Assessing the Long-Term Behaviour of the Industrial Bentonites Employed in a Repository for Radioactive Wastes by Studying Natural Bentonites in the Field
Abstract
:1. Introduction
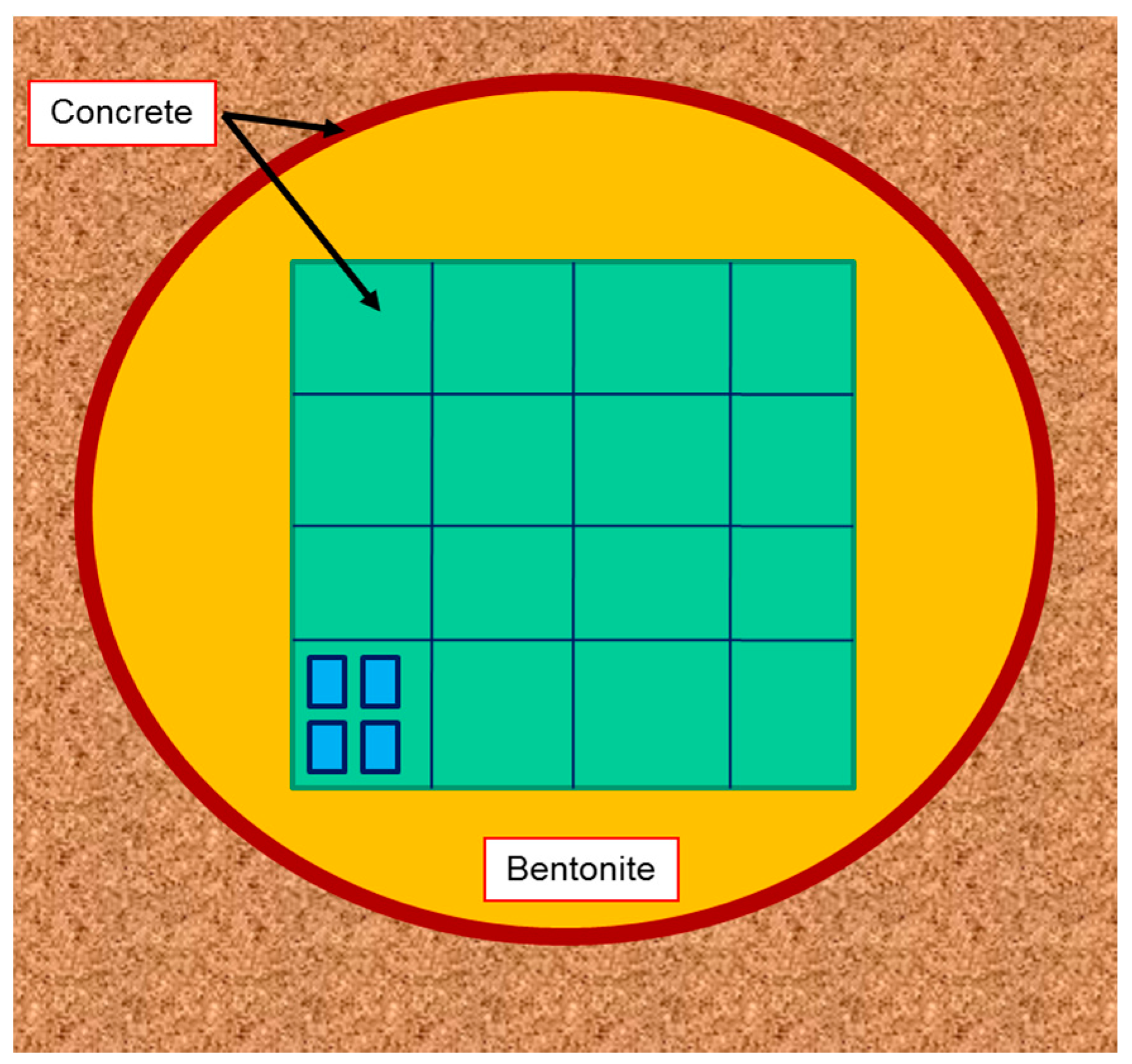
- an appropriately large mass of bentonite is “in place”, rather than just a small plug of material within laboratory apparatus
- an appropriately dense overburden can settle into the bentonite in a manner similar to what would be expected in the repository EBS
- the settlement timescales are of much more relevance to a repository than accelerated laboratory and URL (underground research laboratory) studies
- potential chemical reaction between the overburden and the bentonite can be assessed. Generally, this is assumed to be diffusive transport of solutes (especially OH and Ca) into the body of the bentonite from the overburden/bentonite contact zone, again as would be expected in a repository where OH and Ca from the cementitious materials could interact with the bentonite
- the temperatures of reaction (ca. 10–30 ℃) are also repository-relevant.
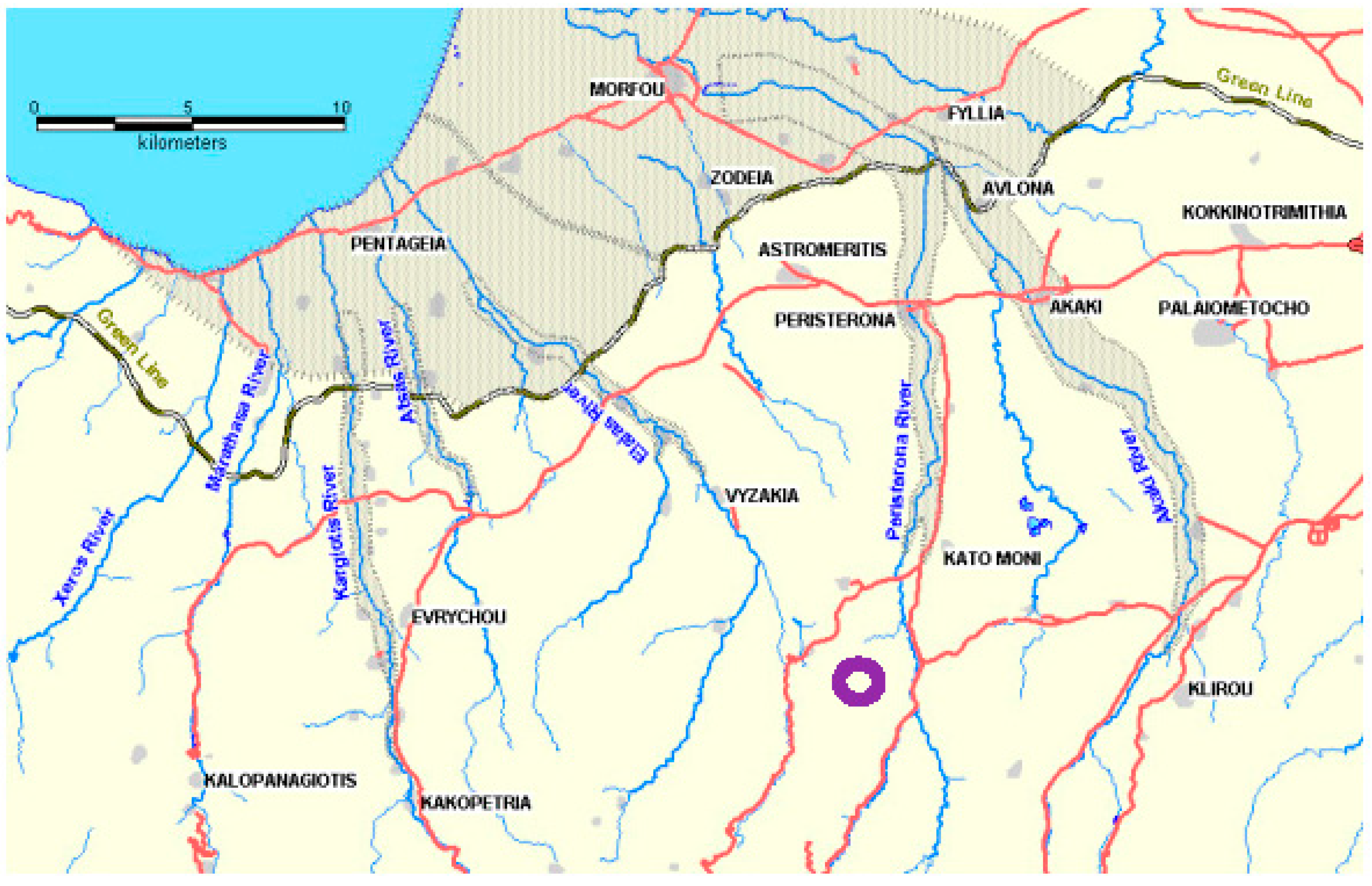
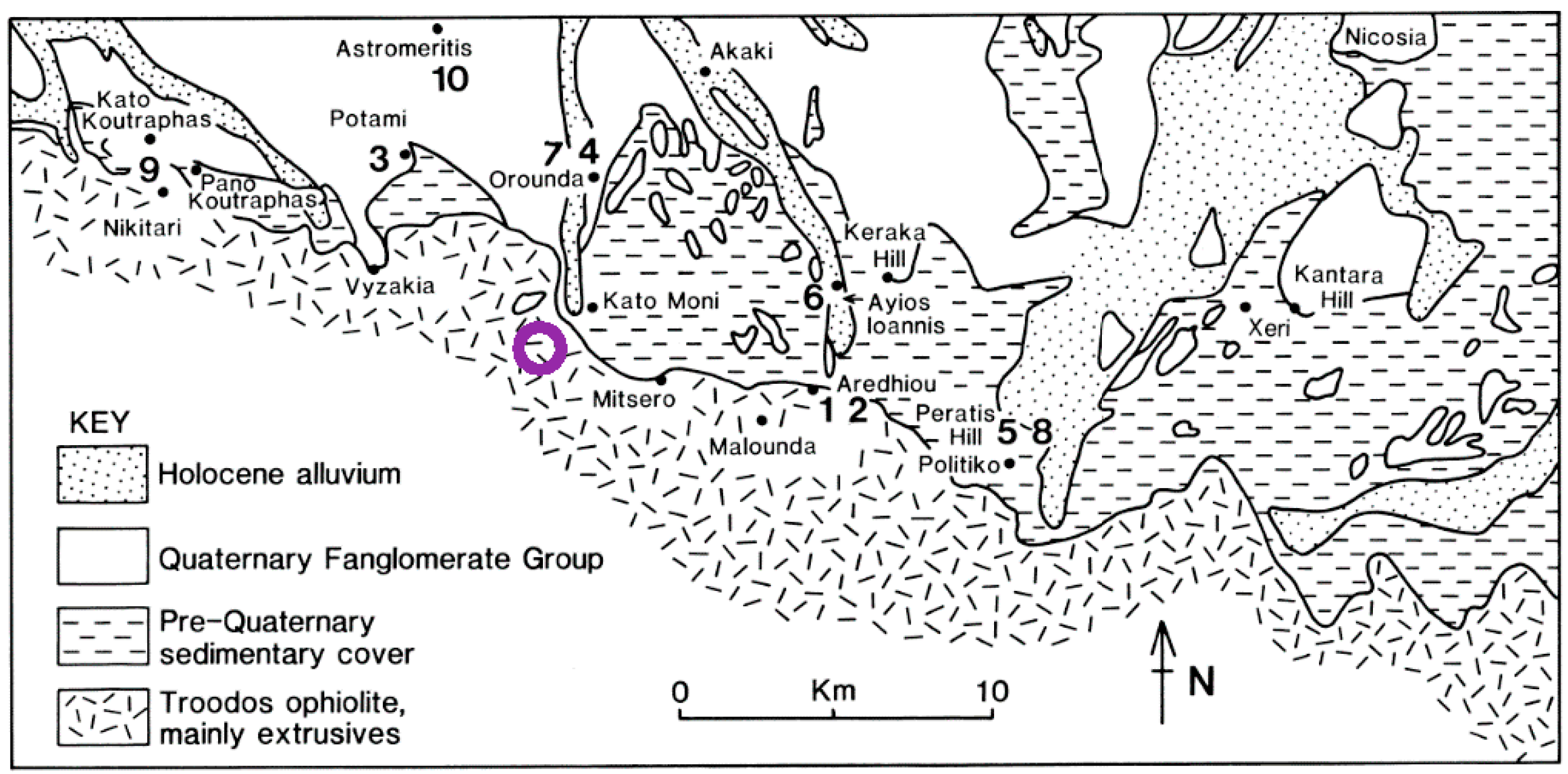
2. Geological Setting of the Study Area
- pre-Quaternary sedimentary cover (Lefkara, Pahkna and Nicosia Formations)
- Quaternary Fanglomarate Group to the north of the KM area
- in the channel area of the Peristerona River, Holocene alluvium deposits
- as the Troodos Massif has been uplifted some 2000 m in ca. 2 Ma, the KM site has probably been exposed for around 0.5 Ma.
- average erosion rates of the limestone overburden could be in the order of 50 m in 1 Ma, suggesting local overburden loss at KM of some 25 m in that time.
- based on the likely main erosion processes onsite, it is suggested that the small valley in which the KM3 borehole was drilled was initiated at least 50 ka ago.
- it is also suggested that the valley sidewalls might retreat by some 6–12 m over a 10 ka time span, indicating that the valley bottom at the KM3 site has been exposed for a few ka at the very most, and possibly much less.
3. Materials and Methods
3.1. Field Sampling
3.2. XRF/XRD
3.2.1. X-ray Fluorescence Analysis
3.2.2. X-ray Diffraction Analysis
- Unoriented and untreated samples (all): To provide an overview of the bulk sample mineralogy, unoriented and untreated samples were measured initially. After air drying at room temperature, all samples were ground in an agate mortar until smooth to the fingertip. The powdered samples were then loaded into the sample holder (aluminium plate depth 1mm diameter 25 mm).
- Oriented and untreated samples (clay): In order to identify the clay mineral composition in detail, oriented and untreated samples were measured. The above powdered samples were diluted with distilled water in a 300 mL beaker and, after standing for 4 h, the upper 5 cm (which is presumed to contain clay particles of less than 2 μm) was collected, centrifuged (1900 rpm, 10 min) and the resulting precipitate placed on a glass slide (two per sample) and then air dried.
- Ethylene glycol treated samples (EG): If peaks were identified near 10 Å or 14 Å in the oriented and untreated sample, the oriented samples were treated with ethylene glycol to determine if they represented smectite or chlorite. One set of oriented and untreated samples had a drop of ethylene glycol added and allowed to stand for about 5 min.
- Hydrochloric acid treated samples (HCl): If peaks were identified near 7 Å or 10 Å in the oriented and untreated samples, the oriented samples were treated with hydrochloric acid to determine if they represented kaolinite or chlorite. The above powdered samples were added to HCl (10 mL of 6 N) in a test tube and placed in a hot water bath for 120 min. Thereafter, they were centrifuged (1900 rpm, 5 min), the supernatant discarded and resaturated with distilled water. This was repeated five times and the final precipitate was then air dried on a slide glass.
3.3. Optical Petrographic Examination
3.4. Bentonite Physical Properties
- Natural Moisture Content: All natural moisture content tests, in accordance with [34], were determined for all samples tested for Unconfined Compressive Strength, as well as for samples tested for Atterberg Limits.
- Specific Gravity/Particle Density: This test was carried out in accordance with [35].
- Bulk and Dry Density: These tests were carried out in accordance with [36].
- Atterberg Limits: Testing was carried out in accordance with the requirements of [37]. The Cone penetration apparatus (method 2B) was adopted for the liquid limit tests. The results are presented graphically on individual test sheets and on the Casagrande Plasticity Classification Chart. All samples tested for Atterberg Limits were also tested for linear shrinkage.
- Unconfined Compression Tests: Eighteen Unconfined Compression tests were carried out on cored samples on the ELE-MULTIPLEX 50 triaxial machine. The treatment and testing of samples was in accordance with [38]. The orientation of the samples when placed in the testing machine was as in situ and the rate of strain applied was 1.00 mm·min−1.
- Triaxial Strength Tests: The Unconsolidated Undrained Triaxial Tests (UU) were carried out at various cell pressures in accordance with the requirements of [39] in order to obtain values of the peak strength, cohesion and angle of shearing resistance (total values).
- Swelling Pressure: The testing was performed in accordance with the procedure given in [40]. They are presented graphically with the cumulative weight vs. square root of time.
3.5. Cation Exchange Capacity (CEC) and Exchangeable Cation Composition (EC) Analysis
3.5.1. Ba—Mg Compulsive Exchange Method for CEC
3.5.2. EC Analysis
3.6. Stable Isotopes
3.7. Natural Decay Series (NDS)
3.7.1. Alpha Spectroscopy
3.7.2. Gamma Spectroscopy
3.8. Smectite Content Estimation Utilising the Methylene Blue Method
4. Results
4.1. XRD/XRF
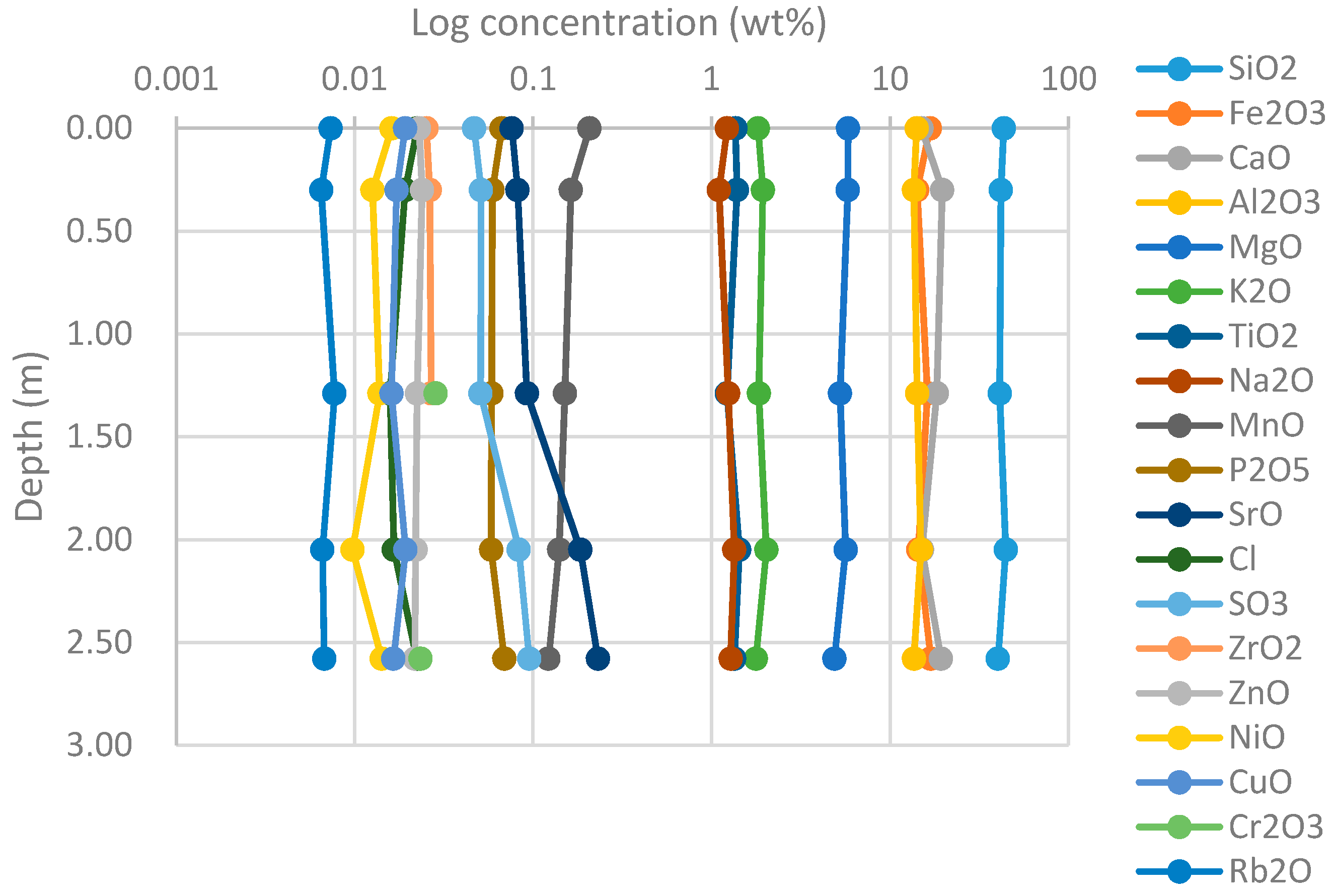
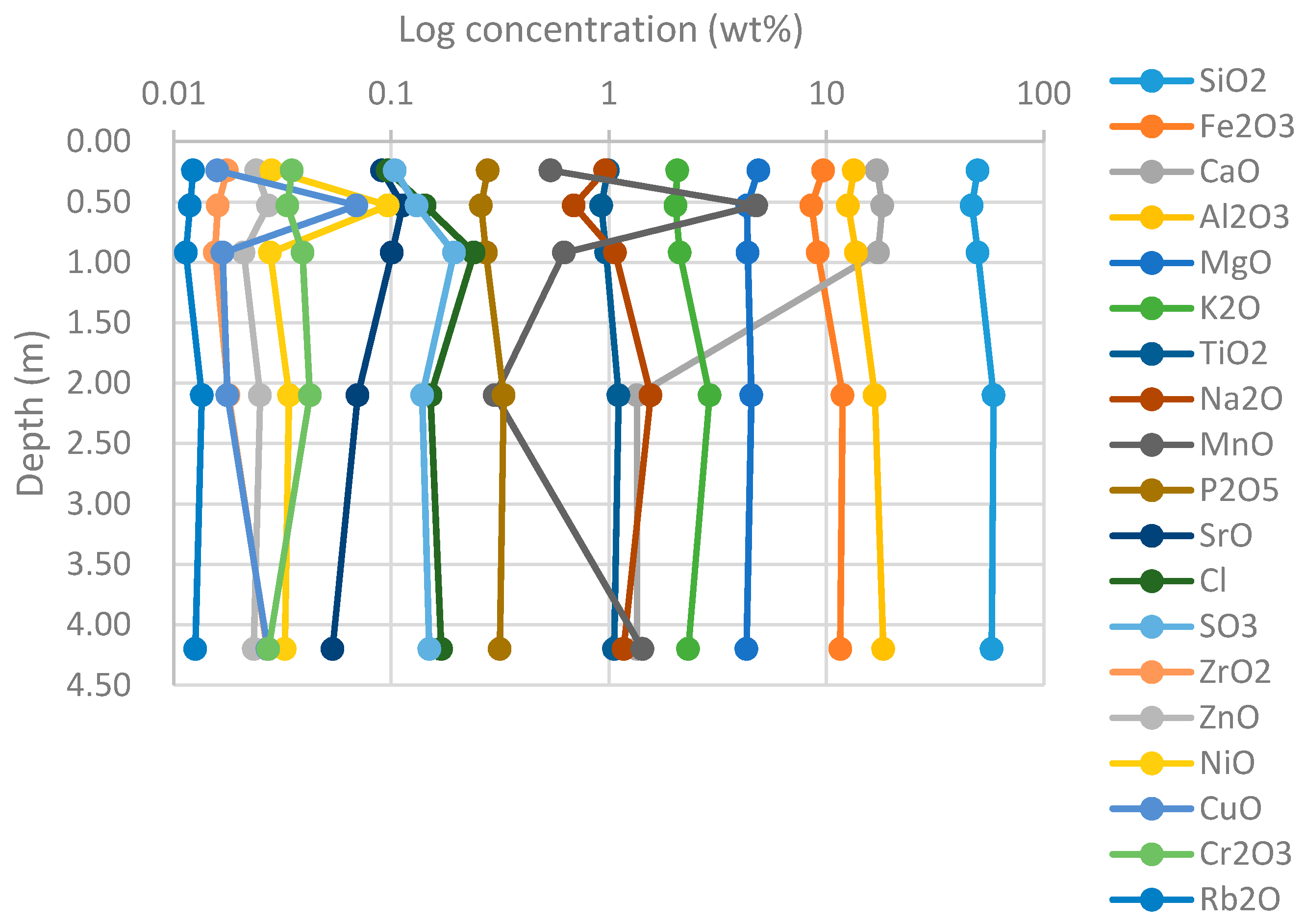
4.1.1. XRF
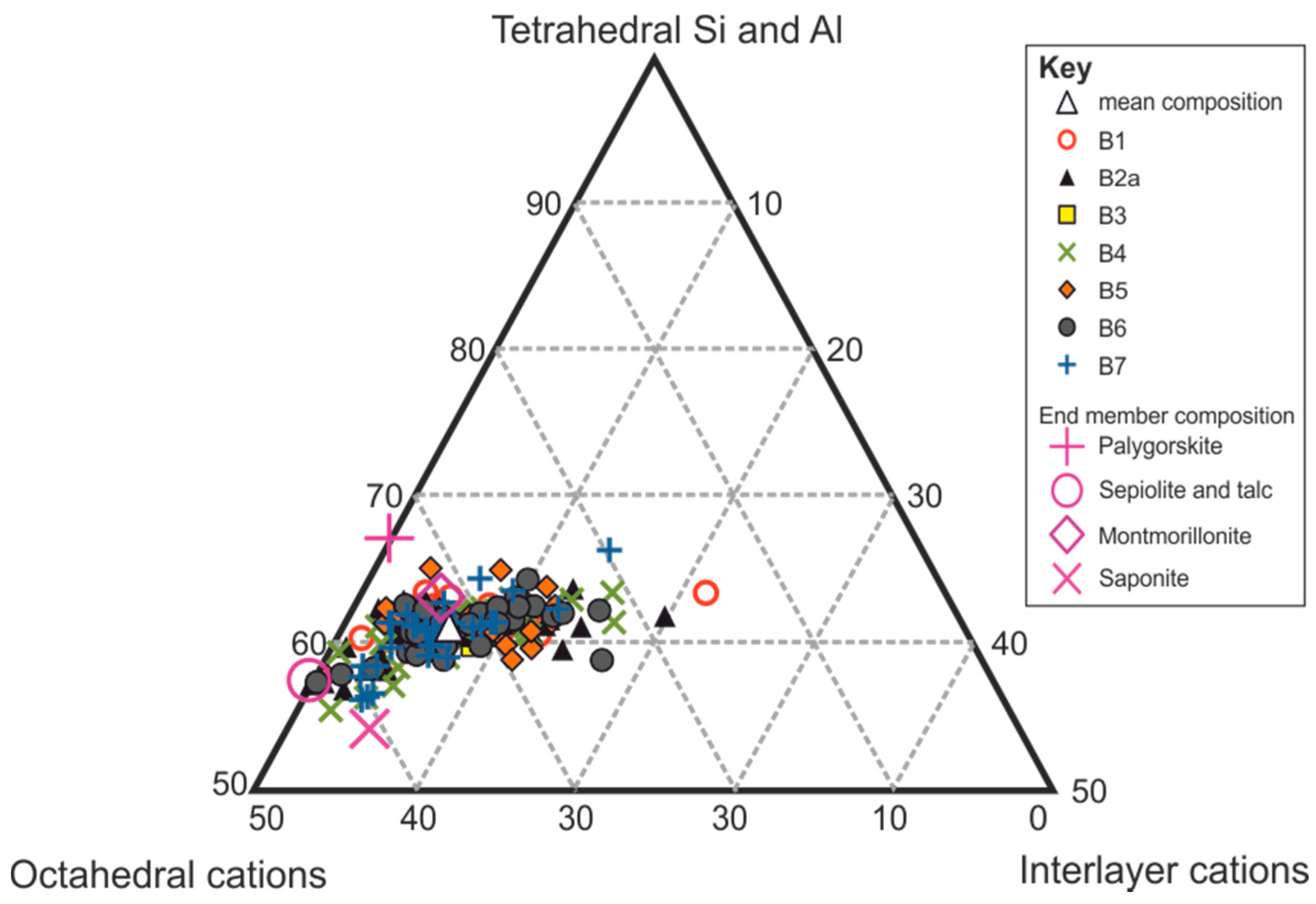
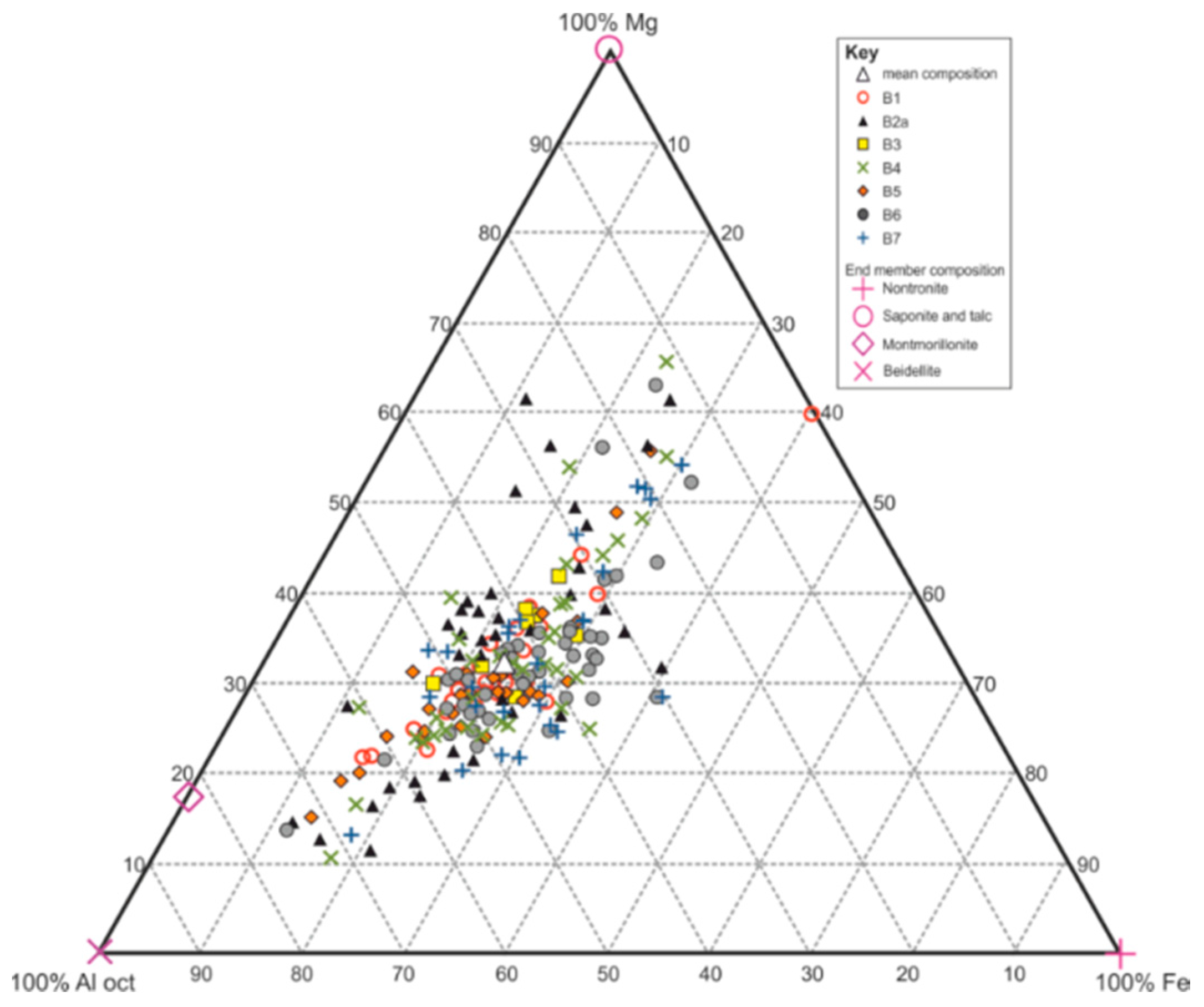
4.1.2. XRD
4.2. Optical Petrographic Examination
4.2.1. Petrography of Boreholes KM1, KM2 and KM3
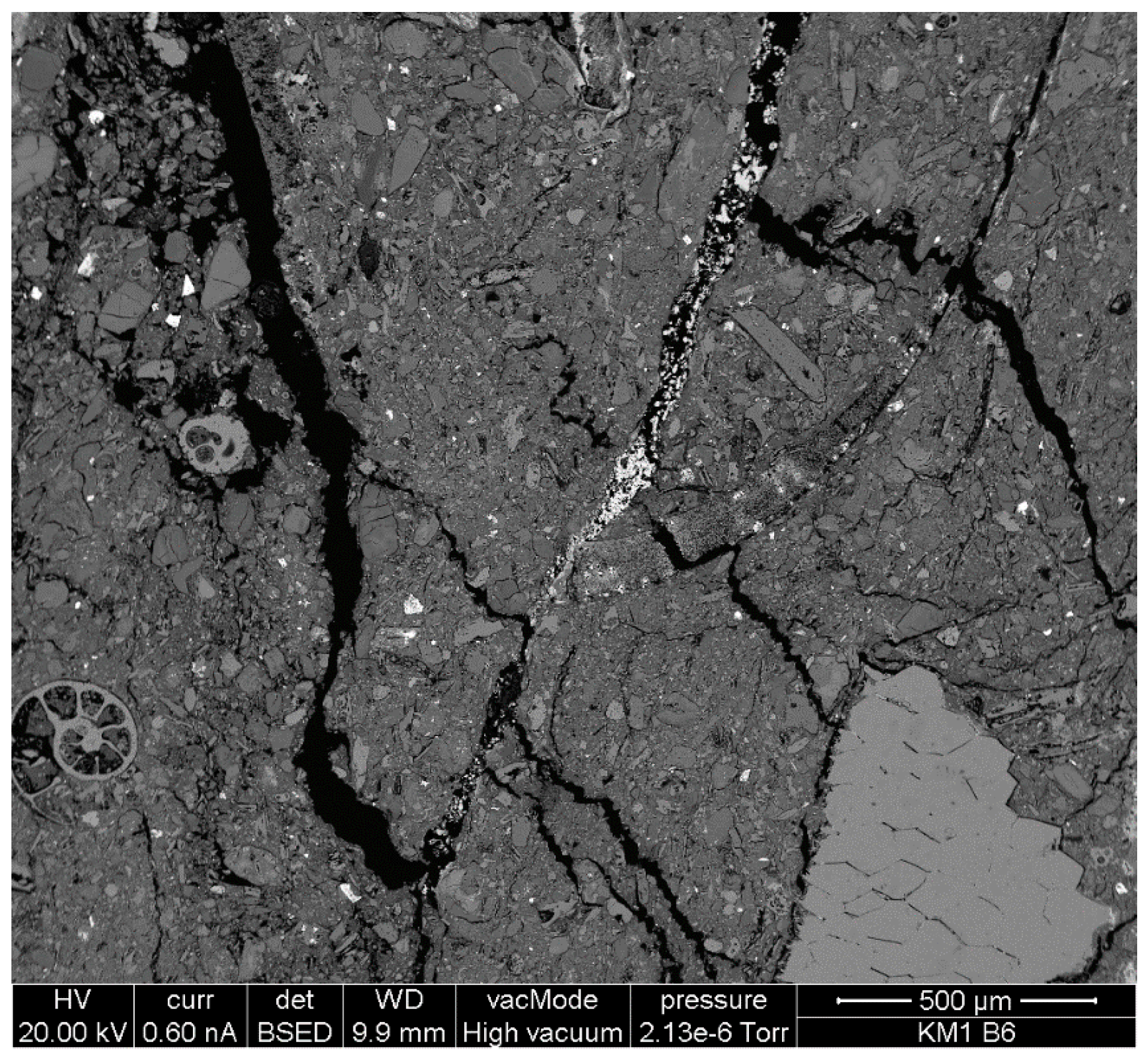
4.2.2. Bentonite Texture
- if the sampling induced “drying”, pull-apart features are ignored, the pattern of faulting indicates that there has been some lateral shear along the bedding planes.
- in most cases, the sense of shear is unclear, so it is not possible to define if the shear is an accommodation of compressive stress by movement on foliation planes—or simply a response to gravitationally-induced movement towards the local valleys around the Kato Moni quarry.
- more importantly, there is evidence of some multi-directional shear in the bentonite. The network conjugate, steeply-inclined, micro-faults that tail-off into shear fabrics suggest movement in at least two directions—i.e., movement unlikely to be gravitationally induced.
- as in boreholes KM1 and KM2, there is evidence of multi-directional shear in the bentonite, only it is more clear in KM3. The S- and C-type shear fabrics present here clearly indicate movement in at least two directions, with the shear zone representing an accommodation of compressive stress by movement on foliation planes (cf. [50]).
- the “chicken wire” network has already been proposed as an analogue of industrial bentonite pellets [49] due to similarities in form between lapilli tuff sourced bentonite and the industrial bentonites. That the shear plane networks are of a different form to those propagated in the more homogenous bentonite of boreholes KM1 and KM2 suggests that these features would be worth further study to assess if the fundamental shear process is different in bentonite pellets. For example, is the load of the overlying limestone being dissipated in a different form due to the pellet structure? That the S- and C-type shear fabrics “reappear” in borehole KM3 at depth when the bentonite fabric is once more homogenous suggests this to be the case.
4.3. Bentonite Physical Properties
- Low hydraulic conductivity
- Sufficient swelling pressure
- Workability
- Sufficient density
- Sufficient thermal conductivity
4.4. CEC and Methyl Blue Adsorption
4.4.1. CEC
4.4.2. Methyl Blue Adsorption
4.5. Stable Isotopes
- Normal marine seawater and limestone: δ13C PDB ~0‰ ± 2‰.
- Marine shells: δ13C PDB typically 0‰ ± 2‰.
- Bacterial sulphate-reduction and oxidadtion of sedimentary organic matter: δ13C PDB typically between −8‰ and −32‰.
- Methane oxidation: δ13C PDB typically less than −35‰.
- Fermentation: δ13C PDB around +5‰ to +10‰.
- Soil carbonates δ13C PDB are around −8‰ to −12‰.
- Meteoric cements δ13C PDB are around: −4‰ to −15‰.
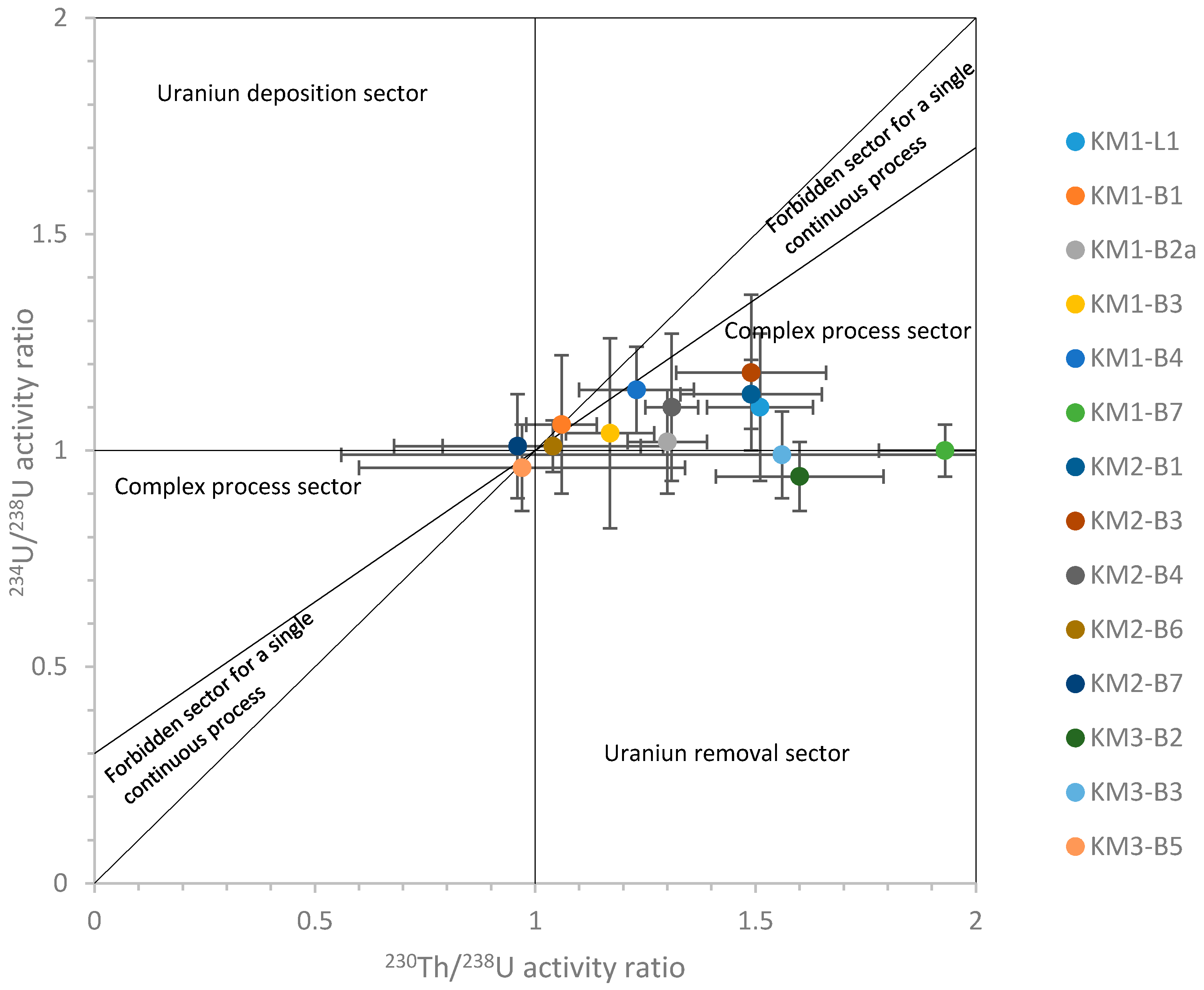
4.6. Natural Decay Series (NDS)
5. Discussion
5.1. Repository Relevance
5.1.1. Site
- despite significant coupled uplift/erosion processes on the north side of the Troodos Massif producing massive sedimentary bodies and deep river valleys, the KM quarry ridge has likely been “protected” throughout much of this period (ca. 0.5 Ma) and has avoided catastrophic change.
- although the limestone overburden is an aquifer regionally, the isolated nature of the ridge suggests it has been uncoupled from the regional flow for some time, so minimising recent physico-chemical disturbance to the bentonite (something confirmed by the petrographic, stable isotope and NDS analyses).
- overall the climatic system in the area would tend to suggest that the site has been historically relatively dry, once again protecting the bentonite from significant external perturbations.
5.1.2. Relevance to Expected Repository Behaviour
- many of the reactions between the limestone and the bentonite (e.g., diffusion of Ca into the clay from the limestone and pillow lavas) would also be expected between the concrete waste packages and waste forms and the bentonite in a repository
- Further, the petrographic data indicate there has been little or no reaction along the older fractures which are present in the bentonite, meaning the site is repository representative insofar that solute transport would appear to be dominated by diffusion
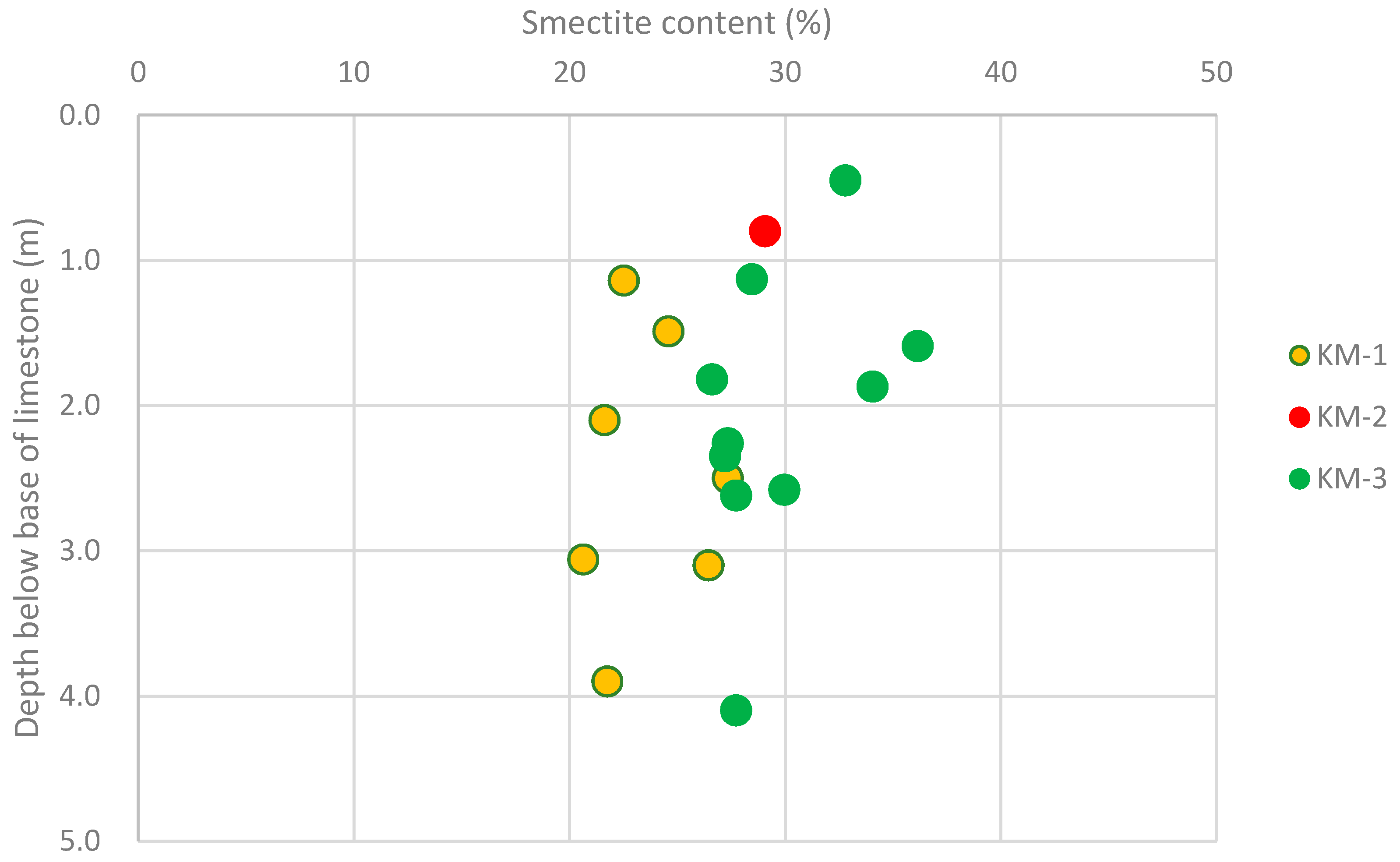
- The smectite content is particularly applicable to repository designs utilising lower quality bentonite or bentonite sand mixtures.
5.2. Bentonite Stability at Kato Moni
5.3. Bentonite Behaviour
- mechanical integrity and confinement of radioactivity in case of a drop accident during the interim storage phase
- degradation of concrete due to carbonation or ground water access
- confinement of radioactivity in the post closure phase
- performance in case of gas generation inside containers
- available experience with container type and materials
- costs
- mechanical strength to:
- -
- withstand stacking forces
- -
- resist damage due to pressurisation by internally generated gases
- -
- ensure that the specified impact accident performance can be achieved
- -
- withstand other loads that may occur during the long-term management of the waste package, as required by the generic Environmental Safety Case
- radiation shielding to ensure that the external dose rate is minimised and that the limits specified for transport are not exceeded
- thermal properties to ensure that the required fire accident performance and other requirements for the thermal performance of the waste package will be achieved
- resistance to degradation to ensure the overall integrity of the waste container is maintained for an adequate period.
6. Conclusions
- swelling pressures/density/Pl, etc., from a range of natural bentonites should be examined to assess the true impact of these parameters on bentonite shear for both L/ILW and SF repository designs. If full saturation cannot be automatically assumed, then models of bentonite shear such as [68] will need to be modified and the potential impact on the bentonite performance re-assessed.
- the sedimentary fabric impacts on the deformation and fracturing behaviour of the bentonite as, where banding or lamination is present, the bentonite displays intense micro-fracturing oriented parallel to the lamination. In addition, the shearing tends to be better developed in the slightly coarser material. This has implications for repository performance as, for example, in those concepts where pre-compressed bentonite blocks are foreseen—is there a difference in the fabric when uniaxially or triaxially compressed materials are employed?
- similarly with the disseminated shear in the parts of core KM3 which contain the pelletised bentonite, what does this imply for those design concepts which utilise bentonite pellets around waste packages?
- all three cores display shearing throughout, but it would be worth assessing if the smectite content has influenced the degree of shearing to any extent. As higher smectite content generally means higher plasticity, can any difference be seen between the lowest smectite containing samples in core KM1 (20.6% smectite) and the highest in core KM3 (36.2%)?
- that shear planes have been found throughout the natural bentonites at KM indicates that the basic conceptual model is correct, but without any sense of direction of these shears, these observations are of only limited value. As such, it is recommended that additional samples be collected at the KM site with a directional coring tool which will allow the sense of movement of the shears to be ascertained.
- it would be also advisable to drill deeper samples, down to the underlying pillow lavas, to obtain a better spatial understanding of the change in the internal structures of the bentonite with depth above the fixed boundary at the base of the bentonite.
- further examination of the “pellet” bentonite in the KM3 borehole would be useful as the inherited structure appears to spread the shear through anastomising shear plane networks, potentially minimising disruption of the bentonite fabric. This could be an alternative bentonite design option, so avoiding a potential redesign of existing waste containers.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFM | triaxial octahedral Al—Fe—Mg cations data plot |
| APFU | atoms per functional unit |
| BSEM | backscattered scanning electron microscopy |
| CEC | cation exchange capacity |
| EBS | engineered barrier system |
| EC | exchangeable cation |
| EDXA | energy-dispersive X-ray microanalysis |
| ESEM | environmental scanning electron microscopy |
| HLW | vitrified high-level waste |
| KM | Kato Moni |
| L/ILW | low- and intermediate-level waste |
| NA | natural analogues |
| NDS | natural decay series |
| PDB | Pee Dee Belemnite |
| PPL | plane-polarized light |
| SF | spent fuel |
| WOB | weight on bit |
| XPL | cross-polarized light |
References
- Alexander, W.R.; McKinley, L.E. Deep Geological Disposal of Radioactive Wastes; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Reijonen, H.M.; Alexander, W.R. Bentonite analogue research related to geological disposal of radioactive waste: Current status and future outlook. Swiss J. Geosci. 2015, 108, 101–110. [Google Scholar] [CrossRef]
- Sidborn, M.; Marsic, N.; Crawford, J.; Joyce, S.; Hartley, L.; Idiart, A.; de Vries, L.M.; Maia, F.; Molinero, J.; Svensson, U.; et al. Potential Alkaline Conditions for Deposition Holes of a Repository in Forsmark as a Consequence of OPC Grouting; SKB R-12-17; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2014. [Google Scholar]
- Alexander, W.R.; Reijonen, H.M.; McKinley, I.G. Natural analogues: studies of geological processes relevant to radioactive waste disposal in deep geological repositories. Swiss J. Geosci. 2015, 108, 75–100. [Google Scholar] [CrossRef]
- Reijonen, H.M.; Alexander, W.R.; Marcos, N.; Lehtinen, A. Complementary considerations in Posiva’s safety case: Support from natural analogues and natural systems. Swiss J. Geosci. 2015, 108, 111–120. [Google Scholar] [CrossRef]
- Åkesson, M.; Kristensson, O.; Börgesson, L.; Dueck, A. THM Modelling of Buffer, Backfill and Other System Components. Critical Processes and Scenarios; SKB TR-10-11; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2010. [Google Scholar]
- Börgesson, L.; Hernelind, J. Canister Displacement in KBS-3V: A Theoretical Study; SKB TR-06-04; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2006. [Google Scholar]
- Svensk Kärnbränslehantering AB (SKB). Barrier Process Report for the Safety Assessment SR-PSU; SKB TR-14-04; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2014. [Google Scholar]
- Alexander, W.R.; Milodowski, A.E. Cyprus Natural Analogue Project (CNAP) Phase IV Final Report; Posiva Working Report WR 2014-02; Posiva: Eurajoki, Finland, 2014. [Google Scholar]
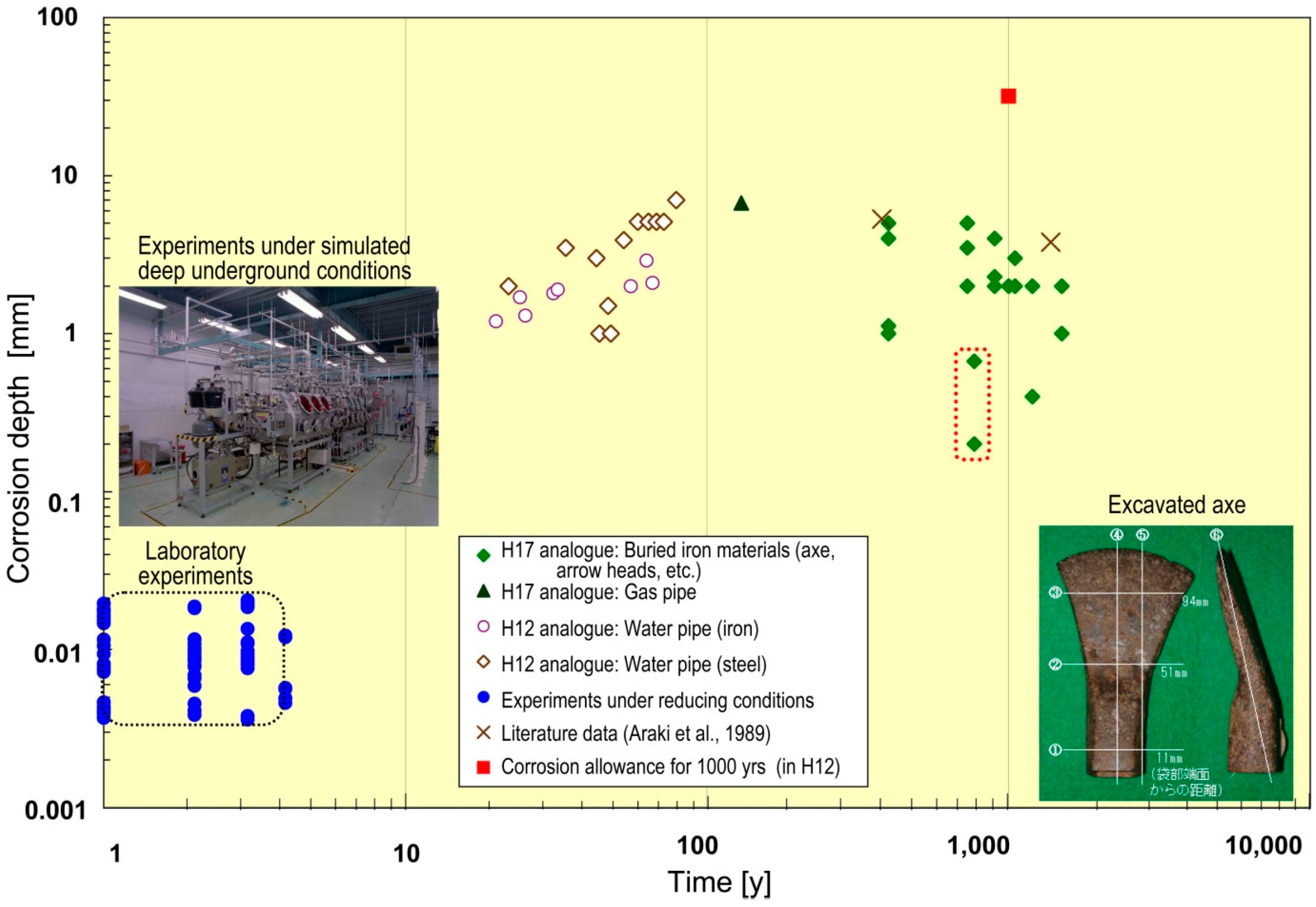
- Takaji, K.; Shigeno, Y.; Takafumi Shimogouchi, T.; Hirai, T.; Shiratake, T. Improvement in Reliability of the Long-Term Mechanical Behaviour of Buffer Material; Japan Atomic Energy Agency (JAEA): Tokai, Japan, 2005. [Google Scholar]
- Petit, J.-C. Reasoning by analogy: Rational foundation of natural analogue studies. Appl. Geochem. 1992, 7, 9–12. [Google Scholar] [CrossRef]
- Alexander, W.R.; McKinley, I.G.; Kawamura, H. The process of defining an optimal natural analogue programme to support national disposal programmes. In Proceedings of the NEA-GRS Workshop on Natural Analogues for Safety Cases of Repositories in Rock Salt, Braunschweig, Germany, 4–6 September 2012; Nuclear Energy Agency/Organisation for Economic Co-operation and Development (NEA/OECD): Paris, France, 2014; pp. 29–43. [Google Scholar]
- McKinley, I.G. Applying natural analogues in predictive performance assessment (1): Principles and requirements; (2): Examples and discussions. In Risk Analysis in Nuclear Waste Management; Klewer Academic Publisher: Dordrecht, The Netherlands, 1989; pp. 357–396. [Google Scholar]
- West, J.M.; Alexander, W.R.; Kaku, K.; McKinley, I.G.; Shimmura, A. Communication of nuclear power concepts to stakeholders. The use of Nature’s own laboratories. In Japan Atomic Energy Research Institute Report, Proceedings of the NUCEF 2001—Scientific Bases for Criticality Safety, Separation Process and Waste Disposal, Tokai, Japan, 31 October to 2 November 2001; Japan Atomic Energy Research (JAEA): Tokai, Japan, 2002; pp. 47–54. [Google Scholar]
- Alexander, W.R.; Arcilla, C.A.; McKinley, I.G.; Kawamura, H.; Takahashi, Y.; Aoki, K.; Miyoshi, S. A new natural analogue study of the interaction of low-alkali cement leachates and the bentonite buffer. Sci. Basis Nucl. Waste Manag. 2008, 56, 493–500. [Google Scholar]
- Alexander, W.R.; Gautschi, A.; Zuidema, P. Thorough testing of performance assessment models: The necessary integration of in situ experiments, natural analogues and laboratory work. Sci. Basis Nucl. Waste Manag. 1998, 21, 1013–1014. [Google Scholar] [CrossRef]
- Miller, W.M.; Alexander, W.R.; Chapman, N.A.; McKinley, I.G.; Smellie, J.A.T. Geological Disposal of Radioactive Wastes and Natural Analogues; Waste Management Series; Pergamon: Amsterdam, The Netherlands, 2000; Volume 2. [Google Scholar]
- Smellie, J.A.T.; MacKenzie, A.B.; Scott, R.D. An analogue validation study of natural radionuclide migration in crystalline rocks using uranium series disequilibrium: preliminary results. Sci. Basis Nucl. Waste Manag. 1985, 9, 91–98. [Google Scholar] [CrossRef]
- Japan Nuclear Cycle Development Institute (JNC). H12: Second Progress Report on R&D for the Geological Disposal of HLW in Japan; Japan Atomic Energy Agency (JAEA): Tokai, Japan, 2000. [Google Scholar]
- Pate, S.M.; McKinley, I.G.; Alexander, W.R. Use of Natural Analogue Test Cases to Evaluate a New Performance Assessment TDB; CEC Report EUR15176EN; European Union (EU): Luxembourg, 1994. [Google Scholar]
- Japan Nuclear Cycle Development Institute (JNC). H17: Development and Management of the Technical Knowledge Base for the Geological Disposal of HLW—Knowledge Management Report; Japan Atomic Energy Agency (JAEA): Tokai, Japan, 2005. [Google Scholar]
- Georgiou, A.; Dörflinger, G. Assessment of Groundwater Resources of Cyprus; Report TCP/CYP/8921; Ministry of Agriculture, Natural Resources and Environment: Lefkosia, Cyprus, 2002. [Google Scholar]
- McCallum, J.E.; Robertson, A.H.F. Sedimentology of two fan-delta systems in the Pliocene-Pleistocene of the Mesaoria Basin, Cyprus. Sediment. Geol. 1994, 98, 215–244. [Google Scholar] [CrossRef]
- McCallum, J.E. Sedimentology and Tectonics of the Plio-Pleistocene of Cyprus. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1989. [Google Scholar]
- Poole, A.J.; Robertson, A.H.F. Pleistocene fanglomerate deposition related to uplift of the Troodos ophiolite, Cyprus. Proc. Ocean Drill. Prog. Sci. Results 1989, 160, 545–566. [Google Scholar]
- Kinnaird, T.C. Tectonic and Sedimentary Response to Oblique and Incipient Continental—Continental Collision the Easternmost Mediterranean (Cyprus). Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2008. [Google Scholar]
- Shuter, E.; Teasdale, W.E. Application of Drilling, Coring and Sampling Techniques to Test Holes and Wells; U.S. Geological Survey, Techniques of Water-Resources Investigations; U.S. Geological Survey (USGS): Renton, WA, USA, 1989.
- Kunimaru, T.; Ota, K.; Alexander, W.R.; Yamamoto, H. Hydrochemistry of the Groundwaters from JAEA’s Horonobe URL: Data Freeze II—Preliminary Evaluation of Boreholes HDB1 to HDB8 and Field Manual for on-Site Practices; JAEA Report 2011-010; Japan Atomic Energy Agency (JAEA): Tokai, Japan, 2011. [Google Scholar]
- Ewy, R.T. Shale/claystone response to air and liquid exposure, and implications for handling, sampling and testing. Int. J. Rock Mech. Min. Sci. 2015, 80, 388–401. [Google Scholar] [CrossRef]
- Japanese Industrial Standards Committee (JISC). Japanese Industrial Standard K 0131—General Rules for X-ray Diffractometric Analysis; JIS: Tokyo, Japan, 1996. [Google Scholar]
- Japanese Geotechnical Society (JGS). Japanese Geotechnical Society 0251–2000—Method for Preparing Samples for Identifying Clay Minerals in Soils; JGS: Tokyo, Japan, 2000. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus Analytical Standard CYS CEN ISO/TS 17892-2004/2014/2015; CYS: Lefkosia, Cyprus, 2015. [Google Scholar]
- British Standards Institute (BSI). Tests for Geometrical Properties of Aggregates. Assessment of Fines. Methylene Blue Test; BSI EN 933-9 Part 9; BSI: London, UK, 2010. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus Analytical Standard CYS CEN ISO/TS 17892-1:2014; CYS: Lefkosia, Cyprus, 2014. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus analytical standard CYS CEN ISO/TS 17892-3:2015; CYS: Lefkosia, Cyprus, 2015. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus Analytical Standard CYS CEN ISO/TS 17892-2:2014; CYS: Lefkosia, Cyprus, 2014. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus Analytical Standard CYS CEN ISO/TS 17892-12:2004; CYS: Lefkosia, Cyprus, 2004. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus analytical standard CYS CEN ISO/TS 17892-7:2004; CYS: Lefkosia, Cyprus, 2004. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus analytical standard CYS CEN ISO/TS 17892-8:2004; CYS: Lefkosia, Cyprus, 2004. [Google Scholar]
- Cyprus Organisation for Standardisation (CYS). Cyprus analytical standard CYS CEN ISO/TS 17892-5:2004; CYS: Lefkosia, Cyprus, 2004. [Google Scholar]
- Amman, L.; Bergaya, F.; Lagaly, G. Determination of the cation exchange capacity of clays with copper complexes revisited. Clay Miner. 2005, 40, 441–453. [Google Scholar] [CrossRef]
- Bascomb, C.L. Rapid method for the determination of cation exchange capacity of calcareous and non-calcareous soils. J. Sci. Food Agric. 1964, 15, 821–823. [Google Scholar] [CrossRef]
- Gillespie, M.R.; Kemp, S.J.; Vickers, B.P.; Waters, C.; Gowing, C.J. Cation-Exchange Capacity (CEC) of Selected Lithologies from England, Wales and Scotland; Environment Agency R&D Technical Report; Environment Agency (EA): Bristol, UK, 2001. [Google Scholar]
- MacKenzie, A.B.; Scott, R.D.; Linsalata, P.; Miekeley, N.; de Jesus, H.C. Inter-laboratory comparison of analytical methods for the determination of natural series uranium and thorium isotopes in rock samples. J. Radioanal. Nucl. Chem. 1994, 182, 21–34. [Google Scholar] [CrossRef]
- Cyprus Organisation for Standardisation (CYS). Cyprus Analytical Standard CYS EN 933-9 Part 9; CYS: Lefkosia, Cyprus, 1998. [Google Scholar]
- Ota, K.; Alexander, W.R. Development and Testing of Radionuclide Transport Models for Fractured Crystalline Rock—An Overview of the Nagra-JNC Radionuclide Retardation Programme; JNC Technical Review No. 11; Japan Atomic Energy Agency (JAEA): Tokai, Japan, 2001. [Google Scholar]
- Milodowski, A.E.; Basahm, I.R.; Hyslop, E.K.; Pearce, J.M. The Uranium Source-Term Mineralogy and Geochemistry at the Broubster Natural Analogue Site, Caithness; British Geological Survey Technical Report; British Geological Survey: Keyworth, UK, 1989. [Google Scholar]
- Phillips, E.R.; Everest, J.; Reeves, J. Micromorphological evidence for subglacial multiphase sedimentation and deformation during over-pressurized fluid flow associated with hydrofracturing. Boreas 2013, 42, 395–427. [Google Scholar] [CrossRef]
- Alexander, W.R. The use of natural, industrial and archaeological analogues in support of the borehole sealing project (Phase 2—Year 2015–2016). In Sealing Site Investigation Boreholes: Phase II Annual Report for 2015/2016; Jefferies, N.L., Joyce, S., Tsitsopoulos, V., Alexander, W.R., Börgesson, L., Karnland, O., Sanden, T., Gaus, I., Vomvoris, S., Metcalfe, R., et al., Eds.; RWM Ltd. Report RWM/03/XYZ; RWM Ltd.: Harwell, UK, 2016; in press. [Google Scholar]
- Bossart, P.; Mazurek, M. Grimsel Test Site: Structural Geology and Water Flow-Paths in the Migration Shear-Zone; Nagra Technical Report NTB91-12; Nagra: Wettingen, Switzerland, 1991. [Google Scholar]
- Ahonen, L.; Korkeakoski, P.; Tiljander, M.; Kivikoski, H.; Laaksonen, R. Quality Assurance of the Bentonite Material; Posiva Working Report WR2008-33; Posiva: Eurajoki, Finland, 2008. [Google Scholar]
- Posiva. Safety Case for the Disposal of Spent Nuclear Fuel at Olkiluoto—Description of the Disposal System 2012; Posiva Report 2012-05; Posiva: Eurajoki, Finland, 2012. [Google Scholar]
- Kiviranta, L.; Kumpulainen, S. Quality Control and Characterization of Bentonite Materials; Posiva Working Report WR 2011-84; Posiva: Eurajoki, Finland, 2011. [Google Scholar]
- Naoi, Y.; Komine, H.; Yasuhara, K.; Murakami, S.; Momose, K.; Sakagami, T. Influence of Seawater on Swelling Characteristics of Bentonite Buffer Material. J. Jpn. Soc. Civ. Eng. 2005, 785, 39–49, (in Japanese). [Google Scholar] [CrossRef]
- Central Research Institute of the Electric Power Industry, CRIEPI Report N50209, 2007. Available online: http://criepi.denken.or.jp/jp/kenkikaku/report/leaflet/N05029.pdf (accessed on 9 January 2017).
- Wilson, J.; Savage, D.; Bond, A.; Watson, S.; Pusch, R.; Bennett, D. Bentonite: A Review of Key Properties, Processes and Issues for Consideration in the UK Context; Quintessa Report; Quintessa Ltd.: Henley-on-Thames, UK, 2011. [Google Scholar]
- Dunn, A.L. Paleoenvironmental interpretation of the messinian Kalavasos Formation in Gypsum Canyon, Kato Moni, Cyprus. In Proceedings of the 9th Keck Research Symposium in Geology, Willamstown, MA, USA, 9–11 April 1996; pp. 213–216.
- Hudson, J.D. Stable isotopes and limestone lithification. J. Geol. Soc. Lond. 1977, 133, 637–660. [Google Scholar] [CrossRef]
- Scott, R.D.; MacKenzie, A.B.; Alexander, W.R. The interpretation of 238U-234U-230Th-226Ra disequilibria produced by rock-water interaction. J. Geochem. Explor. 1992, 46, 323–343. [Google Scholar] [CrossRef]
- Thiel, K.; Vorwerk, R.; Saager, R.S.; Stupp, H.D. 235U fission tracks and 238U-series disequilibria as a means to study Recent mobilization of uranium in Archaean pyritic conglomerates. Earth Planet. Sci. Lett. 1983, 65, 249–262. [Google Scholar] [CrossRef]
- Scott, E.M.; Cook, G.T.; Naysmith, P. Error and uncertainty in radiocarbon measurements. Radiocarbon 2007, 49, 427–440. [Google Scholar] [CrossRef]
- Svensk Kärnbränslehantering AB (SKB). Initial State Report for the Safety Assessment SR-PSU, Updated 2015-10; SKB TR-14-02; SKB: Stockholm, Sweden, 2015. [Google Scholar]
- Radioactive Waste Management (RWM) Ltd. Geological Disposal. Guidance on the Application of the Waste Package: Specifications for Shielded Waste Packages; RWM Report WPS/702/01; RWM Ltd.: Harwell, UK, 2014. [Google Scholar]
- Japan Atomic Energy Research (JAEA). Second Progress Report on R&D for TRU Waste Disposal in Japan; JAEA Review; JAEA: Tokai, Japan, 2007. [Google Scholar]
- Bain, J.A. A plasticity chart as an aid to the identification and assessment of industrial bentonites. Clay Miner. 1971, 9, 1–17. [Google Scholar] [CrossRef]
- Voight, B. Correlation between Atterberg plasticity limits and residual strength of natural soils. Geotechnique 1973, 23, 265–267. [Google Scholar] [CrossRef]
- Olson, R.E. Shearing strength of kaolinite, illite and montmorillonite. J. Geotech. Div. 1974, 1215–1299. [Google Scholar] [CrossRef]
- Börgesson, L.; Dueck, A.; Johannesson, L.-E. Material Model for Shear of the Buffer—Evaluation of Laboratory Test Results; SKB TR-06-04; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2010. [Google Scholar]
- Dueck, A.; Börgesson, L.; Johannesson, L.-E. Stress-Strain Relation of Bentonite at Undrained Shear—Laboratory Tests to Investigate the Influence of Material Composition and Test Technique; SKB TR-10-31; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 2010. [Google Scholar]
- Sakamoto, H.; Asano, H.; Tunaboylu, K.; Mayer, G.; Klubertanz, G.; Kobayashi, S.; Komuro, T.; Wagner, E. Concrete containers for long-term storage and final disposal of TRU waste and long-lived ILW. In Proceedings of the Waste Management’03 Conference, Tucson, AZ, USA, 23–27 February 2003; Waste Management Symposia: Tempe, AZ, USA, 2003. [Google Scholar]
- Villar, M.V. (Ed.) FEBEX Project Final Report: Post-Mortem Bentonite Analysis; Publicación Técnica 05-1/2006; Empresa Nacional de Residuos Radiactivos (ENRESA): Madrid, Spain, 2006.

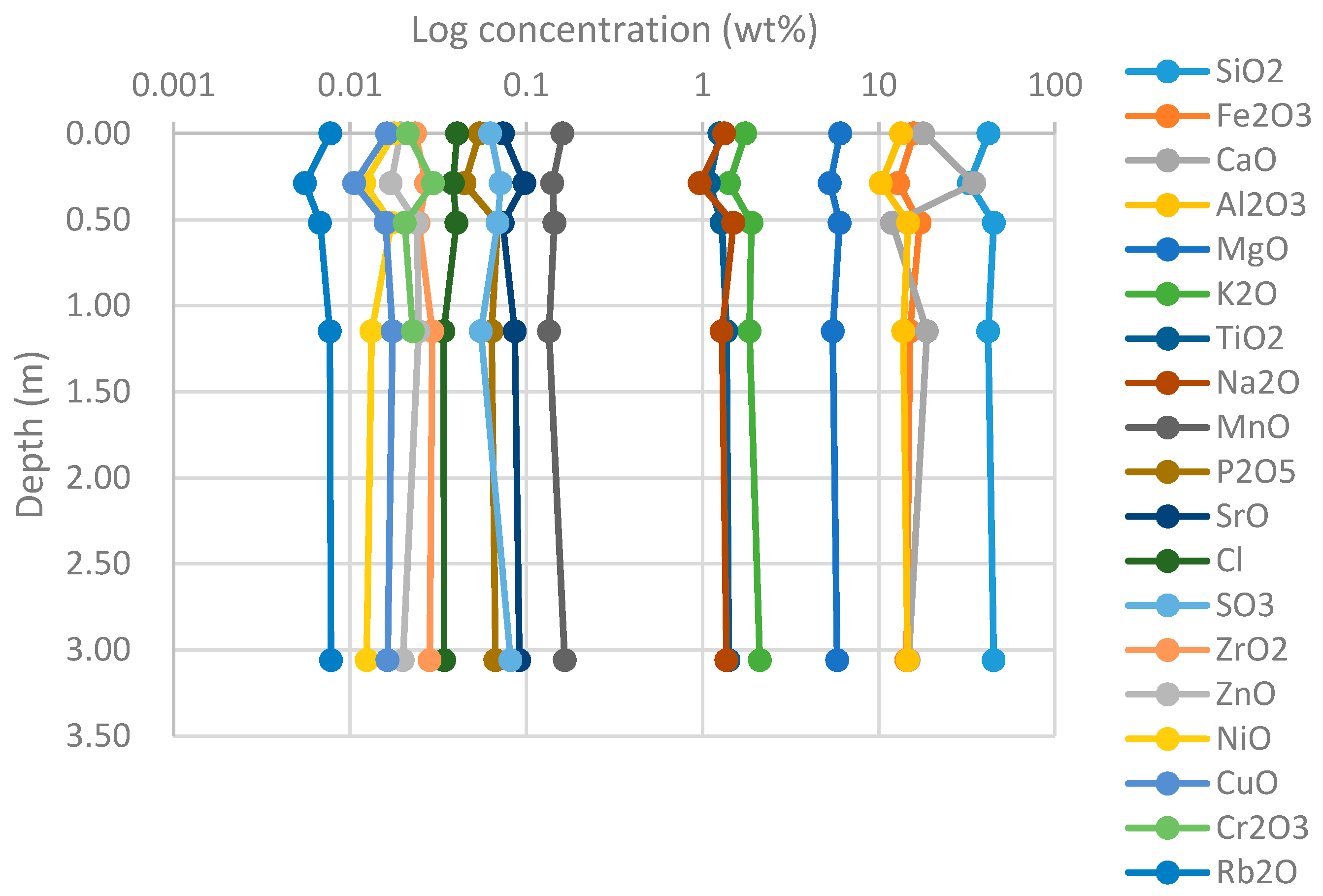


| Borehole ID | Eastings (°) | Northings (°) | Elevation (m.a.s.l.) |
|---|---|---|---|
| KM1 | 0506785 | 3880332 | 439 |
| KM2 | 0506808 | 3880332 | 440 |
| KM3a | 0506844 | 3880391 | 425 |
| KM3b | 0506844 | 3880391 | 425 |
| Sample | Smec-Tite | Mica | Kaol-Inite | Laumontite | Quartz | Plagioclase | Aragonite | Ankerite | Calcite | Pyrite | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KM1 | B1sub | △ | ▪ | △ | ◎ | △ | |||||
| KM1 | B2sub | ▪ | ▪ | ▪ (※1) | ▪ | △ | ◎ | ||||
| KM1 | B3sub | △ | ▪ (※1) | ▪ | △ | △ | △ | ||||
| KM1 | B4sub | △ | ▪ (※1) | ▪ | ▪ | △ | ○ | ▪ (※2) | |||
| KM1 | B7sub | △ | ▪ (※1) | ▪ | ▪ | △ | △ | △ | |||
| KM2 | B1sub | △ | ▪ (※1) | ▪ | ▪ | △ | △ | ||||
| KM2 | B3sub | △ | ▪ (※1) | ▪ | △ | △ | △ | ||||
| KM2 | B4sub | △ | ▪ | ▪ | △ | △ | △ | ▪ (※2) | |||
| KM2 | B6sub | △ | ▪ (※1) | ▪ | ▪ | △ | △ | ▪ | △ | ▪ (※3) | |
| KM2 | B7sub | △ | ▪ | △ | △ | ▪ | △ | ▪ (※3) | |||
| KM3 | B1sub | △ | ▪ | ▪ (※1) | ▪ | △ | ▪ | ▪ | ○ | ||
| KM3 | B2sub | △ | ▪ | ▪ (※1) | ▪ | △ | ▪ | ▪ | ○ | ||
| KM3 | B3sub | ○ | ▪ | ▪ (※1) | ▪ | △ | ▪ | ▪ | △ | ||
| KM3 | B5sub | ○ | ▪ | ▪ | ▪ | △ | △ | ||||
| KM3 | B7sub | ○ | ▪ | ▪ | ▪ | △ | △ | ||||
| Atterberg Limits | UCS | Sp Grav. | Sw Press (kPa) | UU Triaxial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PI (%) | Shrinkage (%) | MC (%) | MC (%) | BD (g/cm3) | DD (g/cm3) | qu (kPa) | Cohesion (kPa) | φο | ||
| 62–132 | 37–103 | 14–29 | 21.4–34.3 | 1.34–1.92 | 1.09–1.58 | 174–581 | 2.499–2.861 | 109.8–367.3 | 25–205 | 9–22 |
| Parameter | Dry Density (kg·m−3) | Water Content (%) | Smectite Content (%) | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index | CEC mEq (100 g−1) | Swelling Pressure (MPa) |
|---|---|---|---|---|---|---|---|---|
| natural Ca-bentonites | ||||||||
| KM1-B6-3 | 1410–1460 | 28.8 | 20.6–27.3 1 | 84 | 25 | 59 | 50.75 2 | 0.20 |
| KM2-B3-2 | 1550 | 23.5 | 29.1 3 | 113 | 20 | 93 | 46.95 | 0.19 |
| KM3a-B5-3 | 1230 | 34.2 | 26.6–36.2 4 | 112 | 32 | 80 | 55.95 5 | 0.34 |
| T5 6 (Parsata) | 1340–1390 | 17–23 | 24–28 | 106–131 | 39–49 | 67–95 | 34–43 | 0.12–0.17 |
| industrial Na-bentonites | ||||||||
| Posiva SF rings 7,8 | 1752 | 17 | >75 | >80 | >60 | |||
| Posiva SF disc blocks 7,8 | 1701 | 17 | >75 | >80 | >60 | |||
| Posiva SF pellets 7,8 | 919 | 17 | >75 | >80 | >60 | |||
| Posiva buffer Ca-bentonite 9 | 13–15 | 65-≥75 | 60-≥80 | 50 | 10-≥30 | 50-≥60 | ≥2 | |
| Posiva buffer Na-bentonite 9 | 13–15 | 65-≥75 | 200-≥250 | 50 | 150-≥200 | 60-≥70 | ≥2 | |
| Kunigel-V1 10 | 2790 | 48 | 473.9 | 26.61 | 447.3 | 73.2 | ||
| MX-80 10,11 | 2880 | 80 | 437.3 | 38.0 | 399.3 | 110.4 | 7.3 12 | |
| NEO-KUNIBOND 10,11 industrial Na-bentonite | 2680 | 76 | 607.5 | 50.69 | 556.8 | 103.5 | ||
| KUNIBOND 1,10 industrial Ca-bentonite | 2.71 | 84 | 128.7 | 38.4 | 90.3 | 79.5 |
| Bentonite | Kato Moni KM3a | Parsata | MX-80 | MX-80 | GEKO/QI | MX-80 | GEKO/QI |
|---|---|---|---|---|---|---|---|
| % clay | 26–33 | 24–50 | 100 | 50 | 30 | 30 | 10 |
| Density (at water saturation) kg·m−3 | 1340–1700 | 1600–1680 | 2000 | 2100 | 2200 | 1950 | 2200 |
| Swelling pressure (MPa in distilled water) | 0.15–0.34 | 0.09–0.17 | 7.3 | 2.0 | 0.9 | 0.2 | 0.1 |
| Sample | Depth (m) | CEC (meq 100 g−1) | Exchangable Cations (meq 100 g−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Na | K | Sr | Mn | Fe | Total Exchangeable Cations (Σ) | |||
| KM1 | ||||||||||
| KM1 B1 | 8.55 | 50.89 | 291.5 | 29.9 | 10.4 | nd | 0.50 | 0.03 | 0.26 | 332.6 |
| KM1 B2a | 8.77 | 43.60 | 373.4 | 27.4 | 8.1 | nd | 0.50 | 0.03 | 0.23 | 409.6 |
| KM1 B3 | 9.00 | 53.01 | 267.0 | 30.6 | 10.3 | nd | 0.54 | 0.05 | 0.32 | 308.8 |
| KM1 B4 | 9.60 | 53.20 | 303.6 | 34.1 | 10.7 | nd | 0.58 | 0.06 | 0.24 | 349.3 |
| KM1 B7 | 11.55 | 50.75 | 299.4 | 31.5 | 10.8 | nd | 0.68 | 0.07 | 0.23 | 342.8 |
| KM2 | ||||||||||
| KM2 B1 | 13.50 | 53.81 | 336.7 | 33.0 | 9.0 | nd | 0.57 | 0.05 | 0.22 | 379.6 |
| KM2 B2 | 13.55 | 54.02 | 329.4 | 33.6 | 9.7 | nd | 0.57 | 0.08 | 0.21 | 373.5 |
| KM2 B3 | 13.65 | 46.95 | 457.1 | 33.5 | 7.7 | nd | 0.58 | 0.06 | 0.23 | 499.2 |
| KM2 B4 | 14.50 | 44.35 | 439.5 | 28.6 | 7.1 | nd | 0.56 | 0.14 | 0.30 | 476.1 |
| KM2 B7 | 16.08 | 44.35 | 516.0 | 27.8 | 6.9 | nd | 0.66 | 0.08 | 0.25 | 551.7 |
| KM3 | ||||||||||
| KM3 B1 | 0.17 | 52.83 | 265.8 | 22.7 | 17.6 | nd | 0.51 | 0.52 | nd | 307.2 |
| KM3 B2 | 0.54 | 54.23 | 366.9 | 22.3 | 21.7 | nd | 0.65 | 0.71 | nd | 412.2 |
| KM3 B3 | 0.82 | 51.51 | 417.8 | 20.4 | 22.7 | nd | 0.84 | 1.76 | nd | 463.6 |
| KM3 B4 | 1.52 | 55.95 | 8.9 | 25.2 | 33.4 | 3.63 | 0.13 | 0.00 | 0.21 | 71.5 |
| KM3 B7 | 4.20 | 57.53 | 13.1 | 25.3 | 25.7 | 3.45 | 0.18 | 0.00 | nd | 67.7 |
| Sample | δ13C | δ18O |
|---|---|---|
| KM1 L1a | −7.48 | −3.36 |
| KM1 L1a2 (repeat analysis) | −7.71 | −3.90 |
| KM1 L1b | −7.57 | −3.80 |
| KM2 B5a | −6.29 | −4.12 |
| KM2 B5b | −6.76 | −4.47 |
| KM2 B7a | −6.20 | −4.17 |
| KM2 B7b | −6.12 | −4.48 |
| BDH calcium carbonate secondary standard (CCS) | ||
| Measured value | −22.29 | −13.24 |
| Preferred value | −22.30 | −13.35 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, W.R.; Reijonen, H.M.; MacKinnon, G.; Milodowski, A.E.; Pitty, A.F.; Siathas, A. Assessing the Long-Term Behaviour of the Industrial Bentonites Employed in a Repository for Radioactive Wastes by Studying Natural Bentonites in the Field. Geosciences 2017, 7, 5. https://doi.org/10.3390/geosciences7010005
Alexander WR, Reijonen HM, MacKinnon G, Milodowski AE, Pitty AF, Siathas A. Assessing the Long-Term Behaviour of the Industrial Bentonites Employed in a Repository for Radioactive Wastes by Studying Natural Bentonites in the Field. Geosciences. 2017; 7(1):5. https://doi.org/10.3390/geosciences7010005
Chicago/Turabian StyleAlexander, W. Russell, Heini M. Reijonen, Gillian MacKinnon, Antoni E. Milodowski, Alistair F. Pitty, and Andreas Siathas. 2017. "Assessing the Long-Term Behaviour of the Industrial Bentonites Employed in a Repository for Radioactive Wastes by Studying Natural Bentonites in the Field" Geosciences 7, no. 1: 5. https://doi.org/10.3390/geosciences7010005
APA StyleAlexander, W. R., Reijonen, H. M., MacKinnon, G., Milodowski, A. E., Pitty, A. F., & Siathas, A. (2017). Assessing the Long-Term Behaviour of the Industrial Bentonites Employed in a Repository for Radioactive Wastes by Studying Natural Bentonites in the Field. Geosciences, 7(1), 5. https://doi.org/10.3390/geosciences7010005





