant prokaryotes. In Evolution of Ionizing Radiation Research; Nenoi, M., Ed.; InTechOpen: London, UK, 2015; pp. 89–106. ISBN 978-953-51-2167-1. [Google Scholar] [CrossRef]
- Vorobyova, E.A.; Soina, V.S.; Mulukin, A.L. Microorganisms and enzyme activity in permafrost after removal of long-term cold stress. Adv. Space Res. 1996, 18, 103–108. [Google Scholar] [CrossRef]
- Vorobyova, E.; Soina, V.; Gorlenko, M.; Minkovskaya, N.; Zalinova, N.; Mamukelashvili, A.; Gilichinsky, D.; Rivkina, E.; Vishnivetskaya, T. The deep cold biosphere: Facts and hypothesis. FEMS Microbiol. Rev. 1997, 20, 277–290. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial biofilms in intertidal systems: An overview. Cont. Shelf Res. 2000, 20, 1257–1273. [Google Scholar] [CrossRef]
- Frösler, J.; Panitz, C.; Wingender, J.; Flemming, H.C.; Rettberg, P. Survival of Deinococcus geothermalis in biofilms under desiccation and simulated space and Martian conditions. Astrobiology 2017, 17, 431–447. [Google Scholar] [CrossRef] [PubMed]
- El-Registan, G.I.; Mulyukin, A.L.; Nikolaev, Y.A.; Suzina, N.E.; Gal’chenko, V.F.; Duda, V.I. Adaptogenic functions of extracellular autoregulators of microorganisms. Microbiology 2006, 75, 380–389. [Google Scholar] [CrossRef]
- Pacelli, C.; Selbmann, L.; Zucconi, L.; Raguse, M.; Moeller, R.; Shuryak, I.; Onofri, S. Survival, DNA integrity, and ultrastructural damage in Antarctic cryptoendolithic eukaryotic microorganisms exposed to ionizing radiation. Astrobiology 2017, 17, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dartnell, L.R.; Desorgher, L.; Ward, J.M.; Coates, A.J. Martian sub-surface ionising radiation: Biosignatures and geology. Biogeosci. Discuss. 2007, 4, 455–492. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Desorgher, L.; Ward, J.M.; Coates, A.J. Modelling the surface and subsurface Martian radiation environment: Implications for astrobiology. Geophys. Res. Lett. 2007, 34, L02207. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Vasilyev, G.; Ostryakov, V.M.; Pavlov, A.K.; Mahaffy, P. Degradation of the organic molecules in the shallow subsurface of Mars due to irradiation by cosmic rays. Geophys. Res. Lett. 2012, 39, L13202. [Google Scholar] [CrossRef]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [PubMed]
- Fairén, A.G.; Davila, A.F.; Lim, D.; Bramall, N.; Bonaccorsi, R.; Zavaleta, J.; Uceda, E.R.; Stoker, C.; Wierzchos, J.; Dohm, J.M.; et al. Astrobiology through the ages of Mars: The study of terrestrial analogues to understand the habitability of Mars. Astrobiology 2010, 10, 821–843. [Google Scholar] [CrossRef] [PubMed]
- Westall, F.; Loizeau, D.; Foucher, F.; Bost, N.; Betrand, M.; Vago, J.; Kminek, G. Habitability on Mars from a microbial point of view. Astrobiology 2013, 13, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.M.; Craddock, R.A. The geological and climatological case for a warmer and wetter early Mars. Nat. Geosci. 2018, 11, 230–237. [Google Scholar] [CrossRef]
- Wordsworth, R.; Kalugina, Y.; Lokshtanov, S.; Vigasin, A.; Ehlmann, B.; Head, J.; Sanders, C.; Wang, H. Transient reducing greenhouse warming on early Mars. Geophys. Res. Lett. 2017, 44, 665–671. [Google Scholar] [CrossRef]
- Wordsworth, R.D.; Kerber, L.; Pierrehumbert, R.T.; Forget, F.; Head, J.W. Comparison of “warm and wet” and “cold and icy” scenarios for early Mars in a 3-D climate model. J. Geophys. Res. Planets 2015, 120, 1201–1219. [Google Scholar] [CrossRef]
- Ramirez, R.M. A warmer and wetter solution for early Mars and the challenges with transient warming. Icarus 2017, 297, 71–82. [Google Scholar] [CrossRef]
- Kurokawa, H.; Kurosawa, K.; Usui, T. A lower limit of atmospheric pressure on early Mars inferred from nitrogen and argon isotopic compositions. Icarus 2018, 299, 443–459. [Google Scholar] [CrossRef]
- Rivkina, E.M.; Kraev, G.N.; Krivushin, K.V.; Laurinavichus, K.S.; Fyodorov-Davydov, D.G.; Kholodov, A.L.; Shcherbakova, V.A.; Gilichinsky, D.A. Methane in permafrost of Northeastern Arctic. Earth Cryosphere 2006, 10, 23–41. (In Russian) [Google Scholar]
- Gilichinsky, D.A.; Vorobyova, E.A.; Erokhina, L.G.; Fyordorov-Dayvdov, D.G.; Chaikovskaya, N.R. Long-term preservation of microbial ecosystems in permafrost. Adv. Space Res. 1992, 12, 255–263. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Polyanskaya, L.M.; Gorlenko, M.V.; Pavlov, A.K.; Lomasov, V.N. Sustainability of extreme microbial ecosystems to the complex impact of physical factors of Martian regolith. Moscow Univ. Soil Sci. Bull. 2018, 3. in press. [Google Scholar]
- Angel, R.; Soares, M.I.M.; Ungar, E.D.; Gillor, O. Biogeography of soil archaea and bacteria along a steep precipitation gradient. ISME J. 2010, 4, 553. [Google Scholar] [CrossRef] [PubMed]
- Singer, A. The Soils of Israel; Springer: Berlin, Germany, 2007; p. 306. ISBN 978-3-540-71734-8. [Google Scholar] [CrossRef]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; NCEA-C-1282; U.S. Environmental Protection Agency, National Exposure Research Laboratory: Washington, DC, USA, 2002; p. 23.
- Cheptsov, V.S.; Vorobyova, E.A.; Gorlenko, M.V.; Manucharova, N.A.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Zvyagintsev, D.G. Influence of gamma radiation, low pressure and low temperature on viability of microbial community of the gray soil as analytical model of Martian regolith. Sovr. Probl. Nauki Obrazov. 2015, 3. Available online: https://science-education.ru/pdf/2015/3/515.pdf (accessed on 10 August 2017). (In Russian).
- Cheptsov, V.S.; Vorobyova, E.A.; Tambiev, A.H.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Zvyagintsev, D.G. Influence of gamma radiation, low pressure and low temperature on catalase activity and reactivity of exometabolites of Kocuria rosea and Arthrobacter polychromogenes. Sovr. Probl. Nauki Obrazov. 2016, 5. Available online: https://science-education.ru/pdf/2016/5/25133.pdf (accessed on 12 June 2018). (In Russian).
- Cheptsov, V.S.; Vorobyova, E.A.; Tambiev, A.H.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N. Influence of gamma irradiation in simulated Martian conditions on catalase activity and reactivity of exometabolites of Kocuria rosea and Arthrobacter polychromogenes. In The Seventh Moscow Solar System Symposium (7M-S3); Russian Academy of Sciences: Moscow, Russia, 2016; pp. 262–264. ISBN 978-5-00015-013-9. Available online: http://ms2016.cosmos.ru/sites/ms2016.cosmos.ru/files/7ms3-2016_abstract_book_www.pdf (accessed on 20 June 2018).
- Gorlenko, M.V.; Majorova, T.N.; Kozhevin, P.A. Disturbances and their influence on substrate utilization patterns in soil microbial communities. In Microbial Communities; Insam, H., Rangger, A., Eds.; Springer: Berlin, Germany, 1997; pp. 84–93. ISBN 978-3-642-60694-6. [Google Scholar] [CrossRef]
- Gorlenko, M.V.; Kozhevin, P.A. Differentiation of soil microbial communities by multisubstrate testing. Microbiology 1994, 63, 158–161. [Google Scholar]
- Garland, J.L.; Mills, A.L. A community-level physiological approach for studying microbial communities. In Beyond the Biomass: Compositional and Functional Analysis of Soil Microbial Communities; Ritz, K., Dighton, J., Giller, K.E., Eds.; Wiley: Chichester, UK, 1994; pp. 77–83. ISBN 978-0471950967. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 2036. ISBN 9781439804087. [Google Scholar]
- Gorlenko, M.V.; Kozhevin, P.A. Multisubstrate Testing of Natural Microbial Communities; MAKS Press: Moscow, Russia, 2005; p. 88. ISBN 5-317-01228-7. (In Russian) [Google Scholar]
- Manaeva, E.S.; Lomovtseva, N.O.; Kostina, N.V.; Gorlenko, M.V.; Umarov, M.M. Biological activity of soils in the settlements of southern (Microtus rossiaemeridionalis) and bank (Clethrionomys glareolus) voles. Biol. Bull. 2014, 41, 80–88. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 1991; pp. 115–175. ISBN 9780471929062. [Google Scholar]
- Gerhardt, F. Methods of General Bacteriology; Mir: Moscow, Russia, 1983; p. 536. (In Russian) [Google Scholar]
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—The consummate survivor. Nat. Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Musilova, M.; Wright, G.; Ward, J.M.; Dartnell, L.R. Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology 2015, 15, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Verseux, C.; Baqué, M.; Cifariello, R.; Fagliarone, C.; Raguse, M.; Moeller, R.; Billi, D. Evaluation of the resistance of Chroococcidiopsis spp. to sparsely and densely ionizing irradiation. Astrobiology 2017, 17, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.; Moeller, R.; Reitz, G.; Sommer, S.; Rettberg, P. Effect of relative humidity on Deinococcus radiodurans’ resistance to prolonged desiccation, heat, ionizing, germicidal, and environmentally relevant UV radiation. Microb. Ecol. 2011, 61, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Gilichinsky, D. Permafrost as a microbial habitat. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wiley: New York, NY, USA, 2002; pp. 932–956. ISBN 978-0-471-35450-5. [Google Scholar]
- Harris, D.R.; Pollock, S.V.; Wood, E.A.; Goiffon, R.J.; Klingele, A.J.; Cabot, E.L.; Schackwitz, W.; Martin, J.; Eggington, J.; Durfee, T.J.; et al. Directed evolution of ionizing radiation resistance in Escherichia coli. J. Bacteriol. 2009, 191, 5240–5252. [Google Scholar] [CrossRef] [PubMed]
- Tesfai, A.T.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Radio-resistance development of DNA repair deficient Escherichia coli DH5α in ground beef subjected to electron beam at sub-lethal doses. Int. J. Radiat. Biol. 2011, 87, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Soina, V.S.; Mulyukin, A.L.; Demkina, E.V.; Vorobyova, E.A.; El-Registan, G.I. The structure of resting bacterial populations in soil and subsoil permafrost. Astrobiology 2004, 4, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Goordial, J.; Davila, A.; Greer, C.W.; Cannam, R.; DiRuggiero, J.; McKay, C.P.; Whyte, L.G. Comparative activity and functional ecology of permafrost soils and lithic niches in a hyper-arid polar desert. Environ. Microbiol. 2017, 19, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.M.; Dhakephalkar, P. Diversity, cold active enzymes and adaptation strategies of bacteria inhabiting glacier cryoconite holes of High Arctic. Extremophiles 2014, 18, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Bajerski, F.; Ganzert, L.; Mangelsdorf, K.; Lipski, A.; Wagner, D. Cryobacterium arcticum sp. nov., a psychrotolerant bacterium from an Arctic soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Kumari, K.; Kishore, K.H.; Pindi, P.K.; Rao, P.S.; Srinivas, T.N.R.; Asthana, R.; Ravindra, R. Vertical distribution of bacteria in a lake sediment from Antarctica by culture-independent and culture-dependent approaches. Res. Microbiol. 2011, 162, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Z.H.; Luo, X.S.; Liu, M.; Huang, Q. Diversity of ionizing radiation-resistant bacteria obtained from the Taklimakan Desert. J. Basic Microbiol. 2015, 55, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.; Peeters, Z.; La Duc, M.T.; Mancinelli, R.; Ehrenfreund, P.; Venkateswaran, K. Effect of shadowing on survival of bacteria under conditions simulating the Martian atmosphere and UV radiation. Appl. Environ. Microbiol. 2008, 74, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Srinivasan, S.; Lim, S.; Joe, M.; Im, S.; Bae, S.I.; Park, S.J.; Han, J.-H.; Park, S.-H.; Joo, B.-M.; et al. Spirosoma radiotolerans sp. nov., a gamma-radiation-resistant bacterium isolated from gamma ray-irradiated soil. Curr. Microbiol. 2014, 69, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, Y.; Gao, P.; Chen, H.; Wen, W.; Sun, Q. Radiation-resistant Micrococcus luteus sc1204 and its proteomics change upon gamma irradiation. Curr. Microbiol. 2016, 72, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Chan, O.W.; Lacap-Bugler, D.C.; Pointing, S.B. Radiation-tolerant bacteria isolated from high altitude soil in Tibet. Indian J. Microbiol. 2016, 56, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Fuma, S.; Tagami, K.; Honma-Takeda, S.; Shikano, S. Responses of the bacterial community to chronic gamma radiation in a rice paddy ecosystem. Int. J. Radiat. Biol. 2011, 87, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Verde, S.C.; Silva, T.; Matos, P. Effects of gamma radiation on wastewater microbiota. Radiat. Environ. Biophys. 2016, 55, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.D.; Eichstadt, S.L.; Kokjohn, T.A.; Martin, E.L. Effects of ultraviolet radiation on the gram-positive marine bacterium Microbacterium maritypicum. Curr. Microbiol. 2007, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, Z.; Gholami, M.; Derikvand, P. UV-resistant bacteria with multiple-stress tolerance isolated from desert areas in Iran. Geomicrobiol. J. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Zamanian, S.N.; Etemadifar, Z. Radical scavengering of pigments from novel strains of Dietzia schimae and Microbacterium esteraromaticum. Prog. Biol. Sci. 2017, 6, 159–170. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Raghu, G.; Sharma, Y.V.R.K.; Biju, A.R.; Rajasekharan, M.V.; Shivaji, S. Increase in oxidative stress at low temperature in an Antarctic bacterium. Curr. Microbiol. 2011, 62, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Øvreås, L. Microbial diversity, life strategies, and adaptation to life in extreme soils. In Microbiology of Extreme Soils; Dion, P., Nautiyal, C.S., Eds.; Springer: Berlin, Germany, 2008; pp. 15–43. ISBN 978-3-540-74231-9. [Google Scholar]
- Stotzky, G. Influence of soil mineral colloids on metabolic processes, growth, adhesion, and ecology of microbes and viruses 1. Interactions of soil minerals with natural organics and microbes. In Interactions of Soil Minerals with Natural Organics and Microbes; Huang, P.M., Schnitzer, M., Eds.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 305–428. ISBN 978-0-89118-912-1. [Google Scholar]
- Hattori, T.; Hattori, R.; McLaren, A.D. The physical environment in soil microbiology: An attempt to extend principles of microbiology to soil microorganisms. CRC Crit. Rev. Microbiol. 1976, 4, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arct. Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Carroll, T.L.; Sundin, G.W. The role of pigmentation, ultraviolet radiation tolerance, and leaf colonization strategies in the epiphytic survival of phyllosphere bacteria. Microb. Ecol. 2005, 49, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, C.; Bryan, R.A.; Onofri, S.; Selbmann, L.; Zucconi, L.; Shuryak, I.; Dadachova, E. The effect of protracted X-ray exposure on cell survival and metabolic activity of fast and slow growing fungi capable of melanogenesis. Environ. Microbiol. Rep. 2018, 10, 255–263. [Google Scholar] [CrossRef] [PubMed]
- El-Registan, G.I.; Mulyukin, A.L.; Nikolaev, Y.A.; Stepanenko, I.Y.; Kozlova, A.N.; Martirosova, E.I.; Shanenko, E.F.; Strakhovskaya, M.G.; Revina, A.A. The role of microbial low-molecular-weight autoregulatory factors (alkylhydroxybenzenes) in resistance of microorganisms to radiation and heat shock. Adv. Space Res. 2005, 36, 1718–1728. [Google Scholar] [CrossRef]
- McNamara, N.P.; Black, H.I.J.; Beresford, N.A.; Parekh, N.R. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 2003, 24, 117–132. [Google Scholar] [CrossRef]
- García-Contreras, R.; Nunez-Lopez, L.; Jasso-Chávez, R.; Kwan, B.W.; Belmont, J.A.; Rangel-Vega, A.; Maeda, T.; Wood, T.K. Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. ISME J. 2015, 9, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chanal, A.; Chapon, V.; Benzerara, K.; Barakat, M.; Christen, R.; Achouak, W.; Barras, F.; Heulin, T. The desert of Tataouine: an extreme environment that hosts a wide diversity of microorganisms and radiotolerant bacteria. Environ. Microbiol. 2006, 8, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Rainey, F.A.; Ray, K.; Ferreira, M.; Gatz, B.Z.; Nobre, M.F.; Bagaley, D.; Rash, B.A.; Park, M.-J.; Earl, A.M.; Shank, N.C.; et al. Extensive diversity of ionizing-radiation-resistant bacteria recovered from Sonoran Desert soil and description of nine new species of the genus Deinococcus obtained from a single soil sample. Appl. Environ. Microbiol. 2005, 71, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Mileikowsky, C.; Cucinotta, F.A.; Wilson, J.W.; Gladman, B.; Horneck, G.; Lindegren, L.; Melosh, J.; Rickman, H.; Valtonen, M.; Zheng, J.Q. Natural transfer of viable microbes in space: 1. From Mars to Earth and Earth to Mars. Icarus 2000, 145, 391–427. [Google Scholar] [CrossRef] [PubMed]
- Homeck, G.; Mileikowsky, C.; Melosh, H.J.; Wilson, J.W.; Cucinotta, F.A.; Gladman, B. Viable transfer of microorganisms in the solar system and beyond. In Astrobiology: The Quest for the Conditions of Life; Horneck, G., Baumstark-Khan, C., Eds.; Springer: Berlin, Germany, 2002; pp. 57–76. ISBN 978-3-642-59381-9. [Google Scholar] [CrossRef]
- Rummel, J.D.; Beaty, D.W.; Jones, M.A.; Bakermans, C.; Barlow, N.G.; Boston, P.J.; Chevrier, V.F.; Clark, B.C.; de Vera, J.-P.P.; Gough, R.V.; et al. A new analysis of Mars “special regions”: Findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 2014, 14, 887–968. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Battista, J.R.; Christner, B.C. Double-strand DNA break repair at −15 °C. Appl. Environ. Microbiol. 2013, 79, 7662–7668. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.K.; Shelegedin, V.N.; Vdovina, M.A.; Pavlov, A.A. Growth of microorganisms in Martian-like shallow subsurface conditions: laboratory modelling. Int. J. Astrobiol. 2010, 9, 51–58. [Google Scholar] [CrossRef]
- Martín-Torres, F.J.; Zorzano, M.P.; Valentín-Serrano, P.; Harri, A.M.; Genzer, M.; Kemppinen, O.; Rivera-Valentin, E.G.; Jun, I.; Wray, J.J.; Madsen, M.B.; et al. Transient liquid water and water activity at Gale crater on Mars. Nat. Geosci. 2015, 8, 357–361. [Google Scholar] [CrossRef]
- Jones, E.G. Shallow transient liquid water environments on present-day Mars, and their implications for life. Acta Astronaut. 2018, 146, 144–150. [Google Scholar] [CrossRef]
- Jones, E.G.; Lineweaver, C.H.; Clarke, J.D. An extensive phase space for the potential Martian biosphere. Astrobiology 2011, 11, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Krivushin, K.; Gilichinsky, D.; Schuerger, A.C. Growth of Carnobacterium spp. from permafrost under low pressure, temperature, and anoxic atmosphere has implications for Earth microbes on Mars. Proc. Nat. Acad. Sci. USA 2013, 110, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; McCoy, L.E.; Kerney, K.R.; Ming, D.W.; Golden, D.C.; Schuerger, A.C. Aqueous extracts of a Mars analogue regolith that mimics the Phoenix landing site do not inhibit spore germination or growth of model spacecraft contaminants Bacillus subtilis 168 and Bacillus pumilus SAFR-032. Icarus 2012, 220, 904–910. [Google Scholar] [CrossRef]
- Beblo-Vranesevic, K.; Huber, H.; Rettberg, P. High tolerance of Hydrogenothermus marinus to sodium perchlorate. Front. Microbiol. 2017, 8, 1369. [Google Scholar] [CrossRef] [PubMed]
- Beblo-Vranesevic, K.; Bohmeier, M.; Perras, A.K.; Schwendner, P.; Rabbow, E.; Moissl-Eichinger, C.; Cockell, C.S.; Pukall, R.; Vannier, P.; Marteinsson, V.T.; et al. The responses of an anaerobic microorganism, Yersinia intermedia MASE-LG-1 to individual and combined simulated Martian stresses. PLoS ONE 2017, 12, e0185178. [Google Scholar] [CrossRef] [PubMed]
- Al Soudi, A.F.; Farhat, O.; Chen, F.; Clark, B.C.; Schneegurt, M.A. Bacterial growth tolerance to concentrations of chlorate and perchlorate salts relevant to Mars. Int. J. Astrobiol. 2017, 16, 229–235. [Google Scholar] [CrossRef]
- Nuding, D.L.; Gough, R.V.; Venkateswaran, K.J.; Spry, J.A.; Tolbert, M.A. Laboratory investigations on the survival of Bacillus subtilis spores in deliquescent salt Mars analog environments. Astrobiology 2017, 17, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Heinz, J.; Schirmack, J.; Airo, A.; Kounaves, S.P.; Schulze-Makuch, D. Enhanced microbial survivability in subzero brines. Astrobiology 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.B.; Muller, J.P.; Neukum, G.; Werner, S.C.; van Gasselt, S.; Hauber, E.; Markiewicz, W.J.; Head, J.W., III; Foing, B.H.; Page, D.; et al. The HRSC Co-Investigator Team. Evidence from the Mars Express High Resolution Stereo Camera for a frozen sea close to Mars’ equator. Nature 2005, 434, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.F.; Cooper, C.D.; Rifkin, M.K. Evidence for recent climate change on Mars from the identification of youthful near-surface ground ice. Nature 2001, 412, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Madeleine, J.B.; Head, J.W.; Forget, F.; Navarro, T.; Millour, E.; Spiga, A.; Colaïtis, A.; Määttänen, A.; Montmessin, F.; Dickson, J.L. Recent ice ages on Mars: The role of radiatively active clouds and cloud microphysics. Geophys. Res. Lett. 2014, 41, 4873–4879. [Google Scholar] [CrossRef]
- Johnsson, A.; Reiss, D.; Hauber, E.; Hiesinger, H.; Zanetti, M. Evidence for very recent melt-water and debris flow activity in gullies in a young mid-latitude crater on Mars. Icarus 2014, 235, 37–54. [Google Scholar] [CrossRef]
- Hahn, C.; Hans, M.; Hein, C.; Mancinelli, R.L.; Mücklich, F.; Wirth, R.; Rettberg, P.; Hellweg, C.E.; Moeller, R. Pure and oxidized copper materials as potential antimicrobial surfaces for spaceflight activities. Astrobiology 2017, 17, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, K.; Rettberg, P.; Pukall, R.; Auerbach, A.; Wink, L.; Barczyk, S.; Perras, A.; Mahnert, A.; Margheritis, D.; Kminek, G.; et al. Microbial biodiversity assessment of the European Space Agency’s ExoMars 2016 mission. Microbiome 2017, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Rettberg, P.; Shimizu, T.; Thoma, M. Cold atmospheric plasma technology for decontamination of space equipment. In Proceedings of the 6th International Conference on Plasma Medicine, Bratislava, Slovakia, 4–9 September 2016. [Google Scholar]
- Dartnell, L.R. Ionizing radiation and life. Astrobiology 2011, 11, 551–582. [Google Scholar] [CrossRef] [PubMed]
- Noffke, N.; Christian, D.; Wacey, D.; Hazen, R.M. Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old dresser formation, Pilbara, Western Australia. Astrobiology 2013, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grosch, E.G.; McLoughlin, N. Reassessing the biogenicity of Earth’s oldest trace fossil with implications for biosignatures in the search for early life. Proc. Natl. Acad. Sci. USA 2014, 111, 8380–8387. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.; Van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Allwood, A.C. Geology: Evidence of life in Earth’s oldest rocks. Nature 2016, 537, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Giardino, G.; Pillitteri, I.; Favata, F.; Micela, G. The X-ray luminosity of solar-mass stars in the intermediate age open cluster NGC 752. Astron. Astrophys. 2008, 490, 113–123. [Google Scholar] [CrossRef]
- Ribas, I.; Guinan, E.F.; Güdel, M.; Audard, M. Evolution of the solar activity over time and effects on planetary atmospheres. I. High-energy irradiances (1–1700 Å). Astrophys. J. 2005, 622, 680–694. [Google Scholar] [CrossRef]
- Cnossen, I.; Sanz-Forcada, J.; Favata, F.; Witasse, O.; Zegers, T.; Arnold, N.F. Habitat of early life: Solar X-ray and UV radiation at Earth’s surface 4–3.5 billion years ago. JGR: Planets 2007, 112, E02008. [Google Scholar] [CrossRef]
- Kasting, J.F. Atmospheric composition of Hadean–early Archean Earth: The importance of CO. In Earth’s Early Atmosphere and Surface Environment; Shaw, G.H., Ed.; Geological Society of America: Boulder, CO, USA, 2014; pp. 19–28. ISBN 9780813725048. [Google Scholar] [CrossRef]
- Karam, P.A.; Leslie, S.A. Calculations of background beta-gamma radiation dose through geologic time. Health Phys. 1999, 77, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.K.; Kalinin, V.L.; Konstantinov, A.N.; Shelegedin, V.N.; Pavlov, A.A. Was Earth ever infected by martian biota? Clues from radioresistant bacteria. Astrobiology 2006, 6, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Obridko, V.N.; Miroshnichenko, L.I.; Ragulskaya, M.V.; Khabarova, O.V.; Khramova, E.G.; Katsova, M.M.; Livshits, M.A. Space factor of the biosphere evolution: new fields of investigation. In Problems of Evolution of the Biosphere; Rozhnov, S.V., Ed.; PIN RAS: Moscow, Russia, 2013; pp. 66–94. ISBN 978-5-903825-31-8. (In Russian) [Google Scholar]
- Obridko, V.N.; Ragulskaya, M.V.; Khabarova, O.V.; Miroshnichenko, L.I.; Khramova, E.G. Cosmophysical factors of evolution of biosphere: new lines of research. Psychosom. Integr. Res. 2015, 1, 0101. (In Russian) [Google Scholar]
| Sample | SN2 | M-1/91 | |
|---|---|---|---|
| Attribute | |||
| pH | 8.11 | 7.51 | |
| NO2−, mg/kg | Traces | Traces | |
| NO3−, mg/kg | 1.03 | 0.89 | |
| NH4+, mg/kg | 4.12 | 3.34 | |
| Cl−, mg/kg | 58.15 | 49.84 | |
| CO32–, mg/kg | 330.41 | 129.29 | |
| Na+, mg/kg | 512.7 | 87.57 | |
| Mn2+, mg/kg | 1348.2 | 3.23 | |
| Mg2+, mg/kg | 136.46 | 170.4 | |
| K+, mg/kg | 877.5 | 40.47 | |
| Fe2++Fe3+, mg/kg | 4.35 | 29.55 | |
| Total organic carbon, % | 1.27 | 0.32 | |
| Sample | Consumption of Substrates of Different Groups, Relative Units | |||||||
|---|---|---|---|---|---|---|---|---|
| Pentoses | Hexoses | Oligoses | Alcohols | Amino Acids | Salts of Carboxylic Acids | Polymers | Amides, Amines, Nucleosides | |
| SN2, Control | 526 | 3950 | 5340 | 4910 | 6186 | 3894 | 2654 | 124 |
| SN2, TPC | 544 | 3972 | 5294 | 4862 | 6268 | 3754 | 2716 | 132 |
| SN2, 10 kGy | 0 | 0 | 0 | 0 | 0 | 0 | 122 | 0 |
| SN2, 100 kGy | 0 | 0 | 0 | 0 | 0 | 0 | 114 | 0 |
| M-1/91, Control | 2150 | 1420 | 1912 | 2562 | 5670 | 4318 | 3642 | 22 |
| M-1/91, 100 kGy | 0 | 0 | 0 | 0 | 0 | 0 | 146 | 0 |
| Sample | Strain—Genbank Accession Number | The Most Closely Related Sequences in Genbank—Similarity, % | Taxonomic Affiliation |
|---|---|---|---|
| SN2, TPC | KBP.AS.531 MH050938 | JX949673 Arthrobacter sp.—100 | Arthrobacter sp. 1 |
| NR_026198 Arthrobacter agilis—99.5 | |||
| NR_148833 Arthrobacter echini—99.3 | |||
| SN2, TPC | KBP.AS.532 MH050939 | JQ419609 Saccharothrix sp.—99.8 | Saccharothrix sp. |
| NR_109447 Saccharothrix ecbatanensis—99.5 | |||
| NR_109103 Saccharothrix hoggarensis—99.5 | |||
| SN2, TPC | KBP.AS.340 MH050940 | KJ578028 Uncultured bacterium clone—99.9 | Phenylobacterium sp. |
| KT191026 Phenylobacterium panacis—99.5 | |||
| NR_117783 Phenylobacterium muchangponense—99.5 | |||
| SN2, TPC | KBP.AS.341 MH050941 | DQ177485 Microbacteriaceae bacterium—99.6 | Cryobacterium sp. |
| KR857373 Cryobacterium sp.—99.4 | |||
| FR691394 Cryobacterium sp.—99.4 | |||
| NR_108605 Cryobacterium arcticum—99.4 | |||
| JX205201 Cryobacterium psychrotolerans—99.2 | |||
| SN2, TPC | KBP.AS.342 MH050942 | KY820858 Streptomyces fulvissimus—99.3 | Streptomyces sp. |
| AJ781354 Streptomyces mediolani—99.3 | |||
| SN2, TPC | KBP.AS.343 MH050943 | NR_119270 Microbacterium saperdae—100 | Microbacterium sp. |
| NR_025405 Microbacterium phyllosphaerae—100 | |||
| SN2, TPC | KBP.AS.344 MH050944 | GQ232449 Bacterium RK91_tank—100 | Micrococcus sp. 2 |
| JN378531 Micrococcus luteus—99.9 | |||
| NR_134088 Micrococcus aloeverae—99.7 | |||
| NR_116578 Micrococcus yunnanensis—99.6 | |||
| SN2, TPC | KBP.AS.345 MH050945 | DQ906916 Uncultured bacterium clone—99.4 | Sphingoaurantiacus sp. |
| NR_147725 Sphingoaurantiacus polygranulatus—96.8 | |||
| NR_148321 Sphingomonas chloroacetimidivorans—95.4 | |||
| KT321369 Sphingoaurantiacus capsulatus—95.3 | |||
| SN2, TPC | KBP.AS.346 MH050946 | JX840970 Arthrobacter oxydans—100 | Arthrobacter sp. 3 |
| NR_108849 Arthrobacter siccitolerans—99.7 | |||
| NR_026192 Arthrobacter polychromogenes—99.6 | |||
| SN2, TPC | KBP.AS.347 MH050947 | NR_117474 Bacillus frigoritolerans—100 | Bacillus sp. |
| NR_114919 Bacillus simplex—100 | |||
| SN2, 10 kGy | KBP.AS.298 MH050948 | NR_113978 Spirosoma rigui—99.8 | Spirosoma sp. 4 |
| EF507901 Spirosoma aquatica—99.8 | |||
| SN2, 10 kGy | KBP.AS.299 MH050949 | AB637009 Uncultured bacterium clone—100 | Rufibacter immobilis |
| HG316123 Rufibacter immobilis—99 | |||
| CP012645 Rufibacter tibetensis—97.8 | |||
| SN2, 10 kGy | KBP.AS.300 MH050950 | NR_042469 Arthrobacter phenanthrenivorans—100 | Arthrobacter sp. 3 |
| NR_117356 Arthrobacter cryotolerans—100 | |||
| NR_041546 Arthrobacter humicola—100 | |||
| SN2, 10 kGy | KBP.AS.533 MH050951 | KY386623 Arthrobacter sp.—100 | Arthrobacter sp. 1 |
| NR_026198 Arthrobacter agilis—100 | |||
| NR_148833 Arthrobacter echini—99.6 | |||
| SN2, 10 kGy | KBP.AS.301 MH050952 | HQ860629 Uncultured bacterium clone—100 | Planomicrobium sp. 5 |
| GQ140340 Planomicrobium okeanokoites—99.9 | |||
| NR_116601 Planomicrobium flavidum—99.7 | |||
| SN2, 10 kGy | KBP.AS.302 MH050953 | HQ860629 Uncultured bacterium clone—100 | Planomicrobium sp. 5 |
| GQ140340 Planomicrobium okeanokoites—99.9 | |||
| NR_116601 Planomicrobium flavidum—99.7 | |||
| SN2, 10 kGy | KBP.AS.303 MH050954 | FR675946 Uncultured bacterium clone—99.5 | Massilia sp. |
| NR_117040 Massilia consociata—99.0 | |||
| NR_042502 Massilia aurea—98.4 | |||
| NR_126273 Massilia kyonggiensis—98.4 | |||
| SN2, 10 kGy | KBP.AS.534 MH050955 | NR_134088 Micrococcus aloeverae—99.8 | Micrococcus sp. 2 |
| NR_116578 Micrococcus yunnanensis—99.8 | |||
| SN2, 100 kGy | KBP.AS.297 MH050956 | NR_113978 Spirosoma rigui—99.8 | Spirosoma sp. 4 |
| EF507901 Spirosoma aquatica—99.8 | |||
| SN2, 100 kGy | KBP.AS.321 MH050957 | AB637009 Uncultured bacterium clone—99.7 | Rufibacter sp. |
| JF417863 Uncultured bacterium clone—97.9 | |||
| HG316124 Rufibacter immobilis—96.8 | |||
| NR_116350 Rufibacter tibetensis—96.3 | |||
| SN2, 100 kGy | KBP.AS.322 MH050958 | HQ910259 Uncultured bacterium clone—99.6 | Microvirga sp. |
| JX504809 Microvirga vignae—98.7 | |||
| NR_114298 Microvirga aerilata—98.7 | |||
| SN2, 100 kGy | KBP.AS.323 MH050959 | KC354446 Arthrobacter sp.—100 | Arthrobacter sp. 3 |
| JX840970 Arthrobacter oxydans—100 | |||
| NR_108849 Arthrobacter siccitolerans—99.7 | |||
| SN2, 100 kGy | KBP.AS.324 MH050960 | KX247636 Microvirga soli—99.7 | Microvirga soli |
| JF295810 Uncultured bacterium clone—99 | |||
| HF954468 Microvirga sp.—98 | |||
| NR_104766 Microvirga subterranea—97.9 | |||
| SN2, 100 kGy | KBP.AS.325 MH050961 | NR_042252 Arthrobacter parietis—100 | Arthrobacter sp. |
| KP125973 Arthrobacter subterraneus—100 | |||
| SN2, 100 kGy | KBP.AS.535 MH050962 | FR691450 Pontibacter sp.—99.1 | Pontibacter sp. |
| NR_148858 Pontibacter amylolyticus—98.1 | |||
| NR_116853 Pontibacter salisaro—97.9 | |||
| SN2, 100 kGy | KBP.AS.536 MH050963 | KX247636 Microvirga soli—99.4 | Microvirga sp. |
| NR_044563 Microvirga guangxiensis—99.1 | |||
| SN2, 100 kGy | KBP.AS.326 MH050964 | AJ863207 Uncultured bacterium clone—99 | Pontibacter sp. |
| KX350156 Pontibacter sp.—99.0 | |||
| JN037908 Uncultured Bacteroidetes bacterium—98.2 | |||
| NR_133822 Pontibacter deserti—97.5 | |||
| NR_109067 Pontibacter populi—96.3 | |||
| M-1/91, 100 kGy | KBP.AS.317 MH050965 | KJ000846 Brevundimonas sp.—99.9 | Brevundimonas sp. |
| NR_116722 Brevundimonas naejangsanensis—99.9 | |||
| NR_113602 Brevundimonas diminuta—99.7 | |||
| M-1/91, 100 kGy | KBP.AS.319 MH050966 | NR_114986 Microbacterium maritypicum—100 | Microbacterium sp. 7 |
| KT899483 Microbacterium oxydans—100 | |||
| M-1/91, 100 kGy | KBP.AS.320 MH050967 | NR_114986 Microbacterium maritypicum—100 | Microbacterium sp. 7 |
| KT899483 Microbacterium oxydans—100 | |||
| M-1/91, 100 kGy | KBP.AS.537 MH050968 | HM811712 Uncultured bacterium—99.5 | Stenotrophomonas rhizophila |
| NR_121739 Stenotrophomonas rhizophila—99.2 | |||
| LT906480 Stenotrophomonas maltophilia—97.9 |
| Sample | Strain | Taxonomic Affiliation | Temperature Limits of Growth, °C | pH-range of Growth | Maximum Salt Concentrations at Which Growth Is Possible, % | |||
|---|---|---|---|---|---|---|---|---|
| NaCl | KCl | MgSO4 | NaHCO3 | |||||
| SN2, TPC | KBP.AS.531 | Arthrobacter sp. | 2–37 | 5–9 | 2 | 2 | 15 | 0 |
| SN2, TPC | KBP.AS.532 | Saccharothrix sp. | 10–37 | 4–12 | 2 | 5 | 20 | 0 |
| SN2, TPC | KBP.AS.340 | Phenylobacterium sp. | 10–37 | 6–12 | 0 | 0 | 5 | 0 |
| SN2, TPC | KBP.AS.341 | Cryobacterium sp. | 2–37 | 7–11 | 20 | 15 | 20 | 0 |
| SN2, TPC | KBP.AS.342 | Streptomyces sp. | 10–45 | 4–12 | 10 | 10 | 20 | 5 |
| SN2, TPC | KBP.AS.343 | Microbacterium sp. | 10–37 | 4–11 | 10 | 15 | 20 | 0 |
| SN2, TPC | KBP.AS.344 | Micrococcus sp. | 10–37 | 5–8 | 0 | 5 | 0 | 0 |
| SN2, TPC | KBP.AS.346 | Arthrobacter sp. | 2–50 | 6–10 | 0 | 0 | 2 | 0 |
| SN2, TPC | KBP.AS.347 | Bacillus sp. | 2–37 | 5–8 | 0 | 5 | 0 | 0 |
| SN2, 10 kGy | KBP.AS.298 | Spirosoma sp. | 10–37 | 5–12 | 20 | 15 | 20 | 0 |
| SN2, 10 kGy | KBP.AS.299 | Rufibacter immobilis | 2–37 | 6–8 | 0 | 0 | 2 | 0 |
| SN2, 10 kGy | KBP.AS.300 | Arthrobacter sp. | 2–37 | 6–8 | 0 | 0 | 2 | 0 |
| SN2, 10 kGy | KBP.AS.533 | Arthrobacter sp. | 10–37 | 4–9 | 10 | 15 | 15 | 0 |
| SN2, 10 kGy | KBP.AS.301 | Planomicrobium sp. | 2–37 | 5–12 | 0 | 0 | 15 | 0 |
| SN2, 10 kGy | KBP.AS.302 | Planomicrobium sp. | 2–37 | 6–12 | 0 | 0 | 15 | 0 |
| SN2, 10 kGy | KBP.AS.303 | Massilia sp. | 10–37 | 5–8 | 0 | 0 | 0 | 0 |
| SN2, 10 kGy | KBP.AS.534 | Micrococcus sp. | 10–37 | 5–12 | 20 | 15 | 20 | 0 |
| SN2, 100 kGy | KBP.AS.297 | Spirosoma sp. | 10–37 | 7–12 | 0 | 0 | 0 | 2 |
| SN2, 100 kGy | KBP.AS.323 | Arthrobacter sp. | 4–37 | 8–12 | 5 | 0 | 0 | 2 |
| SN2, 100 kGy | KBP.AS.324 | Microvirga sp. | 4–37 | 7–12 | 10 | 15 | 20 | 0 |
| SN2, 100 kGy | KBP.AS.325 | Arthrobacter sp. | 2–50 | 5–12 | 0 | 5 | 15 | 0 |
| M-1/91, 100 kGy | KBP.AS.317 | Brevundimonas sp. | 10–37 | 5–12 | 20 | 15 | 20 | 0 |
| M-1/91, 100 kGy | KBP.AS.319 | Microbacterium sp. | 10–37 | 5–8 | 2 | 2 | 2 | 0 |
| M-1/91, 100 kGy | KBP.AS.320 | Microbacterium sp. | 4–50 | 7–12 | 0 | 0 | 2 | 0 |
| M-1/91, 100 kGy | KBP.AS.537 | Stenotrophomonas rhizophila | 2–37 | 6–11 | 0 | 0 | 5 | 2 |
| Sample | Strain Number | Taxonomic Affiliation |
|---|---|---|
| SN2, TPC | KBP.AS.341 | Cryobacterium sp. |
| SN2, TPC | KBP.AS.343 | Microbacterium sp. |
| SN2, TPC | KBP.AS.347 | Bacillus sp. |
| SN2, 10 kGy | KBP.AS.301 | Planomicrobium sp. |
| SN2, 100 kGy | KBP.AS.323 | Arthrobacter sp. |
| SN2, 100 kGy | KBP.AS.324 | Microvirga sp. |
| M-1/91, 100 kGy | KBP.AS.319 | Microbacterium sp. |
| SN 1 | SN_T60 | Kocuria rosea |
| SN 1 | SN_T61 | Arthrobacter polychromogenes |
| The strain obtained from the VKM collection | VKM B-1422T | Deinococcus radiodurans |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheptsov, V.; Vorobyova, E.; Belov, A.; Pavlov, A.; Tsurkov, D.; Lomasov, V.; Bulat, S. Survivability of Soil and Permafrost Microbial Communities after Irradiation with Accelerated Electrons under Simulated Martian and Open Space Conditions. Geosciences 2018, 8, 298. https://doi.org/10.3390/geosciences8080298
Cheptsov V, Vorobyova E, Belov A, Pavlov A, Tsurkov D, Lomasov V, Bulat S. Survivability of Soil and Permafrost Microbial Communities after Irradiation with Accelerated Electrons under Simulated Martian and Open Space Conditions. Geosciences. 2018; 8(8):298. https://doi.org/10.3390/geosciences8080298
Chicago/Turabian StyleCheptsov, Vladimir, Elena Vorobyova, Andrey Belov, Anatoly Pavlov, Denis Tsurkov, Vladimir Lomasov, and Sergey Bulat. 2018. "Survivability of Soil and Permafrost Microbial Communities after Irradiation with Accelerated Electrons under Simulated Martian and Open Space Conditions" Geosciences 8, no. 8: 298. https://doi.org/10.3390/geosciences8080298
APA StyleCheptsov, V., Vorobyova, E., Belov, A., Pavlov, A., Tsurkov, D., Lomasov, V., & Bulat, S. (2018). Survivability of Soil and Permafrost Microbial Communities after Irradiation with Accelerated Electrons under Simulated Martian and Open Space Conditions. Geosciences, 8(8), 298. https://doi.org/10.3390/geosciences8080298





