Applying the Coastal and Marine Ecological Classification Standard (CMECS) to Nearshore Habitats in the Northeastern Gulf of Mexico
Abstract
:1. Introduction
2. Materials and Methods
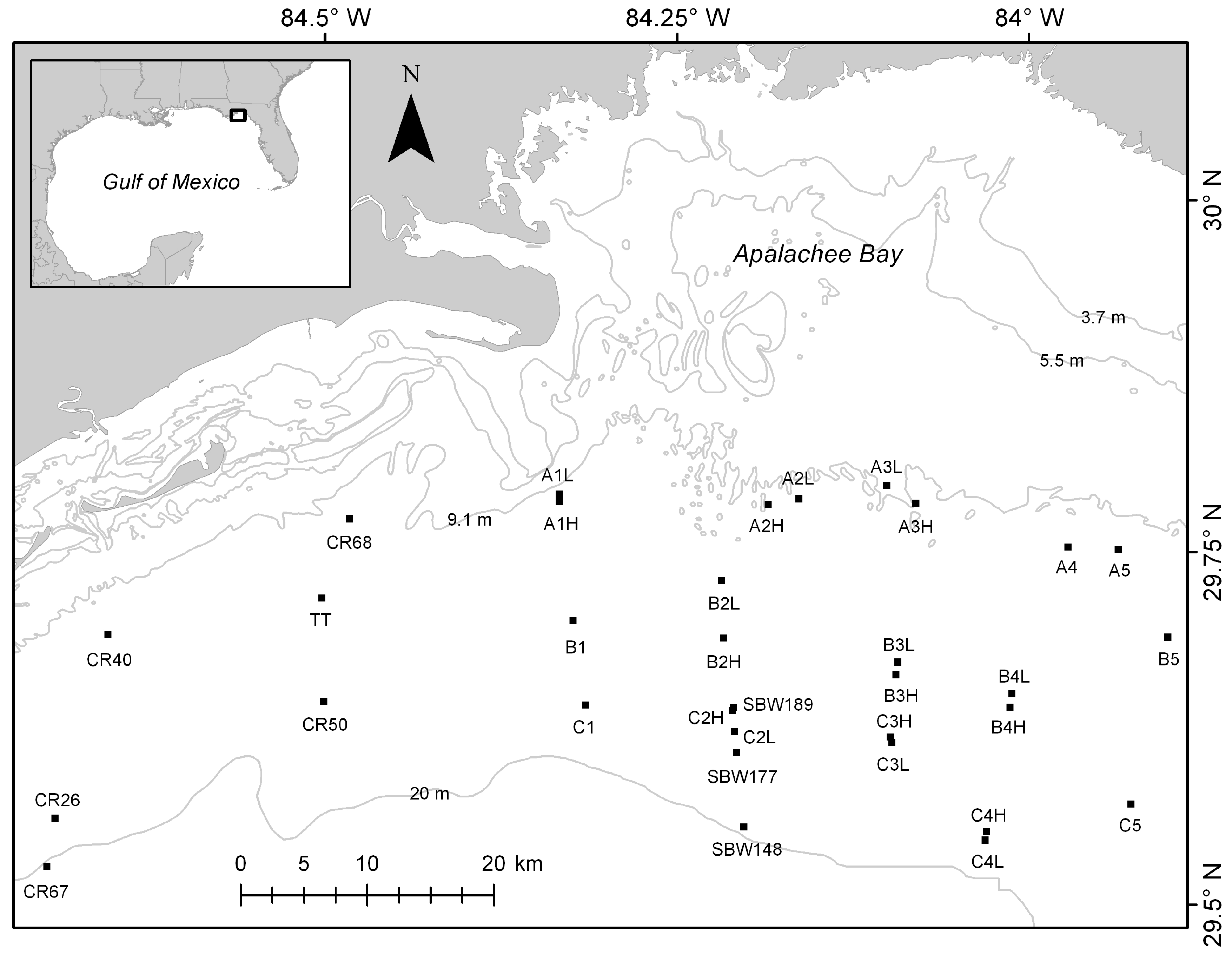
2.1. Study Area
2.2. Methodology
3. Results
3.1. Water Column Component
3.2. Geoform Component
3.3. Substrate Component
3.4. Biotic Component
4. Discussion
4.1. Temporal Scale
4.2. Spatial Scale
4.3. Habitat Heterogeneity
4.4. Ecotones and Seascape Metrics
4.5. Data Acquisition
4.6. Combining Biotic and Abiotic
4.7. Future Work
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Anderson, J.R.; Hardy, E.E.; Roach, J.T. A Land Use and Land Cover Classification System for Use with Remote Sensor Data; U.S. Geological Survey Cire: Reston, VA, USA, 1972.
- Anderson, J.R.; Hardy, E.E.; Roach, J.T.; Witmer, R.E. A Land Use Classification System for Use with Remote-Sensor Data; U.S. Geological Survey Professional Paper 964; U.S. Geological Survey: Reston, VA, USA, 1976.
- Federal Geographic Data Committee (FGDC). Coastal and Marine Ecological Classification Standard. Available online: https://www.fgdc.gov/standards/projects/cmecs-folder/CMECS_Version_06-2012_FINAL.pdf (accessed on 12 January 2018).
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification Revised 2004. Available online: https://www.eea.europa.eu/data-and-maps/data/eunis-habitat-classification/documentation/eunis-2004-report.pdf/download (accessed on 12 January 2018).
- Guarinello, M.L.; Shumchenia, E.J.; King, J.W. Marine habitat classification for ecosystem-based management: A proposed hierarchical framework. Environ. Manag. 2010, 45, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.E.; Cochran, S.A.; Logan, J.B.; Grossman, E.E. Benthic Habitats and Offshore Geological Resources of Kaloko-Honnok–Ohau National Historical Park, Hawai`i. Available online: https://pubs.usgs.gov/sir/2006/5256/sir2006-5256.pdf (accessed on 12 January 2018).
- Greene, H.G.; Yoklavich, M.M.; Starr, R.M.; O’Connell, V.M.; Wakefield, W.W.; Sullivan, D.E.; McRea, J.E.; Cailliet, G.M. A classification scheme for deep seafloor habitats. Oceanol. Acta 1999, 22, 663–678. [Google Scholar] [CrossRef]
- Mumby, P.J.; Harborne, A.R. Development of a systematic classification scheme of marine habitats to facilitate regional management and mapping of Caribbean coral reefs. Biol. Conserv. 1999, 88, 155–163. [Google Scholar] [CrossRef]
- Auster, P.J.; Heinonen, K.B.; Witharana, C.; McKee, M. A Habitat Classification Scheme for the Long Island Sound Region. Available online: http://longislandsoundstudy.net/wp-content/uploads/2010/02/Auster-et-al_EPA-Final-Technical-Report_Habitat-Classification_June-091.pdf (accessed on 12 January 2018).
- Briones, E.E. Current knowledge of benthic communities in the Gulf of Mexico. In Environmental Analysis of the Gulf of Mexico; Withers, K., Nippers, M., Eds.; Texas A&M University: Corpus Christi, TX, USA, 2003. [Google Scholar]
- Kendall, M.S.; Buja, K.R.; Christensen, J.D.; Kruer, C.R.; Monaco, M.E. The seascape approach to coral ecosystem mapping: An integral component of understanding the habitat utilization patterns of reef fish. Bull. Mar. Sci. 2004, 75, 225–237. [Google Scholar]
- Galtsoff, P.S. Gulf of Mexico: Its Origin, Waters, and Marine Life; Fishery Bulletin of the Fish and Wildlife Service: Washington, DC, USA, 1954. [Google Scholar]
- Kendall, M.S.; Jensen, O.P.; Alexander, C.; Field, D.; McFall, G.; Bohne, R.; Monaco, M.E. Benthic mapping using sonar, video transects, and an innovative approach to accuracy assessment: A characterization of bottom features in the Georgia Bight. J. Coast. Res. 2005, 21, 1154–1165. [Google Scholar] [CrossRef]
- Kingon, K.C. Mapping, Classification, and Spatial Variation of Hardbottom Habitats in the Northeastern Gulf of Mexico. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2013. [Google Scholar]
- Peck, J.E. Multivariate Analysis for Community Ecologists: Step-by-Step Using PC-ORD. Available online: https://www.researchgate.net/publication/285753955_Multivariate_Analysis_for_Community_Ecologists_Stepby-_Step_using_PC-ORD (accessed on 12 January 2018).
- Schellinger, J. Hardbottom Sessile Macroinvertebrate Communities of the Apalachee Bay Region of Florida’s Northeastern Gulf of Mexico. Master’s Thesis, Florida State University, Tallahassee, FL, USA, 2013. [Google Scholar]
- Cheney, D.P.; Dyer, J.P. Deep-water benthic algae of the Florida Middle Ground. Mar. Biol. 1974, 27, 185–190. [Google Scholar] [CrossRef]
- Kingon, K.C.; Koenig, C.C.; Brooke, S.; Stallings, C.D.; Wall, K.; Sandon, C. Do artificial reefs sustain communities similar to nearby natural reefs? A seasonal study in the northeastern Gulf of Mexico. In Proceedings of the 67th Annual Meeting of the Gulf and Caribbean Fisheries Institute, Christ Church, Barbados, 3–7 November 2015. [Google Scholar]
- Cochrane, G.R.; Dethier, M.N.; Hodson, T.O.; Kull, K.J.; Golden, N.E.; Ritchie, A.C.; Moegling, C.H.; Pacunski, R.E. Salish Seas Map Series—Admirality Inlet; Geological Survey Open-File Report 2015-1073; U.S. Geological Survey: Reston, VA, USA, 2015.
- Keefer, M.L.; Peery, C.A.; Wright, N.; Daigle, W.R.; Caudill, C.C.; Clabough, T.S.; Griffith, D.W.; Zacharias, M.A. Evaluating the NOAA coastal and marine ecological classification standard in estuarine systems: A Columbia River estuary case study. Estuar. Coast. Shelf Sci. 2008, 78, 89–106. [Google Scholar] [CrossRef]
- Carollo, C.; Allee, R.J.; Yoskowitz, D.W. Linking the Coastal and Marine Ecological Classification Standard (CMECS) to ecosystem services: An application to the US Gulf of Mexico. Int. J. Biodivers. Sci. Ecosyst. Ser. Manag. 2013, 9, 249–256. [Google Scholar] [CrossRef]
- Davies, J.S.; Stewart, H.A.; Narayanaswamy, B.E.; Jacobs, C.; Spicer, J.; Golding, N.; Howell, K.L. Benthic assemblages of the Anton Dohrn Seamount (NE Atlantic): Defining deep-sea biotopes to support habitat mapping and management efforts with a focus on vulnerable marine ecosystems. PLoS ONE 2015, 10, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Seyfabadi, J.; Owfi, F.; Rahimi, M.; Allee, R. Ecological classification of southern intertidal zones of Qeshm Island, based on CMECS model. Iran. J. Fish. Sci. 2014, 13, 1–19. [Google Scholar]
- Zajac, R.N.; Lewis, R.S.; Poppe, L.J.; Twichell, D.C.; Vozarik, J.; DiGiacomo-Cohen, M.L. Responses of infaunal populations to benthoscape structure and the potential importance of transition zones. Limnol. Oceanogr. 2003, 48, 829–842. [Google Scholar] [CrossRef]
- Zajac, R.N. Macrobenthic biodiversity and sea floor landscape structure. J. Exp. Mar. Biol. Ecol. 2008, 366, 198–203. [Google Scholar] [CrossRef]
- Diaz, R.J.; Solan, M.; Valente, R.M. A review of approaches for classifying benthic habitats and evaluating habitat quality. J. Environ. Manag. 2004, 73, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Zajac, R.N. Challenges in marine, soft-sediment benthoscape ecology. Landsc. Ecol. 2008, 23, 7–18. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Thrush, S.E.; Legendre, P.; Funnell, G.A.; Ellis, J.; Morrison, M. Mapping of marine soft-sediment communities: Integrated sampling for ecological interpretation. Ecol. Appl. 2004, 14, 1203–1216. [Google Scholar] [CrossRef]
- Shumchenia, E.J.; King, J.W. Comparison of methods for integrating biological and physical data for marine habitat mapping and classification. Coast. Shelf Res. 2010, 30, 1717–1729. [Google Scholar] [CrossRef]
- Cook, R.R.; Auster, P.J. A Bioregional Classification of the Continental Shelf of Northeastern North America for Conservation Analysis and Planning Based on Representation; Marine Sanctuaries Conservation Series NMSP-0; NOAA’s National Ocean Service: Ginsen, MD, USA, 2007.
| Taxa | |
|---|---|
| brown algae | hard coral |
| red algae | hydroid |
| green algae | gorgonian |
| colonial tunicate | sponge |
| solitary tunicate | bivalve |
| bryozoan | sea urchin |
| Northeastern Gulf of Mexico off Northwest Florida (10–23 m Depths) |
|---|
| Biogeographic Setting |
| Realm: Temperate Northern Atlantic |
| Province: Warm Temperate Northwest Atlantic |
| Ecoregion: Northern Gulf of Mexico |
| Aquatic Setting |
| System: Marine |
| Subsystem: Nearshore |
| Tidal Zone: Subtidal |
| Water Column Component |
| Water Column Layer: Marine Nearshore Lower Water Column |
| Salinity Regime: Euhaline |
| Temperature Regime: Warm |
| Geoform Component |
| Tectonic Setting: Passive Continental Margin |
| Physiographic Setting: Continental Shelf |
| Physiographic Setting: Embayment/Bay |
| Geoform Origin: Geologic |
| Level 1 Geoform: Pavement Area |
| Level 2 Geoform: Ledge |
| Level 2 Geoform: Karren |
| Level 1 Geoform: Outcrop |
| Substrate Component |
| Substrate Origin: Geologic Substrate |
| Substrate Class: Rock Substrate |
| Substrate Subclass: Bedrock (5–76% exposed) |
| Co-occurring Element: Sand (24–88%) |
| Biotic Component |
| Biotic Setting: Benthic/Attached Biota |
| Biotic Class: Faunal Bed |
| Biotic Subclass: Attached Fauna |
| Biotic Group: Diverse Colonizers |
| Biotic Community: Sponge/Hard Coral Colonizers |
| Co-occurring Element: Benthic Macroalgae |
| Water Column Layer | Salinity Regime | Temperature Regime | Hydroform Class | Hydroform | Hydroform Type |
|---|---|---|---|---|---|
| marine nearshore surface Layer | euhaline water | cool water | current | ekman flow | ekman upwelling |
| marine nearshore upper water Column | moderate water | ekman downwelling | |||
| marine nearshore pycnocline | warm water | inertial current | |||
| marine nearshore lower water column | very warm water | langmuir circulation | |||
| hot water | tidal flow | mixed semi-diurnal tidal flow | |||
| wind-driven current | |||||
| front | coastal upwelling front | ||||
| Tidal Front | |||||
| Wave | Coastally Trapped Wave | Shelf Wave | |||
| Storm Surge |
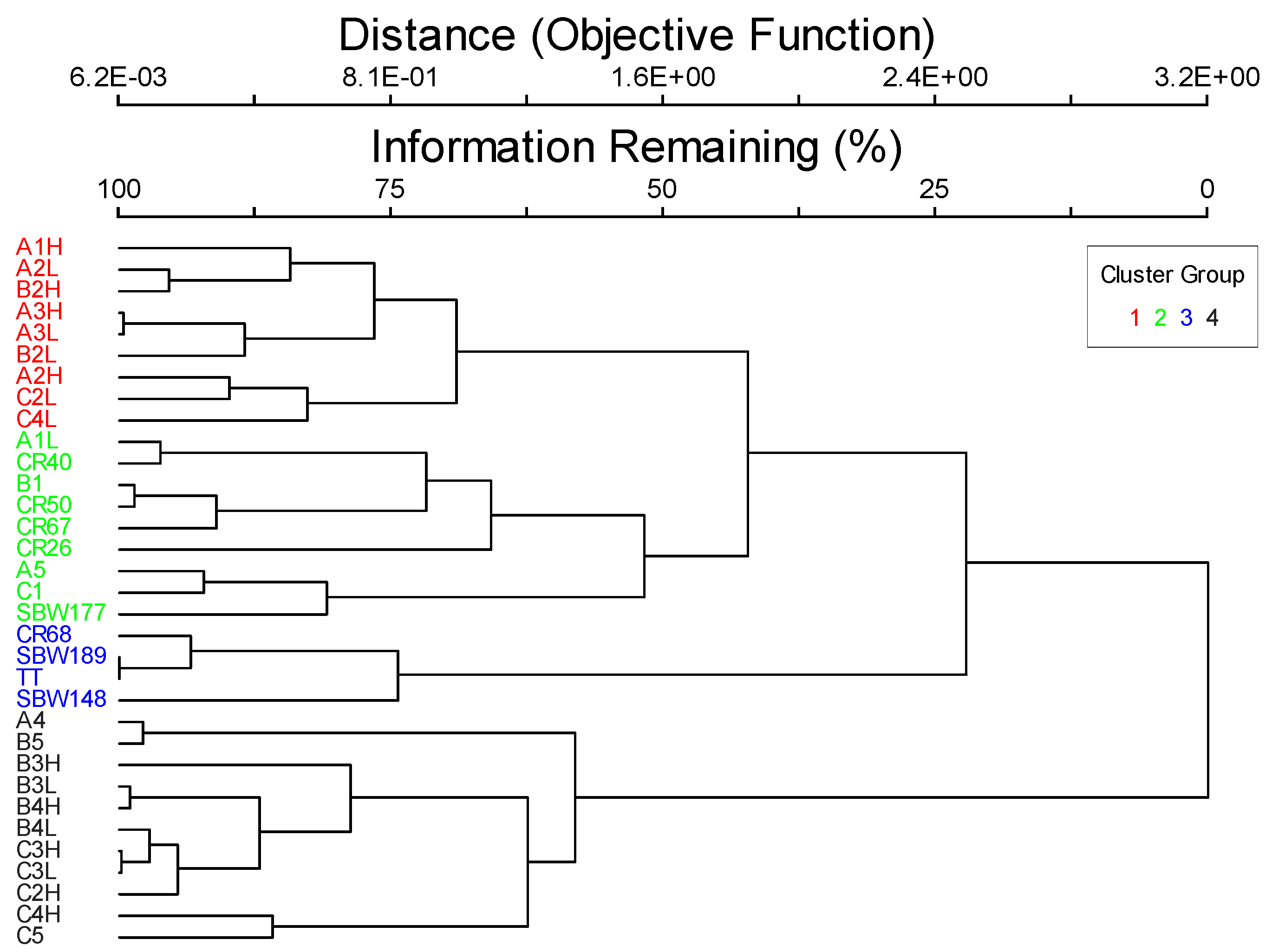
| Taxa | Average | Maximum | Maximum Group | Cluster Group Indicator Values (%) | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| bivalve | 22 | 86 | 1 | 86 | 0 | 0 | 3 |
| bryozoan | 17 | 57 | 1 | 57 | 4 | 0 | 8 |
| green algae | 13 | 33 | 1 | 33 | 1 | 0 | 19 |
| gorgonian | 8 | 19 | 1 | 19 | 0 | 0 | 11 |
| solitary tunicate | 7 | 24 | 1 | 24 | 1 | 0 | 4 |
| colonial tunicate | 12 | 29 | 2 | 18 | 29 | 0 | 3 |
| sponge | 22 | 37 | 2 | 24 | 37 | 19 | 10 |
| sea urchin | 12 | 38 | 2 | 7 | 38 | 0 | 4 |
| hydroid | 13 | 17 | 3 | 9 | 12 | 17 | 15 |
| red algae | 24 | 80 | 3 | 9 | 2 | 80 | 3 |
| brown algae | 25 | 69 | 4 | 29 | 0 | 0 | 69 |
| hard coral | 24 | 33 | 4 | 24 | 11 | 26 | 33 |
| Averages | 17 | 43 | 28 | 11 | 12 | 15 | |
| Taxa | Maximum Group | Observed Indicator Value | p |
|---|---|---|---|
| bivalve | 1 | 85.9 | 0.001 |
| bryozoan | 1 | 57.1 | 0.007 |
| sponge | 2 | 36.9 | 0.092 |
| sea urchin | 2 | 37.7 | 0.060 |
| red algae | 3 | 80.4 | 0.001 |
| brown algae | 4 | 69.3 | 0.001 |
| Cluster Group | Group 1 | Group 2 | Group 3 | Group 4 | ||
|---|---|---|---|---|---|---|
| Variables | Bivalve | Bryozoan | Sponge | Sea Urchin | Red Algae | Brown Algae |
| Latitude | 0.452 | 0.256 | 0.145 | −0.130 | 0.257 | −0.024 |
| Longitude | 0.143 | −0.025 | −0.648 | −0.197 | −0.286 | 0.400 |
| Distance to shore | −0.190 | −0.208 | −0.432 | −0.059 | −0.295 | 0.359 |
| Mean depth | −0.451 | −0.211 | 0.062 | 0.102 | −0.174 | −0.125 |
| Rock percent cover | −0.040 | −0.271 | 0.216 | 0.142 | 0.502 | 0.021 |
| Rock type | 0.168 | −0.496 | −0.120 | −0.028 | 0.193 | 0.106 |
| Mean sand depth | 0.067 | 0.384 | 0.041 | 0.121 | −0.150 | −0.057 |
| Thin sand percent cover | 0.081 | −0.020 | −0.100 | −0.350 | −0.234 | 0.026 |
| Mean bottom temperature | 0.083 | 0.114 | 0.553 | 0.046 | 0.408 | −0.037 |
| Mean vertical visibility | 0.042 | −0.125 | −0.456 | −0.236 | −0.168 | 0.462 |
| Mean horizontal visibility | 0.415 | −0.025 | −0.440 | −0.194 | −0.235 | 0.337 |
| Hardbottom percent cover from the acoustic data | 0.252 | 0.123 | −0.226 | −0.414 | −0.013 | 0.285 |
| Video relief | −0.350 | −0.149 | 0.218 | 0.088 | 0.336 | −0.248 |
| Video heterogeneity | −0.139 | −0.520 | 0.093 | 0.120 | 0.309 | −0.200 |
| Geoform from the acoustic data | −0.466 | −0.028 | 0.093 | 0.060 | 0.043 | −0.182 |
| Heterogeneity from the acoustic data | 0.234 | 0.308 | −0.088 | −0.423 | 0.227 | 0.318 |
| Relief from the acoustic data | −0.386 | −0.040 | 0.224 | 0.145 | 0.127 | <−0.001 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kingon, K. Applying the Coastal and Marine Ecological Classification Standard (CMECS) to Nearshore Habitats in the Northeastern Gulf of Mexico. Geosciences 2018, 8, 22. https://doi.org/10.3390/geosciences8010022
Kingon K. Applying the Coastal and Marine Ecological Classification Standard (CMECS) to Nearshore Habitats in the Northeastern Gulf of Mexico. Geosciences. 2018; 8(1):22. https://doi.org/10.3390/geosciences8010022
Chicago/Turabian StyleKingon, Kelly. 2018. "Applying the Coastal and Marine Ecological Classification Standard (CMECS) to Nearshore Habitats in the Northeastern Gulf of Mexico" Geosciences 8, no. 1: 22. https://doi.org/10.3390/geosciences8010022
APA StyleKingon, K. (2018). Applying the Coastal and Marine Ecological Classification Standard (CMECS) to Nearshore Habitats in the Northeastern Gulf of Mexico. Geosciences, 8(1), 22. https://doi.org/10.3390/geosciences8010022




