Groundwater Natural Contamination by Toluene in Beja and Faro Districts, Portugal
Abstract
1. Introduction
1.1. Toluene
1.2. Unconventional Hydrocarbons
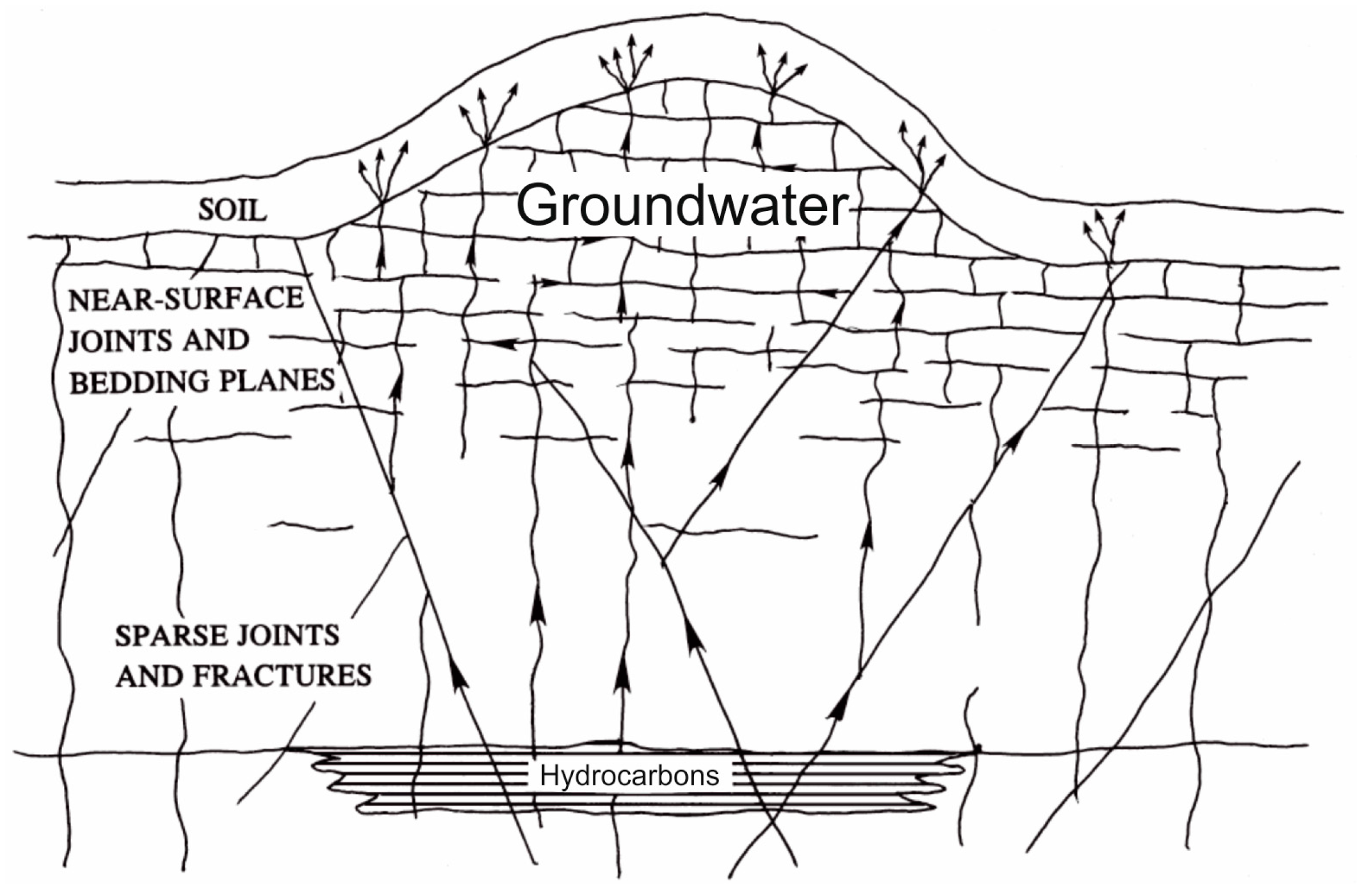
1.3. Hydrocarbon Seeps
- -
- Seeps can be indicators of natural petroleum systems;
- -
- Seeps indicate the occurrence of a fault. According to Etiope [26], gas, in fact, follows preferential pathways of movement (less resistance paths), as determined by fractures and faults;
- -
- Seeps can represent a geo-hazard for societal community and industry;
- -
- Seeps are natural sources of greenhouse gas.
1.4. Objectives
2. Material and Methods
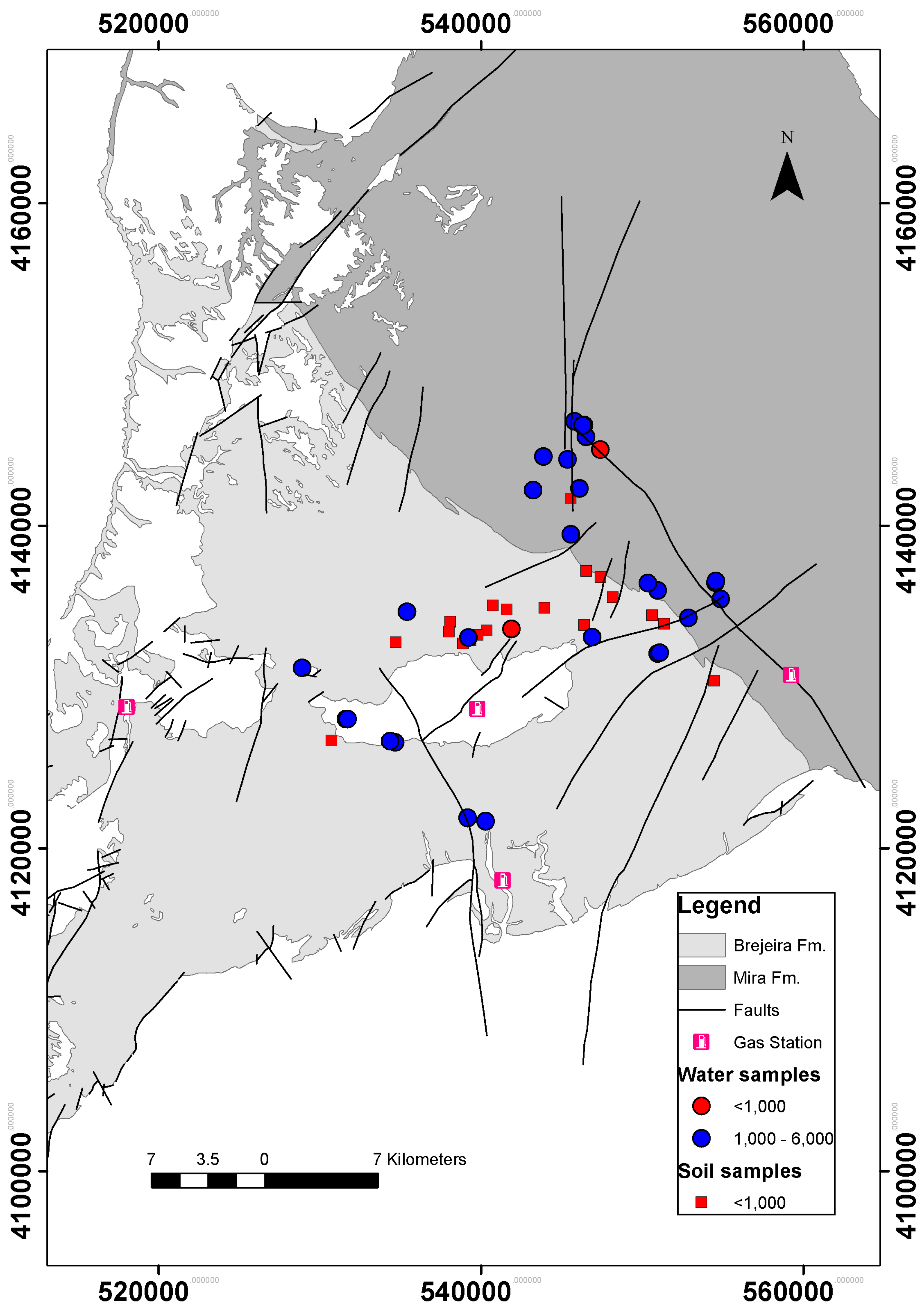
2.1. Study Area
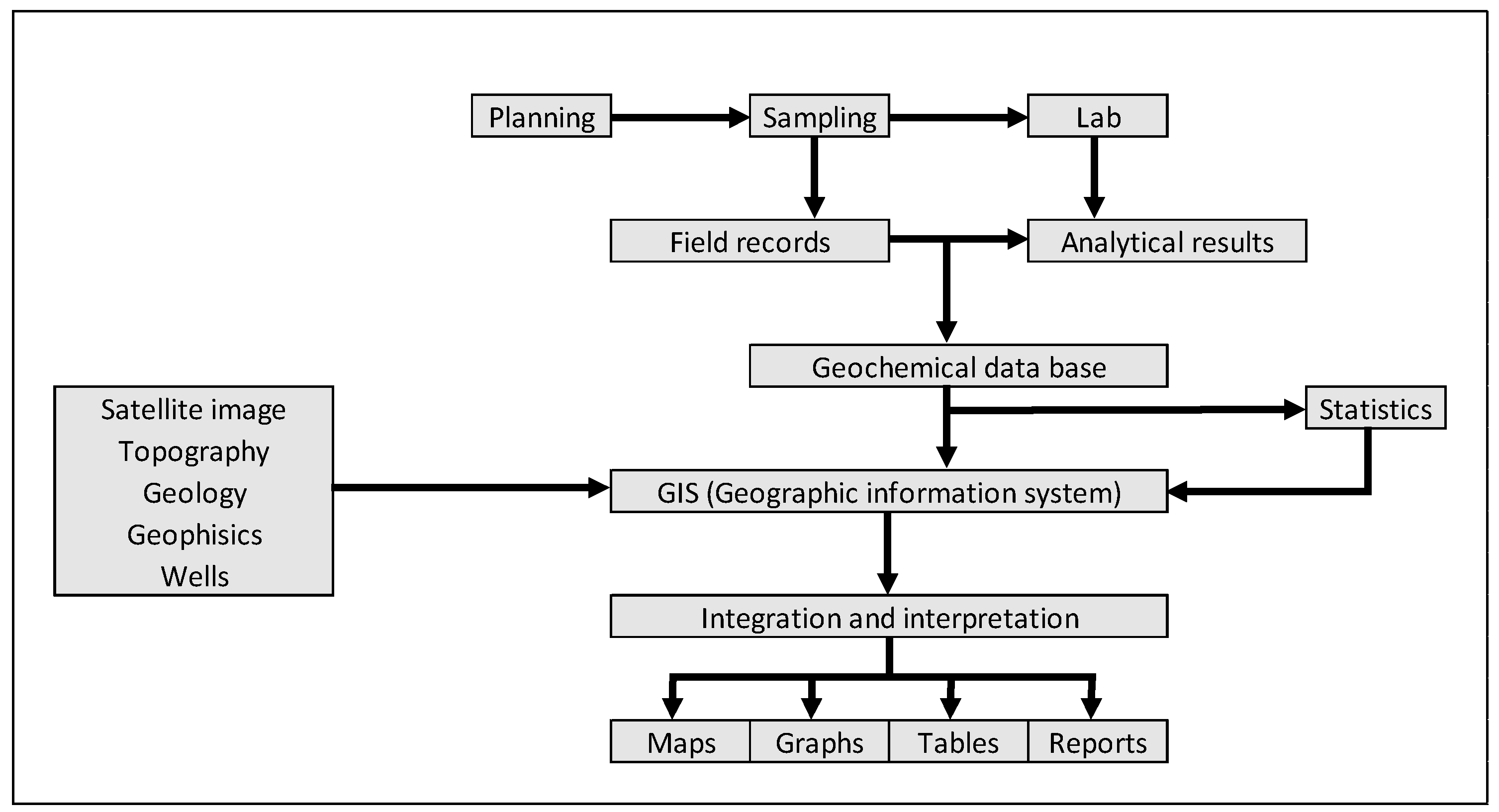
2.2. Surface Geochemical Prospecting
2.3. Analytical Methodology
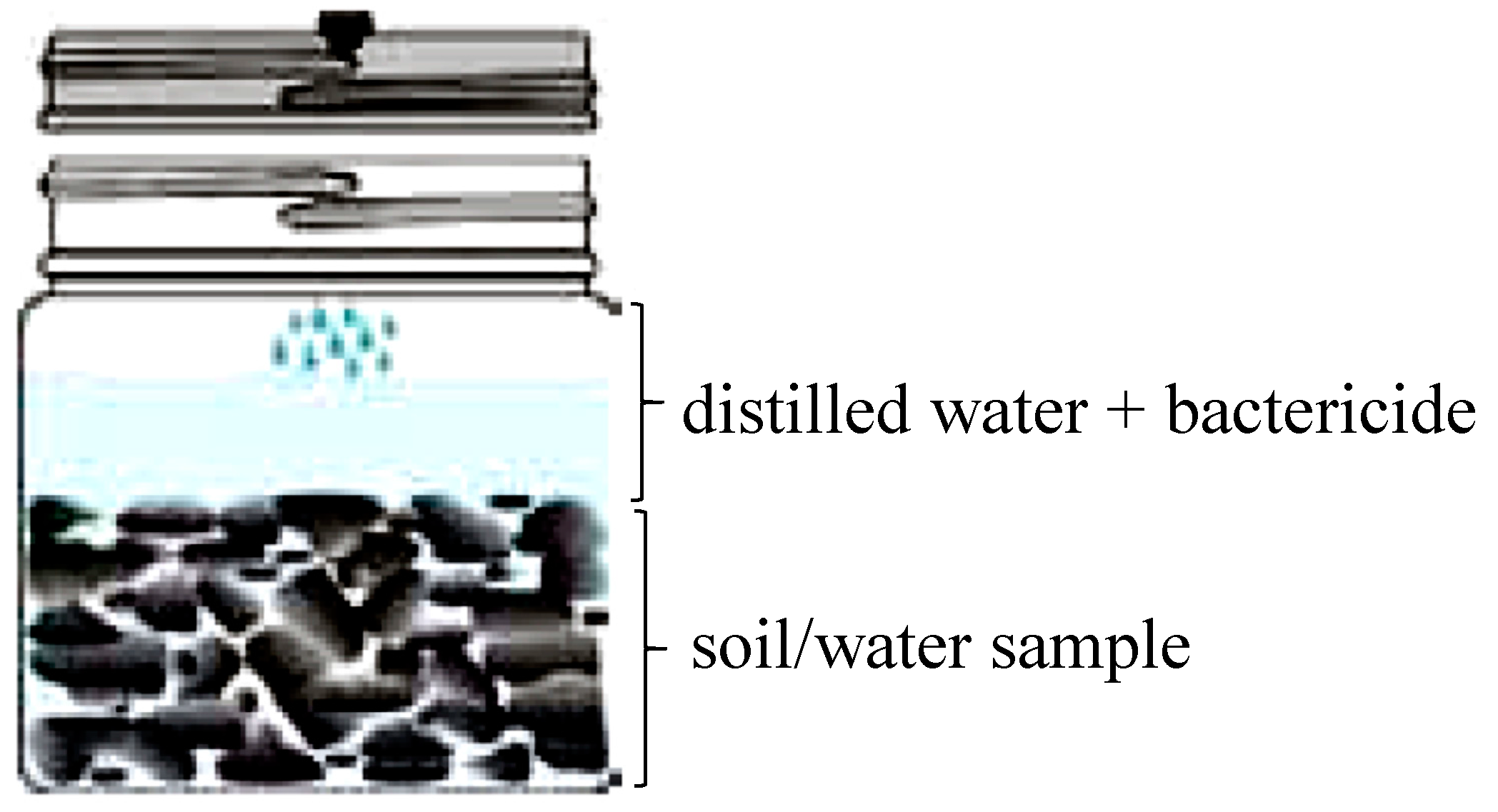
- Put the sample in jar (approximately 1 cup);
- Only add distilled water to reach the line on the label;
- Do not fill the jar with water as the headspace gap is needed to allow gas to desorb into the gap;
- Leave about a 1-inch gap between the water and top of the jar;
- Add 10 drops of the dilute bactericide, Benzalkonium Chloride, to the jar;
- Screw the lid on as tight as possible;
- Tape the lid to keep it tight and from vibrating loose during shipment. Tape in same direction (clockwise) that the lid is screwed on.
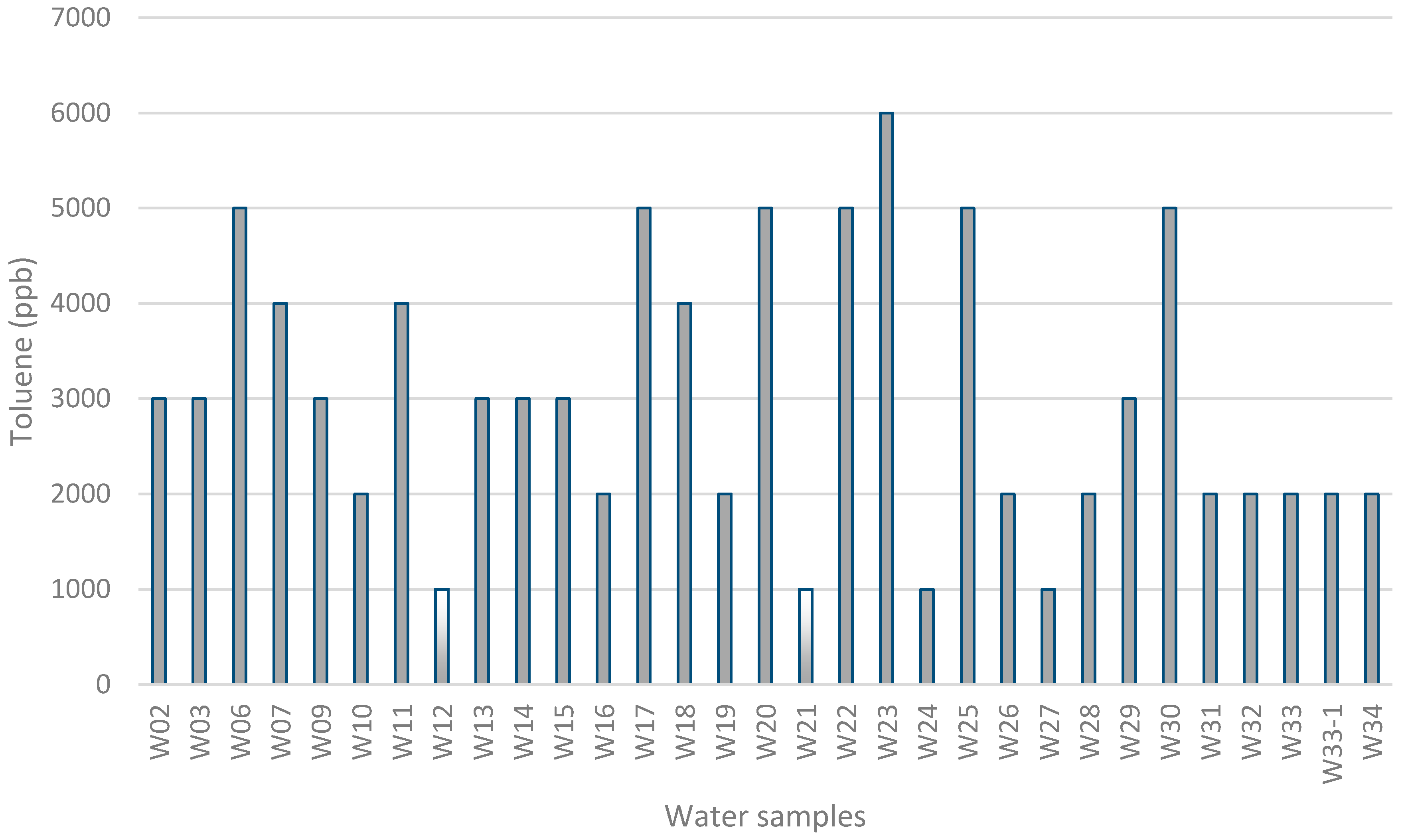
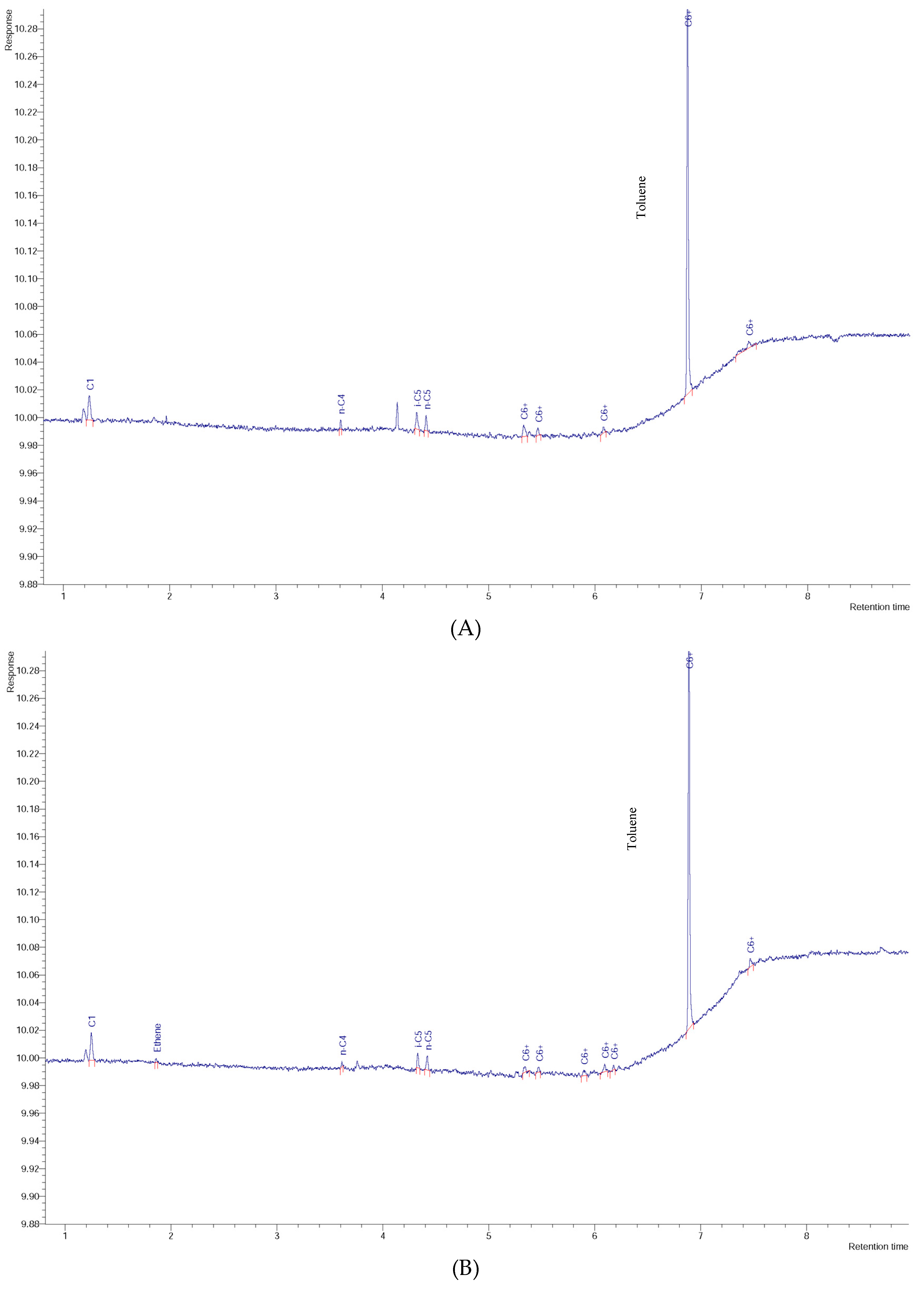
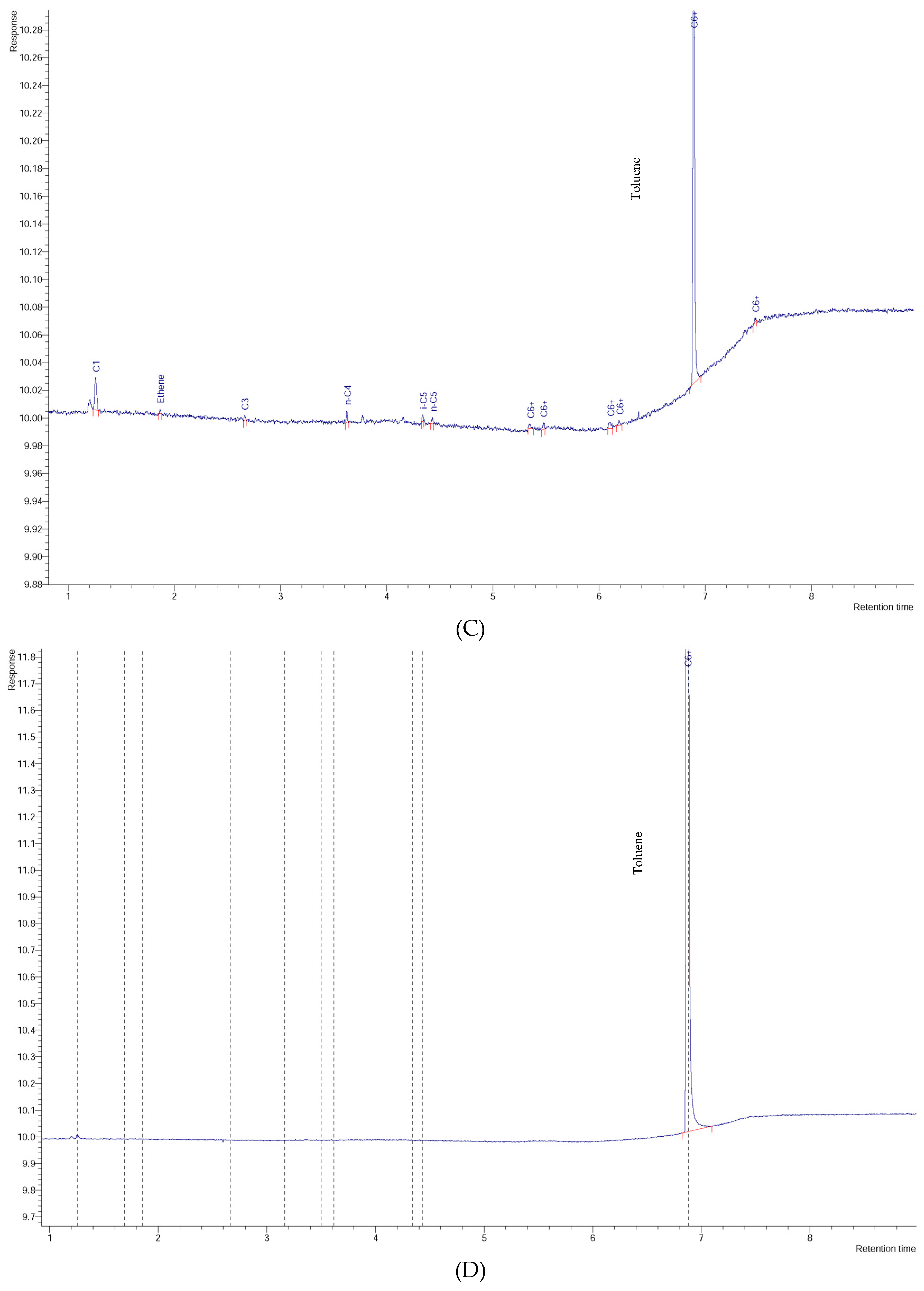
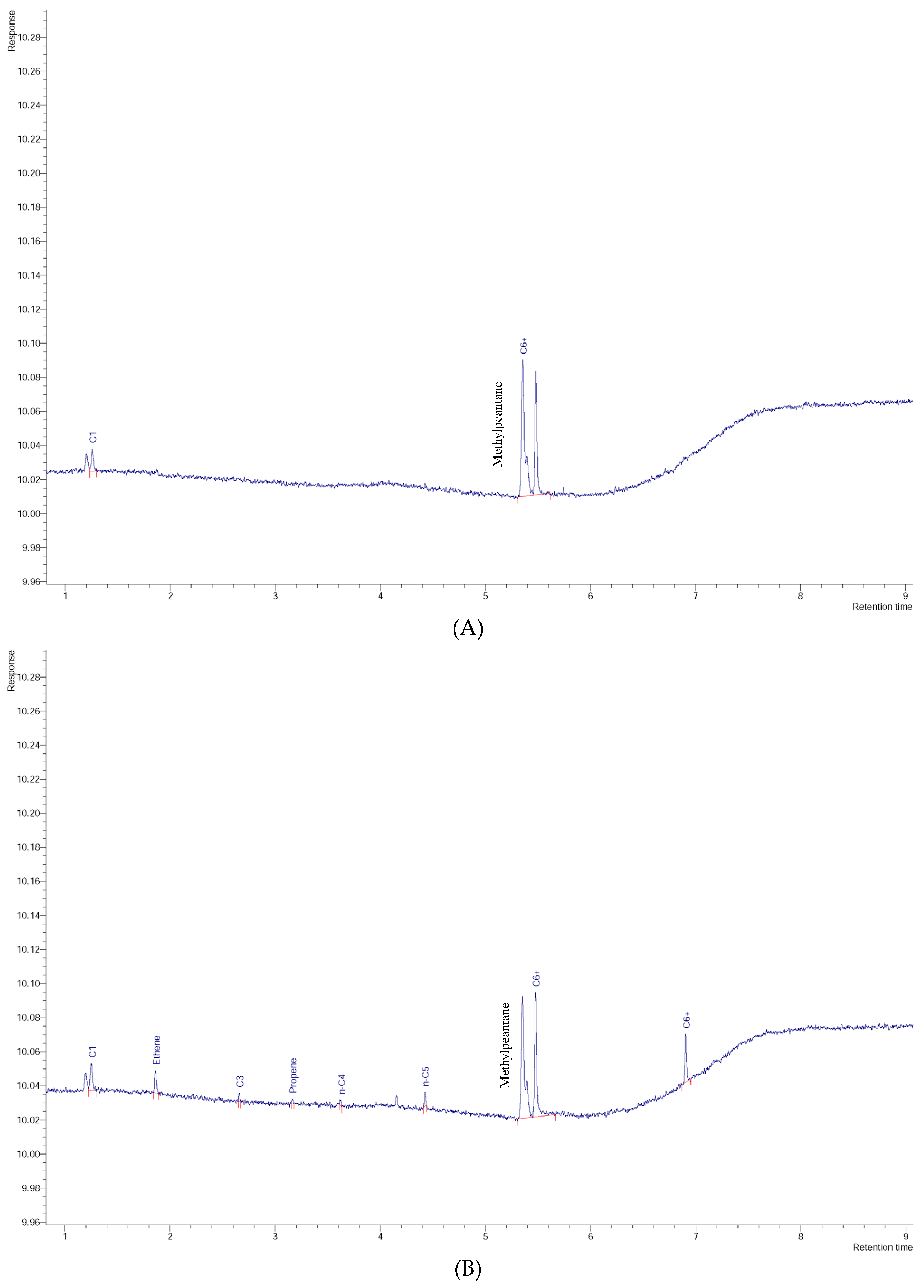
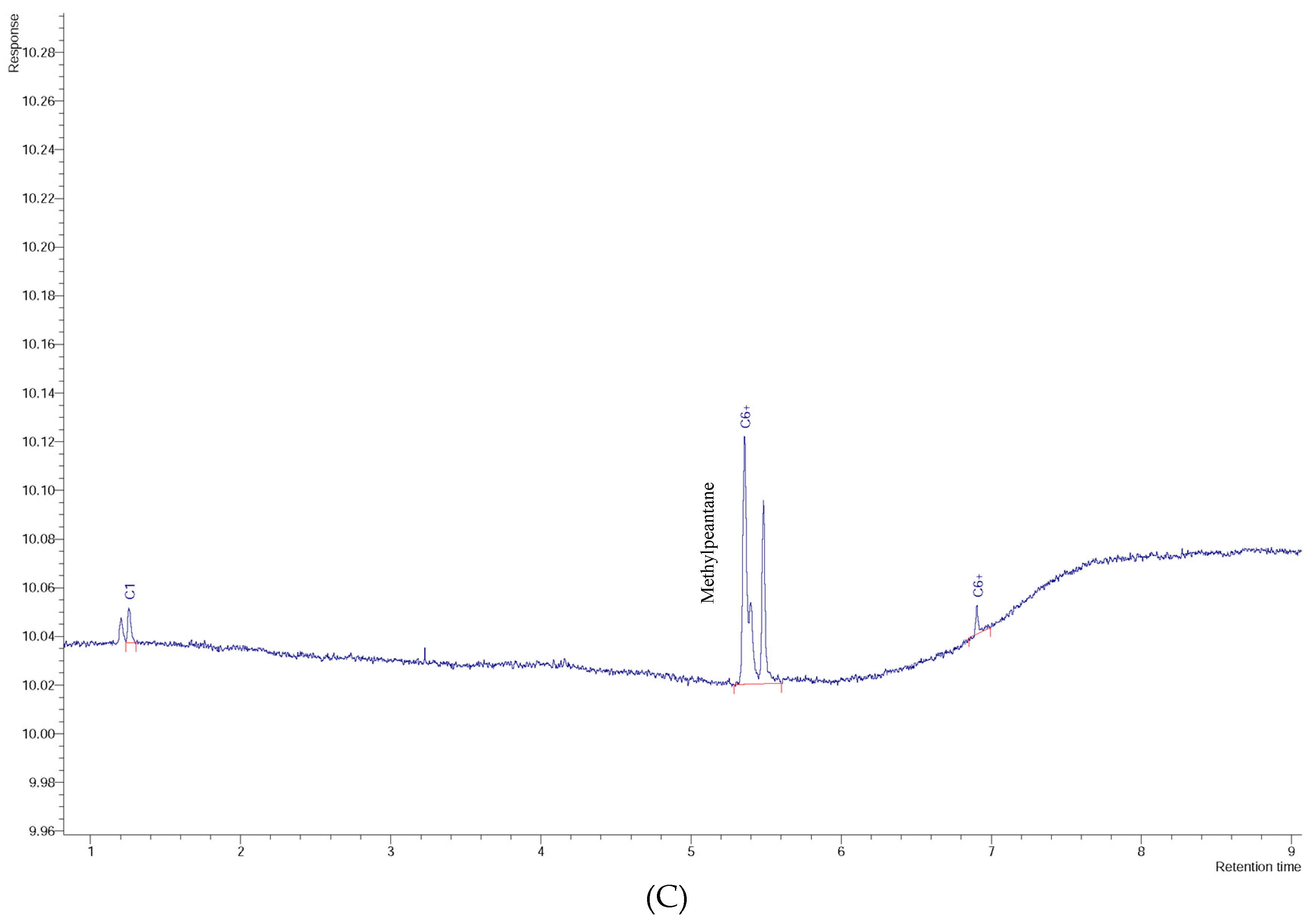
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Toluene. US Department of Health and Human Services; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2017; p. 496.
- World Health Organization (WHO). Toluene in Drinking-Water; Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO Graphics: Geneva, Switzerland, 2004; p. 9. [Google Scholar]
- Stout, S.A.; Wang, Z. Chemical fingerprinting methods and factors affecting petroleum fingerprints in the environment. In Standard Handbook Oil Spill Environmental Forensics; Stout, S.A., Wang, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 61–129. ISBN 9780128096598. [Google Scholar]
- Bandeira de Mello, C.S.; Miller, D.J. Risks to Health from Organic Substances. In Workshop International of Medical Geology; Silva, C.R., Figueiredo, B.R., Capitani, E.M., Cunha, F.G., Eds.; CPRM: Belo Horizonte, Brazil, 2005; p. 206. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Support Document to the 2015 Priority List of Hazardous Substances That Will Be Candidates for Toxicological Profiles; Agency for Toxic Substances and Disease Registry Division of Toxicology and Human Health Sciences: Atlanta, GA, USA, 2015; p. 12.
- Kaplan, I.R.; Galperin, Y.; Lu, S.T.; Lee, R.P. Forensic Environmental Geochemistry: Differentiation of fuel-types, their sources and release time. Org. Geochem. 1997, 27, 289–317. [Google Scholar] [CrossRef]
- Australian Environment Protection Authority (EPA). EPA Guidelines for Environmental Management of On-Site Remediation; Environment Protection Authority: Carlton, Australia, 2006; p. 33. ISBN 192112511X.
- Ramachandra, T.V. Soil and Groundwater Pollution from Agricultural Activities; Commonwealth of Learning Indian Institute of Science: Bangalore, India, 2006; p. 352. [Google Scholar]
- Kirk, R.E.; Othmer, D.F.; Grayson, M.; Eckroth, D. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; John Wiley: New York, NY, USA, 2004; 22950p, ISBN 9780471238966. [Google Scholar]
- Government of Canada. Toluene—Priority Substances List Assessment Report Nº 4; Canadian Environmental Protection Act; Canada Communication Group: Toronto, ON, Canada, 1992; p. 26.
- McGraw-Hill (Ed.) Dictionary of Chemistry, 2nd ed.; McGraw-Hill: New York, NY, USA, 2003; p. 431. ISBN 9780071410465. [Google Scholar]
- Daintith, J. Dictionary of Science, 15th ed.; Oxford University Press: Oxford, UK, 2005; p. 888. ISBN 9780198738374. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality; WHO Graphics: Geneva, Switzerland, 2011; p. 541. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Occurrence of Synthetic Organic Chemicals in Drinking Water, Food, and Air. PB89-192520; Office of Drinking Water: Washington, DC, USA, 1987; p. 175.
- Leusch, F.; Bartkow, M. A Short Primer on Benzene, Toluene, Ethylbenzene and Xylenes (BTEX) in the Environment and in Hydraulic Fracturing Fluids; Griffith University Press: Brisbane, Australia, 2010; p. 8. [Google Scholar]
- Prince, R.C.; Walters, C.C. Biodegradation of oil hydrocarbons and its implications for source identification. In Standard Handbook Oil Spill Environmental Forensics; Stout, S.A., Wang, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 869–916. ISBN 9780128096598. [Google Scholar]
- Zogorski, J.S.; Carter, J.M.; Ivahnenko, T.; Lapham, W.W.; Moran, M.J.; Rowe, B.L.; Squillace, P.J.; Toccalino, P.L. The Quality of Our Nation’s Waters—Volatile Organic Compounds in the Nation’s Ground Water and Drinking-Water Supply Wells; U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Reston, VA, USA, 2006; p. 101.
- Gilbert, D.; Woodruff, C.; Preston, A.; Thomas, R.; Wood, M. Exposure and Risk Assessment for Toluene. PB8S-221505; National Technical Information Service: Springfield, VA, USA, 1983; p. 182.
- Lawrence, S.J. Description, Properties, and Degradation of Selected Volatile Organic Compounds Detected in Ground Water—A Review of Selected Literature; Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2006; p. 54.
- Maier, U.; Mayer, U.; Crathwohl, P. Natural Attenuation of Volatile Hydrocarbons in the Unsaturated Zone—Modeling for the Vaerlose field site. In Bringing Groundwater Quality Research to the Watershed Scale; Thomson, N.R., Ed.; IAHS Public: Hamilton, ON, Canada, 2005; p. 297. [Google Scholar]
- Jarvie, D.M. Shale resources systems for oil and gas: Part 1—Shale-gas resources systems. In Shale Reservoirs—Giant Resources for the 21st Century; Breyer, J.A., Ed.; AAPG: Tulsa, OK, USA, 2012; pp. 69–87. ISBN 9780891813798. [Google Scholar]
- Boyer, C.; Clark, B. Shale Gas: A Global Resource. OilField Rev. 2011, 23, 28–39. [Google Scholar]
- Kent, P.; Lee, J. Unconventional Gas Reservoirs—Tight Gas, Coal Seams, and Shales. Work. Doc. NPC Glob. Oil Gas Study 2007, 29, 54. [Google Scholar]
- Ground Water Protection Council and All Consulting. Modern Shale Gas Development in the United States: A Primer; US Department of Energy Office and Fossil Energy and National Energy Technology Laboratory: Washington, DC, USA, 2009; p. 98.
- Law, B.E.; Curtis, J.B. Introduction to Unconventional Petroleum Systems. AAPG Bull. 2002, 86, 1851–1852. [Google Scholar] [CrossRef]
- Etiope, G. Natural Gas Seepage—The Earth’s Hydrocarbon Degassing; Springer: Cham, Switzerland, 2015; p. 199. ISBN 9783319146010. [Google Scholar]
- Saunders, D.F.; Burson, K.R.; Thompson, C.K. Model for Hydrocarbon Microseepage and Related Near-Surface Alterations. AAPG Bull. 1999, 83, 170–184. [Google Scholar] [CrossRef]
- Abrams, M.A. Best practices for the collection, analysis, and interpretation of seabed geochemical samples to evaluate subsurface hydrocarbon generation and entrapment. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013. [Google Scholar] [CrossRef]
- Abrams, M.A. Significance of hydrocarbon seepage relative to petroleum generation and entrapment. Mar. Pet. Geol. 2005, 22, 457–477. [Google Scholar] [CrossRef]
- Magoon, L.B.; Schmoker, J.W. The Total Petroleum System—The Natural Fluid Network That Constraints the assessment Units. In USGS Digital Data SERIES 60; U.S. Geological Survey: Reston, VA, USA, 2000; p. 31. [Google Scholar]
- Etiope, G. A global dataset of onshore gas and oil seeps: A new tool for hydrocarbon exploration. Oil Gas Bus. 2009, 1–10. Available online: http://hdl.handle.net/2122/6040 (accessed on 22 August 2017).
- Etiope, G.; Klusman, R.W. Geologic emissions of methane to the atmosphere. Chemosphere 2002, 49, 777–789. [Google Scholar] [CrossRef]
- Eltschlager, K.K.; Hawkins, J.W.; Ehler, W.C.; Baldassare, F. Technical Measures for the Investigation and Mitigation of Fugitive Methane Hazards in Areas of Coal Mining; U.S. Department of the Interior—Office of Surface Mining: Pittsburgh, PA, USA, 2001; p. 124.
- Oliveira, J.T. The marine Carboniferous of South Portugal: A stratigraphic and sedimentological approach. In The Carboniferous of Portugal; Lemos de Sousa, M.J., Oliveira, J.T., Eds.; Memórias dos Serviços Geológicos de Portugal: Lisbon, Portugal, 1983; Volume 29, pp. 3–37. (In Portuguese) [Google Scholar]
- Oliveira, J.T.; Relvas, J.; Pereira, Z.; Matos, J.X.; Rosa, C.; Rosa, D.; Munhá, J.M.; Fernandes, P.; Jorge, R.; Pinto, A. Geologia da Zona Sul Portuguesa, com Ênfase na Estratigrafia e Na Vulcanologia Física, Geoquímica e Mineralizações da Faixa Piritosa. In Geologia de Portugal; Dias, R., Araújo, A., Terrinha, P., Kullberg, J.C., Eds.; Escolar Editora: Lisbon, Portugal, 2013; Volume I, pp. 673–767. ISBN 9789725923641. (In Portuguese) [Google Scholar]
- Abad, I.; Mata, M.P.; Nieto, F.; Velilla, N. The Phyllosilicates in diagenetic-metamorphic rocks of the South Portuguese Zone, southwestern Portugal. Can. Mineral. 2001, 39, 1571–1589. [Google Scholar] [CrossRef]
- McCormack, N.; Clayton, G.; Fernandes, P. The thermal history of the Upper Palaeozoic rocks of southern Portugal. Mar. Pet. Geol. 2007, 24, 145–150. [Google Scholar] [CrossRef]
- Fernandes, P.; Musgrave, J.A.; Clayton, G.; Pereira, Z.; Oliveira, J.T.; Goodhue, R.; Rodrigues, B. New evidence concerning the thermal history of Devonian and Carboniferous rocks in the South Portuguese Zone. J. Geol. Soc. Lond. 2012, 169, 647–654. [Google Scholar] [CrossRef]
- Barberes, G.A.; Fonseca, P.E.; Pena dos Reis, R.; Pimentel, N.; Azevedo, M. Preliminary assessment of potential for Shale Gas in South Portuguese Zone carboniferous units. Comunicações Geológicas 2014, 101, 737–741. [Google Scholar]
- Almeida, C.; Mendonça, J.J.L.; Jesus, M.R.; Gomes, A.J. Sistemas Aquíferos de Portugal Continental; Instituto da Água: Lisboa, Portugal, 2000; p. 42. (In Portuguese) [Google Scholar]
- Bandeira de Mello, C.S.; Françolin, J.B.L.; Miller, D.J.; Gonçalves, R.C.S.; Capilla, R.; Lopes, J.P.; Jesuíno, L.S.; Silva Freitas, L.C.; Soares Filho, J.R.S.; Fontes, R.A. Principais metodologias de prospecção geoquímica de hidrocarbonetos em superfície. In Prospecção Geoquímica de Depósitos Minerais Metálicos, Não-Metálicos, Óleo e Gás; Licht, O.A.B., Bandeira de Mello, C.S., Silva, C.R., Eds.; SBGq-CPRM: Rio de Janeiro, Brasil, 2007; p. 788. ISBN 9788574990576. (In Portuguese) [Google Scholar]
- Isotech Laboratories Inc. Procedure for Taking Cuttings Samples in IsoJars®; Isotech Laboratories Inc.: Champaign, IL, USA, 2014. [Google Scholar]
- Tedesco, S.A. Surface Geochemistry in Petroleum Exploration; Springer Science+Business Media Dordrecht: Dordrecht, The Netherlands, 1995; p. 229. ISBN 9781461526605. [Google Scholar]
- Hale, M. Genesis, behaviour and detection of gases in the crust. In Handbook of Exploration Geochemistry—Geochemical Remote Sensing of the Sub-Surface; Govett, G.J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 7, pp. 3–16. ISBN 9780444504395. [Google Scholar]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography—Theory and Practice; Wiley-Interscience: Hoboken, NJ, USA, 2006; p. 349. ISBN 9780471749448. [Google Scholar]
- Restek. A Technical Guide for Static Headspace Analysis Using GC. Lit. Cat. # 59895B; Restek Technical Guide; Restek: Bellefonte, PA, USA, 2000; p. 19. [Google Scholar]
- Tipler, A. An Introduction to Headspace Sampling in Gas Chromatography—Fundamentals and Theory; PerkinElmer Inc.: Waltham, MA, USA, 2013; p. 35. [Google Scholar]
- Loch, J.P.G.; van Dijk-Looyaard, A.; Zoeteman, B.C.J. Organics in groundwater. In The Future for Water Quality in Europe; Wheeler, D., Richardson, M.L., Bridges, J., Eds.; Pergamon Press: Oxford, UK, 1989; pp. 39–55. ISBN 9780080373775. [Google Scholar]
- Merian, E.; Zander, M. Volatile aromatics. In Handbook of Environmental Chemistry. 3 (B), Anthropogenic Compounds; Hutzinger, O., Ed.; Springer: Berlin, Germany, 1982; pp. 117–161. [Google Scholar]
- Gomez-Belinchon, J.I.; Grimalt, J.O. Volatile organic compounds in two polluted rivers in Barcelona (Catalonia, Spain). Water Res. 1991, 25, 577–589. [Google Scholar] [CrossRef]
- Alentejo, A.R.H. Planos de Gestão das Bacias Hidrográficas Integradas nas Regiões Hidrográficas 6 e 7—Região Hidrográfica 6, Volume I (Relatório), Parte 2 (Caracterização e Diagnóstico); Ministério da Agricultura, Mar, Ambiente e Ordenamento do Território: Lisbon, Portugal, 2011; p. 336. (In Portuguese) [Google Scholar]
- Collins, A.G. Geochemistry of OilField Waters; Elsevier: Amsterdam, The Netherlands, 1975; p. 496. ISBN 9780080868554. [Google Scholar]
- Seneshen, D.M.; Chidsey, T.C.; Morgan, C.D.; Vanden Berg, M.D. New Techniques for new Hydrocarbon Discoveries—Surface Geochemical Surveys in the Lisbon and Lightning Draw Southeast Field Areas, San Juan County, Utah; Miscellaneous Publication 2010; Utah Geological Survey: Salt Lake City, UT, USA, 2010; Volume 10, p. 68.
- Bandeira de Mello, C.S.; Graciano Filho, J.G.; Menezes, T.R. The importance of the inclusion of natural organic substances in geomedicine. In Book of Abstracts of the 4th International Symposium Environmental Geochemistry in Tropical Countries; Charlie, R.H., Ed.; Coastal Education and Research Foundation: Lawrence, KS, USA, 2004. [Google Scholar]
- Bandeira de Mello, C.S.; Francolin, J.B.L. Study of oil spills and seeps using geological profiles in a river from the Negro basin, Amazon rain forest, Brazil. In International Symposium Environment 2010: Situation and Perspectives for the European Union, Proceedings of the International Symposium 2010, Porto, Portugal, 6–10 May 2003; Neves, M.V., Neves, A.C.V., Eds.; University of Porto: Porto, Portugal, 2010. [Google Scholar]
- Gülensoy, N.; Alvarez, P.J.J. Diversity and correlation of specific aromatic hydrocarbon biodegradation capabilities. Biodegradation 1999, 10, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; Suflita, J.M. Anaerobic biodegradation of natural gas condensate can be stimulated by the addition of gasoline. Biodegradation 2007, 18, 515–523. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (US EPA). National Primary Drinking Water Regulation. EPA 816-F-09-004; EPA’s Safe Drinking Water; US EPA: Washington, DC, USA, 2009; p. 6.
- Kristiansson, K.; Malmqvist, L. Evidence for non-diffusive transport of 222Rn in the ground and a new geophysical model for the transport. Geophysics 1982, 47, 1444–1452. [Google Scholar] [CrossRef]
- Malmqvist, L.; Kristiansson, K. Experimental evidence for an ascending microflow of geogas in the ground. Earth Planet. Sci. Lett. 1984, 70, 407–416. [Google Scholar] [CrossRef]
- Malmqvist, L.; Kristiansson, K. A physical mechanism for the release of free gases in the lithosphere. Geoexploration 1985, 23, 447–453. [Google Scholar] [CrossRef]


| Guideline Value | 700 µg/L |
|---|---|
| Occurrence | Concentration of a few micrograms per litre have been found in surface water, groundwater and drinking-water;point emissions can lead to higher concentrations in groundwater (up to 1000 µg/L);It may also penetrate plastic pipes from contaminated soil |
| TDI | 223 µg/kg body weight based on a LOAEL of 312 mg/kg body weight per day for marginal hepatotoxic effects observed in a 13-week gavage study in mice, adjusting for daily dosing and using na uncertainty factor for 1000 (100 for interspecies and intraspecies variattion and 10 for the short suration of the study and use of a LOAEL instead of a NOAEL) |
| Limit of Detection | 0.13 µg/L by GC with FID;6 ppb by GC-MS |
| Treatment Perfomance | 1 µg/L should be achievable using air stripping |
| Guideline Value Derivation | |
| Allocation to Water | 10% of TDI |
| Weight | 60 kg adult |
| Consumption | 2 L/day |
| Additional Comments | The guideline value exceeds the lowest reported odour threshold for toluene in water |
| Assessment Date | 2003 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barberes, G.A.; Pena dos Reis, R.; Spigolon, A.L.D.; Fonseca, P.E.; Bandeira de Mello, C.; Barata, M.T. Groundwater Natural Contamination by Toluene in Beja and Faro Districts, Portugal. Geosciences 2018, 8, 9. https://doi.org/10.3390/geosciences8010009
Barberes GA, Pena dos Reis R, Spigolon ALD, Fonseca PE, Bandeira de Mello C, Barata MT. Groundwater Natural Contamination by Toluene in Beja and Faro Districts, Portugal. Geosciences. 2018; 8(1):9. https://doi.org/10.3390/geosciences8010009
Chicago/Turabian StyleBarberes, Gabriel A., Rui Pena dos Reis, André L. D. Spigolon, Paulo E. Fonseca, Carlos Bandeira de Mello, and Maria Teresa Barata. 2018. "Groundwater Natural Contamination by Toluene in Beja and Faro Districts, Portugal" Geosciences 8, no. 1: 9. https://doi.org/10.3390/geosciences8010009
APA StyleBarberes, G. A., Pena dos Reis, R., Spigolon, A. L. D., Fonseca, P. E., Bandeira de Mello, C., & Barata, M. T. (2018). Groundwater Natural Contamination by Toluene in Beja and Faro Districts, Portugal. Geosciences, 8(1), 9. https://doi.org/10.3390/geosciences8010009





