Columbite-Group Minerals from New York Pegmatites: Insights from Isotopic and Geochemical Analyses
Abstract
1. Introduction
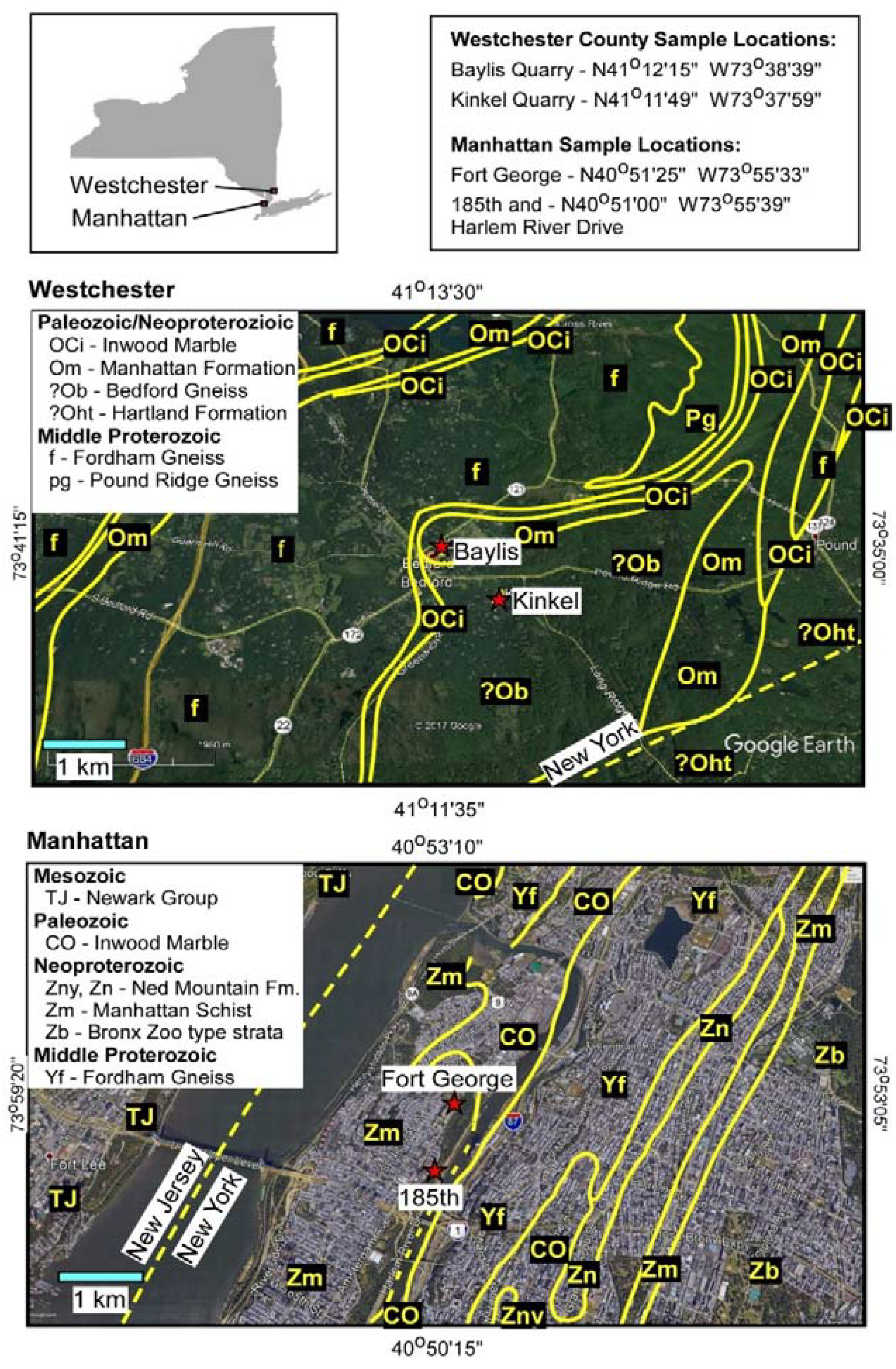
2. Geological Setting
3. Analytical Methods
3.1. Major Elements
3.2. Trace Elements
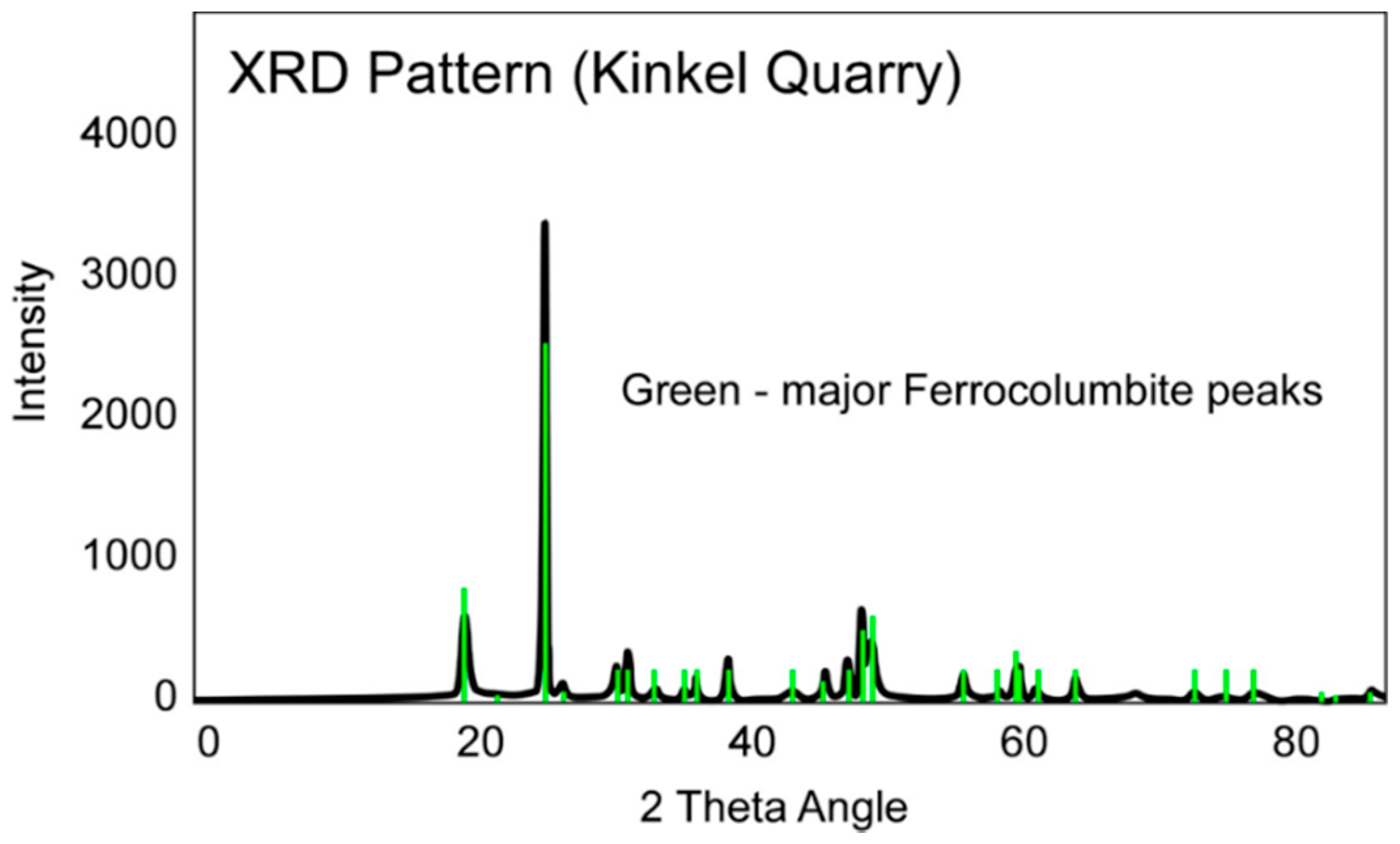
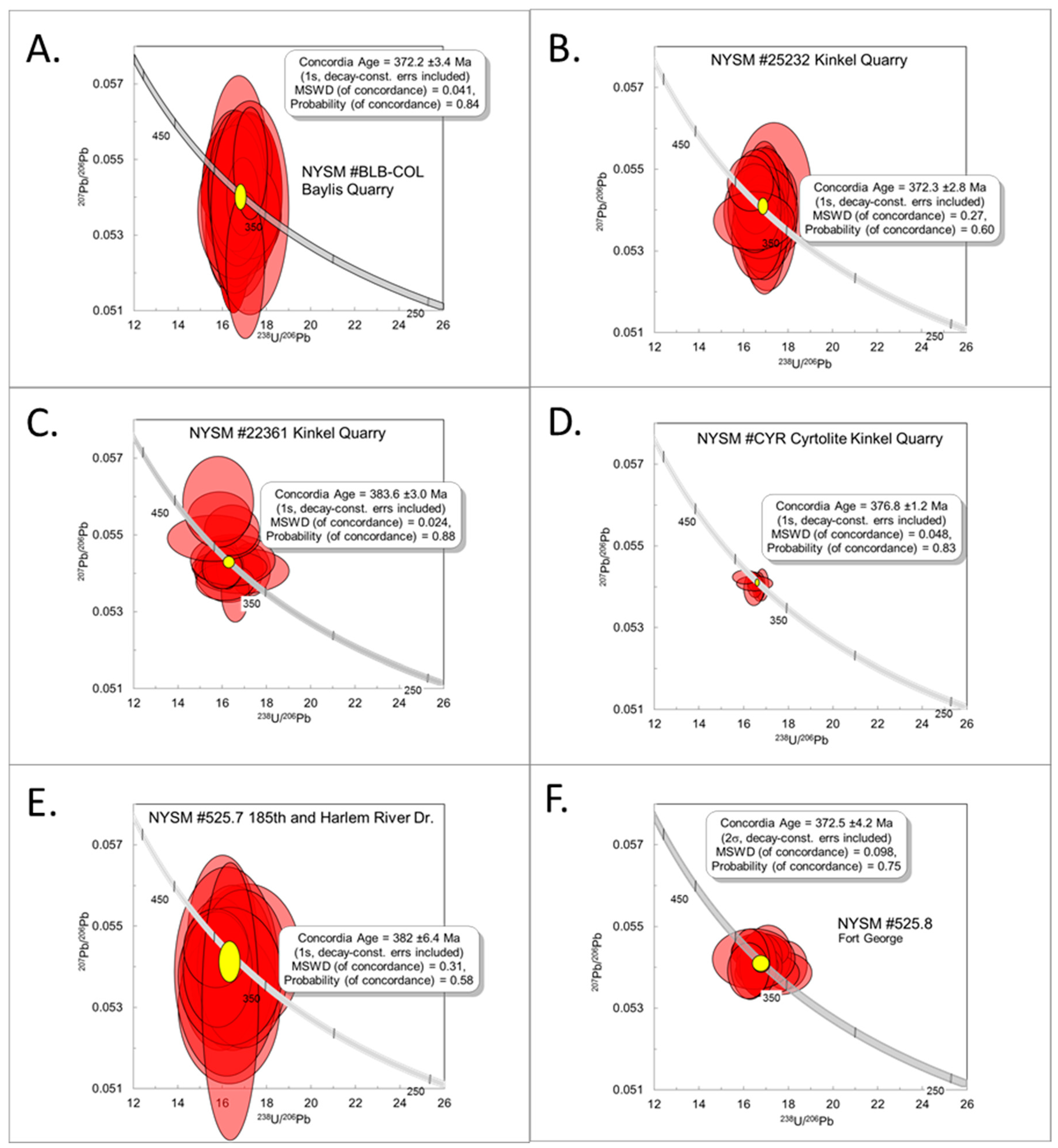
3.3. Geochronology
4. Results
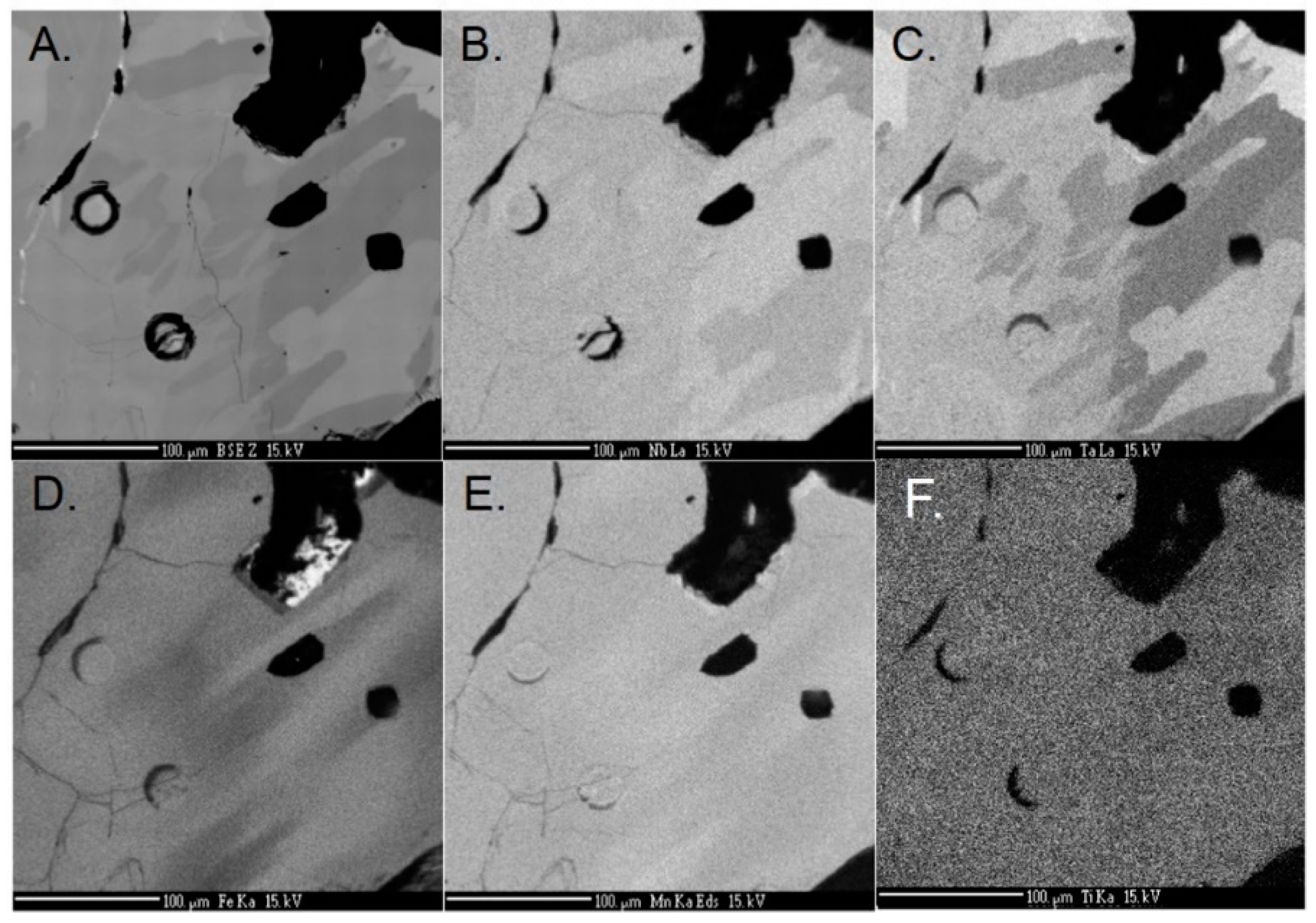
4.1. Major Elements
4.2. Trace Elements
4.3. Geochronology
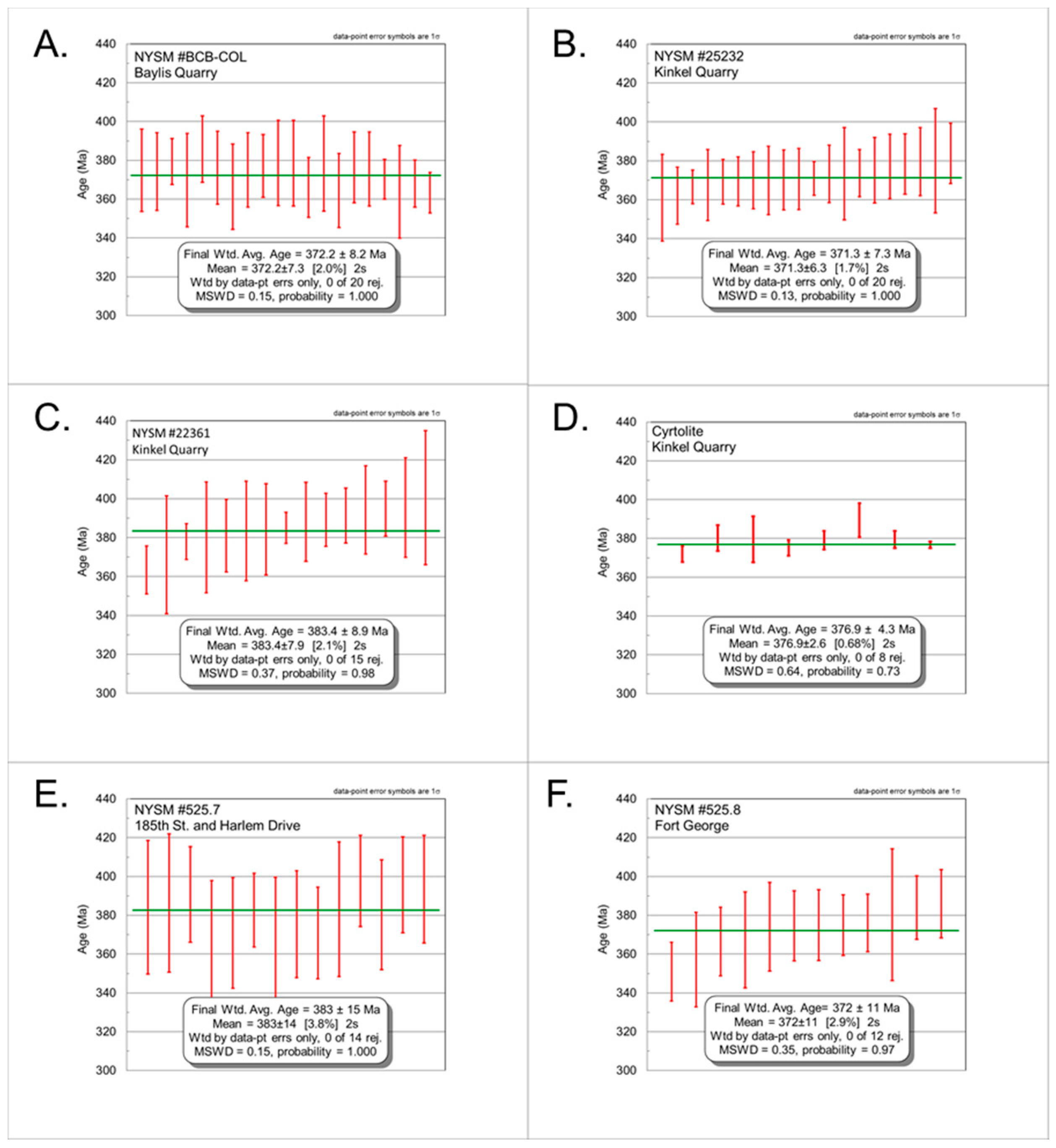
4.3.1. NYSM #BCB-COL
4.3.2. NYSM #25232
4.3.3. NYSM #22361
4.3.4. Zircon- “Cyrtolite” (CYR)
4.3.5. NYSM #525.7
4.3.6. NYSM #525.8
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burke, E.A.J. Tidying up mineral names: An IMA-CNMNC scheme for suffixes, hyphens and diacritical marks. Miner. Rec. 2008, 39, 131–135. [Google Scholar]
- Romer, R.L.; Wright, J.E. U-Pb dating of columbites: A geochronologic tool to date magmatism and ore deposits. Geochim. Cosmochim. Acta 1992, 56, 2137–2142. [Google Scholar] [CrossRef]
- Möller, P. REE (Y), Nb, and Ta enrichment in pegmatites and carbonatite-alkalic rock complexes. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Special Publication No. 7 of the Society for Geology Applied to Mineral Deposits 7; Springer: Berlin/Heidelberg, Germany, 1989; pp. 27–79. [Google Scholar] [CrossRef]
- Černý, P. Characteristics of pegmatite deposits of tantalum. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Special Publication No. 7 of the Society for Geology Applied to Mineral Deposits 7; Springer: Berlin/Heidelberg, Germany, 1989; pp. 195–239. [Google Scholar]
- USGS. Conflict Minerals from the Democratic Republic of the Congo: Global Tungsten Processing Plants, a Critical Part of the Tungsten Supply Chain. 2014. Available online: https://pubs.usgs.gov/fs/2014/3069/pdf/fs2014-3069.pdf (accessed on 19 November 2017).
- Romer, R.L.; Lehmann, B. U-Pb columbite–tantalite age of Neoproterozoic Ta-Nb mineralisation in Burundi. Econ. Geol. 1995, 90, 2303–2309. [Google Scholar] [CrossRef]
- Romer, R.L.; Smeds, S.A. Implications of U-Pb ages of columbite–tantalites from granitic pegmatites for the Paleoproterozoic accretion of 1.90–1.85 Ga magmatic arcs to the Baltic Shield. Precambrian Res. 1994, 67, 141–158. [Google Scholar] [CrossRef]
- Smith, S.R.; Foster, G.L.; Romer, R.L.; Tindle, A.G.; Kelley, S.P.; Noble, S.R.; Horstwood, M.; Breaks, F.W. U-Pb columbite–tantalite chronology of rare-element pegmatites using TIMS and Laser Ablation–Multi Collector–ICP–MS. Contrib. Miner. Petrol. 2004, 147, 549–564. [Google Scholar] [CrossRef]
- Melcher, F.; Sitnikova, M.A.; Graupner, T.; Martin, N.; Oberthür, T.; Henjes-Kunst, F.; Gäbler, E.; Gerdes, A.; Brätz, H.; Davis, D.W.; et al. Fingerprinting of conflict minerals: Columbite-tantalite (“coltan”) ores. SGA News, 23 June 2008. [Google Scholar]
- Aldrich, L.T.; Davis, G.L.; Tilton, G.R.; Wetherill, G.W. Radioactive ages of minerals from the Brown Derby Mine and the Quartz Creek Granite near Gunnison, Colorado. J. Geophys. Res. 1956, 61, 215–232. [Google Scholar] [CrossRef]
- Romer, R.L.; Smeds, S.A. U-Pb columbite ages of pegmatites from Sveconorwegian terranes in southwestern Sweden. Precambrian Res. 1996, 76, 15–30. [Google Scholar] [CrossRef]
- Romer, R.L.; Smeds, S.A. U-Pb columbite chronology of post-kinematic Palaeoproterozoic pegmatites in Sweden. Precambrian Res. 1997, 82, 85–99. [Google Scholar] [CrossRef]
- Dill, H.G.; Gerdes, A.; Weber, B. Cu-Fe-U phosphate mineralization of the Hagendorf-Pleystein pegmatite province, Germany: With special reference to laser-ablation inductively-coupled plasma mass spectrometry (LA-ICP-MS) of limonite-cored torbenite. Miner. Mag. 2007, 71, 371–387. [Google Scholar] [CrossRef]
- Dewaele, S.; Henjes-Kunst, F.; Melcher, F.; Sitnikova, M.; Burgess, R.; Gerdes, A.; Fernandez, M.A.; DeClercq, F.; Muchez, F.; Lehman, B. Late Neoproterozoic overprinting of the cassiterite and columbite-tantalite bearing pegmatites of the Gatumba area, Rwanda (Central Africa). J. Afr. Earth Sci. 2011, 61, 10–26. [Google Scholar] [CrossRef]
- Deng, X.D.; Li, J.W.; Zhao, X.F.; Hu, Z.C.; Hu, H.; Selby, D.; de Souza, Z.S. U-Pb isotope and trace elements analysis of columbite-(Mn) and zircon by laser ablation ICP-MS: Implications for geochronology of pegmatite and associated ore deposits. Chem. Geol. 2013, 344, 1–11. [Google Scholar] [CrossRef]
- Che, X.D.; Wu, F.Y.; Wang, R.C.; Gerdes, A.; Ji, W.Q.; Shao, Z.H.; Yang, J.H.; Zhu, Z.Y. In situ U-Pb dating of columbite-tantalite by LA-ICP-MS. Ore Geol. Rev. 2015, 65, 979–989. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, J.Y.; Zhang, H.; Cai, D.W.; Lv, Z.H.; Liu, Y.L.; Zhang, X. Precise columbite-(Fe) and zircon U-Pb dating of the Nanping No. 31 pegmatite vein in northeastern Cathaysia Block, SE China. Ore Geol. Rev. 2017, 83, 300–311. [Google Scholar] [CrossRef]
- Lupulescu, M.V.; Chiarenzelli, J.R.; Bailey, D.G. Mineralogy, classification, and tectonic setting of the granitic pegmatites of New York, USA. Can. Miner. 2012, 50, 1713–1728. [Google Scholar] [CrossRef]
- Tan, L.-P. Major Pegmatite Deposits of New York State; New York State Museum Science Service, Bulletin; New York State Museum: Albany, NY, USA, 1966; Volume 408, p. 138. [Google Scholar]
- Aleinikoff, J.N. Isotopic and morphologic evidence for the age of the Fordham Gneiss. Am. J. Sci. 1985, 285, 459–479. [Google Scholar] [CrossRef]
- Fisher, D.W.; Isachsen, Y.W.; Rickard, L.V. Geological Map of New York: Hudson-Mohawk Sheet; New York State Museum Science Service Map and Chart Series; The University of the State of New York: Albany, NY, USA, 1970; Volume 15. [Google Scholar]
- Horton, J.D.; San Juan, C.A.; Stoeser, D.B. The State Geological Map Compilation. (SGMC) Geodatabase of the Conterminous United States; United States Geological Survey Data Series 1052.2788749; U.S. Geological Survey: Reston, VA, USA, 2017.
- Luquer, L.M.; Reis, H. The augen-gneiss area, pegmatite veins, and diorite dikes at Bedford, New York. Am. Geol. 1896, 18, 239–261. [Google Scholar]
- Newland, D.H. The Mining and Quarry Industry of New York State 1905; New York State Museum Science Service, Bulletin: Albany, NY, USA, 1905; p. 102. [Google Scholar]
- Bastin, E.S. Economic geology of the feldspar deposits of the United States. U.S. Geol. Surv. Bull. 1910, 420, 54–63. [Google Scholar]
- Newland, D.H. The mineral resources of the State of New York. N. Y. State Mus. Bull. 1921, 223–224, 69–76. [Google Scholar]
- Newland, D.H.; Hartnagel, C.A. The mining and quarry industries of New York State for 1934 to 1936. N. Y. State Mus. Bull. 1939, 319, 18–36. [Google Scholar]
- Scotford, D.M. Metamorphism and axial-plane folding in the Poundridge area, New York. Geol. Soc. Am. Bull. 1956, 67, 1155–1198. [Google Scholar] [CrossRef]
- Manchester, J.G. The minerals of New York City and its environments. N. Y. Miner. Club Bull. 1931, 3, 168. [Google Scholar]
- Frondel, C. Systematic mineralogy of uranium and thorium. U. S. Geol. Surv. Bull. 1958, 1064, 400. [Google Scholar]
- Lupulescu, M.V. Minerals from New York State Pegmatites. Rocks Miner. 2007, 82, 34–38. [Google Scholar] [CrossRef]
- Gehrels, G.E.; Valencia, V.; Ruiz, J. Enhanced precision, accuracy, efficiency, and spatial resolution of U-Pb ages by laser ablation-multicollector inductively coupled plasma-mass spectrometry. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Stacey, J.S.; Kramers, J.D. Approximation of terrestrial lead isotope evolution by a two-stage model. Earth Planet. Sci. Lett. 1975, 26, 207–221. [Google Scholar] [CrossRef]
- Ludwig, K. Isoplot 3.60; Berkeley Geochronology Center: Berkeley, CA, USA, 2008. Available online: https://www.bgc.org/isoplot_etc/isoplot.html (accessed on 4 May 2018).
- Ercit, S.T. The geochemistry and crystal chemistry of columbite-group minerals from granitic pegmatites, southwestern Grenville Province. Can. Miner. 1994, 32, 421–438. [Google Scholar]
- Černý, P.; Ercit, S.T.; Smeds, S.A.; Groat, L.A.; Chapman, R. Zirconium and hafnium in minerals of the columbite and wodginite groups from granitic pegmatites. Can. Miner. 2007, 45, 185–202. [Google Scholar] [CrossRef]
- Muench, O.B. The analysis of cyrtolite for lead and uranium. Am. J. Sci. 1931, 21, 350–357. [Google Scholar] [CrossRef]
- Muench, O.B. The analysis of Bedford cyrtolite for lead and uranium. J. Am. Chem. Soc. 1934, 56, 1536. [Google Scholar] [CrossRef]
- Agar, W.M. The pegmatites of Bedford, New York. In Proceedings of the 16th International Geological Congress, Washington, DC, USA, 22–29 July 1933; pp. 123–128. [Google Scholar]
- Isachsen, Y.W. Geochronology of New York State; Empire State Geogram; New York State Museum Science Service; New York State Museum: New York, NY, USA, 1963; pp. 1–8. [Google Scholar]
- Wise, M.A.; Cerny, P.; Falster, A.U. Scandium substitution in columbite-group minerals and ixiolite. Can. Miner. 1998, 36, 673–680. [Google Scholar]
- Graupner, T.; Melcher, F.; Gabler, H.-E.; Sitnikova, M.; Bratz, H.; Bahr, A. Rare Earth element geochemistry of columbite-group minerals: LA-ICP-MS data. Miner. Mag. 2010, 74, 691–713. [Google Scholar] [CrossRef]
- van Staal, C.R.; Whalen, J.B. Magmatism during the Salinic, Acadian and Neoacadian Orogenies; Geological Society of America Abstracts with Programs; Geological Society of America: Harrisburg, PA, USA, 2006; Volume 38, p. 31. [Google Scholar]
- Bradley, D.; Buchwaldt, R.; Shea, E.; Bowring, S.; O’Sullivan, P.; Benowitz, J.; McCauley, A.; Bradley, L. Geochronology and Orogenic Context of Northern Appalachian Lithium-Cesium-Tantalum Pegmatites; Geological Society of America Abstracts with Programs; Geological Society of America: Breton Woods, NH, USA, 2013; Volume 45, p. 108. [Google Scholar]
- Bradley, D.; Shea, E.; Buchwaldt, R.; Bowring, S.; McCauley, A.; Benowitz, J.; O’Sullivan, P. Geochronology and Orogenic Context of Lithium-Cesium-Tantalum Pegmatites in the Appalachians; Geological Society of America Abstracts with Programs; Geological Society of America: Albany, NY, USA, 2016; Volume 48. [Google Scholar]

| Oxide | NYSM 22361 | NYSM 25232 | BCB-COL | NYSM 525.8 | NYSM 525.7 |
|---|---|---|---|---|---|
| CaO | 0.02 | udl | udl | 0.03 | 0.10 |
| FeO | 14.18 | 14.18 | 14.12 | 6.24 | 6.26 |
| MnO | 6.38 | 6.33 | 6.32 | 13.64 | 12.73 |
| MgO | 0.14 | 0.06 | 0.13 | udl | 0.03 |
| Nb2O5 | 71.50 | 71.53 | 71.74 | 67.85 | 59.89 |
| Ta2O5 | 6.35 | 7.93 | 6.93 | 12.78 | 20.43 |
| TiO2 | 1.62 | 0.82 | 1.69 | 0.45 | 0.64 |
| Total | 100.19 | 100.85 | 100.93 | 100.99 | 100.08 |
| apfu | |||||
| Ca2+ | nd | nd | nd | nd | 0.01 |
| Fe2+ | 0.68 | 0.68 | 0.67 | 0.30 | 0.32 |
| Mn2+ | 0.31 | 0.31 | 0.30 | 0.67 | 0.66 |
| Mg2+ | 0.01 | 0.01 | 0.01 | 0 | nd |
| Nb5+ | 1.85 | 1.85 | 1.84 | 1.79 | 1.65 |
| Ta5+ | 0.10 | 0.12 | 0.11 | 0.20 | 0.34 |
| Ti4+ | 0.07 | 0.04 | 0.07 | 0.02 | 0.03 |
| Mn/(Mn+Fe) | 0.31 | 0.31 | 0.31 | 0.69 | 0.67 |
| Ta/(Ta+Nb) | 0.05 | 0.06 | 0.06 | 0.10 | 0.17 |
| Sample | Zr | Y | Sn | Sc | W | Hf | Pb | U | Th |
|---|---|---|---|---|---|---|---|---|---|
| BCB-COL | 4670 | 170 | 487.2 | 53.8 | 7050 | 480 | 580.8 | 620.5 | 8.7 |
| NYSM 22361 | 4500 | 223.9 | 499.5 | 72.7 | 8391.6 | 476.8 | 882.5 | 647.1 | 10.9 |
| NYSM 25232 | 4524 | 307.4 | 368.8 | 30.4 | 2088 | 556.4 | 392.8 | 315.0 | 2.4 |
| NYSM 525.7 | 500.4 | 3930 | 97.7 | 35.2 | 1726 | 71.9 | 238.6 | 170.1 | 13.9 |
| NYSM 525.8 | 2622 | 2104 | 386.8 | 8.6 | 4496 | 406 | 736 | 500.2 | 20.7 |
| CYR | - | 1948.2 | - | 118.7 | - | 54,100 | 1678.2 | 2327 | 16.9 |
| Sample | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCB-COL | 0.07 | 0.72 | 0.35 | 3.89 | 26.14 | 0.05 | 75.88 | 23.8 | 78.84 | 4.12 | 4.11 | 0.32 | 1.59 | 0.13 | 170 |
| 22361 | 11 | 16 | 2 | 10 | 33 | 0.07 | 99 | 30 | 105 | 6 | 6 | 0.6 | 3 | 0.22 | 223.85 |
| 25232 | 0.02 | 0.3 | 0.2 | 2 | 20 | 0.03 | 82 | 31 | 127 | 10 | 11 | 1 | 5 | 0.35 | 307.4 |
| 525.7 | 2.76 | 8 | 2 | 20 | 76 | 2 | 264 | 112 | 824 | 144 | 397 | 70 | 574 | 75 | 3930 |
| 525.8 | 0.16 | 3 | 2 | 14 | 66 | 1 | 191 | 86 | 534 | 64 | 138 | 23 | 180 | 23 | 2104 |
| CYR | 1.49 | 1.95 | 0.35 | 1.88 | 10.29 | 0.11 | 97.09 | 48.92 | 301.75 | 29.95 | 50.99 | 7.23 | 54.13 | 7.91 | 1948.2 |
| Sample | U (ppm) | 206Pb/204Pb | U/Th | Concordant Age (Ma) | MSDW | PROB | Weighted Mean (Ma) | MSDW | PROB | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NYSM BCB-COL | Mean | 620.1 | 28,730.2 | 117.0 | 372.2 | 0.04 | 0.84 | 372.2 | 0.15 | 1.00 | 20 |
| SD | 26.7 | 1405.3 | 3.2 | 3.4 | 8.2 | ||||||
| NYSM 25232 | Mean | 315.0 | 92,970.4 | 352.3 | 372.3 | 0.27 | 0.60 | 371.3 | 0.13 | 1.00 | 20 |
| SD | 6.1 | 9769.9 | 6.0 | 2.8 | 7.3 | ||||||
| NYSM 22361 | Mean | 647.1 | 77,805.3 | 128.1 | 383.6 | 0.02 | 0.88 | 383.4 | 0.37 | 0.98 | 15 |
| SD | 80.1 | 41,412.8 | 12.0 | 3.0 | 8.9 | ||||||
| NYSM CYR | Mean | 2230 | 238,925 | 239 | 376.8 | 0.05 | 0.83 | 376.9 | 0.64 | 0.73 | 8 |
| SD | 525 | 164,168 | 36 | 1.2 | 4.3 | ||||||
| NYSM 525.7 | Mean | 165.5 | 106,213.2 | 32.7 | 382 | 0.31 | 0.58 | 383.0 | 0.15 | 1.00 | 14 |
| SD | 49.5 | 72,993.9 | 8.7 | 6.4 | 15 | ||||||
| NYSM 525.8 | Mean | 500.2 | 28,854.1 | 80.5 | 372.4 | 0.03 | 0.86 | 372 | 0.35 | 0.97 | 12 |
| SD | 4.5 | 5479.9 | 2.5 | 3.9 | 11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupulescu, M.V.; Chiarenzelli, J.R.; Pecha, M.E.; Singer, J.W.; Regan, S.P. Columbite-Group Minerals from New York Pegmatites: Insights from Isotopic and Geochemical Analyses. Geosciences 2018, 8, 169. https://doi.org/10.3390/geosciences8050169
Lupulescu MV, Chiarenzelli JR, Pecha ME, Singer JW, Regan SP. Columbite-Group Minerals from New York Pegmatites: Insights from Isotopic and Geochemical Analyses. Geosciences. 2018; 8(5):169. https://doi.org/10.3390/geosciences8050169
Chicago/Turabian StyleLupulescu, Marian V., Jeffrey R. Chiarenzelli, Mark E. Pecha, Jared W. Singer, and Sean P. Regan. 2018. "Columbite-Group Minerals from New York Pegmatites: Insights from Isotopic and Geochemical Analyses" Geosciences 8, no. 5: 169. https://doi.org/10.3390/geosciences8050169
APA StyleLupulescu, M. V., Chiarenzelli, J. R., Pecha, M. E., Singer, J. W., & Regan, S. P. (2018). Columbite-Group Minerals from New York Pegmatites: Insights from Isotopic and Geochemical Analyses. Geosciences, 8(5), 169. https://doi.org/10.3390/geosciences8050169




