The Role of Magmatic and Hydrothermal Fluids in the Formation of the Sasa Pb-Zn-Ag Skarn Deposit, Republic of Macedonia
Abstract
1. Introduction
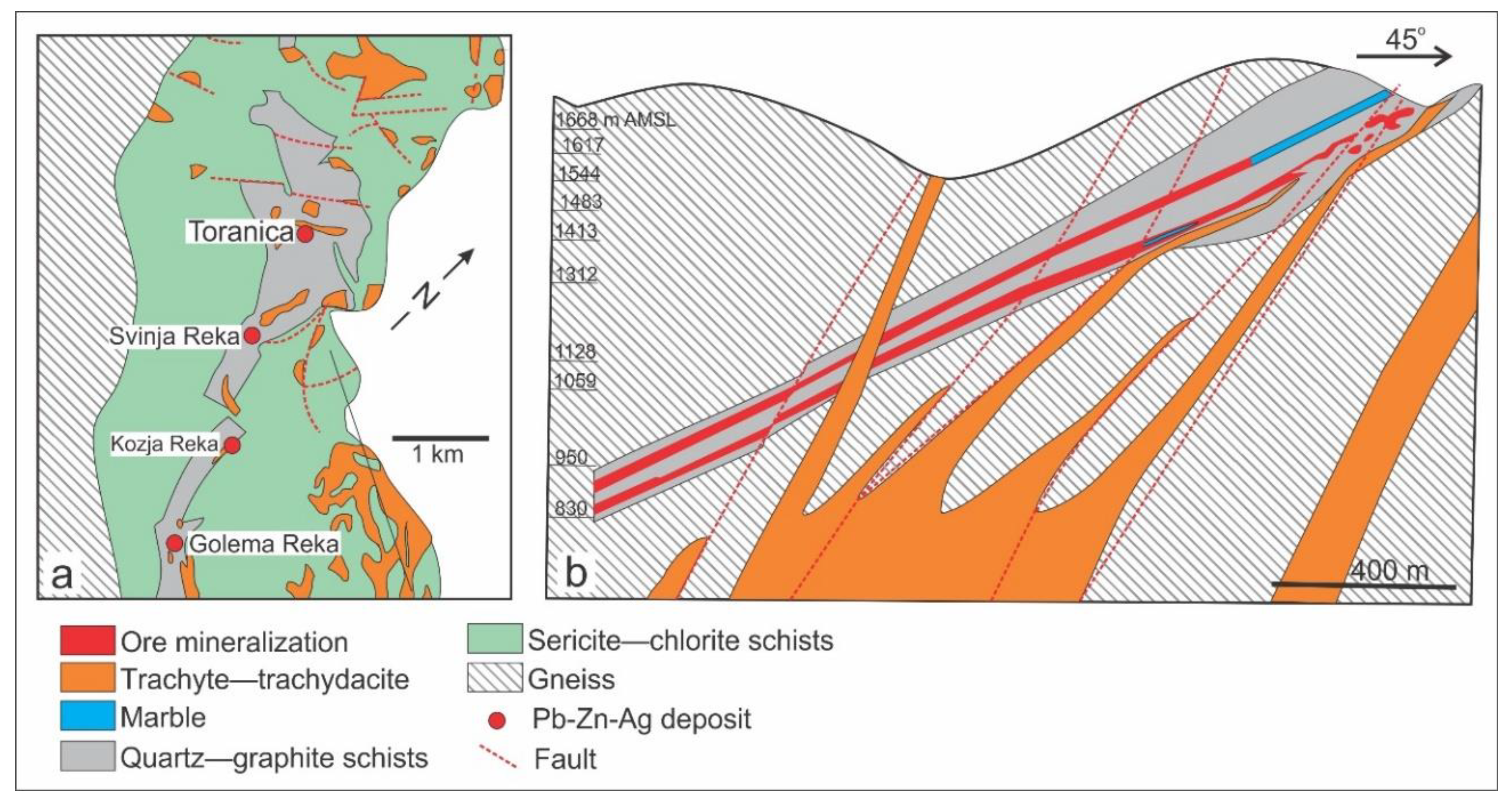
2. Geological Setting
2.1. Regional Geology
2.2. Geology of the Deposit
3. Materials and Methods
4. Results
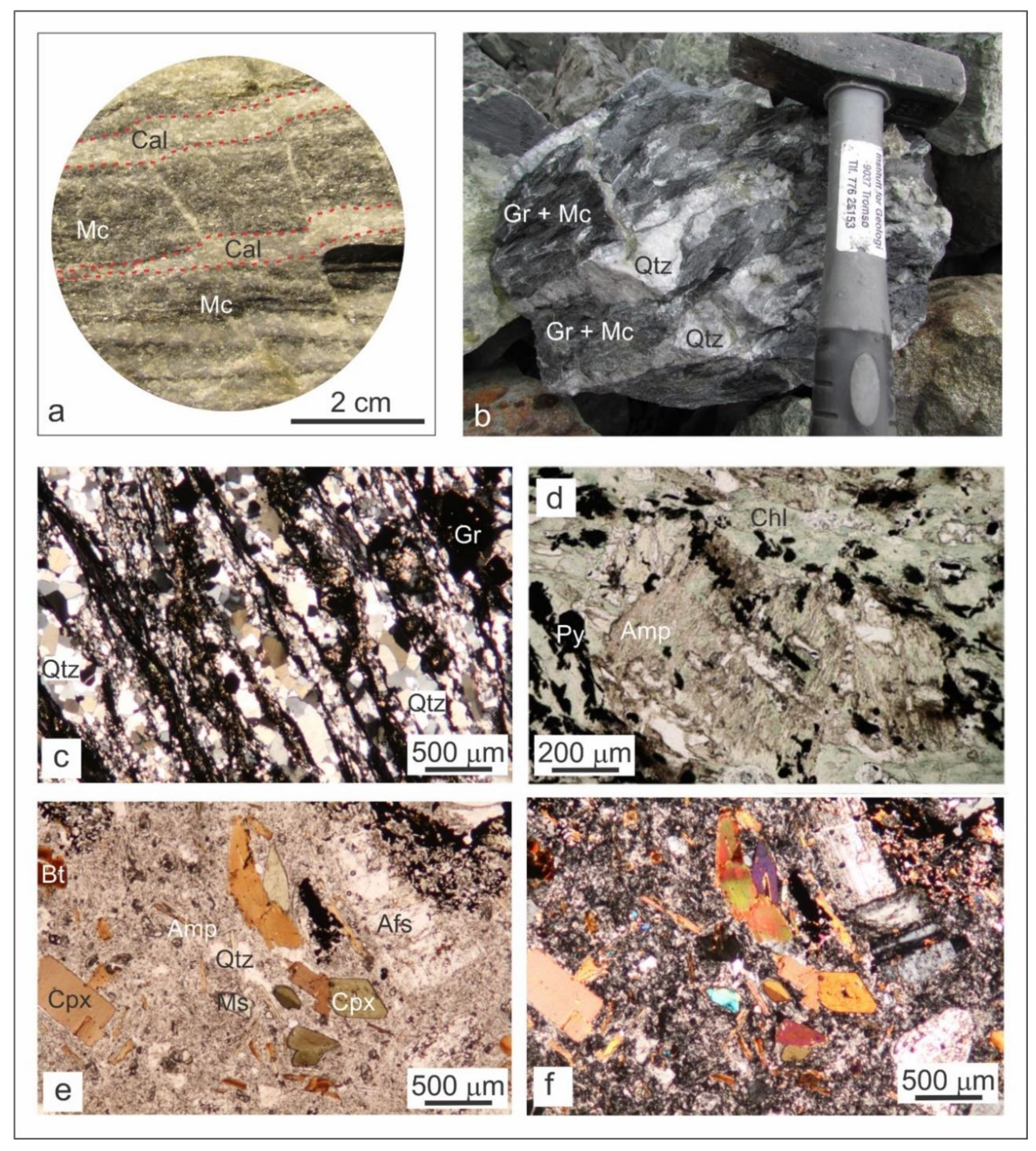
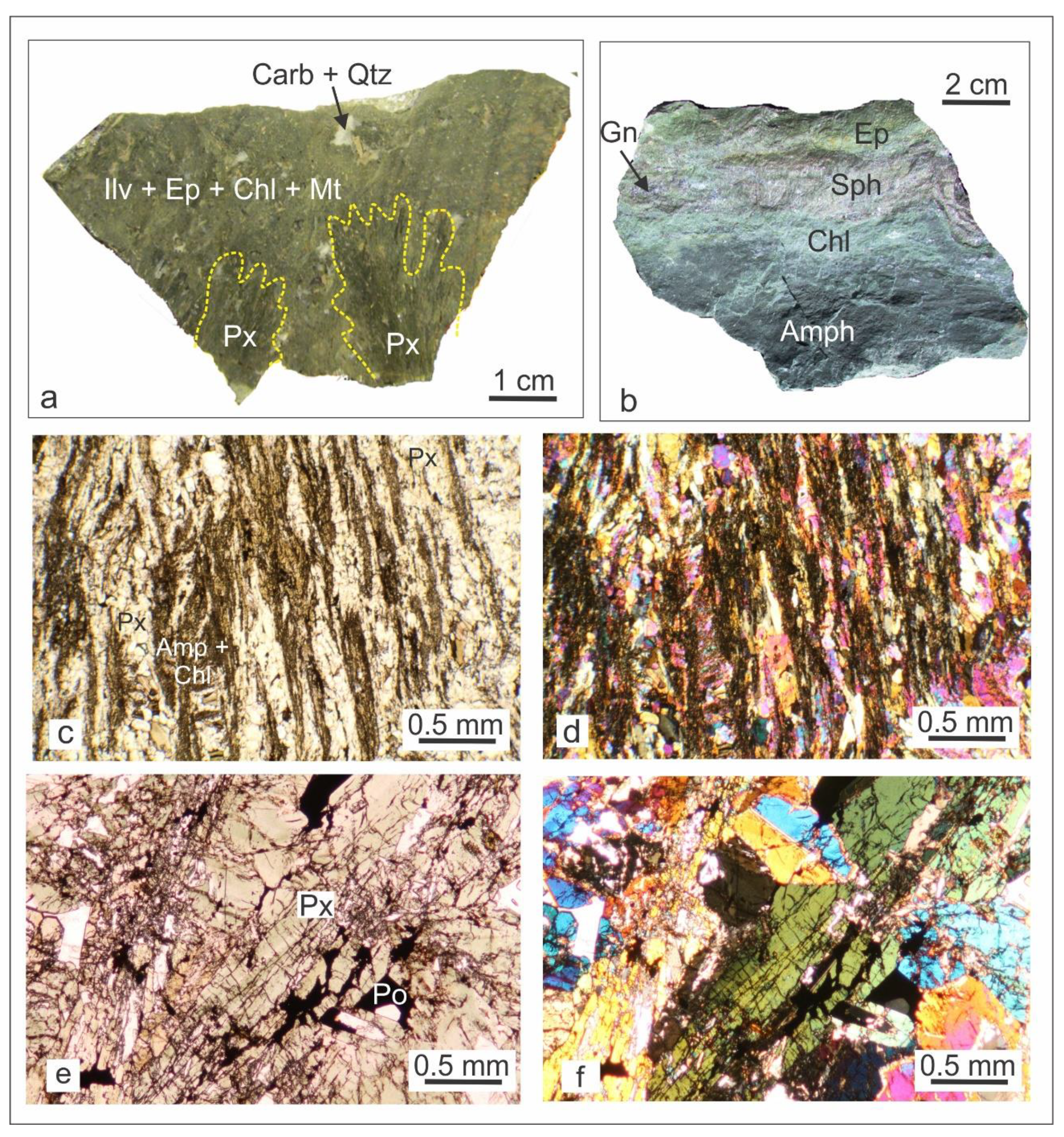
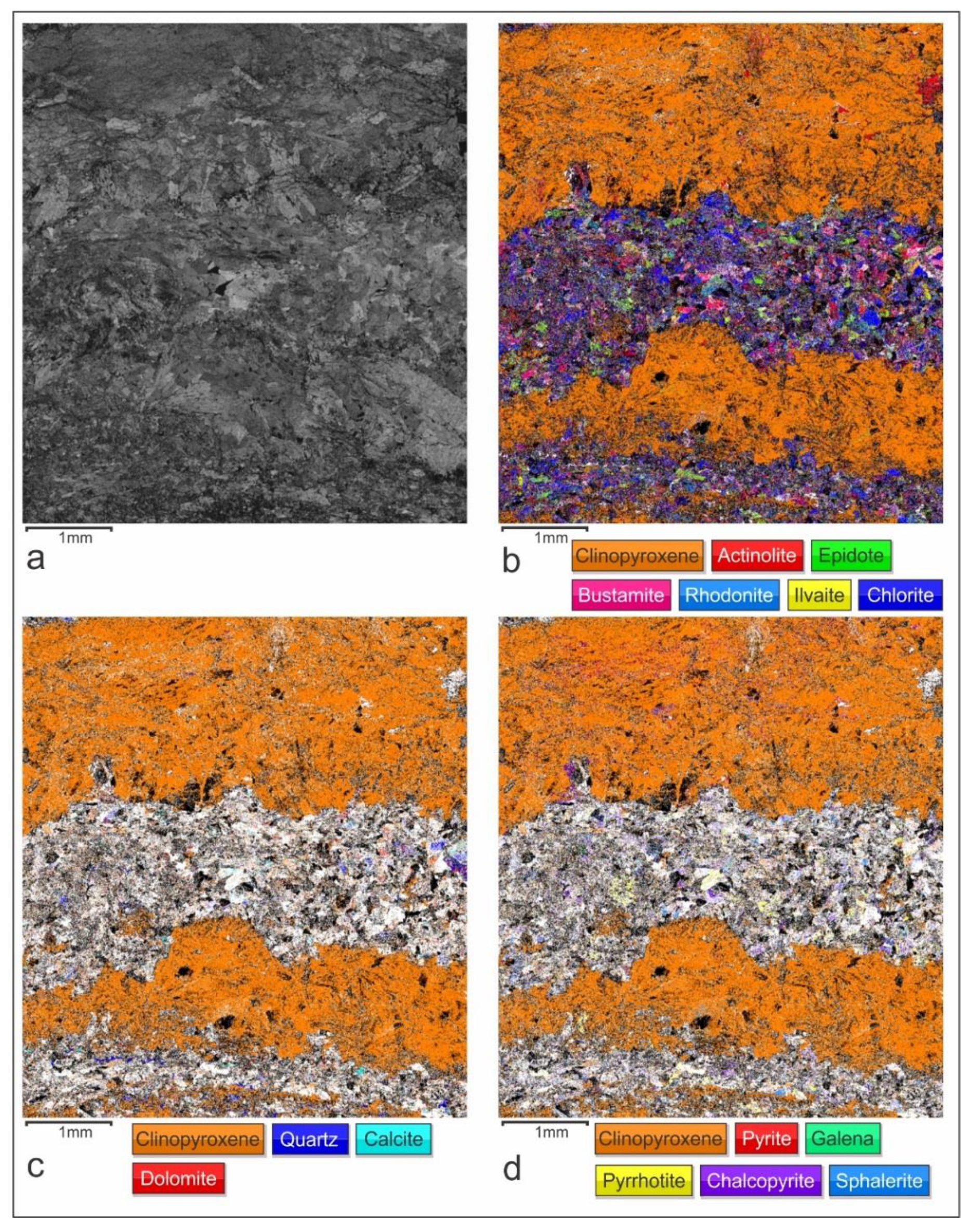
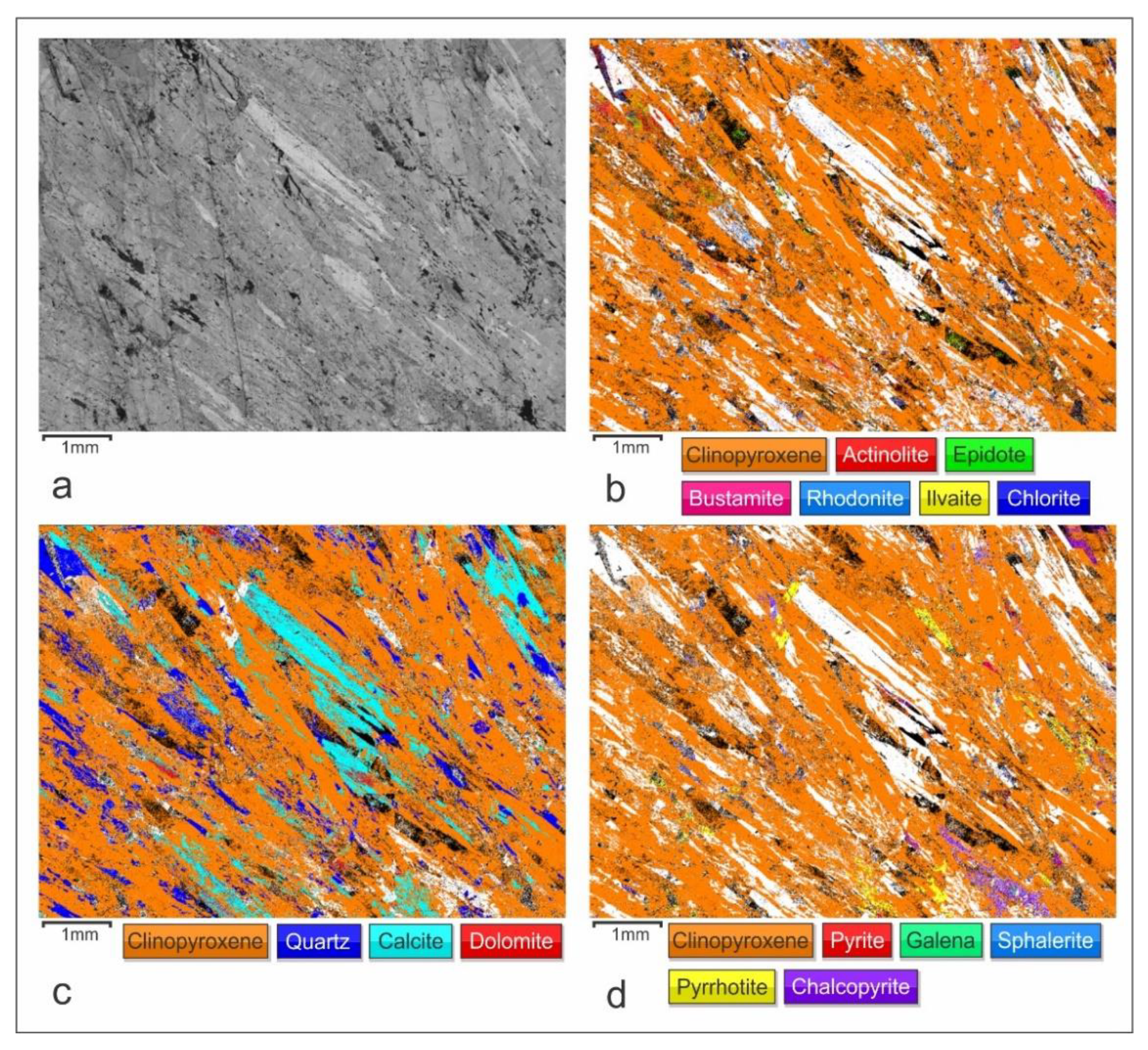
4.1. Petrography
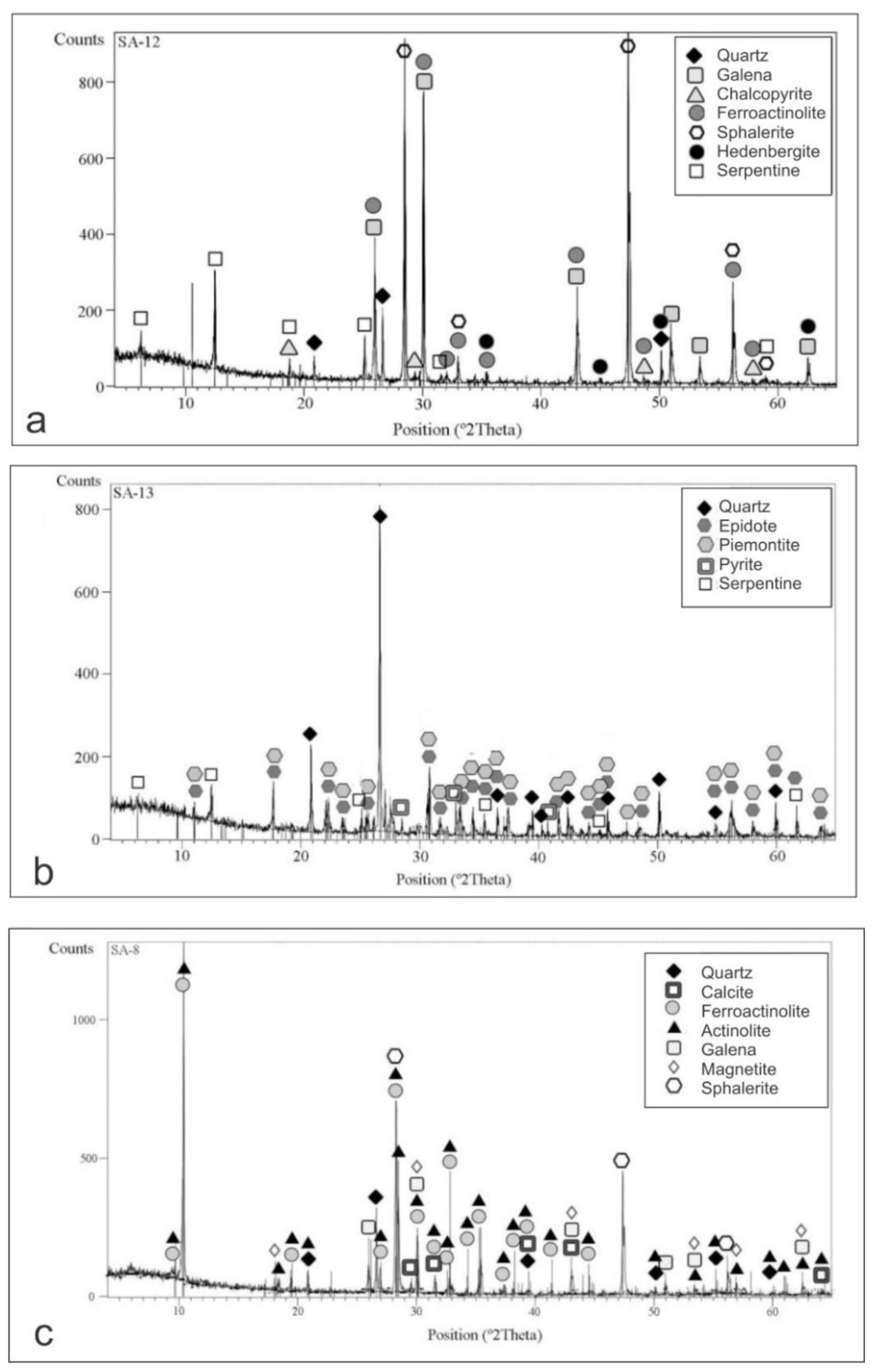
4.2. X-ray Diffraction (XRD)
4.3. Electron Back Scattered Diffraction (EBSD)
4.4. Mineral Chemistry
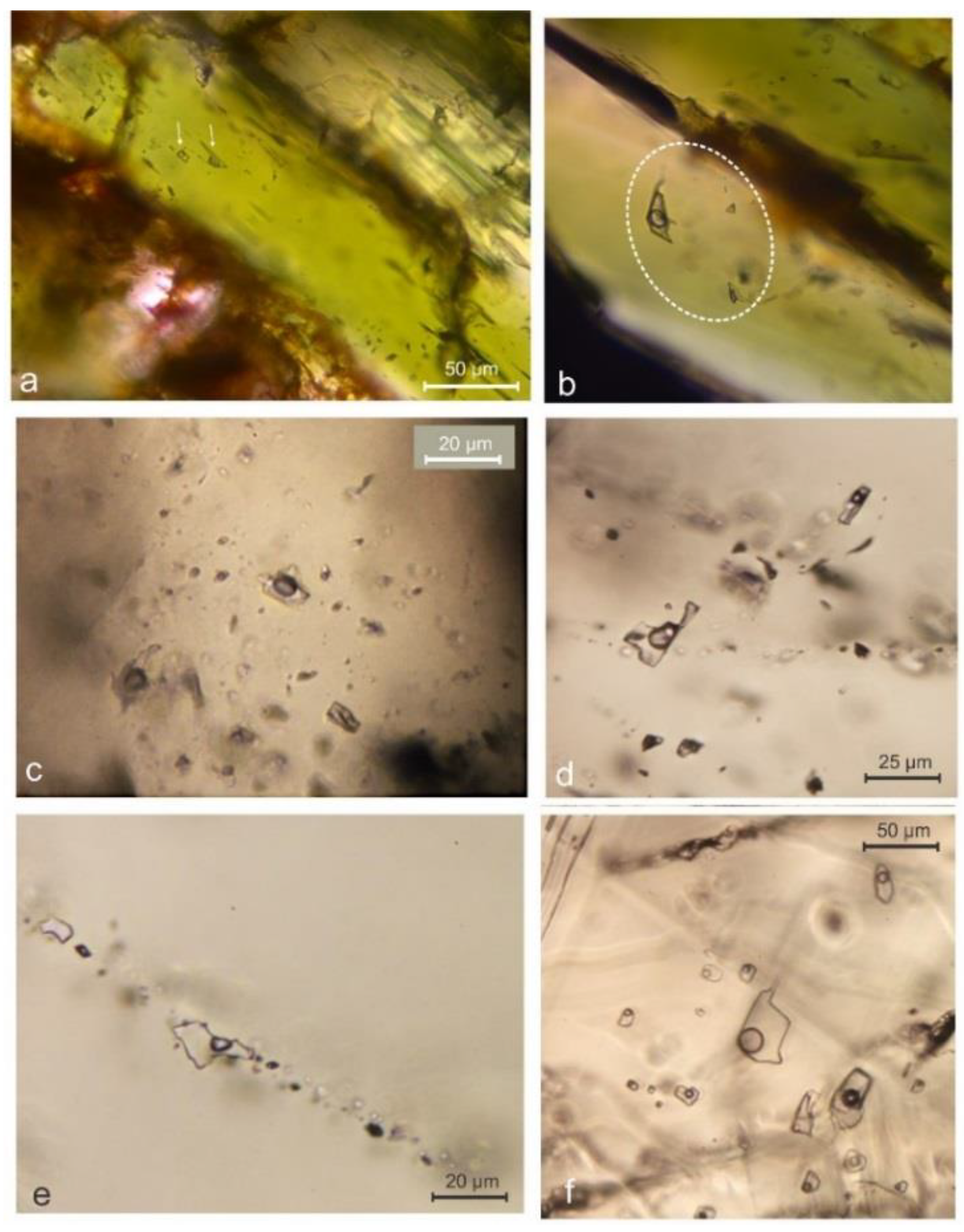
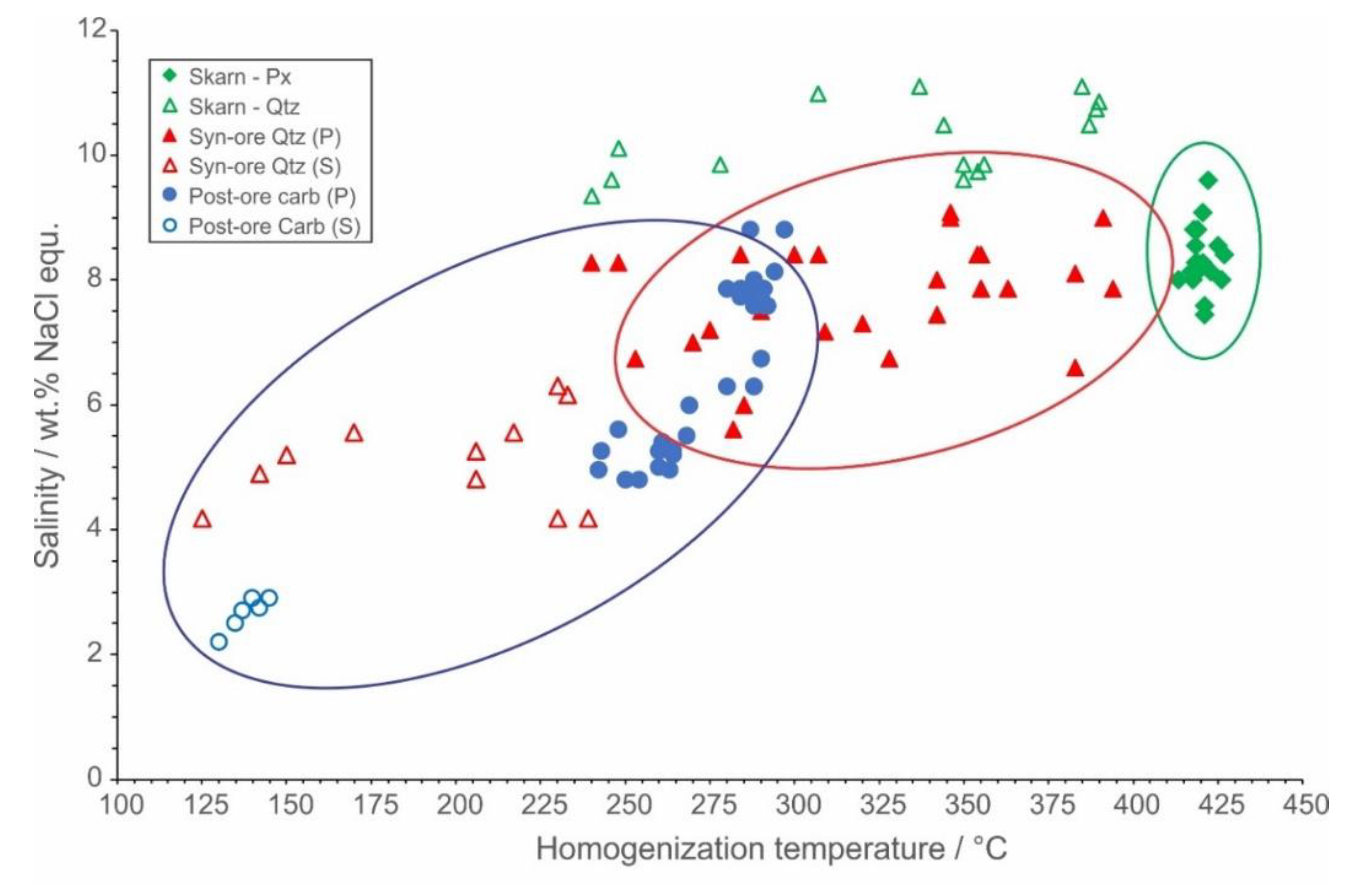
4.5. Fluid Inclusion Studies
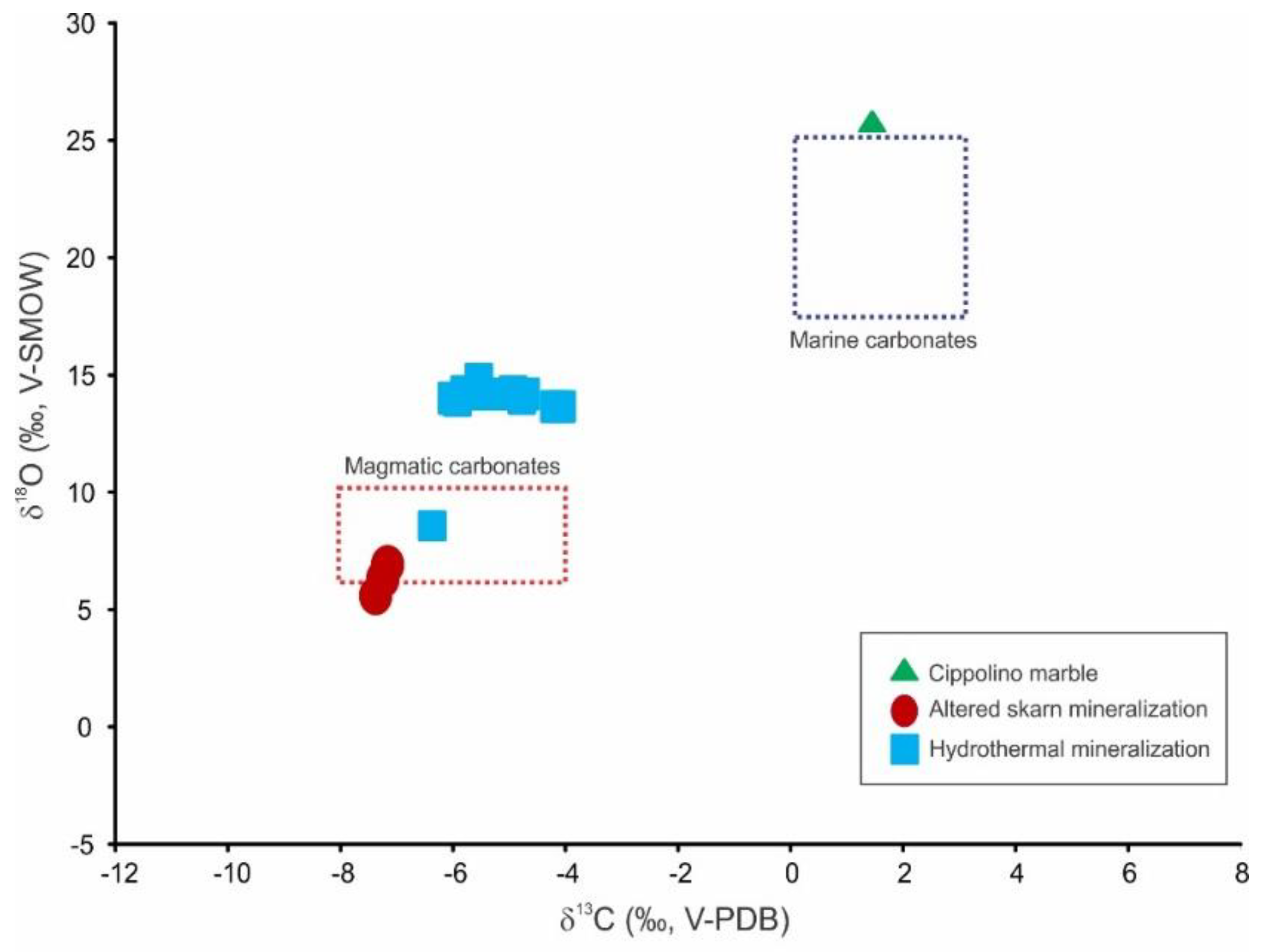
4.6. Stable Isotope Data
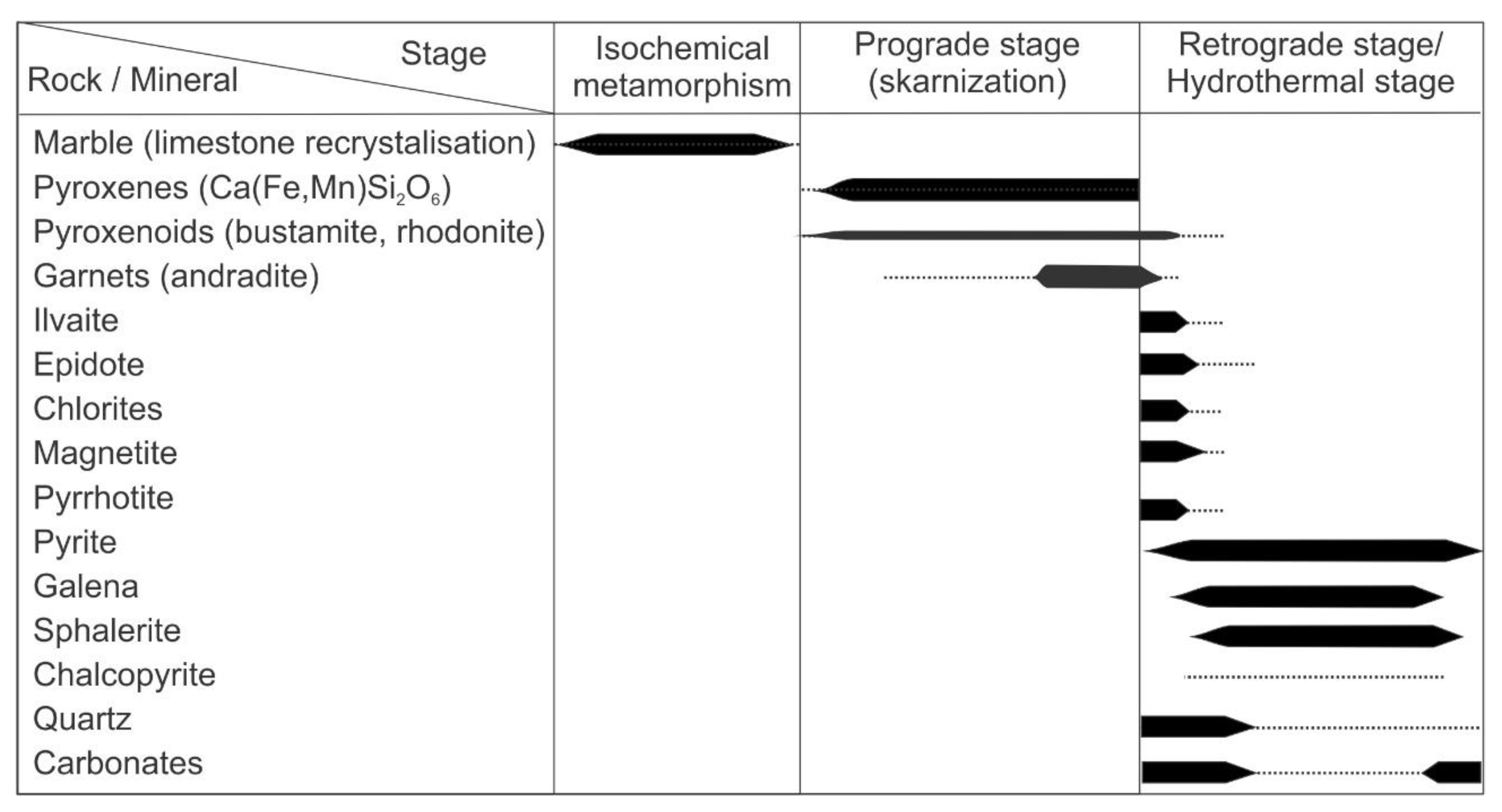
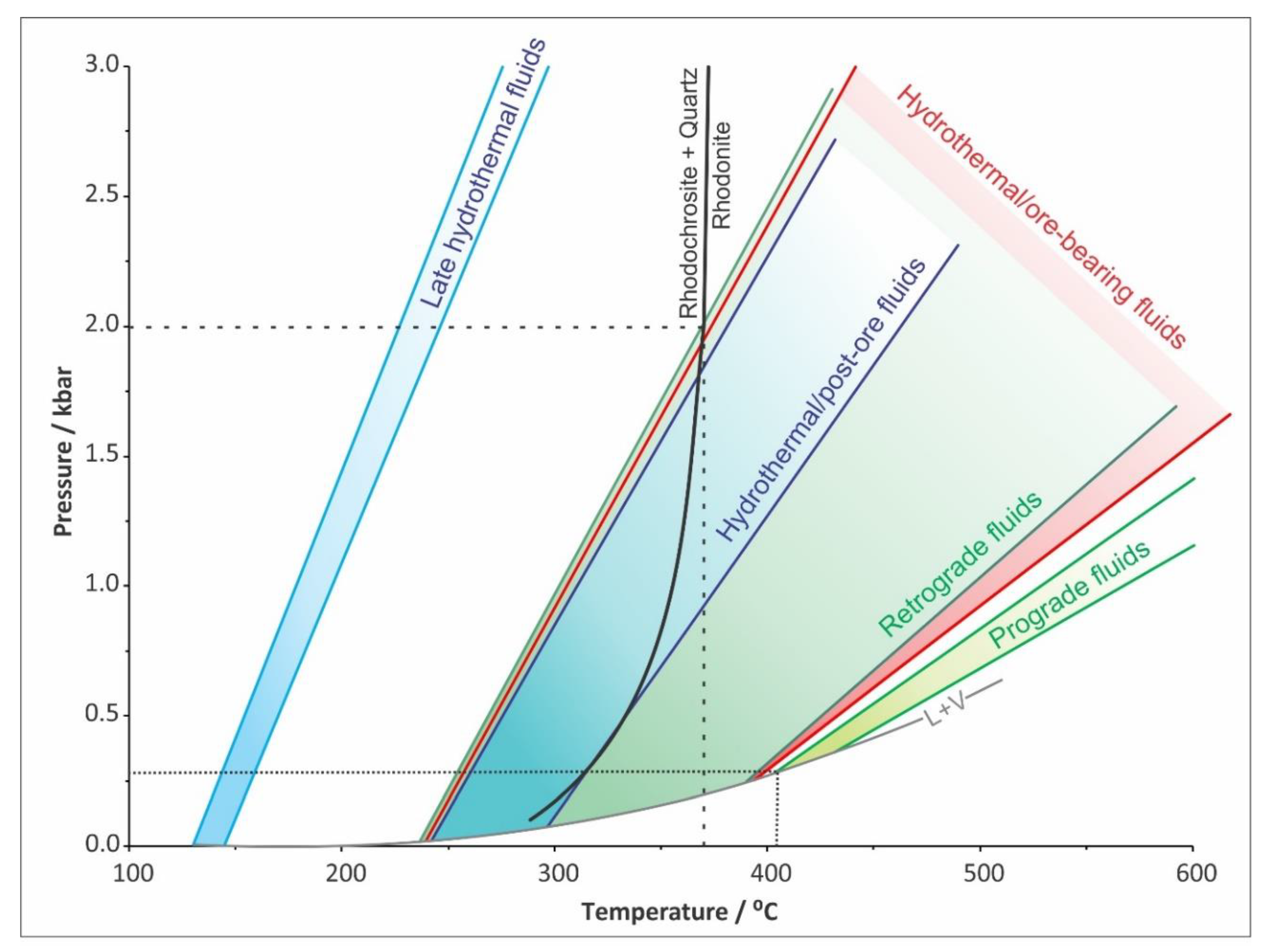
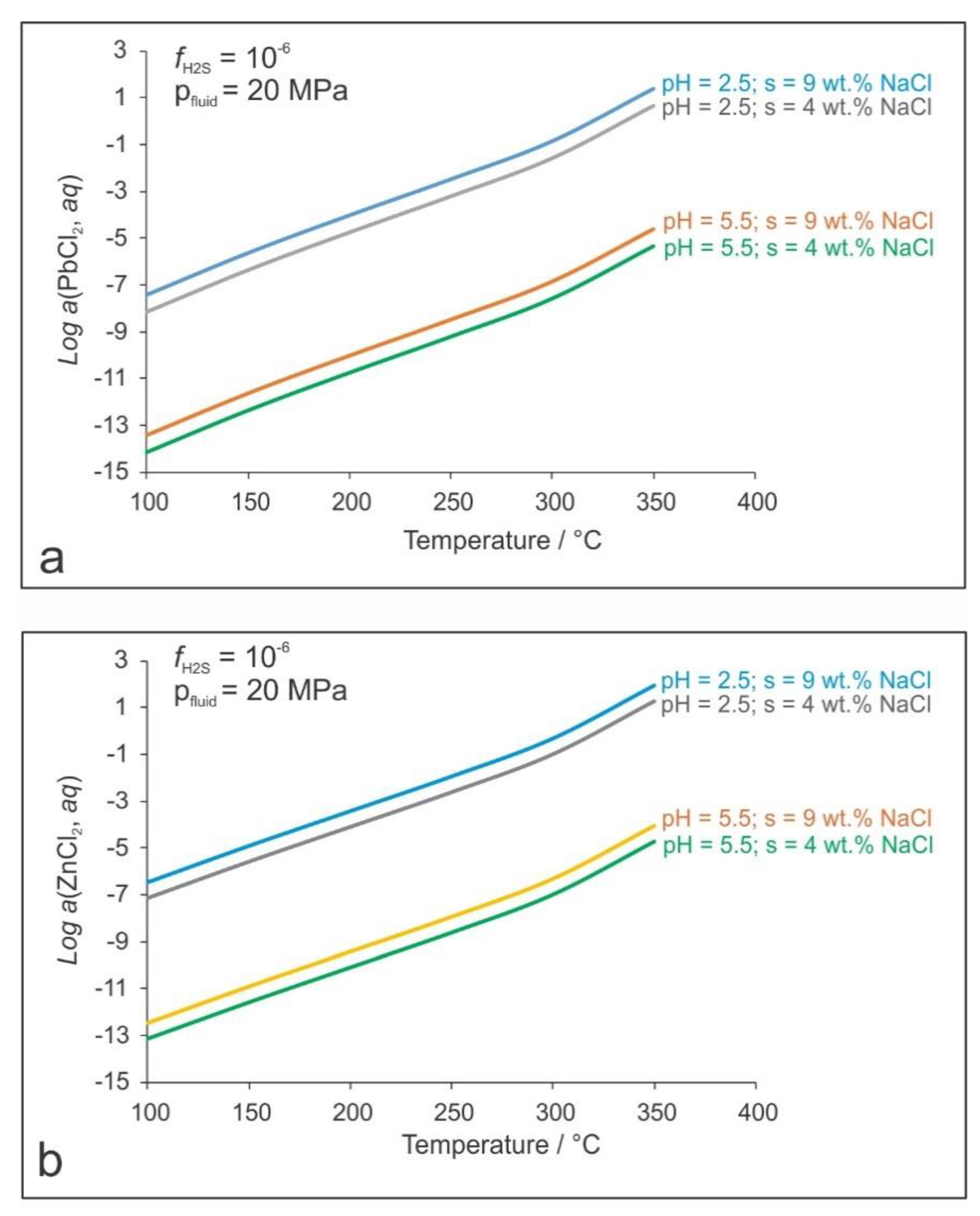
5. Discussion
 CaFeSi2O6 (hedenbergite) + 3 H2O + CO2 + 2 H+
CaFeSi2O6 (hedenbergite) + 3 H2O + CO2 + 2 H+
 CaMnSi2O6 (johansennite) + 3 H2O + CO2 + 2 H+
CaMnSi2O6 (johansennite) + 3 H2O + CO2 + 2 H+
 CaMnSi2O6 (johannsenite) + Fe2+
CaMnSi2O6 (johannsenite) + Fe2+rH25 °C, 1atm = −7 kJ/mole *
 3 Al2Si2O5(OH)4 (mica) + 2 K+
3 Al2Si2O5(OH)4 (mica) + 2 K+
 Ca2Fe5Si8O22(OH)2 (ferroactinolite) + 3 CaCO3 (calcite)+ 2 SiO2 (quartz)
Ca2Fe5Si8O22(OH)2 (ferroactinolite) + 3 CaCO3 (calcite)+ 2 SiO2 (quartz)
 Ca2Fe3(SiO4)3(OH) (piemontite) + CaCO3 (calcite) + 3 SiO2 (quartz) + 3 H+
Ca2Fe3(SiO4)3(OH) (piemontite) + CaCO3 (calcite) + 3 SiO2 (quartz) + 3 H+
 3 CaCO3 (calcite)+ FeO·Fe2O3 (magnetite) + 6 SiO2 (quartz)
3 CaCO3 (calcite)+ FeO·Fe2O3 (magnetite) + 6 SiO2 (quartz)
 Mg3Si2O5(OH)4 (chrysotile) + 3 CaCO3 (calcite)+ 4 SiO2 (quartz)
Mg3Si2O5(OH)4 (chrysotile) + 3 CaCO3 (calcite)+ 4 SiO2 (quartz)
 3 Mg5Al(AlSi3)O10(OH)8 (clinochlore) +15 CaCO3 (calcite) + 25 SiO2 (quartz)
3 Mg5Al(AlSi3)O10(OH)8 (clinochlore) +15 CaCO3 (calcite) + 25 SiO2 (quartz)
 3 Ca2FeAl2Si3O12(OH) (epidote) + FeO·Fe2O3 (magnetite) + 9 SiO2 (quartz)
3 Ca2FeAl2Si3O12(OH) (epidote) + FeO·Fe2O3 (magnetite) + 9 SiO2 (quartz)
 PbS + 2 H+ + 2 Cl−
PbS + 2 H+ + 2 Cl−
 ZnS + 2 H+ + 2 Cl−
ZnS + 2 H+ + 2 Cl−
 PbS + 2 HCO3− + 2 Ca2+ + 2 Cl−
PbS + 2 HCO3− + 2 Ca2+ + 2 Cl−
 ZnS + 2 HCO3− + 2 Ca2+ + 2 Cl−
ZnS + 2 HCO3− + 2 Ca2+ + 2 Cl−
6. Comparison with Other Distal Pb-Zn-Ag Skarn Deposits
- (1)
- Although the prograde mineralization of the Sasa Pb-Zn-Ag deposit can be described as a Ca-Fe-Mg-Mn-system, the retrograde mineralization shows a significant hydrothermal input of Al, plausibly reflecting a contribution of the aluminosilicate component within the host cipollino marble.
- (2)
- Prograde skarn mineralization of the great majority of skarn deposits indicates a dominance of high temperature hypersaline magmatic fluids, whereas the later retrograde stage usually reflects mixing with lower temperature and lower salinity fluids of a meteoric origin [4,62,72,73]. However, fluid inclusions and stable isotope data obtained from the retrograde mineral assemblage of the Sasa Pb-Zn-Ag deposit suggest that in this deposit, magmatic fluids played a significant role during the retrograde stage.
- (3)
- The transition from the prograde to the retrograde stage in different skarn deposits can be triggered by various reasons, including brecciation [31] and reactivation of old fractures [62]. In the Sasa Pb-Zn-Ag deposit, the transition was initiated by cooling of the system below 400 °C and the resulting ductile-to-brittle transition.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meinert, L.D.; Hedenquist, J.W.; Satoh, H.; Matsuhisa, Y. Formation of anhydrous and hydrous skarn in Cu-Au ore deposits by magmatic fluids. Econ. Geol. 2003, 98, 147–156. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/98/1/147/22314/formation-of-anhydrous-and-hydrous-skarn-in-cu-au?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Baker, T.; Van Achterberg, E.; Ryan, C.G.; Lang, J.R. Composition and evolution of ore fluids in a magmatic-hydrothermal skarn deposit. Geology 2004, 32, 117–120. Available online: https://pubs.geoscienceworld.org/gsa/geology/article-abstract/32/2/117/103697/composition-and-evolution-of-ore-fluids-in-a?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Shu, Q.; Lai, Y.; Sun, Y.; Wang, C.; Meng, S. Ore genesis and hydrothermal evolution of the Baiyinnuo’er zinc-lead skarn deposit, northeast China: Evidence from isotopes (S, Pb) and fluid inclusions. Econ. Geol. 2013, 108, 835–860. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/108/4/835/128539/ore-genesis-and-hydrothermal-evolution-of-the?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Strmić Palinkaš, S.; Palinkaš, L.A.; Mandić, M.; Roller-Lutz, Z.; Pécskay, Z.; Maliqi, G.; Bermanec, V. Origin and K-Ar age of the phreatomagmatic breccia at the Trepča Pb-Zn-Ag skarn deposit, Kosovo: Implications for ore-forming processes. Geol. Croat. 2016, 69, 121–142. Available online: http://www.geologia-croatica.hr/ojs/index.php/GC/article/view/GC.2016.10 (accessed on 28 September 2018). [CrossRef]
- Janković, S. Types of copper deposits related to volcanic environment in the Bor district, Yugoslavia. Geol. Rundsch. 1990, 79, 467–478. Available online: https://link.springer.com/article/10.1007/BF01830639 (accessed on 28 September 2018). [CrossRef]
- Janković, S. The Carpatho-Balkanides and adjacent area: A sector of the Tethyan Eurasian metallogenic belt. Miner. Depos. 1997, 32, 426–433. Available online: https://link.springer.com/article/10.1007/s001260050110 (accessed on 28 September 2018). [CrossRef]
- Heinrich, C.A.; Neubauer, F. Cu–Au–Pb–Zn–Ag metallogeny of the Alpine–Balkan–Carpathian–Dinaride geodynamic province. Miner. Depos. 2002, 37, 533–540. Available online: https://link.springer.com/article/10.1007%2Fs00126-002-0271-x (accessed on 28 September 2018). [CrossRef]
- Lehmann, S.; Barcikowski, J.; von Quadt, A.; Gallhofer, D.; Peytcheva, I.; Heinrich, C.A.; Serafimovski, T. Geochronology, geochemistry and isotope tracing of the Oligocene magmatism of the Buchim–Damjan–Borov Dol ore district: Implications for timing, duration and source of the magmatism. Lithos 2013, 180, 216–233. Available online: https://www.sciencedirect.com/science/article/pii/S0024493713002909 (accessed on 28 September 2018). [CrossRef]
- Kroll, T.; Müller, D.; Seifert, T.; Herzig, P.M.; Schneider, A. Petrology and geochemistry of the shoshonite-hosted Skouries porphyry Cu–Au deposit, Chalkidiki, Greece. Miner. Depos. 2002, 37, 137–144. Available online: https://link.springer.com/article/10.1007/s00 (accessed on 28 September 2018). [CrossRef]
- Janković, S.; Serafimovski, T.; Aleksandrov, M. The Besna Kobila–Osogovo metallogenic zone. Geol. Maced. 1995, 9, 39–50. [Google Scholar]
- Peltekovski, Z. Modeling of Mining Reserves in Svinja Reka Deposit Sasa. Master Thesis, University Goce Delcev, Štip, Republic of Macedonia, 2012. (In Macedonian). [Google Scholar]
- Sijakova-Ivanova, T.; Boev, B.; Mircovski, V. Metamorphism of the skarn rocks from the Sasa ore field. Geol. Maced. 2012, 26, 65–70. Available online: http://js.ugd.edu.mk/index.php/GEOLMAC/article/view/651 (accessed on 28 September 2018).
- Dimitrijevic, M.D. Dinarides and the Vardar Zone: A short review of the geology. Acta Vulcanologica 2001, 13, 1000–1008. Available online: https://eurekamag.com/research/018/743/018743766.php (accessed on 28 September 2018).
- Karamata, S. The geological development of the Balkan Peninsula related to the approach, collision and compression of Gondwanan and Eurasian units. Geol. Soc. Lond. Spec. Publ. 2006, 260, 155–178. Available online: http://sp.lyellcollection.org/content/260/1/155 (accessed on 28 September 2018). [CrossRef]
- Meinhold, G.; Kostopoulos, D.; Frei, D.; Himmerkus, F.; Reischmann, T. U–Pb LA-SF-ICP-MS zircon geochronology of the Serbo-Macedonian Massif, Greece: Palaeotectonic constraints for Gondwana-derived terranes in the Eastern Mediterranean. Int. J. Earth. Sci. 2010, 99, 813–832. [Google Scholar] [CrossRef]
- Antić, M.D.; Kounov, A.; Trivić, B.; Spikings, R.; Wetzel, A. Evidence of Variscan and Alpine tectonics in the structural and thermochronological record of the central Serbo-Macedonian Massif (south-eastern Serbia). Int. J. Earth. Sci. 2017, 106, 1665–1692. Available online: https://link.springer.com/article/10.1007/s00531-016-1380-6 (accessed on 28 September 2018). [CrossRef]
- Antić, M.D.; Kounov, A.; Trivić, B.; Wetzel, A.; Peytcheva, I.; von Quadt, A. Alpine thermal events in the central Serbo-Macedonian Massif (southeastern Serbia). Int. J. Earth. Sci. 2016, 105, 1485–1505. Available online: https://link.springer.com/article/10.1007/s00531-015-1266-z (accessed on 28 September 2018). [CrossRef]
- Antić, M.; Peytcheva, I.; von Quadt, A.; Kounov, A.; Trivić, B.; Serafimovski, T.; Tasev, G.; Gerdjikov, I.; Wetzel, A. Pre-Alpine evolution of a segment of the North Gondwanan margin: Geochronological and geochemical evidence from the central Serbo-Macedonian Massif. Gondwana Res. 2016, 19, 523–544. Available online: https://www.sciencedirect.com/science/article/pii/S1342937X15002051 (accessed on 28 September 2018). [CrossRef]
- Zagorčev, I.S.; Bončeva, I. Indications of Devonian basic volcanism in Southwest Bulgaria. Geol. Balcanica 1988, 18, 55–63. [Google Scholar]
- Vasković, N. Petrology and P-T condition of white mica-chlorite schists from Vlasina series—Surdulica, SE Serbia. Ann. Geol. Penins. Balk. 2002, 64, 199–220. Available online: https://www.ingentaconnect.com/content/doaj/03500608/2002/00002002/00000064/art00013 (accessed on 28 September 2018). [CrossRef]
- Cvetković, V.; Prelević, D.; Downes, H.; Jovanović, M.; Vaselli, O.; Pécskay, Z. Origin and geodynamic significance of Tertiary postcollisional basaltic magmatism in Serbia (central Balkan Peninsula). Lithos 2004, 73, 161–186. Available online: https://www.sciencedirect.com/science/article/pii/S0024493703002408 (accessed on 28 September 2018). [CrossRef]
- Prelević, D.; Foley, S.F.; Romer, R.L.; Cvetković, V.; Downes, H. Tertiary ultrapotassic volcanism in Serbia: Constraints on petrogenesis and mantle source characteristics. J. Petrol. 2005, 46, 1443–1487. Available online: https://academic.oup.com/petrology/article/46/7/1443/1546674 (accessed on 28 September 2018). [CrossRef]
- Borojević Šoštarić, S.; Cvetković, V.; Neubauer, F.; Palinkaš, L.A.; Bernroider, M.; Genser, J. Oligocene shoshonitic rocks of the Rogozna Mts.(Central Balkan Peninsula): Evidence of petrogenetic links to the formation of Pb–Zn–Ag ore deposits. Lithos 2012, 148, 176–195. Available online: https://www.sciencedirect.com/science/article/pii/S0024493712002204 (accessed on 28 September 2018). [CrossRef]
- Melfos, V.; Voudouris, P. Cenozoic metallogeny of Greece and potential for precious, critical and rare metals exploration. Ore Geol. Rev. 2017, 89, 1030–1057. Available online: https://www.sciencedirect.com/science/article/pii/S0169136816304206 (accessed on 28 September 2018). [CrossRef]
- Lips, A.L.; Herrington, R.J.; Stein, G.; Kozelj, D.; Popov, K.; Wijbrans, J.R. Refined timing of porphyry copper formation in the Serbian and Bulgarian portions of the Cretaceous Carpatho-Balkan Belt. Econ. Geol. 2004, 99, 601–609. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/99/3/601/22445/refined-timing-of-porphyry-copper-formation-in-the?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Serafimovski, T.; Stefanova, V.; Volkov, A.V. Dwarf copper-gold porphyry deposits of the Buchim-Damjan-Borov Dol ore district, Republic of Macedonia (FYROM). Geol. Ore Deposits 2010, 52, 179–195. Available online: https://link.springer.com/content/pdf/10.1134 2FS1075701510030013 (accessed on 10 October 2018). [CrossRef]
- Serafimovski, T.; Tasev, G.; Strmić Palinkaš, S.; Palinkaš, L.A.; Gjorgjiev, L. Porphyry Cu mineralizations related with the small Tertiary volcanic intrusions in the Bučim ore deposit, Eastern Macedonia. Geol. Croat. 2016, 69, 101–119. Available online: https://hrcak.srce.hr/155678 (accessed on 28 September 2018). [CrossRef]
- Lips, A.L. Correlating magmatic-hydrothermal ore deposit formation over time with geodynamic processes in SE Europe. Geol. Soc. Lond. Spec. Publ. 2002, 204, 69–79. Available online: http://sp.lyellcollection.org/content/specpubgsl/204/1/69 (accessed on 10 October 2018). [CrossRef]
- Neubauer, F. Contrasting late cretaceous with neogene ore provinces in the Alpine-Balkan-Carpathian-Dinaride collision belt. Geol. Soc. Lond. Spec. Publ. 2002, 204, 81–102. Available online: http://sp.lyellcollection.org/content/204/1/81 (accessed on 28 September 2018). [CrossRef]
- Radosavljević, S.A.; Stojanović, J.N.; Radosavljević-Mihajlović, A.S.; Vuković, N.S. (Pb–Sb)-bearing sphalerite from the Čumavići polymetallic ore deposit, Podrinje Metallogenic District, East Bosnia and Herzegovina. Ore Geol. Rev. 2016, 72, 253–268. Available online: https://www.sciencedirect.com/science/article/pii/S0169136815001857 (accessed on 28 September 2018). [CrossRef]
- Strmić Palinkaš, S.; Palinkaš, L.A.; Renac, C.; Spangenberg, J.E.; Lüders, V.; Molnar, F.; Maliqi, G. Metallogenic model of the Trepča Pb-Zn-Ag Skarn Deposit, Kosovo: Evidence from fluid inclusions, rare earth elements, and stable isotope data. Econ. Geol. 2013, 108, 135–162. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article/108/1/135/128446/metallogenic-model-of-the-trepca-pb-zn-ag-skarn (accessed on 28 September 2018). [CrossRef]
- Borojević Šoštarić, S.; Palinkaš, L.A.; Neubauer, F.; Hurai, V.; Cvetkovic, V.; Roller-Lutz, Z.; Mandić, M.; Genser, J. Silver-base metal epithermal vein and listwanite hosted deposit Crnac, Rogozna Mts., Kosovo, part II: A link between magmatic rocks and epithermal mineralization. Ore Geol. Rev. 2013, 50, 98–117. Available online: https://www.sciencedirect.com/science/article/pii/S0169136812002120 (accessed on 28 September 2018). [CrossRef]
- Veselinovic-Williams, M. Characteristics and Origin of Polymetallic Mineralisation in the Kopaonik Region of Serbia and Kosovo, with Particular Reference to the Belo Brdo Pb-Zn (Ag) Deposit. Ph.D. Thesis, Kingston University, London, UK, 2011. [Google Scholar]
- Serafimovski, T.; Tasev, G. Sulfur isotope composition of some polymetallic deposits in the Republic of Macedonia. Geol. Maced. 2005, 19, 1–11. Available online: http://eprints.ugd.edu.mk/1557/ (accessed on 28 September 2018).
- Kalogeropoulos, S.I.; Kilias, S.P.; Bitzios, D.C.; Nicolaou, M.; Both, R.A. Genesis of the Olympias carbonate-hosted Pb-Zn (Au, Ag) sulfide ore deposit, eastern Chalkidiki Peninsula, northern Greece. Econ. Geol. 1989, 84, 1210–1234. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/84/5/1210/20675/genesis-of-the-olympias-carbonate-hosted-pb-zn-au?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Clark, A.H.; Ullrich, T.D. 40 Ar-39 Ar age data for andesitic magmatism and hydrothermal activity in the Timok Massif, eastern Serbia: Implications for metallogenetic relationships in the Bor copper-gold subprovince. Miner. Depos. 2004, 39, 256–262. Available online: https://link.springer.com/article/10.1007/s00126-003-0370-3 (accessed on 28 September 2018). [CrossRef]
- Frei, R. Evolution of mineralizing fluid in the porphyry copper system of the Skouries Deposit, Northeast Chalkidiki (Greece); evidence from combined Pb-Sr and stable isotope data. Econ. Geol. 1995, 90, 746–762. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/90/4/746/21443/evolution-of-mineralizing-fluid-in-the-porphyry?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Tasev, G.; Serafimovski, T.; Lazarov, P. New K-Ar, 87Sr/86 Sr, REE, and XRF data for Tertiary volcanic rocks in the Sasa-Toranica ore district, Macedonia. In Mineral Deposit Research: Meeting the Global Challenge; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 837–840. [Google Scholar]
- Aleksandrov, M. Metalogenetske karakteristike polimetalicnog rudnog polja Sase-lstocna Makedonija. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 1992. (In Serbian). [Google Scholar]
- Serafimovski, T. Metallogeny of the Lece-Chalkidiki Zone. Ph.D. Thesis, University Goce Delcev, Štip, Republic of Macedonia, 1990. (In Macedonian). [Google Scholar]
- Marchev, P.; Raicheva, R.; Downes, H.; Vaselli, O.; Chiaradia, M.; Moritz, R. Compositional diversity of Eocene–Oligocene basaltic magmatism in the Eastern Rhodopes, SE Bulgaria: Implications for genesis and tectonic setting. Tectonophysics 2004, 393, 301–328. Available online: https://www.sciencedirect.com/science/article/pii/S0040195104002823 (accessed on 28 September 2018). [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1994; p. 199. [Google Scholar]
- Goldstein, R.H. Fluid inclusions in sedimentary and diagenetic systems. Lithos 2001, 55, 159–193. Available online: https://www.sciencedirect.com/science/article/pii/S002449370000044X?via%3Dihub (accessed on 28 September 2018). [CrossRef]
- Bodnar, R.J. Introduction to Fluid Inclusions; Mineralogical Association of Canada: Quebec, QC, Canada, 2003; pp. 1–8. [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, G.M. World Skarn Deposits. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Elsevier: Amsterdan, The Netherlands, 2005; pp. 299–336. [Google Scholar]
- Wang, L.; Tang, J.; Bagas, L.; Wang, Y.; Lin, X.; Li, Z.; Li, Y. Early Eocene Longmala skarn Pb-Zn-Cu deposit in Tibet, China: Geochemistry, fluid inclusions, and HOS-Pb isotopic compositions. Ore Geol. Rev. 2017, 88, 99–115. Available online: https://www.sciencedirect.com/science/article/pii/S0169136817300215?via%3Dihub (accessed on 28 September 2018). [CrossRef]
- Soloviev, S.G.; Kryazhev, S.G.; Dvurechenskaya, S.S. Geology, mineralization, stable isotope, and fluid inclusion characteristics of the Vostok-2 reduced W-Cu skarn and Au-W-Bi-As stockwork deposit, Sikhote-Alin, Russia. Ore Geol. Rev. 2017, 86, 338–365. Available online: https://www.sciencedirect.com/science/article/pii/S0169136816302591 (accessed on 28 September 2018). [CrossRef]
- Ciobanu, C.L.; Cook, N.J. Skarn textures and a case study: The Ocna de Fier-Dognecea orefield, Banat, Romania. Ore Geol. Rev. 2004, 24, 315–370. Available online: https://www.sciencedirect.com/science/article/pii/S0169136803000593 (accessed on 28 September 2018). [CrossRef]
- Borisenko, A.S. Cryometric technique applied to studies of the saline composition of solution in gaseous fluid inclusions in minerals. Geol. Geofiz. 1997, 8, 16–27. [Google Scholar]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. Available online: https://www.sciencedirect.com/science/article/pii/001670379390378A?via%3Dihub (accessed on 28 September 2018). [CrossRef]
- Veizer, J.; Hoefs, J. The nature of O18/O16 and C13/C12 secular trends in sedimentary carbonate rocks. Geochim. Cosmochim. Acta 1976, 40, 1387–1395. Available online: https://www.sciencedirect.com/science/article/pii/0016703776901290 (accessed on 10 October 2018). [CrossRef]
- Taylor, H.P.; Frechen, J.; Degens, E.T. Oxygen and carbon isotope studies of carbonatites from the Laacher See District, west Germany and the Alno District, Sweden. Geochim. Cosmochim. Acta 1967, 31, 407–430. Available online: https://www.sciencedirect.com/science/article/pii/0016703767900518 (accessed on 10 November 2018). [CrossRef]
- Serafimovski, T.; Tasev, G.; Dolenec, T. Petrological and geochemical features of the Neogene volcanites of the Osogovo mountains, eastern Macedonia. RMZ Mater. Geoenviron. 2006, 52, 523–534. Available online: http://www.rmz-mg.com/letniki/rmz52/rmz52_0523-0534.pdf (accessed on 28 September 2018).
- Strmić Palinkaš, S.; Hofstra, A.H.; Percival, T.J.; Borojević Šoštarić, S.; Palinkaš, L.; Bermanec, V.; Boev, B.; Pécskay, Z. Comparison of the Allchar Au-As-Sb-Tl Deposit, Republic of Macedonia, with Carlin-Type Gold Deposits. In Diversity of Carlin-Style Gold Deposits; Muntean, J.L., Ed.; Society of Economic Geologists: Littleton, CO, USA, 2018; pp. 335–363. [Google Scholar]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19, 145–162. Available online: https://journals.lib.unb.ca/index.php/GC/article/view/3773/0 (accessed on 28 September 2018). [CrossRef]
- Jansson, N.F.; Allen, R.L. Multistage ore formation at the Ryllshyttan marble and skarn-hosted Zn–Pb–Ag–(Cu)+ magnetite deposit, Bergslagen, Sweden. Ore Geol. Rev. 2015, 69, 217–242. Available online: https://www.sciencedirect.com/science/article/pii/S016913681500061X?via%3Dihub (accessed on 28 September 2018). [CrossRef]
- Gustafson, W.I. The stability of andradite, hedenbergite, and related minerals in the system Ca-Fe-Si-O-H. J. Petrol. 1974, 15, 455–496. Available online: https://academic.oup.com/petrology/article-abstract/15/3/455/1441509?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Sijakova-Ivanova, T.; Boev, B. Mineralogical characteristics of johannsenite in the Sasa ore field. Geol. Maced. 1997, 11, 45–49. Available online: http://eprints.ugd.edu.mk/2977/ (accessed on 28 September 2018).
- Helgeson, H.C.; Delany, J.M.; Nesbitt, H.W.; Bird, D.K. Summary and critique of the thermodynamic properties of rock-forming minerals. Am. J. Sci. 1978, 278, 1–229. [Google Scholar]
- Angel, R.J. The experimental determination of the johannsenite-bustamite equilibrium inversion boundary. Contrib. Mineral. Petr. 1984, 85, 272–278. Available online: https://link.springer.com/article/10.1007/BF00378105 (accessed on 28 September 2018). [CrossRef]
- Kerrick, D.M. The genesis of zoned skarns in the Sierra Nevada, California. J. Petrol. 1971, 18, 144–181. Available online: https://academic.oup.com/petrology/article-abstract/18/1/144/1514509?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Logan, M.A.V. Mineralogy and geochemistry of the Gualilán skarn deposit in the Precordillera of western Argentina. Ore Geol. Rev. 2000, 17, 113–138. Available online: https://www.sciencedirect.com/science/article/pii/S0169136800000093 (accessed on 28 September 2018). [CrossRef]
- Canet, C.; González-Partida, E.; Camprubí, A.; Castro-Mora, J.; Romero, F.M.; Prol-Ledesma, R.M.; Linares, C.; Romero-Guadarrama, J.A.; Sánchez-Vargas, L.I. The Zn-Pb-Ag skarns of Zacatepec, northeastern Oaxaca, Mexico: A study of mineral assemblages and ore-forming fluids. Ore Geol. Rev. 2001, 39, 277–290. Available online: https://www.sciencedirect.com/science/article/pii/S0169136811000357 (accessed on 28 September 2018). [CrossRef]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The chemistry of metal transport and deposition by ore-forming hydrothermal fluids. In Treatise on Geochemistry, 2nd ed.; Holland, H., Turekian, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 29–57. [Google Scholar]
- Fournier, R.O.; Potter, R.W. An equation correlating the solubility of quartz in water from 25 °C to 900 °C at pressures up to 10,000 bars. Geochim. Cosmochim. Acta 1982, 46, 1969–1973. Available online: https://www.sciencedirect.com/science/article/pii/0016703782901351 (accessed on 28 September 2018). [CrossRef]
- Fournier, R.O. Hydrothermal processes related to movement of fluid from plastic into brittle rock in the magmatic-epithermal environment. Econ. Geol. 1999, 94, 1193–1211. Available online: https://pubs.geoscienceworld.org/segweb/economicgeology/article-abstract/94/8/1193/21899/hydrothermal-processes-related-to-movement-of?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Strmić Palinkaš, S.; Wegner, R.; Čobić, A.; Palinkaš, L.A.; Barreto, S.D.B.; Váczi, T.; Bermanec, V. The role of magmatic and hydrothermal processes in the evolution of Be-bearing pegmatites: Evidence from beryl and its breakdown products. Am. Mineral. 2014, 99, 424–432. Available online: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/99/2-3/424/46089/the-role-of-magmatic-and-hydrothermal-processes-in?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Webster, J.D.; Vetere, F.; Botcharnikov, R.E.; Goldoff, B.; McBirney, A.; Doherty, A.L. Experimental and modeled chlorine solubilities in aluminosilicate melts at 1 to 7000 bars and 700 to 1250 °C: Applications to magmas of Augustine Volcano, Alaska. Am. Mineral. 2015, 100, 522–535. Available online: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/100/2-3/522/40399/experimental-and-modeled-chlorine-solubilities-in?redirectedFrom=fulltext (accessed on 28 September 2018). [CrossRef]
- Botcharnikov, R.E.; Holtz, F.; Behrens, H. Solubility and fluid–melt partitioning of H2O and Cl in andesitic magmas as a function of pressure between 50 and 500 MPa. Chem. Geol. 2015, 418, 117–131. Available online: https://www.sciencedirect.com/science/article/pii/S0009254115003381 (accessed on 28 September 2018). [CrossRef]
- Serafimovski, T.; Tasev, G. Sulfur isotope study of sulfides from some mineral deposits from the Lece-Chalkidiki metallogenic zone. In Proceedings of the 2006 National Conference With International Participation Publishing House Bulgarian Geological Society, Sofia, Bulgaria, 30 November–1 December 2006; pp. 251–254. [Google Scholar]
- Burgisser, A.; Alletti, M.; Scaillet, B. Simulating the behavior of volatiles belonging to the C–O–H–S system in silicate melts under magmatic conditions with the software D-Compress. Comput. Geosci. 2015, 79, 1–14. Available online: https://www.sciencedirect.com/science/article/pii/S0098300415000503 (accessed on 28 September 2018). [CrossRef]
- Samson, I.M.; Williams-Jones, A.E.; Ault, K.M.; Gagnon, J.E.; Fryer, B.J. Source of fluids forming distal Zn-Pb-Ag skarns: Evidence from laser ablation–inductively coupled plasma–mass spectrometry analysis of fluid inclusions from El Mochito, Honduras. Geology 2008, 36, 947–950. Available online: https://pubs.geoscienceworld.org/gsa/geology/article/36/12/947/29724/source-of-fluids-forming-distal-zn-pb-ag-skarns (accessed on 10 October 2018). [CrossRef]
- Yang, Y.L.; Ye, L.; Bao, T.; Gao, W.; Li, Z.L. Mineralization of Luziyuan Pb–Zn skarn deposit, Baoshan, Yunnan Province, SW China: Evidence from petrography, fluid inclusions and stable isotopes. Geol. Mag. 2018, 1–20. Available online: https://www.cambridge.org/core/journals/geological-magazine/article/mineralization-of-luziyuan-pbzn-skarn-deposit-baoshan-yunnan-province-sw-china-evidence-from-petrography-fluid-inclusions-and-stable isotopes/E549FFF8C9B403F9FF6F6CBE33573D43 (accessed on 10 October 2018). [CrossRef]


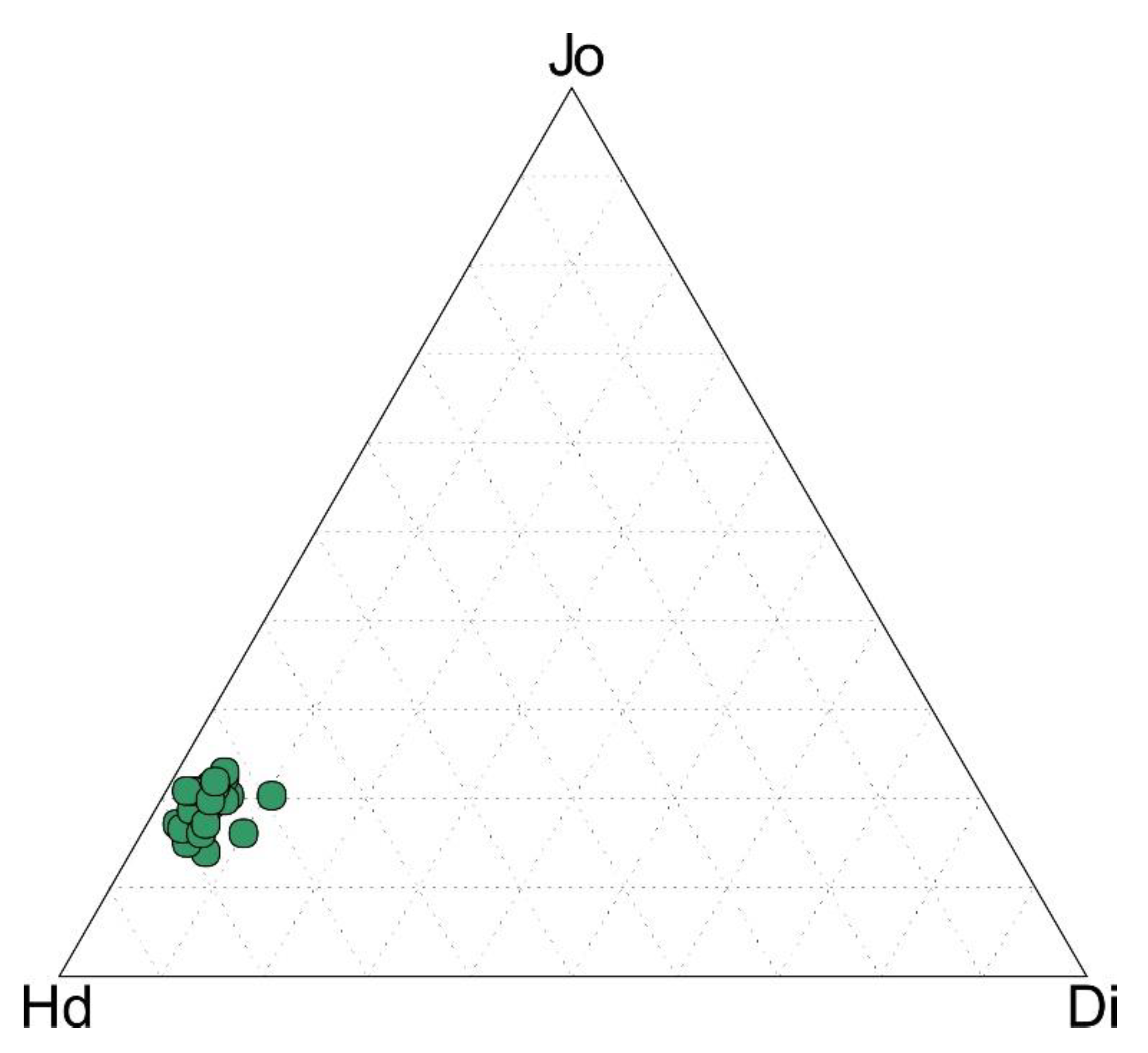
| Sample | Point | CaO | FeO | MnO | MgO | SiO2 | Hd | Jo | Di |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | % | ||||||||
| SA-101 | Px-1 | 24.6 | 22.2 | 5.1 | 0.5 | 47.6 | 79.8 | 18.4 | 1.8 |
| Px-2 | 25.1 | 20.5 | 4.3 | 1.1 | 49.0 | 79.1 | 16.5 | 4.4 | |
| Px-3 | 24.4 | 21.8 | 4.7 | 0.8 | 48.3 | 79.8 | 17.2 | 3.0 | |
| Px-4 | 22.8 | 21.2 | 5.6 | 1.0 | 49.4 | 76.3 | 20.1 | 3.7 | |
| Px-5 | 25.0 | 21.2 | 5.7 | 0.7 | 47.5 | 77.0 | 20.6 | 2.4 | |
| Px-6 | 24.0 | 22.8 | 5.1 | 0.7 | 47.4 | 79.7 | 17.9 | 2.4 | |
| Px-7 | 23.2 | 19.7 | 4.9 | 0.8 | 51.5 | 77.5 | 19.5 | 3.0 | |
| Px-8 | 23.1 | 21.2 | 6.0 | 0.8 | 49.0 | 75.7 | 21.3 | 3.0 | |
| Px-9 | 23.9 | 20.2 | 5.2 | 1.0 | 49.6 | 76.4 | 19.8 | 3.8 | |
| Px-10 | 22.7 | 19.1 | 5.2 | 1.6 | 51.5 | 73.9 | 20.1 | 6.0 | |
| SA-102 | Px-11 | 25.2 | 21.1 | 5.2 | 0.6 | 47.9 | 78.6 | 19.2 | 2.1 |
| Px-12 | 25.4 | 19.8 | 5.3 | 0.7 | 48.8 | 76.8 | 20.5 | 2.8 | |
| Px-13 | 25.4 | 18.9 | 5.3 | 0.7 | 49.6 | 75.7 | 21.4 | 2.9 | |
| Px-14 | 23.9 | 21.2 | 5.6 | 0.4 | 48.8 | 77.9 | 20.4 | 1.6 | |
| Px-15 | 24.0 | 21.0 | 5.5 | 0.4 | 49.1 | 78.2 | 20.4 | 1.3 | |
| Px-16 | 24.6 | 20.4 | 4.6 | 0.9 | 49.4 | 78.9 | 17.8 | 3.4 | |
| Px-17 | 23.4 | 20.3 | 4.6 | 1.6 | 50.1 | 76.5 | 17.5 | 5.9 | |
| Px-18 | 23.0 | 22.8 | 5.3 | 1.0 | 48.0 | 78.4 | 18.1 | 3.4 | |
| Px-19 | 24.6 | 19.8 | 5.0 | 0.7 | 49.9 | 77.3 | 19.7 | 2.9 | |
| Px-20 | 22.5 | 19.8 | 5.4 | 0.7 | 51.6 | 76.6 | 20.8 | 2.5 | |
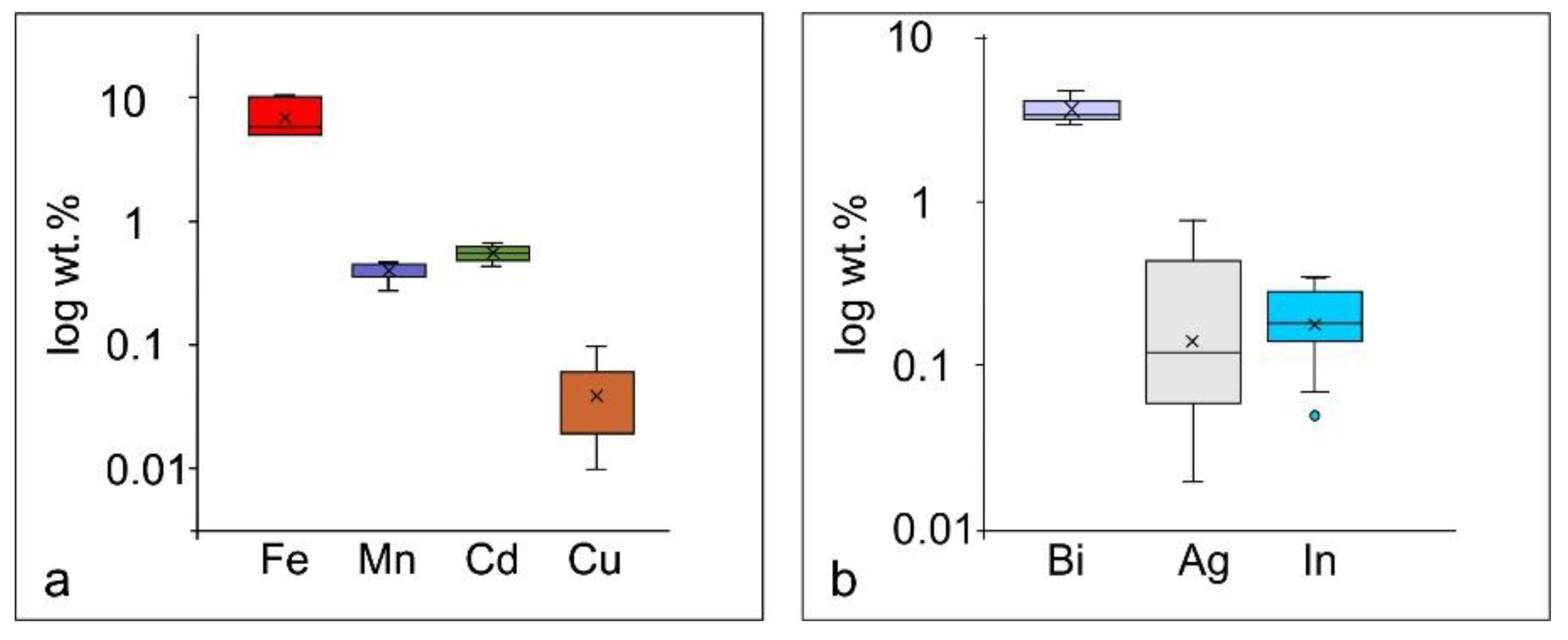
| Sample | Mineralogy | n | Element | Minimum | Maximum | Mean | STD |
|---|---|---|---|---|---|---|---|
| Sasa-17 | Galena | 7 | wt.% | ||||
| Pb | 77.76 | 82.26 | 80.23 | 1.52 | |||
| Bi | 4.02 | 4.69 | 4.36 | 0.23 | |||
| Ag | 0.02 | 0.62 | 0.16 | 0.21 | |||
| In | 0.15 | 0.31 | 0.21 | 0.06 | |||
| S | 12.12 | 12.57 | 12.37 | 0.18 | |||
| Total | 94.30 | 99.22 | 97.33 | 1.69 | |||
| Sasa-17 | Sphalerite | 4 | |||||
| Zn | 58.44 | 59.34 | 58.86 | 0.39 | |||
| Fe | 10.18 | 10.38 | 10.25 | 0.09 | |||
| Mn | 0.28 | 0.34 | 0.32 | 0.03 | |||
| Cd | 0.43 | 0.58 | 0.51 | 0.08 | |||
| Cu | <d.l. | <d.l. | |||||
| S | 28.37 | 28.93 | 28.62 | 0.26 | |||
| Total | 97.85 | 99.55 | 98.56 | 0.79 | |||
| Sasa-20 | Galena | 11 | |||||
| Pb | 83.12 | 84.75 | 83.96 | 0.59 | |||
| Bi | 2.97 | 3.55 | 3.25 | 0.19 | |||
| Ag | <d.l. | 0.77 | 0.36 | 0.27 | |||
| In | <d.l. | 0.28 | 0.12 | 0.08 | |||
| S | 12.48 | 12.69 | 12.55 | 0.07 | |||
| Total | 99.33 | 101.13 | 100.25 | 0.59 | |||
| Sasa-20 | Sphalerite | 12 | |||||
| Zn | 57.56 | 59.95 | 58.85 | 0.92 | |||
| Fe | 5.11 | 5.83 | 5.47 | 0.34 | |||
| Mn | 0.43 | 0.47 | 0.45 | 0.01 | |||
| Cd | 0.45 | 0.66 | 0.55 | 0.07 | |||
| Cu | <d.l. | 59.92 | 5.01 | 17.29 | |||
| S | 32.15 | 32.73 | 32.51 | 0.21 | |||
| Total | 96.53 | 98.89 | 97.88 | 0.81 | |||
| Sasa-24 | Galena | 7 | |||||
| Pb | 82.06 | 83.73 | 82.87 | 0.56 | |||
| Bi | 3.15 | 3.85 | 3.44 | 0.24 | |||
| Ag | 0.03 | 0.14 | 0.09 | 0.04 | |||
| In | 0.16 | 0.35 | 0.28 | 0.07 | |||
| S | 12.36 | 12.55 | 12.44 | 0.08 | |||
| Total | 98.47 | 100.25 | 99.11 | 0.68 | |||
| Sasa-24 | Sphalerite | 4 | |||||
| Zn | 55.21 | 57.95 | 56.39 | 1.17 | |||
| Fe | 9.76 | 10.48 | 10.02 | 0.32 | |||
| Mn | 0.35 | 0.43 | 0.39 | 0.03 | |||
| Cd | 0.58 | 0.63 | 0.61 | 0.02 | |||
| Cu | <d.l. | 0.10 | 0.03 | 0.08 | |||
| S | 30.21 | 31.19 | 30.49 | 0.47 | |||
| Total | 96.24 | 100.50 | 97.92 | 1.83 | |||
| Mineralization Type | Prograde | Retrograde | Hydrothermal | |||
|---|---|---|---|---|---|---|
| Host Mineral | Px | Qtz | Syn-ore Qtz | Syn-ore Qtz | Post-ore Cc | Post-ore Cc |
| Fluid Inclusion Type | Primary | Primary | Primary | Secondary | Primary | Secondary |
| Phases (at 25 °C) | L+V | L+V | L+V | L+V | L+V | L+V |
| F (at 25 °C) | 0.6 | 0.7–0.8 | 0.7 | 0.8–0.9 | 0.7–0.8 | 0.9 |
| Composition | NaCl-CaCl2-H2O | NaCl-MgCl2-H2O | NaCl-MgCl2-H2O or FeCl2-H2O | NaCl-CaCl2-H2O | NaCl-CaCl2-H2O | NaCl-CaCl2-H2O |
| Salinity (wt% NaCl equ.) | 7.5–9.6 | 9.3–10.9 | 3.9–9.1 | 4.2–6.3 | 4.8–8.8 | 2.2–2.9 |
| Th (°C) | 403–433 | 237–390 | 240–394 | 125–239 | 242–297 | 130–145 |
| Density (g/cm3) | 0.5270.598 | 0.680–0.899 | 0.612–0.890 | 0.852–0.966 | 0.820–0.856 | 0.944–0.951 |
| Sample | Type of Mineralization | δ13C (‰. V-PDB) | δ18O (‰. V-SMOW) |
|---|---|---|---|
| Sa-1-C | Cippolino marble | 1.4 | 26.3 |
| Sa-101 | Altered skarn | −7.4 | 5.7 |
| Sa-101-1 | Altered skarn | −7.3 | 6.4 |
| Sa-102 | Altered skarn | −7.2 | 7.0 |
| Sa-103 | Altered skarn | −7.3 | 6.4 |
| Sa-15 | Hydrothermal ore | −4.7 | 14.6 |
| Sa-15-2 | Hydrothermal ore | −4.8 | 14.4 |
| Sa-15-3 | Hydrothermal ore | −4.8 | 14.6 |
| Sa-16-C | Hydrothermal ore | −5.1 | 14.7 |
| Sa-17 | Hydrothermal ore | −6.0 | 14.3 |
| Sa-17-0 | Hydrothermal ore | −5.6 | 15.4 |
| Sa-17-1 | Hydrothermal ore | −5.8 | 14.7 |
| Sa-17-M1 | Hydrothermal ore | −4.1 | 13.9 |
| Sa-17-M2 | Hydrothermal ore | −4.2 | 13.9 |
| Sa-17-C | Hydrothermal ore | −5.6 | 14.7 |
| Sa-18-O | Hydrothermal ore | −6.4 | 8.3 |
| Sa-19 | Hydrothermal ore | −6.0 | 14.4 |
| Sa-19-C | Hydrothermal ore | −5.0 | 14.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palinkaš, S.S.; Peltekovski, Z.; Tasev, G.; Serafimovski, T.; Šmajgl, D.; Rajič, K.; Spangenberg, J.E.; Neufeld, K.; Palinkaš, L. The Role of Magmatic and Hydrothermal Fluids in the Formation of the Sasa Pb-Zn-Ag Skarn Deposit, Republic of Macedonia. Geosciences 2018, 8, 444. https://doi.org/10.3390/geosciences8120444
Palinkaš SS, Peltekovski Z, Tasev G, Serafimovski T, Šmajgl D, Rajič K, Spangenberg JE, Neufeld K, Palinkaš L. The Role of Magmatic and Hydrothermal Fluids in the Formation of the Sasa Pb-Zn-Ag Skarn Deposit, Republic of Macedonia. Geosciences. 2018; 8(12):444. https://doi.org/10.3390/geosciences8120444
Chicago/Turabian StylePalinkaš, Sabina Strmić, Zlatko Peltekovski, Goran Tasev, Todor Serafimovski, Danijela Šmajgl, Kristijan Rajič, Jorge E. Spangenberg, Kai Neufeld, and Ladislav Palinkaš. 2018. "The Role of Magmatic and Hydrothermal Fluids in the Formation of the Sasa Pb-Zn-Ag Skarn Deposit, Republic of Macedonia" Geosciences 8, no. 12: 444. https://doi.org/10.3390/geosciences8120444
APA StylePalinkaš, S. S., Peltekovski, Z., Tasev, G., Serafimovski, T., Šmajgl, D., Rajič, K., Spangenberg, J. E., Neufeld, K., & Palinkaš, L. (2018). The Role of Magmatic and Hydrothermal Fluids in the Formation of the Sasa Pb-Zn-Ag Skarn Deposit, Republic of Macedonia. Geosciences, 8(12), 444. https://doi.org/10.3390/geosciences8120444





