Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites and Sampling Procedure
2.2. Laboratory Methods and Calculations
3. Results
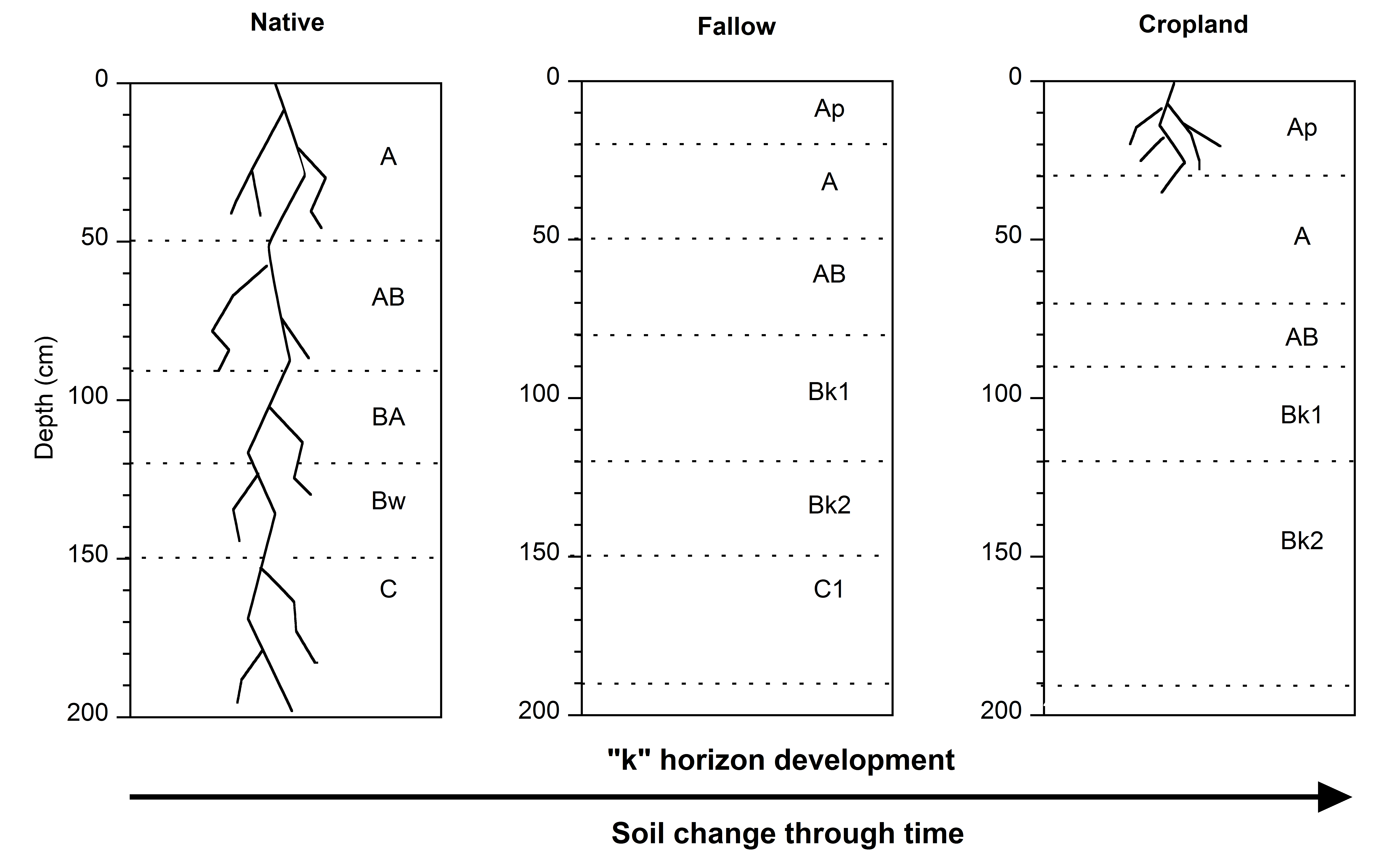
3.1. Soil Types under Different Uses
3.2. Soil Organic Carbon (SOC) under Different Uses
3.3. Soil Inorganic Carbon (SIC) under Different Uses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torn, M.S.; Lapenis, A.G.; Timofeev, A.; Fischer, M.L.; Babikov, B.V.; Harden, J.W. Organic carbon and carbon isotopes in modern and 100-year-old-soil archives of the Russian steppe. Glob. Chang. Biol. 2002, 8, 941–953. [Google Scholar] [CrossRef]
- Willms, W.; Adams, B.; McKenzie, R. Overview: Anthropogenic Changes of Canadian Grasslands. In Arthropods of Canadian Grasslands (Volume 2): Inhabitants of a Changing Landscape; Floate, K.D., Ed.; Biological Survey of Canada: Ottawa, ON, Canada, 2011; pp. 1–22. ISBN 978-0-9689321-5-5. [Google Scholar]
- Paul, E.A.; Follett, R.F.; Leavitt, S.W.; Halvorson, A.; Peterson, G.A.; Lyon, D.J. Radiocarbon dating for determination of soil organic matter pool sizes and dynamics. Soil Sci. Soc. Am. J. 1997, 61, 1058–1067. [Google Scholar] [CrossRef]
- Scharpenseel, H.W.; Becker-Heidmann, P. Twenty-five years of radiocarbon dating soils: Paradigm of erring and learning. Radiocarbon 1992, 34, 541–549. [Google Scholar] [CrossRef]
- Rethemeyer, J.; Grootes, P.M.; Bruhn, F.; Andersen, N.; Nadeau, M.J.; Kramer, C.; Gleixner, G. Age heterogeneity of soil organic matter. Nucl. Instrum. Methods Phys. Res. B 2004, 223–224, 521–527. [Google Scholar] [CrossRef]
- Rethemeyer, J.; Kramer, C.; Gleixner, G.; John, B.; Yamashita, T.; Flessa, H.; Andersen, N.; Nadeau, M.; Grootes, P.M. Transformation of organic matter in agricultural soils: Radiocarbon concentration versus soil depth. Geoderma 2005, 128, 94–105. [Google Scholar] [CrossRef]
- Chendev, Y.G.; Khokhlova, O.S.; Alexandrovskiy, A.L. Agrogenic evolution of automorphic Chernozems in the forest-steppe zone (Belgorod Oblast). Eurasian Soil Sci. 2017, 5, 499–514. [Google Scholar] [CrossRef]
- Wang, H.; Stumf, A.J.; Kumar, P. Radiocarbon and stable isotopes of labile and inert organic carbon in the Critical Zone Observatory in Illinois, USA. Radiocarbon 2018, 60, 989–999. [Google Scholar] [CrossRef]
- Wang, Y.; Amundson, R.; Trumbore, S. Radiocarbon dating of soil organic matter. Quat. Res. 1996, 45, 282–288. [Google Scholar] [CrossRef]
- Perrin, R.M.S.; Willis, E.H.; Hodge, D.A.H. Dating of humus podzols by residual radiocarbon activity. Nature 1964, 202, 165–166. [Google Scholar] [CrossRef]
- Scharpenseel, H.W. Radiocarbon dating of soils. Sov. Soil Sci. 1971, 3, 76–83. [Google Scholar]
- Scharpenseel, H.W. Radiocarbon dating of soils-problems, troubles, hopes. In Paleopedology—Origin, Nature and Dating of Paleosols; Yaalon, D.H., Ed.; International Society of Soil Science and Israel Univ. Press: Jerusalem, Israel, 1971; pp. 77–87. [Google Scholar]
- Scharpenseel, H.W. Natural radiocarbon measurement on soil organic matter fractions and on soil profiles of different pedogenesis. In Proceedings of the 8th International Conference on Radiocarbon Dating, Lower Hutt, New Zealand, 18–25 October 1972; Volume 1, pp. 382–394. [Google Scholar]
- Scharpenseel, H.W. Soil fraction dating. In Radiocarbon Dating; Berger, R., Suess, H.E., Eds.; Univ. of California Press: Berkeley, CA, USA, 1976; pp. 277–283. [Google Scholar]
- Cherkinsky, A.E.; Brovkin, V.A. A model of humus formation in soils based on radiocarbon data of natural ecosystems. Radiocarbon 1991, 33, 186–187. [Google Scholar]
- Rutberg, R.L.; Schimel, D.S.; Hajdas, I.; Broecker, W.S. The effect of tillage on soil organic matter using 14C: A case study. Radiocarbon 1996, 38, 209–217. [Google Scholar] [CrossRef]
- Fazle Rabbi, S.M.; Hua, Q.; Daniel, H.; Lockwood, P.V.; Wilson, B.R.; Young, I.M. Mean residence time of soil organic carbon in aggregates under contrasting land uses based on radiocarbon measurements. Radiocarbon 2013, 55, 127–139. [Google Scholar] [CrossRef]
- Orlova, L.A.; Panychev, V.A. The reliability of radiocarbon dating buried soils. Radiocarbon 1993, 35, 369–377. [Google Scholar] [CrossRef]
- Wang, Y.; McDonald, E.; Amundson, R.; McFadden, L.; Chadwick, O. An isotopic study of soils in chronological sequences of alluvial deposits, Providence Mountains, California. Geol. Soc. Am. Bull. 1996, 108, 379–391. [Google Scholar] [CrossRef]
- Bowler, I.M.; Polach, H.A. Radiocarbon analyses of soil carbonates: An evaluation from paleosols in southeastern Australia. In Paleopedology: Origin, Nature and Dating of Paleosols; Yaalon, D.H., Ed.; International Society of Soil Science: Jerusalem, Israel, 1971; pp. 97–108. [Google Scholar]
- Groshans, G.R.; Mikhailova, E.A.; Post, C.J.; Schlautman, M.A. Accounting for soil inorganic carbon in the ecosystem services framework for the United Nations sustainable development goals. Geoderma 2018, 324, 37–46. [Google Scholar] [CrossRef]
- Goddard, M.A.; Mikhailova, E.A.; Post, C.J.; Schlautman, M.A. Atmospheric Mg2+ wet deposition within the continental United States and implications for soil inorganic carbon sequestration. Tellus Ser. B 2007, 59, 50–56. [Google Scholar] [CrossRef]
- Goddard, M.A.; Mikhailova, E.A.; Post, C.J.; Schlautman, M.A.; Galbraith, J.M. Continental United States atmospheric wet calcium deposition and soil inorganic carbon stocks. Soil Sci. Soc. Am. J. 2009, 73, 989–994. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Goddard, M.A.; Post, C.J.; Schlautman, M.A.; Galbraith, J.M. Potential contribution of combined atmospheric Ca2+ and Mg2+ wet deposition within the continental U.S. to soil inorganic carbon sequestration. Pedosphere 2013, 23, 808–814. [Google Scholar] [CrossRef]
- Monger, H.C.; Rachal, D.M. Soil and landscape memory of climate change: How sensitive, how connected? In New Frontiers in Paleopedology and Terrestrial Paleoclimatology: Paleosols and Soil Surface Analog Systems; Driese, S.G., Nordt, L.C., Eds.; Society for Sedimentary Geology Special Publication 104; Society for Sedimentary Geology: Tulsa, OK, USA, 2013; pp. 63–70. [Google Scholar]
- Monger, C.H. Pedogenic carbonate: Links between biotic and abiotic CaCO3. 2002, p. 897–891 to 897-9. In Proceedings of the 17th WCSS (World Congress of Soil Science), Bangkok, Thailand, 14–21 August 2002. [Google Scholar]
- Schlesinger, W.H. The formation of caliche in soils of the Mojave Desert, California. Geochim. Cosmochim. Acta 1985, 49, 57–66. [Google Scholar] [CrossRef]
- Wang, J.; Monger, C.; Wang, X.; Serena, M.; Leinauer, B. Carbon sequestration in response to grassland-shrubland-turfgrass conversions and a test for carbonate biomineralization in desert soils, New Mexico, USA. Soil Sci. Soc. Am. J. 2016, 80, 1591–1603. [Google Scholar] [CrossRef]
- Landi, A.; Mermut, A.R.; Anderson, D.W. Origin and rate of pedogenic carbonate accumulation in Saskatchewan soils, Canada. Geoderma 2003, 117, 143–156. [Google Scholar] [CrossRef]
- Stevenson, B.A.; Kelly, E.F.; McDonald, E.V.; Busacca, A.J. The stable carbon isotope composition of soil organic carbon and pedogenic carbonates along a bioclimatic gradient in the Palouse region, Washington State, USA. Geoderma 2005, 124, 37–47. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Post, C.J.; Magrini-Bair, K.; Castle, J.W. Pedogenic carbonate concretions in the Russian Chernozem. Soil Sci. 2006, 171, 981–991. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Post, C.J. Effects of land use on soil inorganic carbon stocks in the Russian Chernozem. JEQ 2006, 35, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wood, Y.; Jiang, P.; Li, L.; Pan, G.; Lu, J.; Chang, A.C.; Enloe, H.A. Carbon sequestration and dynamics of two irrigated agricultural soils in California. Soil Sci. Soc. Am. J. 2008, 72, 808–814. [Google Scholar] [CrossRef]
- Han, X.; Gao, G.; Chang, R.; Li, Z.; Ma, Y.; Wang, S.; Wang, C.; Lü, Y.; Fu, B. Changes in soil organic and inorganic carbon stocks in deep profiles following cropland abandonment along a precipitation gradient across the Loess Plateau of China. Agric. Ecosyst. Environ. 2018, 258, 1–13. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421. [Google Scholar] [CrossRef]
- Mayer, S.; Schwindt, D.; Steffens, M.; Völkel, J.; Kögel-Knabner, I. Drivers of organic carbon allocation in a temperate slope-floodplain catena under agricultural use. Geoderma 2018, 327, 63–72. [Google Scholar] [CrossRef]
- Hallsworth, E.G.; Crawford, D.V. Experimental Pedology; Butterworths: London, UK, 1965. [Google Scholar]
- Mikhailova, E.A.; Bryant, R.B.; Vassenev, I.I.; Schwager, S.J.; Post, C.J. Cultivation effects on soil carbon and nitrogen contents at depth in the Russian Chernozem. Soil Sci. Soc. Am. J. 2000, 64, 738–745. [Google Scholar] [CrossRef]
- Chichagova, O.A. Radiocarbon Dating of Humus of Soils: Methods and Applications in Pedology and Palaeogeography; Gerasimov, I.P., Targuljan, V.O., Eds.; Iszd-vo Nauka: Moscow, Russia, 1985; 155p. (In Russian) [Google Scholar]
- Margolina, N.Y.; Alexandrovski, A.L.; Ilichev, B.A.; Cherkinsky, A.E.; Chichagova, O.A. Age and Evolution of Chernozems; Iszd-vo Nauka: Moscow, Russia, 1988; 144p. (In Russian) [Google Scholar]
- Köppen, W. Klassification der Klimate nach Temperatur, Niederschlag and Jahreslauf. Petermanns Geographische Mitteilungen 1918, 64, 193–203, 243–248. Available online: http://koeppen-geiger.vu-wien.ac.at/koeppen.htm (accessed on 20 July 2018).
- Alekhin, V.V. Central-Chernozem Biosphere State Reserve. Letopis’ Prirody (Annual Scientific Reports) 1947–1997; Central-Chernozem Biosphere State Reserve: Kursk, Russia, 1997; Volume 1–63. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 8th ed.; USDA-NRCS: Washington, DC, USA, 1998.
- Stolbovoi, V. Soils of Russia: Correlated with the Revised Legend of the FAO Soil Map of the World and World Reference Base for Soil Resources; International Institute for Applied Systems Analysis: Laxenburg, Austria, 2000. [Google Scholar]
- Pietsch, D. Krotovinas—Soil archives of steppe landscape history. Catena 2013, 104, 257–264. [Google Scholar] [CrossRef]
- Vinogradov, B.V. Aerospace studies of protected natural areas in the USSR. In Conservation, Science and Society (Natural Resources Research, XXI, Volmue 2); UNESCO-UNEP: London, UK, 1984; pp. 435–448. [Google Scholar]
- Ryabov, V.A. Climatological characteristic of the Central-Chernozem Reserve. In Transactions of the Central-Chernozem State Biosphere Reserve; Alekhin, V.V., Ed.; Gidrometeoizdat: Leningrad, Israel, 1979; Volume 12, pp. 5–72. (In Russian) [Google Scholar]
- Mikhailova, E.A.; Bryant, R.B.; Cherney, D.J.R.; Post, C.J.; Vassenev, I.I. Botanical composition, soil and forage quality under different management regimes in Russian grasslands. Agric. Ecosyst. Env. 2000, 80, 213–226. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Bryant, R.B.; DeGloria, S.D.; Post, C.J.; Vassenev, I.I. Modeling soil organic matter dynamics after conversion of native grassland to long-term continuous fallow using the CENTURY model. Ecol. Model. 2000, 132, 247–257. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2, 2nd ed.; Agronomy Monograph 9; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Nelson, R.E. Carbonate and gypsum. In Methods of Soil Analysis. Part 2, 2nd ed.; Agronomy Monograph 9; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 181–197. [Google Scholar]
- Linick, T.W.; Jull, A.J.T.; Toolin, L.J.; Donahue, D.J. Operation of the NSF-Arizona accelerator facility for radioisotope analysis and results from selected collaborative research projects. In Proceedings of the International 14C Conference, 12th Proceedings Radiocarbon, Trondheim, Norway, 24–28 June 1985; Stuiver, M., Kra, R.S., Eds.; Volume 28, pp. 522–533. [Google Scholar]
- Donahue, D.J.; Linick, T.W.; Jull, A.J.T. Isotope-ratio and background corrections for accelerator mass spectrometry radiocarbon measurements. Radiocarbon 1990, 32, 135–142. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H. Reporting of 14C data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Reimer, P.J.; Brown, T.A.; Reimer, R.W. Discussion: Reporting and calibration of post-bomb 14C data. Radiocarbon 2004, 46, 1299–1304. [Google Scholar] [CrossRef]
- Salomons, W.; Mook, W.G. Isotope geochemistry of carbonate dissolution and reprecipitation in soils. Soil Sci. 1976, 122, 15–24. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Bartlett, H.H. Radiocarbon datability of peat, marl, caliche, and archaeological materials. Science 1951, 114, 55–56. [Google Scholar] [CrossRef]
- Cerling, T.E.; Quade, J.; Wang, Y.; Bowman, J.R. Carbon isotopes in soils and paleosols as ecology and paleoecology indicators. Nature 1989, 341, 138–139. [Google Scholar] [CrossRef]
- Broecker, W.S.; Walton, A. The geochemistry of C14 in fresh-water systems. Geochim. Cosmochim. Acta 1959, 16, 15–38. [Google Scholar] [CrossRef]
- Cerling, T.E. The stable isotopic composition of modern soil carbonate and its relationship to climate. Earth Planet. Sci. Lett. 1984, 71, 229–240. [Google Scholar] [CrossRef]
- Deines, P.; Langmuir, D.; Harmon, R.S. Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate ground waters. Geochim. Cosmochim. Acta 1974, 1147–1164. [Google Scholar] [CrossRef]
- Quade, J.; Cerling, T.E.; Bowman, J.R. Systematic variations in the carbon and oxygen isotopic composition of pedogenic carbonate along elevation transects in the southern Great Basin, United States. Geol. Soc. Am. Bull. 1989, 101, 464–475. [Google Scholar] [CrossRef]
- Wang, Y.; Amundson, R.; Trumbore, S. A model for soil 14CO2 and its implications for using 14C to date pedogenic carbonate. Geochim. Cosmochim. Acta 1994, 58, 393–399. [Google Scholar]
- Amundson, R.G.; Lund, L.J. The stable isotope chemistry of a native and irrigated Typic Natrargid in the San-Joaquin Valley of California. Soil Sci. Soc. Am. J. 1987, 51, 761–767. [Google Scholar] [CrossRef]
- Targulian, V.O.; Goryachkin, S.V. Soil memory: Types of record, carriers, hierarchy and diversity. Rev. Mex. Cienc. Geol. 2004, 21, 1–8. [Google Scholar]
- Gerasimova, M.; Lebedeva, M. Contribution of micromorphology to classification of aridic soils. In New Trends in Soil Micromorphology; Kapur, S., Stoops, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 151–162. [Google Scholar]
- Washbourne, C.-L.; Renforth, P.; Manning, D.A.C. Investigating carbonate formation in urban soils as a method for capture and storage of atmospheric carbon. Sci. Total Environ. 2012, 431, 166–175. [Google Scholar] [CrossRef]
- Conry, M.J. Pedological evidence of man’s role in soil profiles modification in Ireland. Geoderma 1972, 8, 139–146. [Google Scholar] [CrossRef]
- Sandor, J.A.; Eash, N.S. Ancient agricultural soils in the Andes of Southern Peru. Soil Sci. Soc. Am. J. 1995, 59, 170–179. [Google Scholar] [CrossRef]

| Depth (cm) | δ13C (‰) | 14C Age for Soil without Carbonates (y BP) |
|---|---|---|
| 10–20 | −25.75 | 1680 ± 60 |
| 30–40 | −26.20 | 2950 ± 80 |
| 50–60 | −25.88 | 2970 ± 110 |
| 70–80 | −24.92 | 4020 ± 90 |
| 120–130 | - | 6100 ± 200 |
| 140–150 | - | 6700 ± 100 |
| Site/Horizon | Depth (cm) | n | pH in Water | Sand (%) | Silt (%) | Clay (%) | USDA Textural Class a |
|---|---|---|---|---|---|---|---|
| Native | |||||||
| A | 0–50 | 5 | 6.1 ± 0.2 | 2.0 ± 0.5 | 66.8 ± 0.3 | 31.2 ± 0.6 | SiCL b |
| AB | 50–90 | 4 | 6.3 ± 0.0 | 0.8 ± 0.1 | 66.9 ± 0.5 | 32.3 ± 0.5 | SiCL |
| BA | 90–120 | 3 | 6.8 ± 0.5 | 0.8 ± 0.1 | 67.8 ± 0.9 | 31.4 ± 1.0 | SiCL |
| Bw | 120–150 | 3 | 7.0 ± 0.4 | 0.9 ± 0.2 | 70.1 ± 3.5 | 29.0 ± 3.6 | SiCL |
| C | 150–200 | 5 | 8.5 ± 0.3 | 1.1 ± 0.2 | 76.8 ± 1.4 | 22.1 ± 1.3 | SiL |
| Fallow | |||||||
| Ap | 0–20 | 2 | 5.9 ± 0.2 | 0.7 ± 0.1 | 65.6 ± 0.1 | 33.7 ± 0.0 | SiCL |
| A | 20–50 | 3 | 6.9 ± 0.4 | 1.1 ± 0.7 | 64.9 ± 0.4 | 34.0 ± 0.6 | SiCL |
| AB | 50–80 | 3 | 7.6 ± 0.3 | 1.0 ± 0.1 | 66.8 ± 2.5 | 32.2 ± 2.4 | SiCL |
| Bk1 | 80–120 | 4 | 8.3 ± 0.1 | 1.2 ± 0.6 | 72.3 ± 3.5 | 26.5 ± 3.2 | SiL |
| Bk2 | 120–150 | 3 | 8.4 ± 0.1 | 1.8 ± 0.6 | 69.4 ± 2.1 | 28.8 ± 1.8 | SiCL |
| C1 | 150–190 | 4 | 8.4 ± 0.1 | 2.4 ± 0.4 | 67.1 ± 2.8 | 30.5 ± 3.3 | SiCL |
| C2 | 190–210 | 2 | 8.5 ± 0.0 | 4.7 ± 1.4 | 73.3 ± 6.2 | 22.0 ± 4.8 | SiL |
| Cropland | |||||||
| Ap | 0–30 | 3 | 7.9 ± 0.1 | 1.1 ± 0.4 | 69.0 ± 0.3 | 29.9 ± 0.3 | SiCL |
| A | 30–70 | 4 | 8.4 ± 0.2 | 0.9 ± 0.3 | 80.4 ± 3.1 | 18.7 ± 3.2 | SiL |
| AB | 70–90 | 2 | 8.6 ± 0.1 | 1.3 ± 0.6 | 77.4 ± 6.7 | 21.3 ± 6.1 | SiL |
| Bk1 | 90–120 | 3 | 8.6 ± 0.0 | 1.5 ± 0.3 | 74.3 ± 1.1 | 24.2 ± 1.1 | SiL |
| Bk2 | 120–190 | 7 | 8.6 ± 0.1 | 1.7 ± 0.4 | 73.7 ± 1.9 | 24.6 ± 2.1 | SiL |
| Ck | 190–200 | 1 | 8.4 | 1.2 | 75.6 | 23.2 | SiL |
| Site/Horizon | Depth (cm) | Munsell Color (Moist) | Munsell Color (Dry) | CaCO3 in Clay Fraction (%) a,b | CaCO3 Equivalent (g kg−1) b | Presence of Carbonates (−/+) |
|---|---|---|---|---|---|---|
| Native | ||||||
| A | 0–50 | 10YR 2/1 | 10YR 3/1 | 0 | 0 | - |
| AB | 50–90 | 10YR 2/1 | 10YR 3/2 | 0 | 0 | - |
| BA | 90–120 | 10YR 3/2 | 10YR 4/2 | 0 | 0 | - |
| Bw | 120–150 | 10YR 3/3 | 10YR 5/4 | 0 | 0 | - |
| C | 150–200 | 10YR 5/3 | 10YR 6/4 | 14.5 | 62 | + w.t.f.m. c |
| Fallow | ||||||
| Ap | 0–20 | 10YR 2/1 | 10YR 3/1 | 0 | 0 | - |
| A | 20–50 | 10YR 2/1 | 10YR 3/1 | 0 | 0 | - |
| AB | 50–80 | 10YR 3/2 | 10YR 3/2 | 0 | 0 | - |
| Bk1 | 80–120 | 10YR 5/6 | 10YR 4/2 | 20.3 | 61 | + w.t.f.m. |
| Bk2 | 120–150 | 10YR 5/6 | 10YR 6/4 | 23.3 | 109 | ± |
| C1 | 150–190 | 10YR 5/6 | 10YR 6/4 | 15.3 | 77 | + w.s.c. d |
| C2 | 190–210 | 10YR 6/6 | 10YR 6/4 | 13.8 | 53 | ± |
| Cropland | ||||||
| Ap | 0–30 | 10YR 2/1 | 10YR 3/2 | 0 | 0 | - |
| A | 30–70 | 10YR 2/1 | 10YR 3/1 | 11.9 | 45 | + w.t.f.m. |
| AB | 70–90 | 10YR 3/2 | 10YR 4/2 | 21.4 | 83 | + w.t.f.m. |
| Bk1 | 90–120 | 10YR 5/3 | 10YR 5/3 | 26.7 | 99 | + w.t.f.m. |
| Bk2 | 120–190 | 10YR 4/2 | 10YR 5/3 | 18.0 | 93 | + w.t.f.m. |
| Ck | 190–200 | 10YR 4/6 | 10YR 5/8 | 16.8 | 79 | + w.s.c. |
| Cambic Horizon (Bw) | Calcic Horizon (Bk) |
|---|---|
| A cambic horizon is the result of physical alterations, chemical transformations, or removals or of a combination of two or more of these processes. | The calcic horizon is an illuvial horizon in which secondary calcium carbonate or other carbonates have accumulated to a significant extent |
| Required Characteristics: The cambic horizon is an altered horizon 15 cm or thicker. If it is composed of lamellae, the combined thickness of the lamellae must be 15 cm or more. In addition, the cambic horizon must meet all of the following: 1. Has a texture class of very fine sand, loamy very fine sand, or finer; and 2. Shows evidence of alteration in one of the following forms: a. Aquic conditions within 50 cm of the soil surface or artificial drainage and all the following: (1) Soil structure or the absence of rock structure, including fine stratifications (5 mm or less thick), in more than one-half of the volume; and (2) Colors that do not change on exposure to air; and (3) Dominant color, moist, on faces of peds or in the matrix as follows: (a) Value of 3 or less and neutral colors with no hue (N) and zero chroma; or (b) Value of 4 or more and chroma of 1 or less; or (c) Any value, chroma of 2 or less, and redox concentrations; or b. Does not have the combination of aquic conditions within 50 cm of the soil surface or artificial drainage and colors, moist, as defined in item 2-a-(3) above, and has soil structure or the absence of rock structure, including fine stratifications (5 mm or less thick), in more than one-half of the volume and one or more of the following properties: (1) Higher chroma, higher value, redder hue, or higher clay content than the underlying horizon or an overlying horizon; or (2) Evidence of the removal of carbonates or gypsum; and 3. Has properties that do not meet the requirements for an anthropic, histic, folistic, melanic, mollic, plaggen, or umbric epipedon, a duripan or fragipan, or an argillic, calcic, gypsic, natric, oxic, petrocalcic, petrogypsic, placic, salic, spodic, or sulfuric horizon; and 4. Is not part of an Ap horizon and does not have a brittle manner of failure in more than 60 percent of the matrix. | Required Characteristics: The calcic horizon: 1. Is 15 cm or thicker; and 2. Has one or more of the following: a. 15 percent or more (by weight, fine-earth fraction) CaCO3 equivalent, and its CaCO3 equivalent is 5 percent or more (absolute) higher than that of an underlying horizon; or b. 15 percent or more (by weight, fine-earth fraction) CaCO3 equivalent and 5 percent or more (by volume) identifiable secondary carbonates; or c. 5 percent or more (by weight, fine-earth fraction) calcium carbonate equivalent and: (1) Has less than 18 percent clay in the fine-earth fraction; and (2) Meets the criteria for a sandy, sandy-skeletal, coarse loamy, or loamy-skeletal particle-size class (defined in chapter 17); and (3) Has 5 percent or more (by volume) identifiable secondary carbonates or a calcium carbonate equivalent (by weight, fine-earth fraction) that is 5 percent or more (absolute) higher than that of an underlying horizon; and 3. Is not cemented or indurated in any part by carbonates, with or without other cementing agents, or is cemented in some part and the cemented part satisfies one of the following: a. It is characterized by so much lateral discontinuity that roots can penetrate through non-cemented zones or along vertical fractures with a horizontal spacing of less than 10 cm; or b. The cemented layer is less than 1 cm thick and consists of a laminar cap underlain by a lithic or paralithic contact; or c. The cemented layer is less than 10 cm thick. |
| Site/Horizon | Depth (cm) | n | Total C (%) a | Organic C (%) a | Inorganic C (%) a | Pedogenic Carbonate (%) b |
|---|---|---|---|---|---|---|
| Native | ||||||
| A | 0–50 | 5 | 4.49 ± 0.97 | 4.49 ± 0.97 | 0 | 0 |
| AB | 50–90 | 4 | 2.70 ± 0.43 | 2.70 ± 0.43 | 0 | 0 |
| BA | 90–120 | 3 | 1.53 ± 0.23 | 1.53 ± 0.23 | 0 | 0 |
| Bw | 120–150 | 3 | 1.20 ± 0.16 | 1.12 ± 0.11 | 0 | 0 |
| C | 150–200 | 5 | 1.71 ± 0.14 | 0.61 ± 0.14 | 1.10 ± 0.20 | 27 ± 8 |
| Fallow | ||||||
| Ap | 0–20 | 2 | 3.38 ± 0.28 | 3.38 ± 0.28 | 0 | 0 |
| A | 20–50 | 3 | 3.09 ± 0.33 | 3.09 ± 0.33 | 0 | 0 |
| AB | 50–80 | 3 | 2.15 ± 0.10 | 2.08 ± 0.21 | 0 | 0 |
| Bk1 | 80–120 | 4 | 2.33 ± 0.38 | 1.14 ± 0.31 | 1.19 ± 0.64 | 53 ± 23 |
| Bk2 | 120–150 | 3 | 2.30 ± 0.22 | 0.66 ± 0.02 | 1.64 ± 0.21 | 53 ± 7 |
| C1 | 150–190 | 4 | 1.45 ± 0.08 | 0.46 ± 0.02 | 0.99 ± 0.07 | 68 ± 13 |
| C2 | 190–210 | 2 | 1.52 ± 0.28 | 0.31 ± 0.13 | 1.22 ± 0.15 | 72 ± 3 |
| Cropland | ||||||
| Ap | 0–30 | 3 | 3.32 ± 0.09 | 3.32 ± 0.09 | 0 | 0 |
| A | 30–70 | 4 | 3.19 ± 0.17 | 2.40 ± 0.15 | 0.79 ± 0.17 | 85 ± 16 |
| AB | 70–90 | 2 | 3.21 ± 0.01 | 1.91 ± 0.08 | 1.30 ± 0.07 | 81 ± 1 |
| Bk1 | 90–120 | 3 | 2.95 ± 0.18 | 1.20 ± 0.16 | 1.75 ± 0.08 | 54 ± 14 |
| Bk2 | 120–190 | 7 | 2.25 ± 0.25 | 0.71 ± 0.14 | 1.55 ± 0.13 | 20 ± 11 |
| Ck | 190–200 | 1 | 1.82 | 0.51 | 1.31 | 10 |
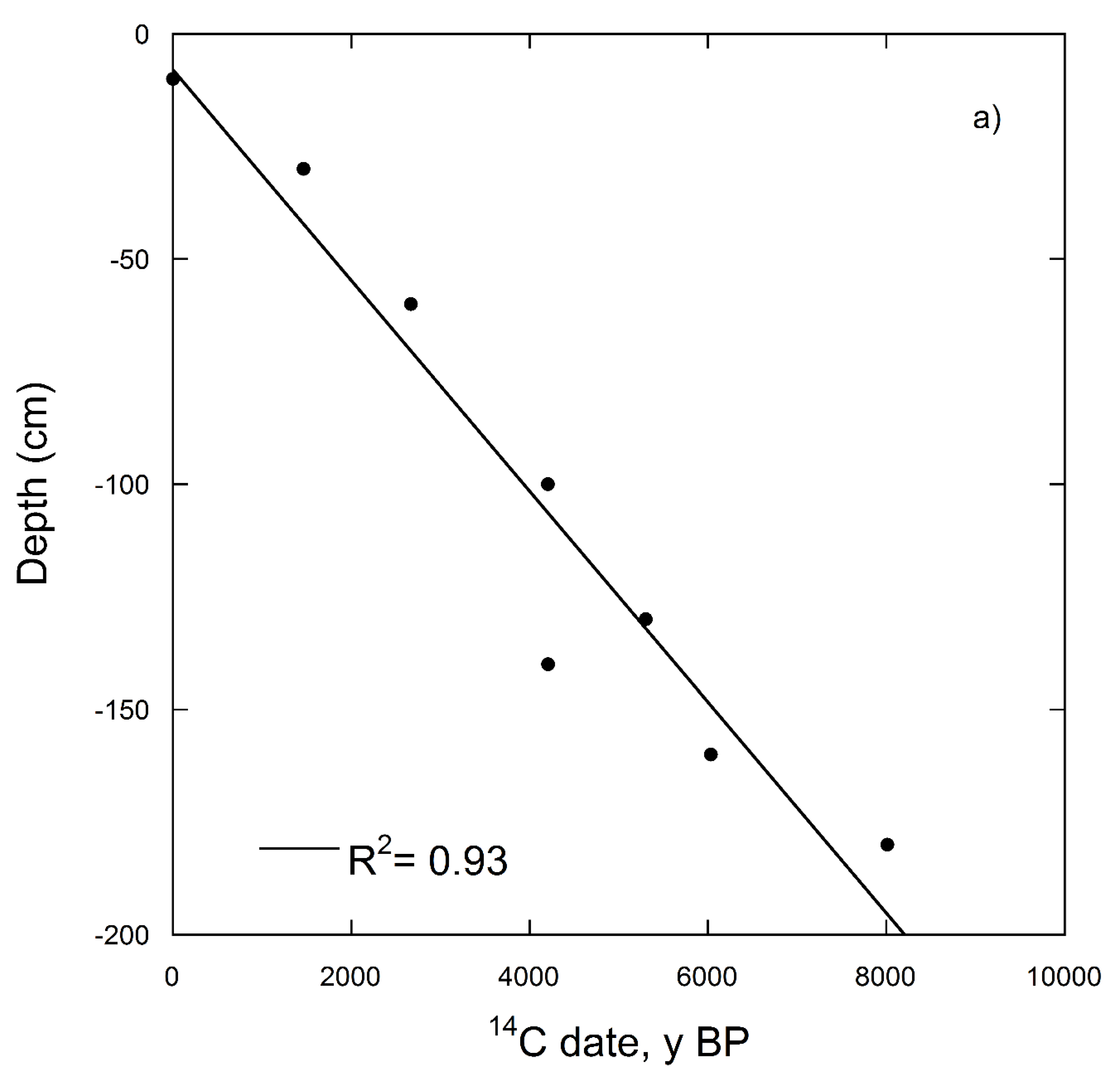
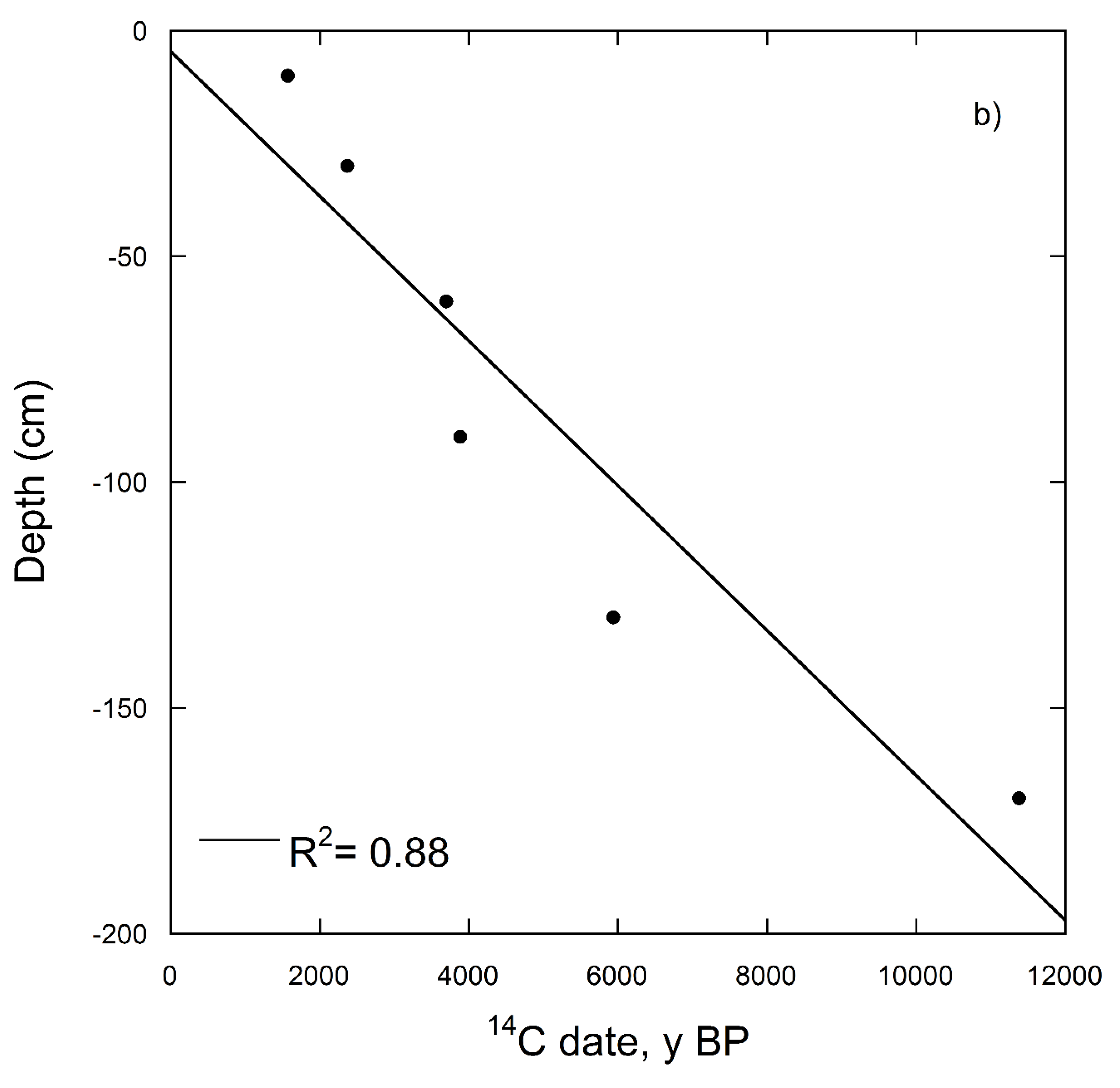
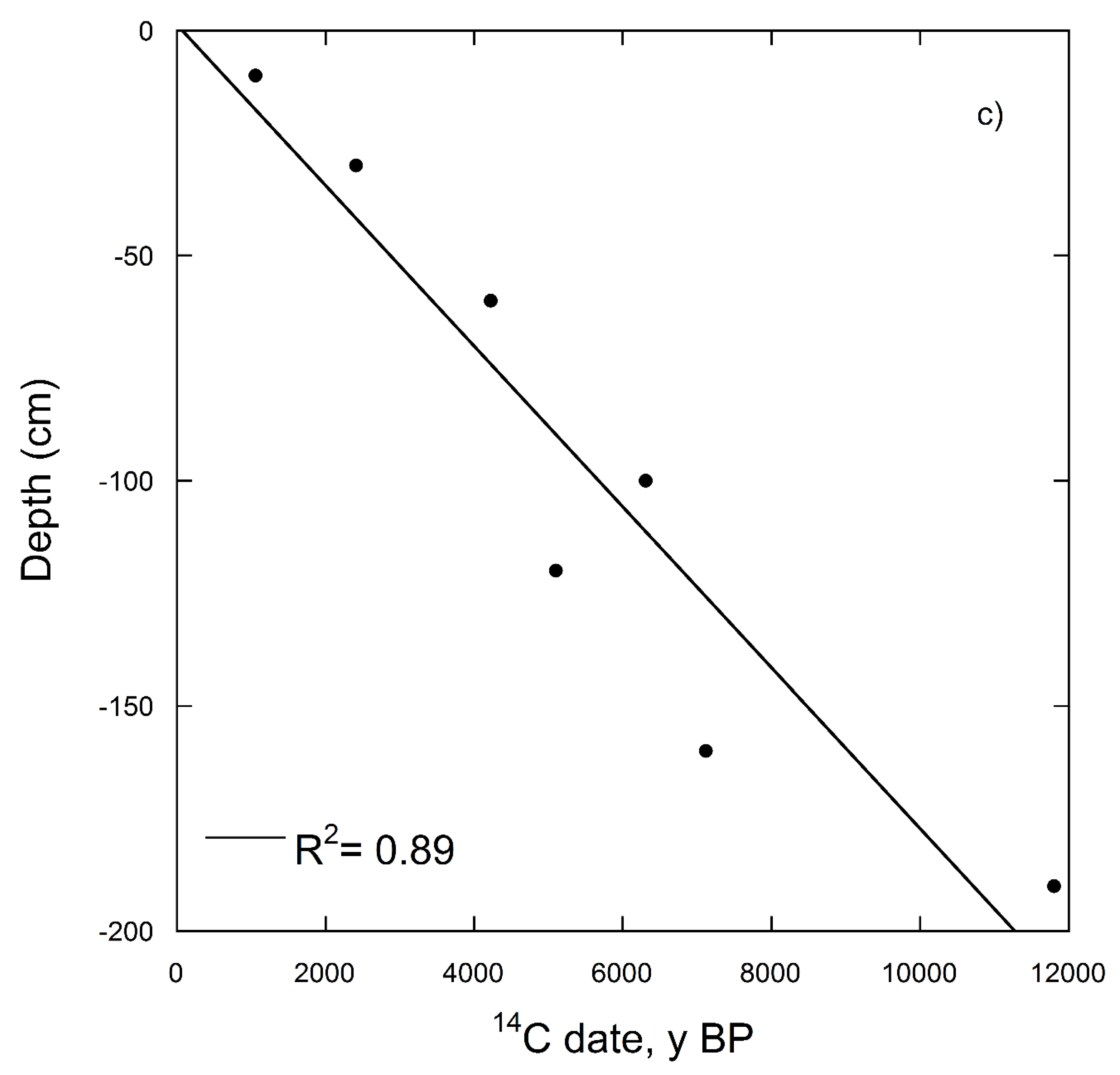
| Depth (cm) | Soil Horizon | 14C Date for Soil without Carbonates (14C y BP) | Percent Modern (%) | Δ14C (‰) | 14C date for Bulk Soil (with Carbonates) (14C y BP) | Percent Modern (%) | Δ14C (‰) |
|---|---|---|---|---|---|---|---|
| Native | |||||||
| 0–10 | A | Post-bomb | 103 | 29.3 | - | - | - |
| 30–40 | A | 1465 ± 39 | 83 | −172.3 | - | - | - |
| 60–70 | AB | 2669 ± 41 | 71 | −287.5 | - | - | - |
| 100–110 | BA | 4206 ± 44 | 59 | −411.5 | - | - | - |
| 130–140 | Bw | 5302 ± 46 | 51 | −486.6 | - | - | - |
| 140–150 | Bw | 4207 ± 44 | 59 | −411.5 | 9700 ± 100 | 30 | −703.1 |
| 160–170 | C | 6031 ± 48 | 47 | −531.1 | 10,950 ± 63 | 25 | −745.8 |
| 180–190 | C | 8011 ± 54 | 37 | −633.5 | 11,436 ± 65 | 24 | −760.8 |
| Fallow | |||||||
| 0–10 | Ap | 1569 ± 41 | 82 | −182.9 | - | - | - |
| 30–40 | A | 2369 ± 41 | 74 | −260.3 | - | - | - |
| 60–70 | AB | 3697 ± 42 | 63 | −373.1 | - | - | - |
| 90–100 | Bk1 | 3883 ± 43 | 61 | −384.4 | 8298 ± 81 | 35 | −646.5 |
| 130–140 | Bk2 | 5937 ± 54 | 47 | −526.6 | 8747 ± 54 | 33 | −665.6 |
| 170–180 | C1 | 11,380 ± 180 | 24 | −758.9 | 10,902 ± 63 | 26 | −744.3 |
| 200–210 | C2 | 7820 ± 190 | 38 | −624.8 | 9198 ± 70 | 32 | −683.9 |
| Cropland | |||||||
| 0–10 | Ap | 1055 ± 38 | 87 | −128.9 | - | - | - |
| 30–40 | A | 2410 ± 41 | 74 | −264.0 | 2675 ± 45 | 71 | −288.0 |
| 60–70 | A | 4224 ± 49 | 59 | −412.9 | 3964 ± 46 | 61 | −393.5 |
| 100–110 | Bk1 | 6308 ± 49 | 45 | −547.0 | 7888 ± 53 | 37 | −627.9 |
| 120–130 | Bk2 | 5098 ± 46 | 53 | −473.4 | 8511 ± 54 | 34 | −655.7 |
| 160–170 | Bk2 | 7118 ± 48 | 41 | −590.4 | 11,043 ± 64 | 25 | −748.8 |
| 190–200 | Ck | 11,805 ± 68 | 23 | −771.5 | 10,991 ± 68 | 25 | −747.2 |
| Site/Depth (cm) | Soil Horizon | δ13C (‰) | Pedogenic Carbonate (%) | 14C Age (14C y BP) | Percent Modern (%) | Δ14C (‰) |
|---|---|---|---|---|---|---|
| Fallow | ||||||
| Concretion (outside) | ||||||
| 190–200 | C2 | −9.49 | 98 | 1909 ± 40 | 78 | −216.8 |
| Concretion (inside) | ||||||
| 190–200 | C2 | −9.69 | 100 | 1693 ± 39 | 80 | −195.4 |
| Cropland | ||||||
| Concretion | ||||||
| 180–190 | Bk2 | −9.68 | 100 | 2285 ± 40 | 75 | −252.6 |
| Soil Inorganic Carbon (SIC) (%) | Difference in Soil Inorganic Carbon (SIC) (%) | |||||
|---|---|---|---|---|---|---|
| Depth (cm) | Native (n = 5) | Fallow (n = 5) | Cropland (n = 5) | Fallow-Native | Cropland-Native | Cropland-Fallow |
| 0–10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 | 0 | 0 |
| 10–20 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 | 0 | 0 |
| 20–30 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 | 0 | 0 |
| 30–40 | 0.13 ± 0.27 | 0 ± 0 | 0.32 ± 0.27 | −0.13 | 0.20 | 0.32 |
| 40–50 | 0.09 ± 0.17 | 0.02 ± 0.04 | 0.47 ± 0.30 | −0.07 | 0.39 | 0.46 |
| 50–60 | 0.16 ± 0.31 | 0.17 ± 0.38 | 0.50 ± 0.21 | 0.01 | 0.34 | 0.33 |
| 60–70 | 0.12 ± 0.24 | 0.28 ± 0.63 | 0.56 ± 0.29 | 0.16 | 0.44 | 0.28 |
| 70–80 | 0.09 ± 0.19 | 0.29 ± 0.54 | 0.93 ± 0.35 | 0.20 | 0.84 | 0.64 |
| 80–90 | 0.13 ± 0.26 | 0.59 ± 0.68 | 1.05 ± 0.37 | 0.46 | 0.92 | 0.46 |
| 90–100 | 0.13 ± 0.27 | 1.02 ± 0.65 | 1.43 ± 0.27 | 0.89 | 1.30 | 0.41 |
| 100–110 | 0.15 ± 0.31 | 1.31 ± 0.35 | 1.55 ± 0.16 | 1.16 | 1.40 | 0.24 |
| 110–120 | 0.13 ± 0.25 | 1.61 ± 0.16 | 1.67 ± 0.22 | 1.48 | 1.54 | 0.06 |
| 120–130 | 0.38 ± 0.50 | 1.65 ± 0.16 | 1.74 ± 0.14 | 1.27 | 1.36 | 0.09 |
| 130–140 | 0.47 ± 0.51 | 1.59 ± 0.15 | 1.62 ± 0.16 | 1.12 | 1.16 | 0.04 |
| 140–150 | 0.61 ± 0.40 | 1.45 ± 0.20 | 1.57 ± 0.06 | 0.84 | 0.96 | 0.12 |
| 150–160 | 1.06 ± 0.18 | 1.41 ± 0.32 | 1.49 ± 0.17 | 0.35 | 0.43 | 0.08 |
| 160–170 | 1.29 ± 0.13 | 1.41 ± 0.24 | 1.49 ± 0.05 | 0.12 | 0.20 | 0.09 |
| 170–180 | 1.23 ± 0.18 | 1.23 ± 0.18 | 1.44 ± 0.09 | −0.01 | 0.20 | 0.21 |
| Depth to carbonates (cm) | 112 ± 47 | 76 ± 23 | 34 ± 5 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, E.A.; Bryant, R.B.; Galbraith, J.M.; Wang, Y.; Post, C.J.; Khokhlova, O.S.; Schlautman, M.A.; Cope, M.P.; Shen, Z. Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem. Geosciences 2018, 8, 458. https://doi.org/10.3390/geosciences8120458
Mikhailova EA, Bryant RB, Galbraith JM, Wang Y, Post CJ, Khokhlova OS, Schlautman MA, Cope MP, Shen Z. Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem. Geosciences. 2018; 8(12):458. https://doi.org/10.3390/geosciences8120458
Chicago/Turabian StyleMikhailova, Elena A., Ray B. Bryant, John M. Galbraith, Yang Wang, Christopher J. Post, Olga S. Khokhlova, Mark A. Schlautman, Michael P. Cope, and Zhixiong Shen. 2018. "Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem" Geosciences 8, no. 12: 458. https://doi.org/10.3390/geosciences8120458
APA StyleMikhailova, E. A., Bryant, R. B., Galbraith, J. M., Wang, Y., Post, C. J., Khokhlova, O. S., Schlautman, M. A., Cope, M. P., & Shen, Z. (2018). Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem. Geosciences, 8(12), 458. https://doi.org/10.3390/geosciences8120458






