Oxygen-Dependent Morphogenesis of Modern Clumped Photosynthetic Mats and Implications for the Archean Stromatolite Record
Abstract
:1. Introduction
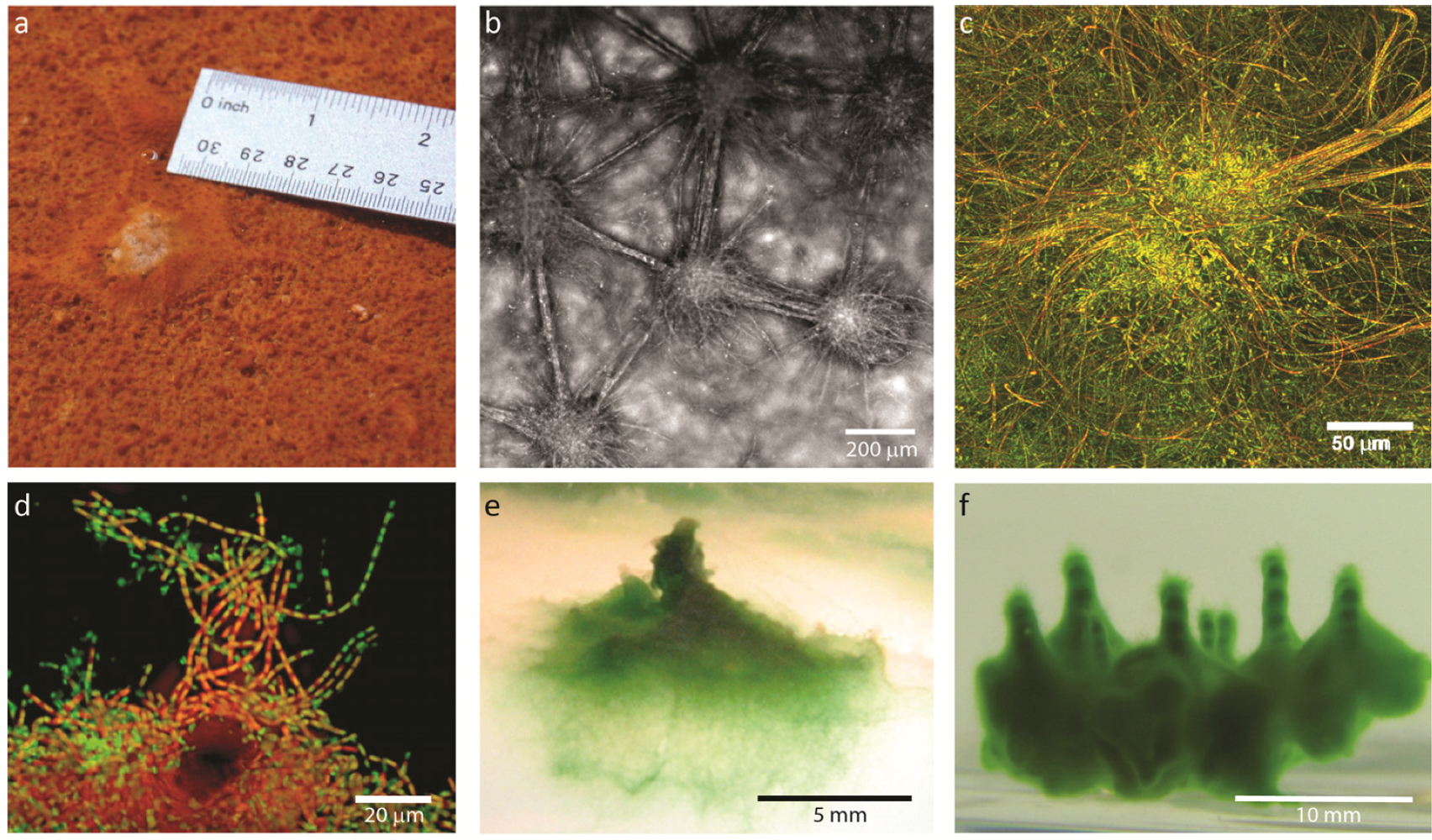
2. Results and Discussion
2.1. Results
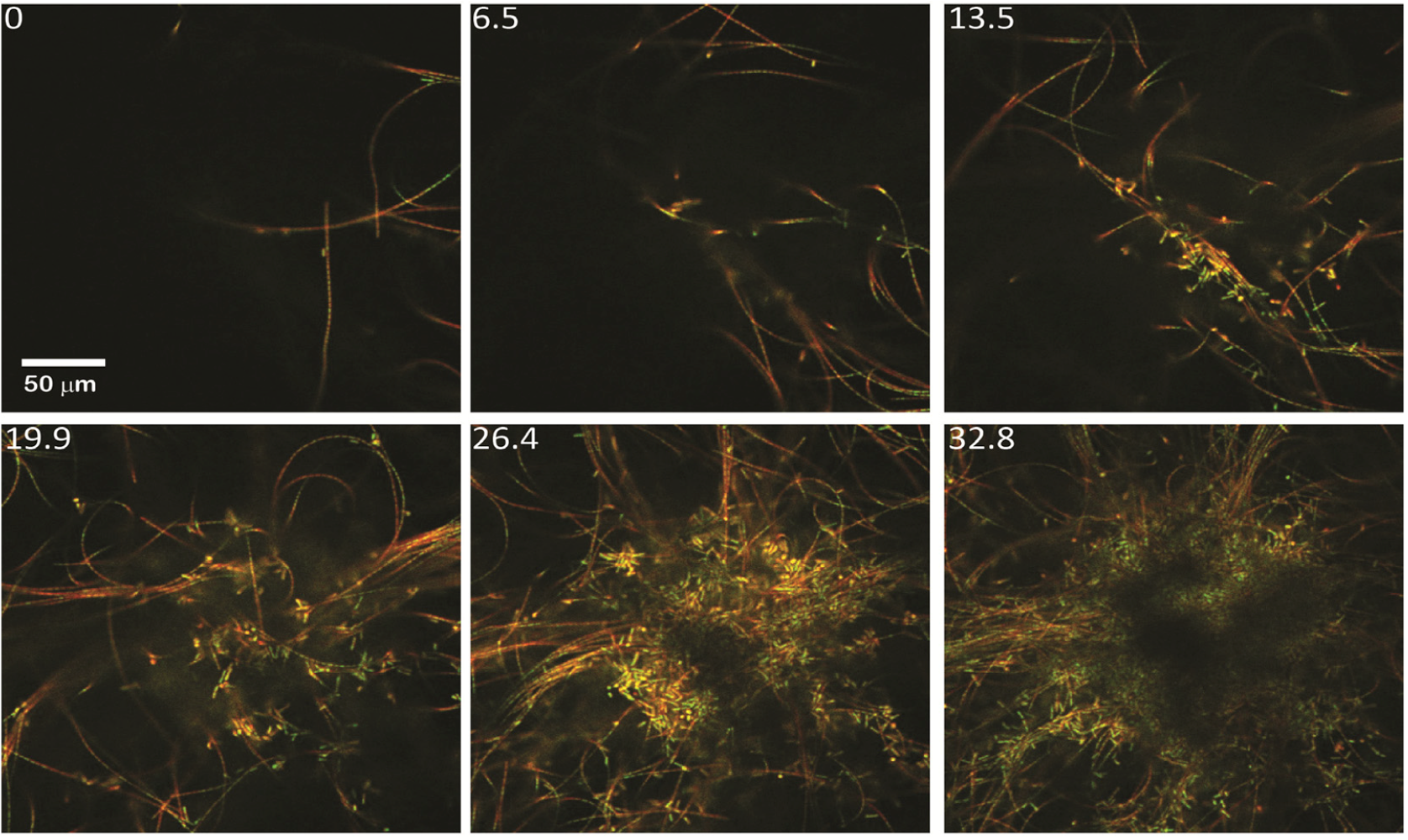
2.1.1. Morphological Stages in the Growth of Cone-forming Microbial Mats
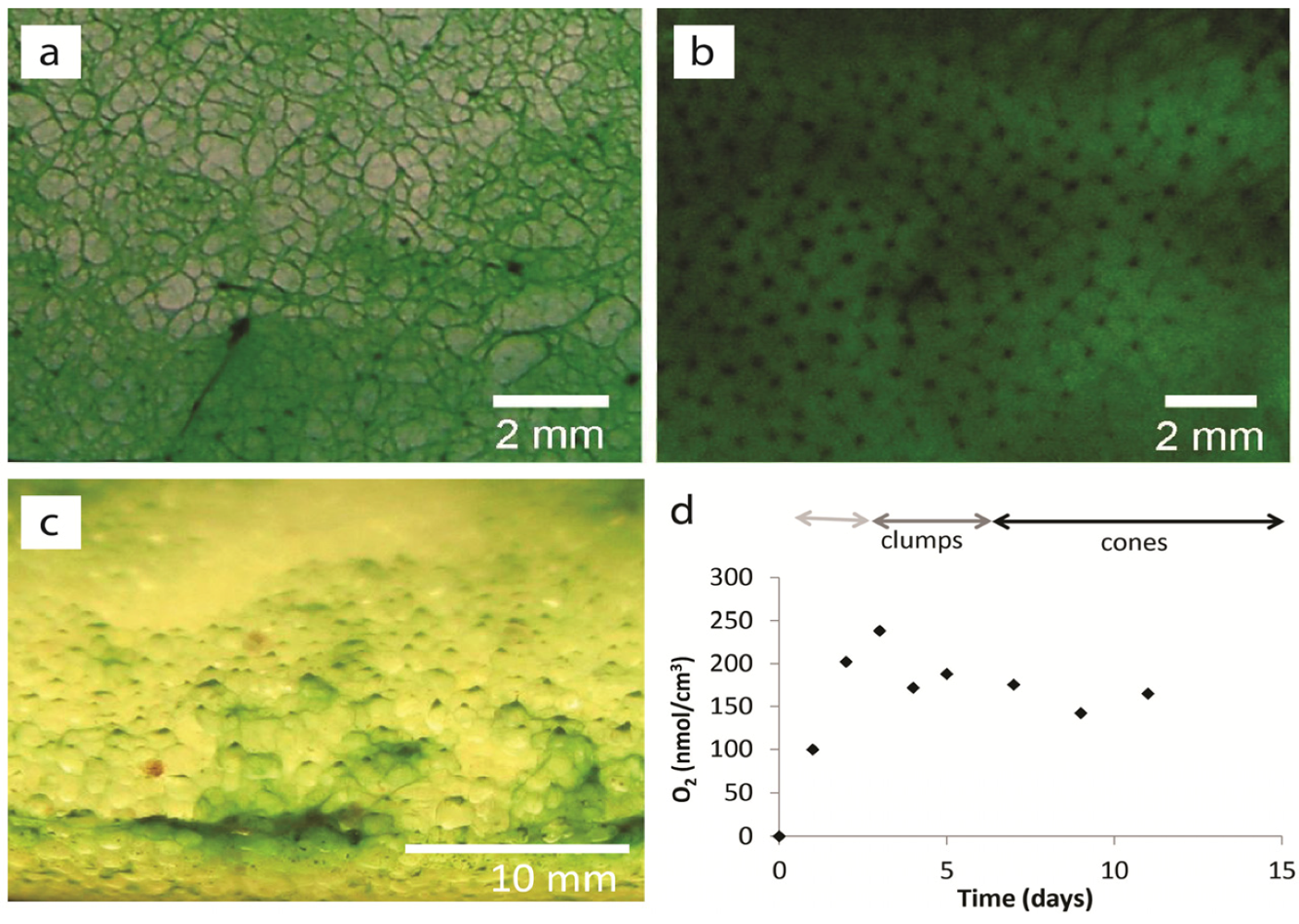
2.1.2. Dispersal of Clumps as a Function of O2 and DIC
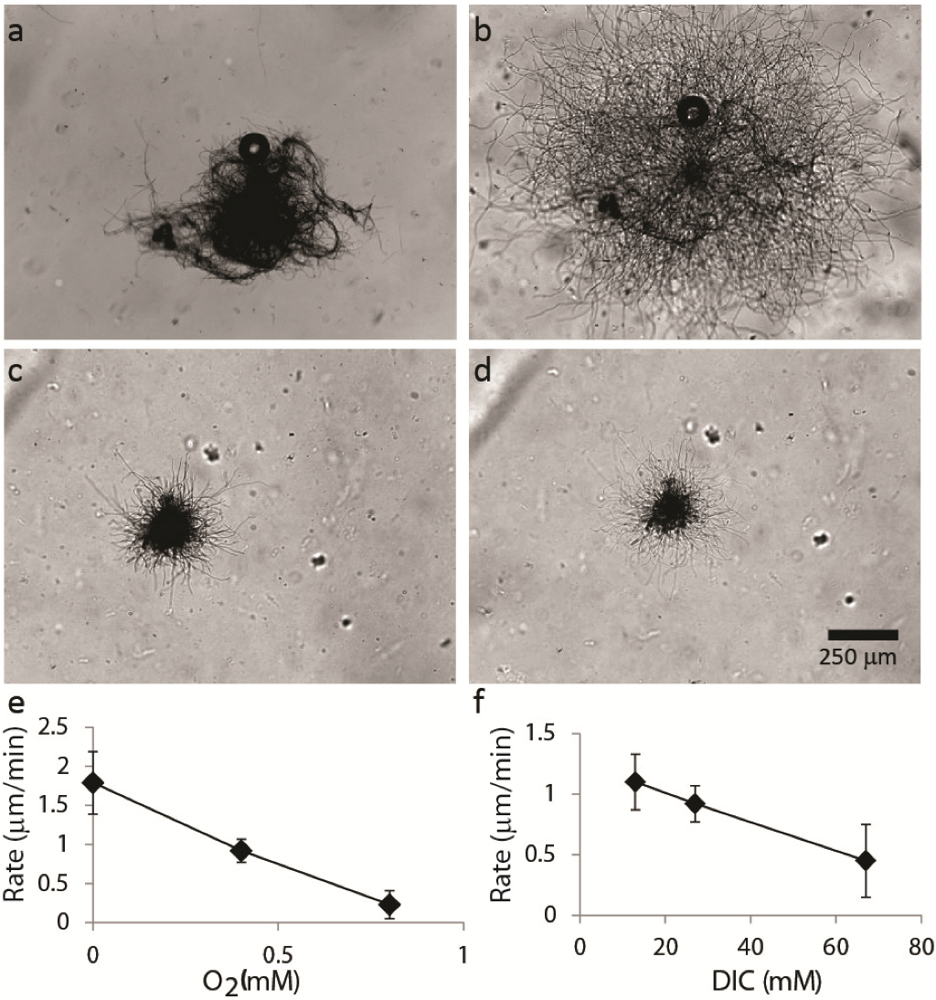
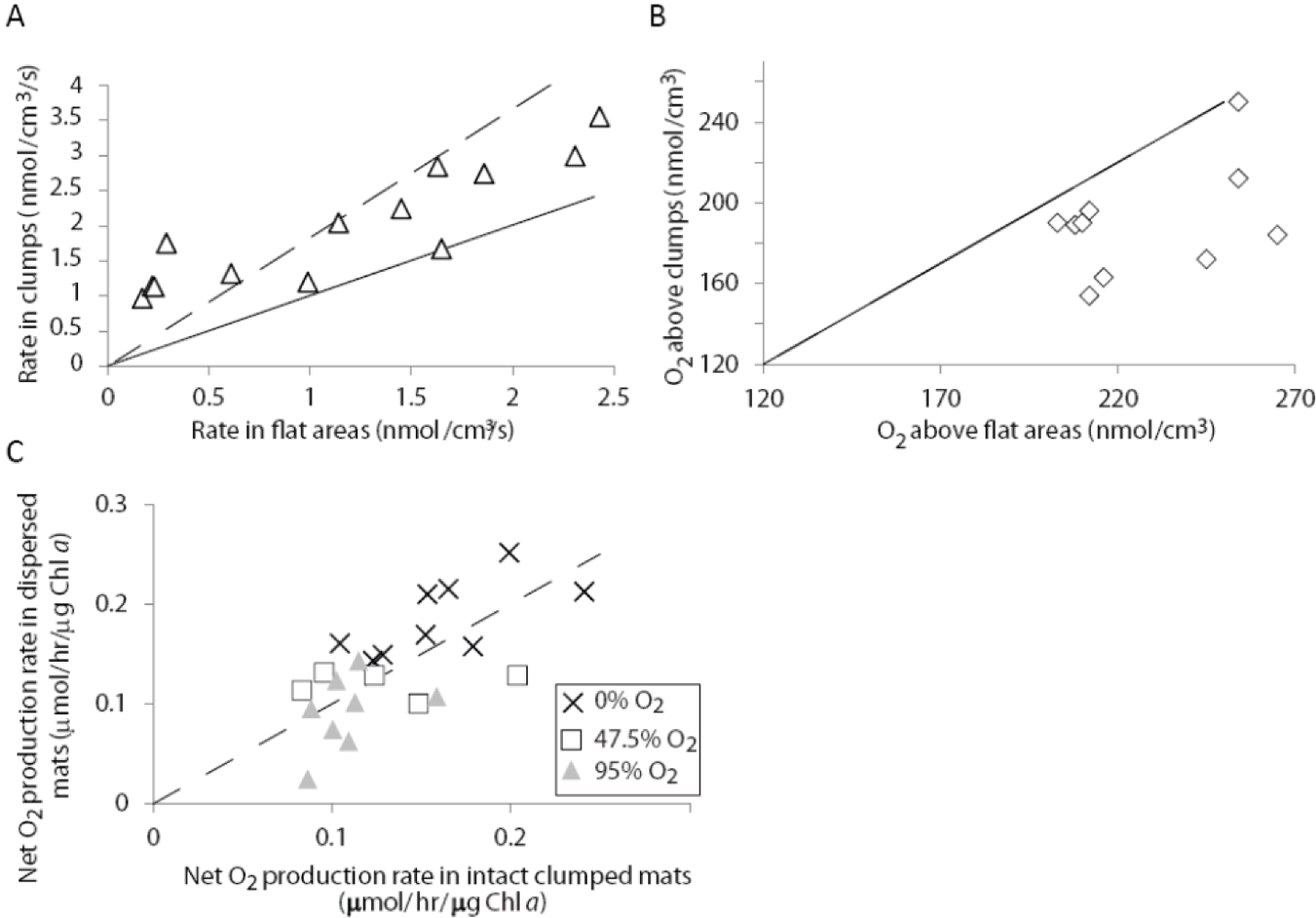
2.1.3. Oxygen Cycling in Clumps and Mats


2.2. Discussion
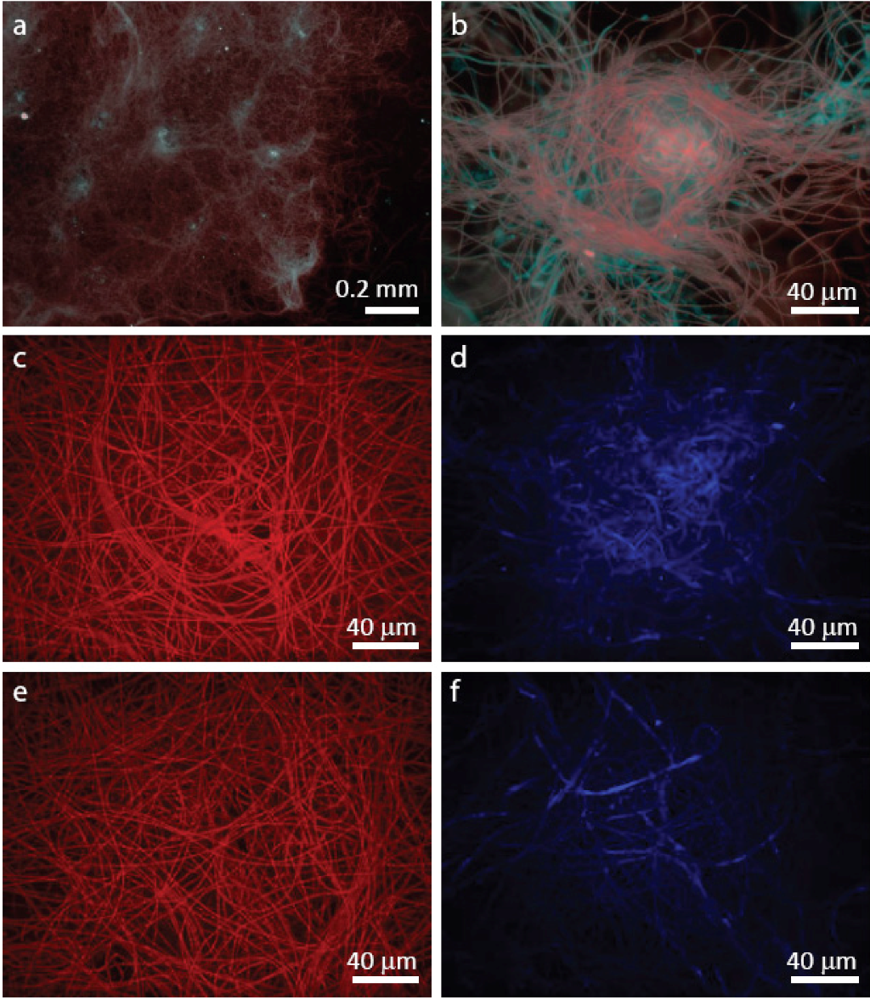
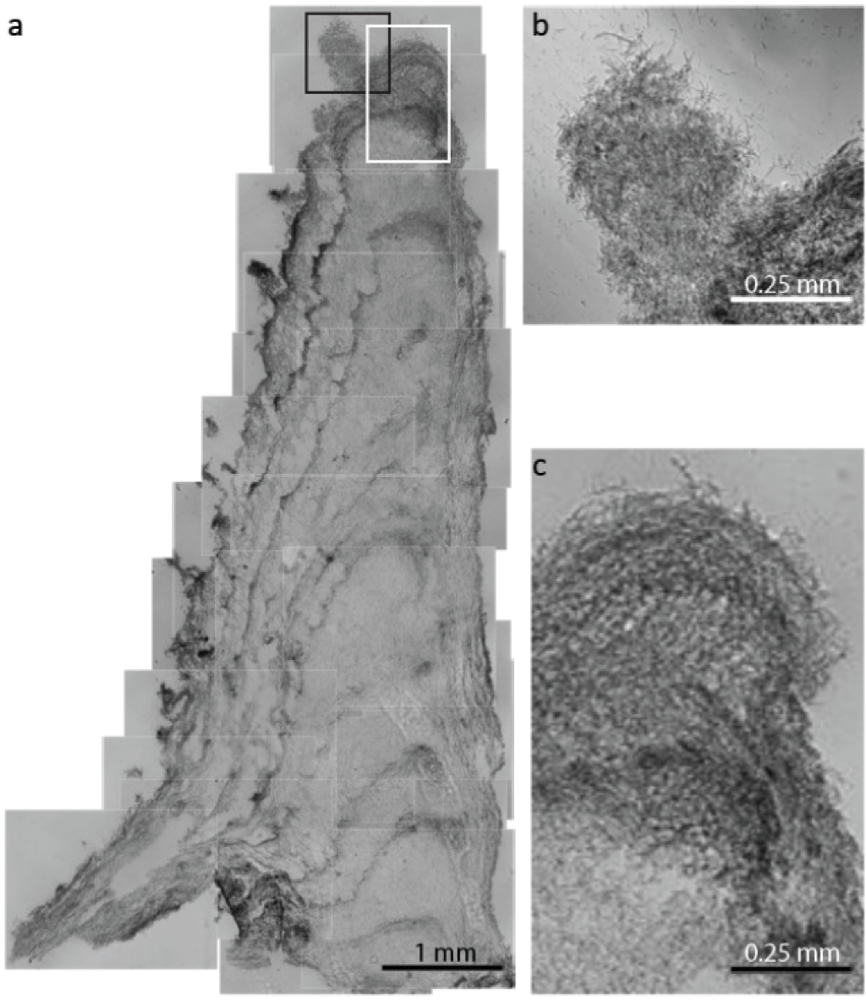
2.2.1. Initiation of Clumping





2.2.2. Cycling of Carbon and Oxygen
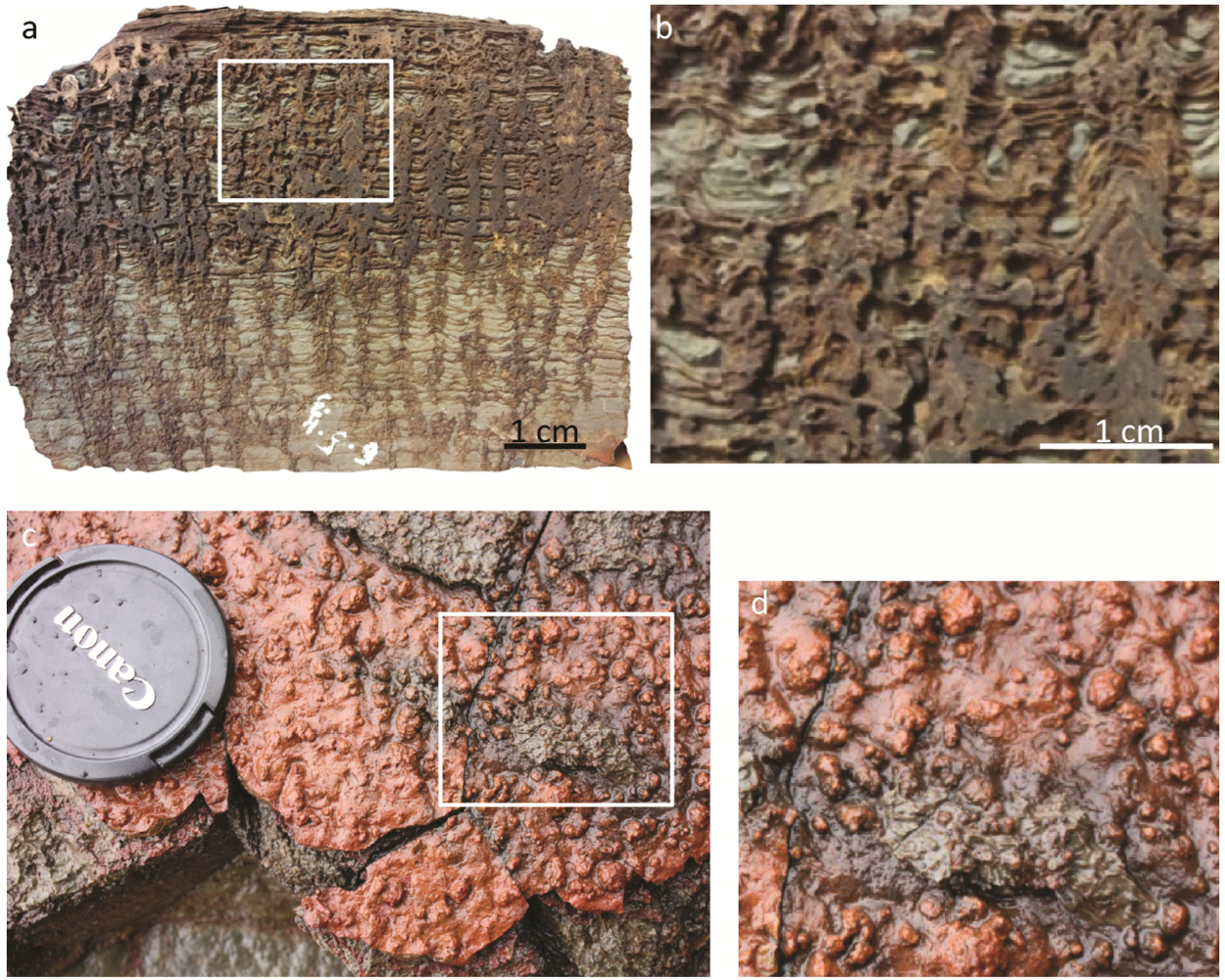
2.2.3. Interpretations of the Stromatolite Record
3. Experimental Section
3.1. Sampling and Culturing Conditions
3.2. Staining of Exopolymeric Substances (EPS)
3.3. Measurements of Clump Spacing and Dispersal
3.4. Measurements of Oxygen, Photosynthetic Activity and Glycolate


3.5. Statistical Treatments
4. Conclusions
Acknowledgments
References
- Raymond, J.; Segré, D. The effect of oxygen on biochemical networks and the evolution of complex life. Science 2006, 311, 1764–1767. [Google Scholar] [CrossRef]
- Bekker, A.; Holland, H.D.; Wang, P.L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef]
- Konhauser, K.; Lalonde, S.; Planavsky, N.; Pecoits, E.; Lyons, T.; Mojzsis, S.; Rouxel, O.; Fralick, P.; Barley, M.; Kump, L.; et al. Aerobic bacterial pyrite oxidation and acid rock drainage during the Great Oxidation Event. Nature 2011, 478, 369–373. [Google Scholar] [CrossRef]
- Farquhar, J.; Zerkle, A.; Bekker, A. Gological constraints on the origin of oxygenic photosynthesis. Photosynth. Res. 2011, 107, 11–36. [Google Scholar] [CrossRef]
- Kopp, R.E.; Kirschvink, J.L.; Hilburn, I.A.; Nash, C.Z. The Paleoproterozoic snowball Earth: A climate disaster triggered by the evolution of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 11131–11136. [Google Scholar]
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef]
- Eigenbrode, J.L.; Freeman, K.H. Late Archean rise of aerobic microbial ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 15759–15764. [Google Scholar] [CrossRef]
- David, L.A.; Alm, E.J. Rapid evolutionary innovation during an Archean genetic expansion. Nature 2011, 469, 93–96. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, Y.-Y.; Kim, K.M.; Qu, G.; Ji, H.F.; Mittenthal, J.E.; Zhang, H.-Y.; Caetano-Anollés, G. A universal molecular clock of protein folds and its power in tracing the early history of aerobic metabolism and planet oxygenation. Mol. Biol. Evol. 2011, 28, 567–582. [Google Scholar] [CrossRef]
- Bosak, T.; Knoll, A.H.; Petroff, A.P. The meaning of stromatolites. Annu. Rev. Earth Planet. Sci. 2012. in submission. [Google Scholar]
- Buick, R. The antiquity of oxygenic photosynthesis: Evidence from stromatolites in sulphate-deficient Archaean lakes. Science 1992, 255, 74–77. [Google Scholar]
- DesMarais, D.J. When did photosynthesis emerge on Earth? Science 2000, 289, 1703–1705. [Google Scholar]
- Flannery, D.T.; Walter, M.R. Archean tufted microbial mats and the Great Oxidation Event: New insights into an ancient problem. Austral. J. Earth Sci. 2012, 59, 1–11. [Google Scholar] [CrossRef]
- Tice, M.; Thornton, D.C.O.; Pope, M.C.; Olszewski, T.D.; Gong, Y. Early microbial communities. Annu. Rev. Earth Planet. Sci. 2011, 39, 297–319. [Google Scholar] [CrossRef]
- Walter, M.R. Archean Stromatolites–Evidence of the Earth’s Earliest Benthos. In Earth’s Earliest Biosphere: Its Origins and Evolution; Schopf, W.J., Ed.; Princeton University Press: Princeton, NJ, USA, 1983; pp. 187–213. [Google Scholar]
- Walter, M.R.; Bauld, J.; Brock, T.D. Siliceous algal and bacterial stromatolites in hot spring and geyser effluents of Yellowstone national park. Science 1972, 27, 402–405. [Google Scholar]
- Bosak, T.; Liang, B.; Sim, M.S.; Petroff, A.P. Morphological record of oxygenic photosynthesis in conical stromatolites. Proc. Natl. Acad. Sci. USA 2009, 106, 10939–10943. [Google Scholar]
- Bosak, T.; Bush, J.; Flynn, M; Liang, B.; Ono, S.; Petroff, A.P.; Sim, M.S. Formation and stability of oxygen-rich bubbles that shape photosynthetic mats. Geobiology 2010, 8, 45–55. [Google Scholar] [CrossRef]
- Mata, S.A.; Harwood, C.L.; Corsetti, F.A.; Stork, N.J.; Eilers, K.; Berelson, W.M.; Spear, J.R. Influence of gas production and filament orientation on stromatolite microfabric. Palaios 2012, 27, 206–219. [Google Scholar] [CrossRef]
- Murphy, M.A.; Sumner, D.Y. Tube structures of probable microbial origin in the Neoarchean Carrawine Dolomite, Hammersley Basin, Western Australia. Geobiology 2008, 6, 83–93. [Google Scholar]
- Hofmann, H.J. Archean Stromatolites as Microbial Archives. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer-Verlag: Berlin, Germany, 2000; pp. 315–327. [Google Scholar]
- Schopf, J.W. Fossil evidence of Archaean life. Philos. Trans. R. Soc. B 2006, 361, 869–885. [Google Scholar] [CrossRef]
- Macalady, J.; McCauley, R.L.; Kakuk, B.; Schaperdoth, I. Extremely low-light adapted phototrophic biofilm community in a Bahamian blue hole. In Proceedings of Astrobiology Science Conference, Atlanta, GA, USA, 15–19 April 2012. Abstract Number 4043.
- Walter, M.R.; Bauld, J.; Brock, T.D. Microbiology and Morphogenesis of Columnar Stromatolites (Conophyton, Vacerrilla) from Hot Springs in Yellowstone National Park. In Developments in Sedimentology, Stromatolites; Walter, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 273–310. [Google Scholar]
- Castenholz, R.W. The behavior of Oscillatoria terebriformis in hot springs. J. Phycol. 1968, 4, 132–139. [Google Scholar] [CrossRef]
- Richardson, L.L.; Castenholz, R. Chemokinetic motility responses of the cyanobacterium Osillatoria terebriformis. Appl. Environ. Microbiol. 1989, 55, 261–263. [Google Scholar]
- Castenholz, R.W.; Jorgensen, B.B.; D’Amelio, E.; Bauld, J. Photosynthetic and behavioral versatility of the cyanobacterium Oscillatoria boryana in a sulfide-rich microbial mat. FEMS Microbiol. Ecol. 1991, 86, 43–58. [Google Scholar] [CrossRef]
- Castenholz, R.W. Cyanobacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd; Boone, D.R., Castenholz, R.W., Garrity, G.M., Eds.; Springer-Verlag: New York, NY, USA, 2001; Volume 1, pp. 473–599. [Google Scholar]
- Malin, G.; Walsby, A.E. Chemotaxis of a cyanobacterium on concentration gradient of carbon dioxide, bicarbonate, and oxygen. J. Gen. Microbiol. 1985, 131, 2643–2652. [Google Scholar]
- Mǿller, M.M.; Nielsen, L.P.; Jǿrgensen, B.B. Oxygen responses and mat formation by Beggiatoa spp. Appl. Environ. Microbiol. 1985, 50, 373–382. [Google Scholar]
- Gerdes, G. Structures left by Modern Microbial Mats in their Host Sediments. In Atlas of Microbial Mat Features Preserved within the Siliciclastic Rock Record; Schieber, J., Bose, P.K., Eriksson, P.G., Banerjee, S., Sarkar, S., Altermann, W., Catuneanu, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 5–38. [Google Scholar]
- Browne, K.M.; Golubic, S.; Seong-Joo, L. Shallow Marine Microbial Carbonate Deposits. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer-Verlag: Berlin, Germany, 2000; pp. 233–249. [Google Scholar]
- Weller, D.; Doemel, W.; Brock, T.D. Requirement of low oxidation-reduction potential for photosynthesis in a blue-green alga (Phormidium sp.). Arch. Microbiol. 1975, 104, 7–13. [Google Scholar] [CrossRef]
- Bosak, T.; Liang, B.; Wu, T.-D.; Templer, S.; Evans, A.; Vali, H.; Guerquin-Kern, J.-L.; Klepac-Ceraj, V.; Sim, M.S.; Mui, J. Cyanobacterial composition and activity in modern conical microbialites. Geobiology 2012. [Google Scholar]
- Zevenboom, W.; de Groot, G.J.; Mur, L.R. Effect of light on nitrate-limited Oscillatoria agardhii in chemostat cultures. Arch. Microbiol. 1980, 125, 59–65. [Google Scholar] [CrossRef]
- Van de Wall, D.B.; Verspagen, J.M.H.; Finke, J.F.; Vournazou, V.; Immers, A.K.; Kardinaal, W.E.A.; Tonk, L.; Becker, S; van Donk, E.; Visser, P.M.; et al. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME J. 2011, 5, 1438–1450. [Google Scholar] [CrossRef]
- McGregor, G.B.; Rasmussen, J.P. Cyanobacterial composition of microbial mats from an Australian thermal spring: A polyphasic evaluation. FEMS Microbiol. Ecol. 2007, 63, 23–35. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Ferris, F.G.; Sherwood-Lollar, B.; Gerits, J.P. Ultrastructure and seasonal growth patterns of microbial mats in a temperate climate saline-alkaline lake: Goodenough Lake, British Columbia, Canada. Can. J. Microbiol. 1996, 42, 147–161. [Google Scholar] [CrossRef]
- Kaplan, A.; Tarazi-Ronen, M.; Zer, H.; Schwarz, R.; Tchernov, D.; Bonfil, D.J.; Schatz, D.; Vardi, A.; Hassidim, M.; Reinhold, L. The inorganic carbon-concentrating mechanism in cyanobacteria: Induction and ecological significance. Can. J. Bot. 1998, 76, 917–924. [Google Scholar]
- Eisenhut, M.; Ruth, W.; Haimowich, M.; Bauwe, H.; Kaplan, A.; Hagemann, M. The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17199–17204. [Google Scholar]
- Glud, R.N.; Ramsing, N.B.; Revsbech, N.P. Photosynthesis and photosynthesis-coupled respiration in natural biofilms quantified with oxygen microsensors. J. Phycol. 1992, 28, 51–60. [Google Scholar]
- Petroff, A.P.; Sim, M.S.; Maslov, A.; Krupenin, M.; Rothman, D.H.; Bosak, T. Biophysical basis for the geometry of conical stromatolites. Proc. Natl. Acad. Sci. USA 2010, 107, 9956–9961. [Google Scholar]
- Schulz, H.N.; Jǿrgensen, B.B. Big bacteria. Annu. Rev. Microbiol. 2001, 55, 105–137. [Google Scholar] [CrossRef]
- Shepard, R.N.; Sumner, D.Y. Undirected motility of cyanobacteria produces reticulate mats. Geobiology 2010, 8, 179–190. [Google Scholar] [CrossRef]
- Wall, D.; Kaiser, D. Alignment enhances the cell-to-cell transfer of the pilus phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3054–3058. [Google Scholar] [CrossRef]
- Petroff, A.P.; Wu, T.-D.; Liang, B.; Mui, J.; Guerkin-Kern, J.-L.; Vali, H.; Rothman, D.H.; Bosak, T. Reaction-diffusion model of nutrient uptake in a biofilm: Theory and experiment. J. Theor. Biol. 2011, 289, 90–95. [Google Scholar] [CrossRef]
- Jǿrgensen, B.B.; DesMarais, D.J. The diffusive boundary layer of sediments: Oxygen microgradients over a microbial mat. Limnol. Oceanogr. 1990, 35, 1343–1355. [Google Scholar] [CrossRef]
- Villanueva, L.; del Campo, J.; Guerrero, R. Diversity and physiology of polyhydroxyalkanoate-producing and degrading strains in microbial mats. FEMS Microbiol. Ecol. 2010, 74, 42–54. [Google Scholar] [CrossRef]
- Dicks, J.M.; Aston, W.J.; Davis, D.; Turner, A.P.F. Mediated amperometric biosensor for D-galactose, glycolate, and L-amino acids based on ferrocene-modified carbon paste electrode. Anal. Chim. Acta 1986, 182, 103–112. [Google Scholar] [CrossRef]
- Nelson, D.C.; Jannasch, H.W. Chemoautotrophic growth of a marine Beggiatoa in sulfide gradient cultures. Arch. Microbiol. 1983, 136, 262–269. [Google Scholar] [CrossRef]
- Pierson, B.K.; Castenholz, R.W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 1974, 100, 5–24. [Google Scholar] [CrossRef]
- Doemel, W.N.; Brock, T.D. Bacterial stromatolites: Origins of laminations. Science 1977, 184, 1083–1086. [Google Scholar]
- Gibson, J.; Pfennig, N.; Waterbury, J.B. Chloroherpeton thalassium gen. nov. et spec. nov., a non-filamentous, flexing and gliding green sulfur bacterium. Arch. Microbiol. 1984, 138, 96–101. [Google Scholar] [CrossRef]
- Hanada, S.; Hiraishi, A.; Shimada, K.; Matsuura, K. Chloroflexus aggregans sp. nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int. J. Syst. Bacteriol. 1995, 145, 676–681. [Google Scholar]
- Hanada, S.; Shimada, K.; Matsuura, K. Active and energy-dependent rapid formation of cell aggregates in the thermophilic photosynthetic bacterium Chloroflexus aggregans. FEMS Microbiol. Lett. 2002, 208, 275–279. [Google Scholar] [CrossRef]
- Barbieri, R.; Cavalazzi, B. Microbial fabrics from Neogene cold seep carbonates, Northern Apennine, Italy. Palaeogeogr. Palaeoclim. Palaeoecol. 2005, 227, 143–155. [Google Scholar] [CrossRef]
- Fukui, M.; Teske, A.; Aßmus, B.; Muyzer, G.; Widdel, F. Physiology, phylogenetic relationships, and ecology of filamentous sulfate-reducing bacteria (genus Desulfonema). Arch. Microbiol. 1999, 172, 193–203. [Google Scholar] [CrossRef]
- Gerdes, G.; Klenke, T.; Noffke, N. Microbial signatures in peritidal siliciclastic sediments: A catalogue. Sedimentology 2000, 47, 279–308. [Google Scholar]
- Horodyski, R.J. Lyngbya mats at Laguna Mormona, Baja California, Mexico; comparison with Proterozoic stromatolites. J. Sed. Res. 1977, 47, 1305–1320. [Google Scholar]
- Park, R.K. The preservation potential of some recent stromatolites. Sedimentology 1977, 24, 485–506. [Google Scholar] [CrossRef]
- Vlasov, F.Y. Anatomy and Morphology of Stromatolites of the Early and Middle Proterozoic of the Southern Urals. In Materialy po Paleontologii Urala; UFIGiG AN SSSR: Sverdlovsk, Russia, 1970; pp. 152–175. [Google Scholar]
- Vlasov, F.Y. Precambrian Stromatolites from the Satka Formation of Southern Urals (in Russian). In Materialy po paleontologii Srednego Paleozoya Urala I, Sibiri; UrO AN SSSR: Sverdlovsk, Russia, 1977; pp. 101–124. [Google Scholar]
- Hofmann, H.J. New stromatolites from the Aphebian Mistassini Group, Quebec. Can. J. Earth Sci. 1978, 15, 571–585. [Google Scholar] [CrossRef]
- Maeda, H.; Ishida, N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J. Biochem. 1967, 62, 276–278. [Google Scholar]
- Hageage, G.J.; Harrington, B.J. Use of Calcofluor White in clinical mycology. Lab. Med. 1984, 15, 109–112. [Google Scholar]
- Revsbech, N.P. An oxygen microelectrode with a guard cathode. Limnol. Oceanogr. 1989, 34, 474–478. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jorgensen, B.B. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: Capabilities and limitations of the method. Limnol. Oceanogr. 1983, 28, 749–756. [Google Scholar] [CrossRef]
- Glud, R.N.; Ramsing, N.B.; Revsbech, N.P. Photosynthesis and photosynthesis-coupled respiration in natural biofilm quantified with oxygen microsensors. J. Phycol. 1992, 28, 51–60. [Google Scholar]
- Revsbech, N.P.; Jorgensen, B.B.; Brix, O. Primary production of microalgae in sediments measured by oxygen microprofile, H14CO3− fixation, and oxygen exchange methods. Limnol. Oceanogr. 1981, 26, 717–730. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. OIKOS 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Waffenschmidt, S.; Knittler, M.; Jaenicke, L. Characterization of a sperm lysine of Volvox carteri. Sex. Plant Rep. 1990, 3, 1–6. [Google Scholar]
- Calkins, V. Microdetermination of glycolic and oxalic acids. Ind. Eng. Chem. Anal. Ed. 1943, 15, 762–763. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sim, M.S.; Liang, B.; Petroff, A.P.; Evans, A.; Klepac-Ceraj, V.; Flannery, D.T.; Walter, M.R.; Bosak, T. Oxygen-Dependent Morphogenesis of Modern Clumped Photosynthetic Mats and Implications for the Archean Stromatolite Record. Geosciences 2012, 2, 235-259. https://doi.org/10.3390/geosciences2040235
Sim MS, Liang B, Petroff AP, Evans A, Klepac-Ceraj V, Flannery DT, Walter MR, Bosak T. Oxygen-Dependent Morphogenesis of Modern Clumped Photosynthetic Mats and Implications for the Archean Stromatolite Record. Geosciences. 2012; 2(4):235-259. https://doi.org/10.3390/geosciences2040235
Chicago/Turabian StyleSim, Min Sub, Biqing Liang, Alexander P. Petroff, Alexander Evans, Vanja Klepac-Ceraj, David T. Flannery, Malcolm R. Walter, and Tanja Bosak. 2012. "Oxygen-Dependent Morphogenesis of Modern Clumped Photosynthetic Mats and Implications for the Archean Stromatolite Record" Geosciences 2, no. 4: 235-259. https://doi.org/10.3390/geosciences2040235
APA StyleSim, M. S., Liang, B., Petroff, A. P., Evans, A., Klepac-Ceraj, V., Flannery, D. T., Walter, M. R., & Bosak, T. (2012). Oxygen-Dependent Morphogenesis of Modern Clumped Photosynthetic Mats and Implications for the Archean Stromatolite Record. Geosciences, 2(4), 235-259. https://doi.org/10.3390/geosciences2040235



