Abstract
Nacre was previously thought to be primitive in the Mollusca, but no convincing Cambrian examples are known. This aragonitic microstructure with crystal tablets that grow within an organic framework is thought to be the strongest, most fracture-resistant type of shell microstructure. Fossils described herein from the Ordovician of Iowa, Indiana, and Ohio provide supporting evidence for the hypothesis that sometime between the middle Cambrian and late Ordovician, nacre originated in cephalopod, bivalve, and possibly gastropod lineages. The correlation of independent origins of fracture-resistant nacre with increasing shell-crushing abilities of predators during the Cambrian-Ordovician suggests an early pulse in the evolutionary arms race between predators and molluscan prey.
Keywords:
nacre; Ordovician; Isorthoceras; cephalopod; shell microstructure; Maquoketa; Arnheim; Cyclora; Ohio; Iowa 1. Introduction
One of the most dramatic evolutionary events in the history of life on Earth is the rapid diversification of animals beginning around 540 million years ago. This event, known widely as the Cambrian Explosion, marks a major transition in Earth history when complex multicellular animals became morphologically disparate, abundant, and widespread. The Cambrian Explosion was followed by three major extinction events and then the Great Ordovician Biodiversification Event (GOBE), a continuation of the earliest diversification of animals.
During the Early Palaeozoic radiations of metazoans, the range of shell microstructures in molluscs expanded in step with increasing disparity of overall shell form [,,]. In at least some lineages the evolutionary transition in the shell was to more fracture-resistant crystal/organic configurations [], correlating with an apparent increasing selective pressure from progressively more efficient predators.
Nacre is considered to have the highest fracture resistance [,] as well as cost of formation [,], and so a transition to this type of shell microstructure may indicate an increase in shell-crushing selective pressures. Nacre was once thought to be primitive in the Mollusca, but a closer analysis of purported Cambrian molluscs with nacre revealed the evidence is equivocal at best [].
When then did nacre originate? Unfortunately no locality from the late Cambrian through middle Ordovician is known to preserve good details of mollusc shell microstructure, and thus there is a gap in understanding about the shell structure of molluscs from that time interval. Nevertheless there are some encouraging Late Ordovician sedimentary deposits, and we have examined material from some of the most promising rock units, from the Midwestern United States (Figure 1).
The Maquoketa Formation is of interest because at the Graf section it contains coquinas of nautiloids (“Nautiloid beds”) whose fossil shells gleam with mother-of-pearl or a remarkably similar material (Figure 1.2 and Figure 2.1). Moreover, Grégoire [] and Mutvei [,] had earlier examined the microstructure of fossil shells from this sedimentary deposit, and both authors classified the texture as a unique version of nacre replicated by apatite. Carter et al. [] tentatively accepted Mutvei’s claim but highlighted the dissimilarities between the disc-shaped tablets in the fossils and those of nacre in modern molluscs. Mutvei [,] considered the phosphatic discs to be exact replicas of the original aragonitic nacre tablets, even though the fossil discs have a radiating ultrastructure unlike any modern nacre. Also, it is perplexing why the same singular type of preserved nacre would appear in cephalopods, gastropods, and a bivalve, as shown by Mutvei []. Plus, in cases of such complex microtextures, there is intrinsic uncertainty in distinguishing what is original from what is diagenetic. Therefore, to better assess the hypothesis that the nautiloid coquinas within the Maquoketa Formation preserve the earliest recorded nacre, we have examined new specimens of bivalves, gastropods, and cephalopods from those unique sedimentary beds.
The Kope, Fairview, and “Arnheim” Formations of Ohio, Kentucky, and Indiana contain gastropod-rich layers (“Cyclora beds”) that are part of the rich Ordovician fossil record of the Cincinnati region [,,]. These beds, informally named for the abundant gastropod Cyclora Hall, 1845 within, were originally described from the upper part (“Oregonia Member”) of the “Arnheim Formation” [,], but similar deposits have subsequently been found by Heimbrock and others in the other formations listed above, and these layers are all referred to herein as “Cyclora beds”. These beds are rich in phosphatic steinkerns of small molluscs, brachiopods, and bryozoans [,]. The “Cyclora beds” hold promise for the preservation of mollusc shell microstructure because: (1) they contain a diversity of molluscs, including bivalves, gastropods, cephalopods, and bellerophonts; (2) they contain a high density of fossils, plus outcrops of the deposits are numerous and accessible; (3) the type of preservation (fine-grained calcium phosphate moulds and partial replacements) is similar to Cambrian deposits that record shell microstructure data; and (4) some localities show very high fidelity of preservational detail. The gastropod Cyclora is small and it may represent the juvenile shell of the larger gastropod Cyclonema Hall, 1852, a common fossil in the Ordovician of the Cincinnati region. However, we retain use of the term “Cyclora bed” because of uncertainty about this synonymy and the historical usage of that informal stratigraphic name.
The stratigraphic terms “Arnheim Formation” and its traditional constituent units, the “Sunset Member” and “Oregonia Member” likewise were used historically, but their applicability to rocks of the Cincinnati Arch is somewhat in question [,]. Because the stratigraphic nomenclature is still in flux, we use historic names but in quotation marks.
By collecting data on early mollusc shell microstructures, we aim to fill gaps in knowledge about controls on the mollusc shell and the nature of the early diversification of animals, parts of a larger project of ours (Vendrasco and Checa). In the first steps in the pursuit of this goal we have utilized fossils from the rocks described above. Heimbrock and Baumann contributed material from, and their expertise about, Cyclora beds around Cincinnati, Ohio, and nautiloid beds in the Maquoketa Shale of Iowa, respectively.
Herein we describe the results of a preliminary examination of this promising material, describe the significance of our early observations, and summarize the state of knowledge about shell microstructures and evolutionary escalation between predator and prey during the early Palaeozoic.
2. Materials and Methods
Specimens of the cephalopod Isorthoceras sociale (Hall, 1862) from the Lower Maquoketa shale were collected by Baumann from the Graf section of Dubuque County, Iowa (Figure 1.1 and Figure 1.3; 42°29'20" N, 90°52'33" W), approximately 0.4 km southwest of Graf, Iowa []. The specimens were collected from the famous “Nautiloid beds” at this section (Figure 1.2 and Figure 1.3) [,]. In addition to Baumann’s material, we examined other fossils from the Graf section of the Maquoketa Formation, including gastropods, bivalves, and specimens of I. sociale from the Los Angeles County Museum of Natural History, Invertebrate Paleontology collections (LACMIP) and the Smithsonian National Museum of Natural History (USNM), as well as specimens purchased from Geological Enterprises (Ardmore, OK, USA). In total we examined via SEM 18 cephalopods, 25 bivalves, 25 gastropods, one bellerophont, and one hyolith from the Graf section of the Elgin Member of the Maquoketa Formation.
The Elgin Member in Iowa belongs to the Late Edenian to Early Maysvillian Stages. The cephalopod beds of the Iowa Graf Section are stratigraphically equivalent to the top of the Kope Formation and the base of the Dillsboro Formation in eastern Indiana. In southwest Ohio and in Kentucky they are equivalent to the lower third of the Fairview Formation and the upper third of the Kope Formation. No equivalent beds exist in southern Illinois, but the Graf beds correlate with the Cape La Croix Formation (a thin limestone) of southeast Missouri. The lithology of the Graf section is dominantly a yellowish brown dolostone interbedded with brown shale. The main beds of Isorthoceras Flower, 1962 occur about three to six meters above the top of the Galena Group (the Trenton and Lexington Limestones in Indiana, Kentucky, and Ohio). A pearly lustre is visible in places on many specimens of Isorthoceras from these beds, corresponding to the preservation of the type of original and/or diagenetic microtextures that Grégoire [] and Mutvei [] described.
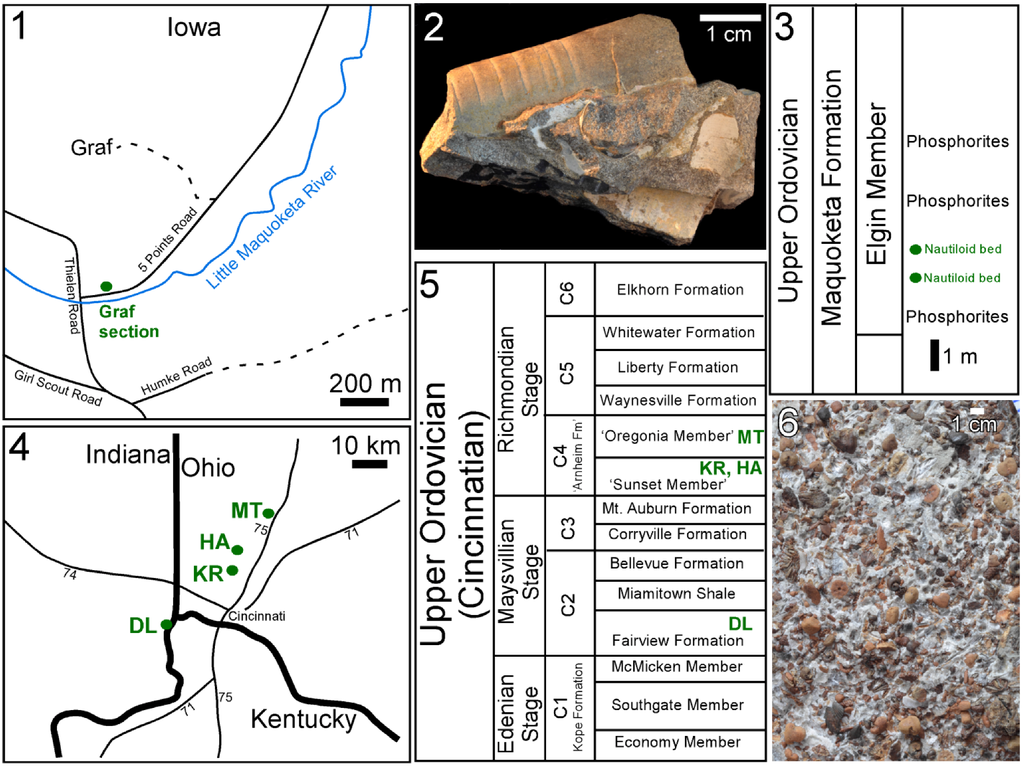
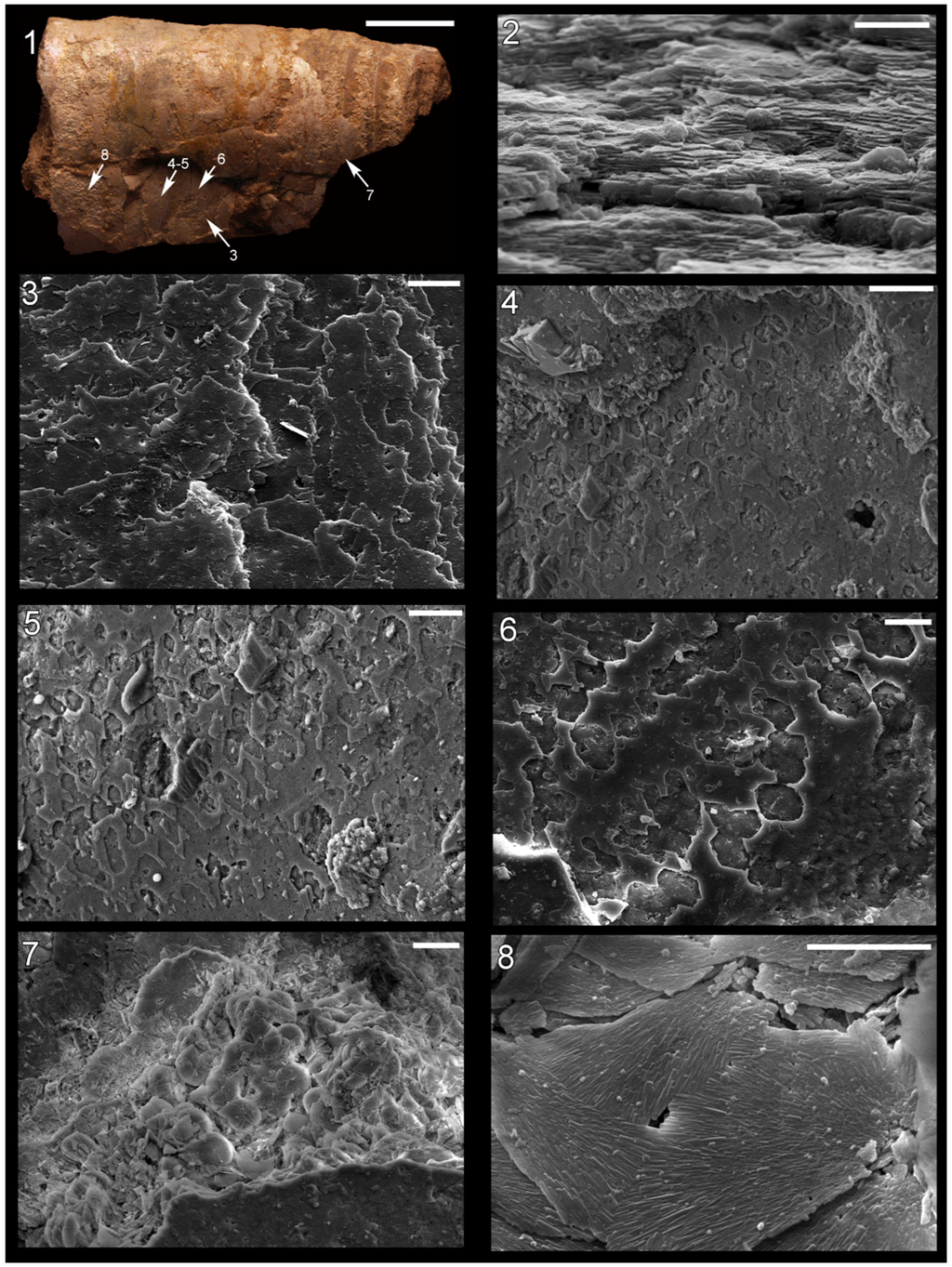
Figure 1.
Localities, stratigraphic units, and fossils featured herein. (1) Location of Graf section; (2) Typical sample from the “Nautiloid beds” of the Maquoketa Formation at Graf, showing remains of multiple cephalopods; (3) Position of “Nautiloid beds” at Graf section from which Isorthoceras was recovered; (4) Locations where Cyclora beds of phosphatic internal moulds of molluscs were recovered; (5) Stratigraphic distribution of the Cyclora beds that yielded fossils illustrated herein; (6) Etched surface of Cyclora bed from Kemper Road, Cincinnati, OH. Key: DL = Dearborn/Lawrenceburg, IN; HA = Hamilton, OH; KR = Kemper Road, OH; MT = Middletown, OH. Sequence stratigraphic cycles (C1–C6) shown in (5) are based on Holland [].
The Cyclora bed is a konzentrat (concentration) lagerstätte rich in phosphatic steinkerns of molluscs that was originally described from the “Arnheim Formation”, but similar Cyclora beds also occur in the Fairview and Kope Formations of Kentucky, Indiana, and Ohio. Specimens of gastropods such as Cyclora and bivalves such as Palaeoconcha Miller, 1889 and Nuculites Conrad, 1841 were recovered from these beds (Figure 1.6) at numerous localities (Figure 1.4 and Figure 1.5). Vendrasco accompanied Heimbrock on a few collecting trips and extracted a few hundred fossils from these rocks but the vast majority of collecting and initial processing was done by Heimbrock.
Fossils were collected from numerous Cyclora-bed localities in Indiana, Kentucky, and Ohio, including Bon Well Hill [] and South Gate Hill [], Indiana. Specimens shown herein are from the following localities:
Dearborn/Lawrenceburg, Indiana (39°05'53" N, 84°52'37" W): Farview Formation.Hamilton, OH (39°24'31" N, 84°30'18" W): the fossils appear to be from the “Arnheim Formation”, but the mixture of index fossils at this site indicates this locality probably consists of fill from different formations.Kemper Road, Cincinnati, OH (39°17'43" N, 84°32'27" W): “Arnheim Formation”, “Sunset Member”, 3 m below the base of the Retrosirostra Schuchert and Cooper, 1931 brachiopod marker bed.Middletown, OH (39°32'35" N, 84°20'42" W): “Arnheim formation”, bottom of “Oregonia Member”, just above the Retrosirostra brachiopod marker bed.
The mollusc steinkern assemblages from the Kope, Fairview, and “Arnheim” Formations are similar in composition and preservation, but appear to differ in depositional environment. The beds in the “Arnheim” and possibly the Fairview Formation occur above breaks in carbonate sedimentation, with apparent transportation of material of similar size in a dense accumulation of mollusc and other fossils. On the other hand, the related deposit in the Kope Formation is mostly composed of calcitic crinoid columnals. Only the mollusc shells in that bed were filled with phosphate.
Heimbrock used repeated washing to extract fossils from the calcareous clay matrix of the Cyclora beds. In addition, Vendrasco extracted fossils from the Kemper Road and Hamilton localities via maceration using dilute acetic acid. Vendrasco and Checa examined these and other phosphatized Ordovician micromolluscs from the “Arnheim Formation” housed in the USNM collections, including from: “Cincinnati, OH” (USNM 49687); near Tottys Bend near Centerville, Hickman County, Tennessee (USNM 25364); and “near Oxford Ohio” (no locality number). Overall we examined, by scanning electron microscope (SEM), the following number of fossils from Cyclora beds at various localities: 157 bivalves, 236 gastropods, 17 cephalopods, 11 bellerophonts, one rostroconch, seven hyoliths, six brachiopods, and two trilobites.
Modern molluscs whose photographs appear herein include: (1) Seguenzia Jeffreys, 1876, MNHN 8528, 825–827 m, Zambeze transect, Mozambique Channel, MAINBAZA CC 3155, Mozambique, 19°36' S, 36°48' E; (2) Arene Adams, 1845, SBMNH 230515, 20 m, Caribbean, Cochino Pequeño, 15°57' N, 86°30' W; and (3) Nautilus pompilius Linnaeus, 1758, Philippine islands, personal collection of MV. A bit of the shell of Nautilus pompilius was chipped away at the aperture, the fragment was dipped in dilute acetic acid for 30 s, the fragment dried and then coated with gold, and the inner shell surface was observed via SEM. For the other two modern molluscs, the whole shell was coated with gold and the inner shell surface as well as shell cross-sections at breaks were examined.
Most of the specimens were gold coated and photographed with a Zeiss EVO XVP scanning electron microscope at the Santa Barbara Museum of Natural History (SBMNH). Some specimens of Isorthoceras were coated with carbon (Hitachi UHS evaporator) and later observed with the Zeiss LEO Gemini 1530 and Zeiss Auriga field emission SEMs (FESEMs) at the Universidad de Granada. Specimens photographed herein are reposited at the LACMIP, USNM, the Santa Barbara Museum of Natural History (SBMNH), and the Muséum National d’Histoire Naturelle (MNHN).
3. Results and Discussion
3.2. Bivalves and Gastropods from the Ordovician Maquoketa Formation of Iowa
Mutvei [] mentioned that gastropods from the Maquoketa Formation show the same texture as what occurs in cephalopods from that rock unit; in another publication he [] provided one SEM photograph each of a bivalve and gastropod that show a similar structure. We examined 25 gastropods, 25 bivalves, and one bellerophont and found a few subtle traces of the Isorthoceras-like shell microstructure in these other taxa.
Some patches on a gastropod, Holopea Hall, 1847, contain adjoining discs that have a radiating ultrastructure (Figure 7.5). Other gastropods show a distinct laminar texture but with the constituent crystallites unclear (Figure 7.6). The merged tablets in the gastropod Murchisonia d’Archiac and E.P. de Verneuil, 1841 [] (fig. 2) are clearly very similar to what occurs in Isorthoceras. However Mutvei [,] did not include images of vertical sections and we were unable to find textures in the Maquoketa gastropods we examined that had the level of detail that characterizes the cephalopods from that unit.
The Maquoketa bivalve Palaeoneilo Hall and Whitfield, 1869 appears to have had a laminar shell microstructure (Figure 7.1), and many fossil bivalves (and gastropods) from these beds contain diagenetic rosettes (Figure 7.3 and Figure 7.4). The one image of shell microstructure in a bivalve from the Maquoketa Formation provided by Mutvei [] (fig. 3) records tablet-like groupings of elongate crystals that are similar to discs in Isorthoceras. These bundles are not as well organized as is usual for specimens of Isorthoceras, but they are consistent with what occurs in some regions of these fossils. Carboniferous specimens of Palaeoneilo oweni (McChesney, 1859) show a prismatic outer shell layer, porcelaneous middle layer, and fine complex crossed lamellar (CCL), matted, and/or homogenous inner shell layer []. The Ordovician specimens of Palaeoneilo described herein show laminae but equivocal tablets and thus may have had a matted shell microstructure consisting of laminae without tablets, as in P. oweni. Such a shell microstructure has been postulated to be an evolutionary transition between nacre and fine CCL [] and so it might be expected to comprise much of the middle and inner shell layers in the Ordovician representatives of this genus.
Mutvei [] described differences between shell microstructure in the bivalve Palaeoconcha with what occurs in both Isorthoceras and the gastropod Murchisonia from the same formation. Palaeoconcha, unlike the others, appeared to lack pores (or accumulations in the centre of discs) as well as vertical stacks of discs. This may be due to incomplete preservation, or perhaps another type of shell microstructure besides nacre is preserved here and similarities with textures in Isorthoceras are due to a similar diagenetic history. Unfortunately, as for the gastropods, vertical sections confirming a nacre-like configuration of the discs are currently unknown. However, Carter [] described prismatic and nacreous shell layers in the Silurian Praenucula faba Liljedahl, 1994, a taxon that, like Palaeoconcha, is a member of the Praenuculidae. Carter’s [] observation corroborates the conclusion that nacre is present in Palaeoconcha from the Ordovician. We likewise agree with Carter [] that Palaeoconcha from the Maquoketa Formation shows nacre, and that phosphatization increased the size of discs from that of the original tablets.
Combining the data here with photographs in Mutvei [], we tentatively conclude that gastropods and bivalves from the Maquoketa Formation show a similar fossil microstructure to Isorthoceras that represents phosphatized nacre. Our model that this texture is largely diagenetic, while still revealing nacre, helps explain why three different classes of mollusc have a similar, unusual fossil shell texture.
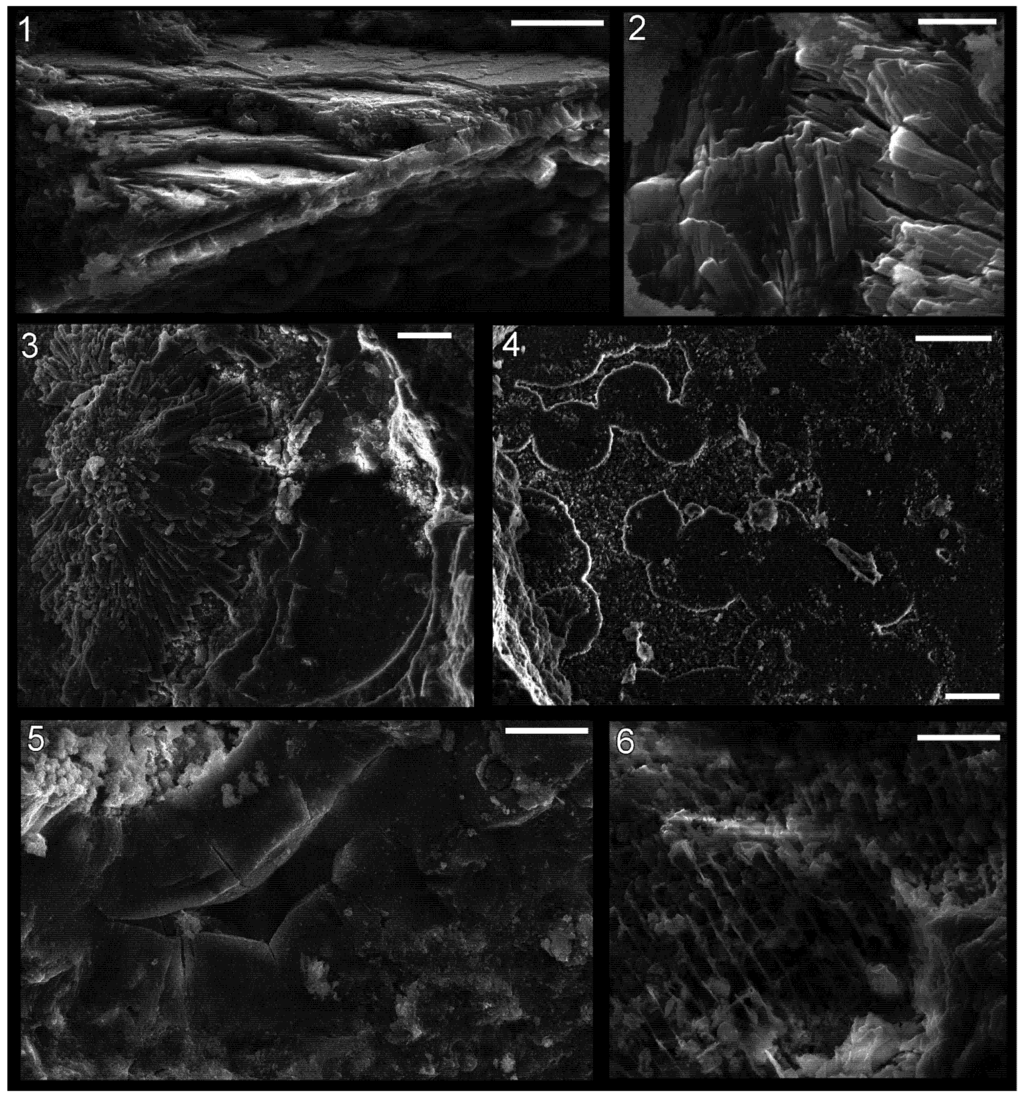
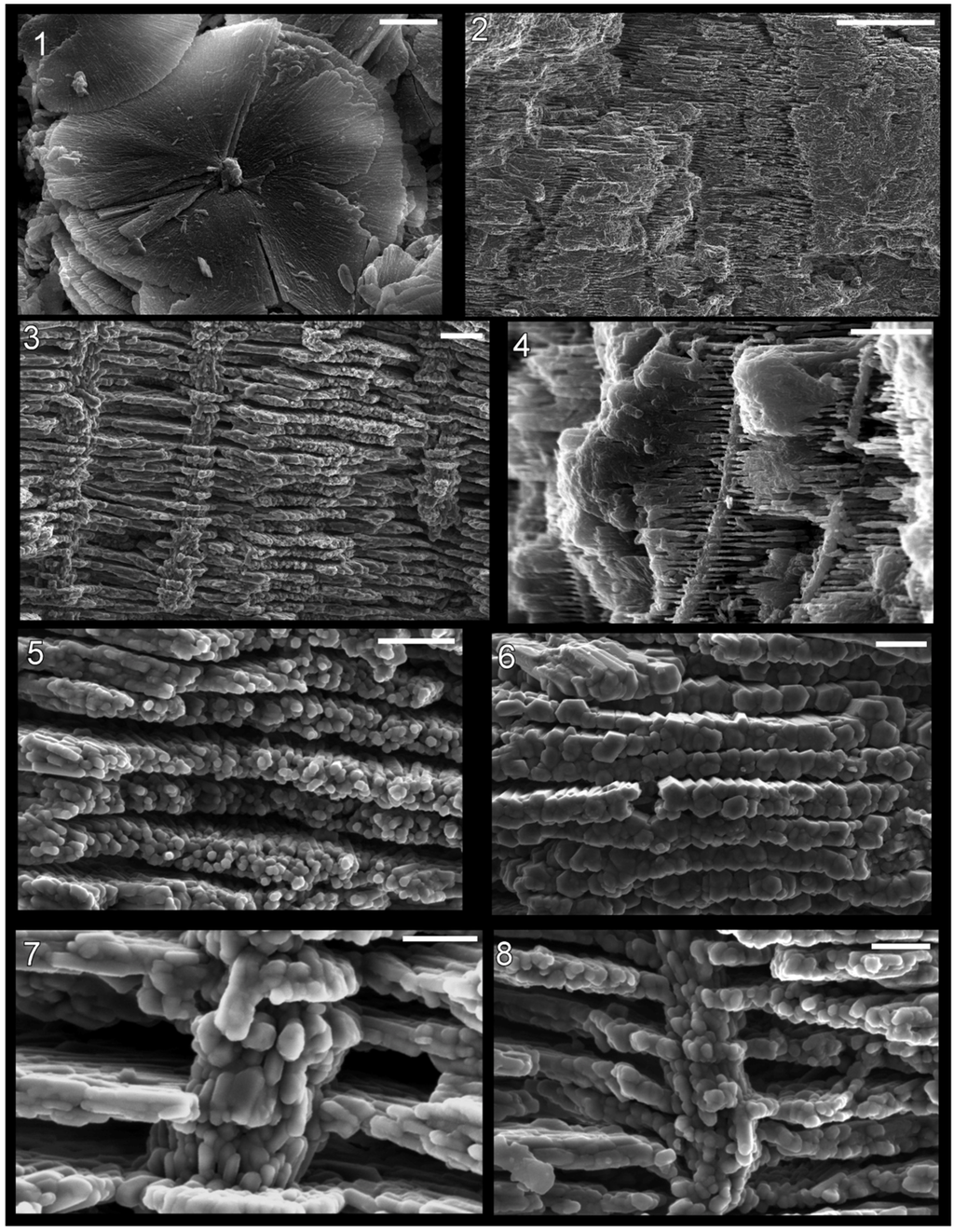
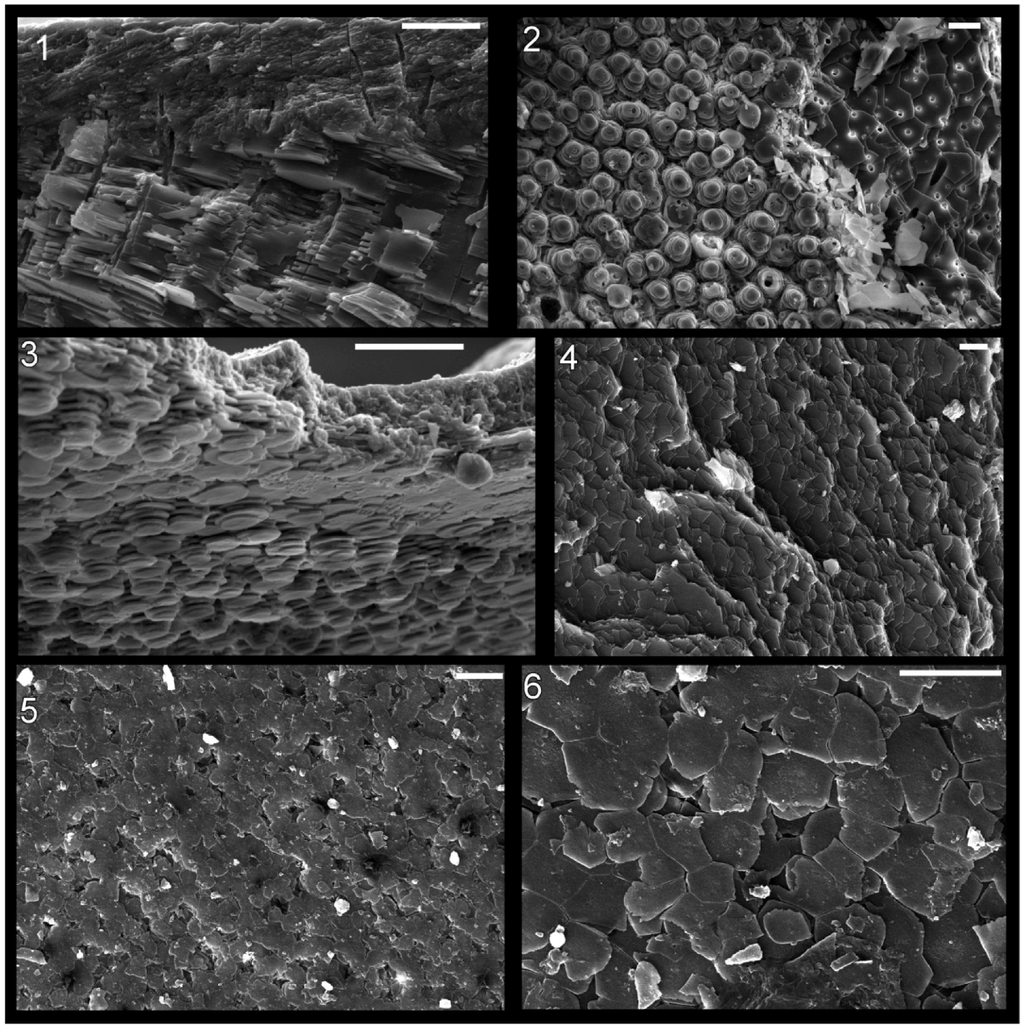
Figure 7.
Shell microstructure in specimens of the bivalve Palaeoneilo sp. (1)–(4) and the gastropod Holopea sp. (5) and (6) from the Ordovician Maquoketa Formation. (1) and (2) LACMIP 14441: (1) semi-transverse section of a portion of replaced shell near midpoint of valve with umbo towards top of photo; (2) horizontal section of replaced shell near aperture; (3) LACMIP 14442, horizontal section of replaced shell in region between valve midpoint and aperture; (4) LACMIP 14436, horizontal section of inferred inner shell layer, near midpoint of valve; (5) USNM 553688, horizontal section of shell replacement; (6) LACMIP 14443, semi-horizontal view of external shell surface (outer shell layer) along lateral region of shell. Scale bars: 20 µm for (1), (3)–(5); 5 µm for (2); 10 µm for (6).
3.3. Molluscs from the Ordovician Cyclora beds of Indiana and Ohio
In mollusc fossils from the Cyclora beds of the Kope, Fairview, and “Arnheim” Formations distinct shell microstructure was rarely observed. In contrast, imprints of fine microstructure are preserved in great detail in most internal moulds of the brachiopod Zygospira (Hall, 1862) and in fossil trilobite spines (Figure 8), and thus the fidelity of preservation in Cyclora beds is sufficient to preserve shell microstructure imprints in molluscs. However, of the more than 400 mollusc fossils examined via SEM, only a few showed unambiguous textures. Nevertheless, the details on some of these specimens were distinct enough to allow inferences to be made about original shell microstructure.
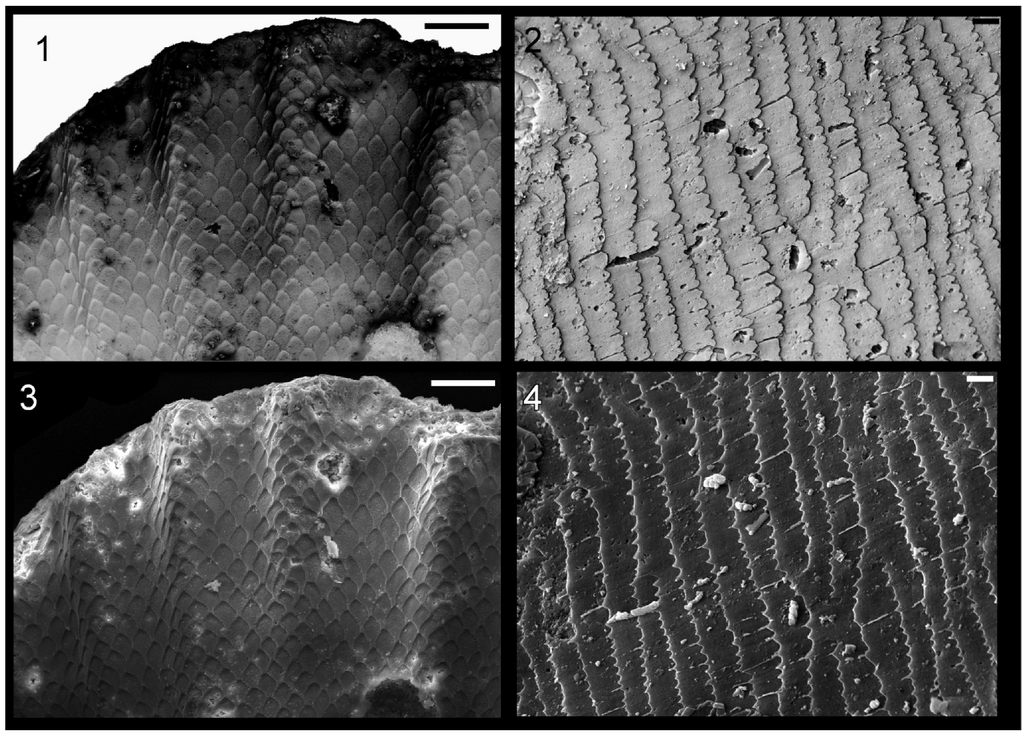
Figure 8.
Imprints of shell microstructure in the brachiopod Zygospira (1) and (3) and a trilobite spine (2) and (4) from the “Arnheim Formation” Cyclora beds of Ohio. Images (1) and (2) are electronically inverted versions of the originals in (3) and (4), to show what the crystal structures imprinted in the fossils originally looked like. (1) and (3) Zygospira sp., LACMIP 14448, from the “Arnheim Formation” near Kemper Road, Cincinnati, Ohio, horizontal surface of internal mould; and (2) and (4) trilobite spine, LACMIP 14449, possibly from the “Arnheim Formation”, Hamilton, Ohio, horizontal surface of internal mould. Scale bars: 50 µm for (1) and (3); and 5 µm for (2) and (4).
Bivalves from the Cyclora beds show the most distinct shell microstructure of any type of mollusc in the deposits. In some specimens of Palaeoconcha, sub-circular and polygonal imprints occur throughout the surface of the internal mould (Figure 9.3, Figure 9.4, Figure 9.5, Figure 9.6 and Figure 9.7). The range of shapes is consistent with growing nacre. Measurements of interfacial angles could be made in six cases, and these ranged from 113° to 132° (mean 123.2°), close to the 122° angle expected in nacre. Widths of the moulds of tablets ranged from 4 µm to 13.5 µm (mean 8.3 µm; median 8.6 µm; standard deviation 2.3 µm; n = 25). These measurements are similar to those of tablets in modern nacre (mean = 6.0 µm; median = 5.5 µm; standard deviation = 2.9 µm; n = 95). Moreover, in some regions of these fossils (Figure 9.6), lines in high relief can be seen intersecting the polygonal depressions. These lines appear to correspond to the boundaries between merged tablets of the underlying lamella (more external in the original shell). These observations support the hypothesis that nacre was present in bivalves at this time, corroborated by Mutvei’s [] observations of this genus from the Maquoketa Formation, and Carter’s [] observations of Silurian representatives of the family.
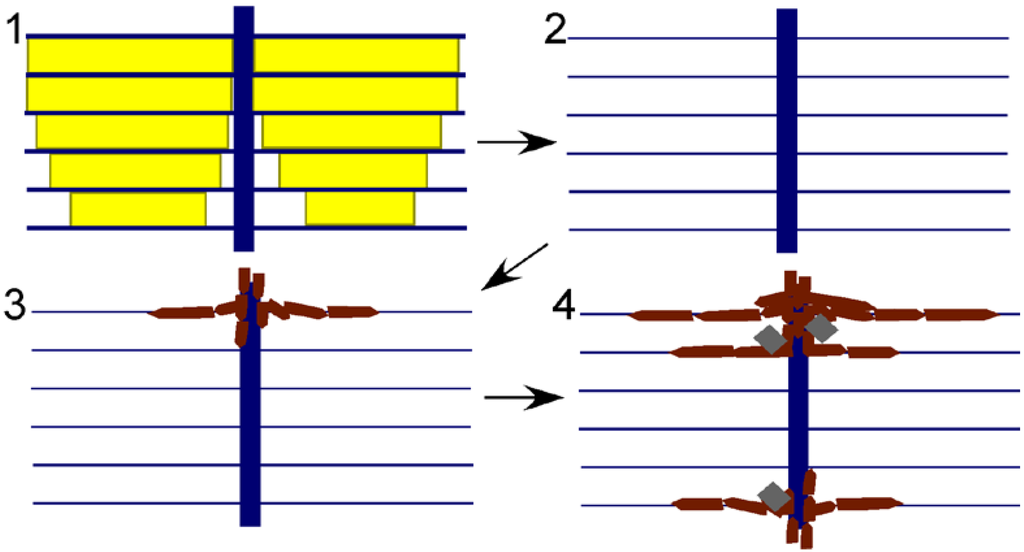
Imprints of shell microstructure in internal moulds of bivalves from the Ordovician of the Midwest are distinct from those of the Cambrian bivalves Pojetaia Jell, 1980 and Fordilla Barrande, 1881. Namely the crystallites in Pojetaia/Fordilla are elongate blade-like laths, whereas the sub-units of the Ordovician bivalves are roughly equidimensional tablets. This microstructure of elongate blades of aragonite arranged in layers was termed “foliated aragonite” by Checa et al. [,], although Carter et al. [] suggested the proper term for this shell microstructure should be “semi-foliated aragonite”. In addition to the blade-like laths, in some regions of fossils of Pojetaia, large, isolated, rounded tablets also occur [], a type of microstructure that Carter et al. [] classified as “large tablet, imbricated nacre” (p. 88). Carter [] previously suggested that this type of microstructure was transitional to nacre. Thus it may be that the transition from foliated (or semi-foliated) aragonite to nacre began in Pojetaia and its kin, and was finalized in the close ancestors of the Cyclora-bed bivalves. This transition from foliated aragonite to nacre was hypothesized by Vendrasco et al. [] and is illustrated in Figure 10.
Internal moulds of gastropods from the Cyclora beds show distinct layered textures, indicating that the innermost shell microstructure was laminar. However, details of the crystalline constituents of the lamellae have not yet been seen, and so it is unclear which type of laminar microstructure is preserved here. In general, coiled shells from Cambrian deposits tend to have lamello-fibrillar shell microstructure [,], where “the horizontal fibers in successive laminae differ in orientation by irregularly varying angles” (p. 611) [].
A small region near the aperture of one gastropod fossil contains a texture that appears to show nacre very clearly (Figure 9.1 and Figure 9.2). However, this texture seems to be below the surface of the internal mould. Although there are scenarios whereby part of the original shell might have been broken and collapsed inward, producing the pattern here, the lack of such textures elsewhere on the surface of the internal mould or on partial casts must call into question the classification of the texture as nacre. If it is not nacre, it will provide a cautionary tale about the over-interpretation of tantalizing but rare textures on fossils. This possibility highlights the need for finding the same textures in multiple specimens of the same species. An alternative interpretation is that this patch represents a fragment of a smaller shell lodged within the empty gastropod chamber prior to infill with phosphate. Such occurrences are common in other deposits of phosphatized molluscs []. If this latter hypothesis is correct, then some unknown member of the Cyclora fauna had nacreous shell microstructure.
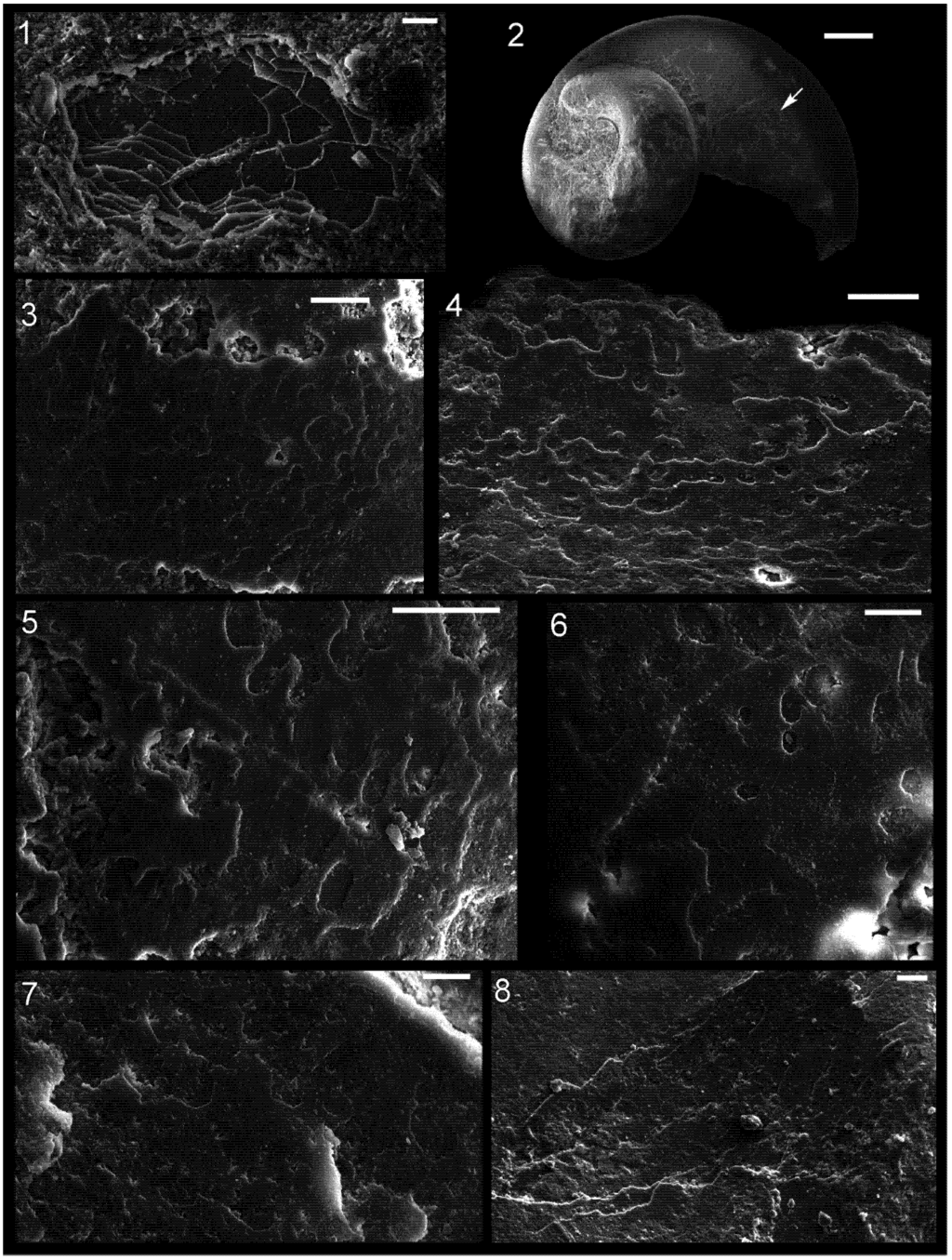
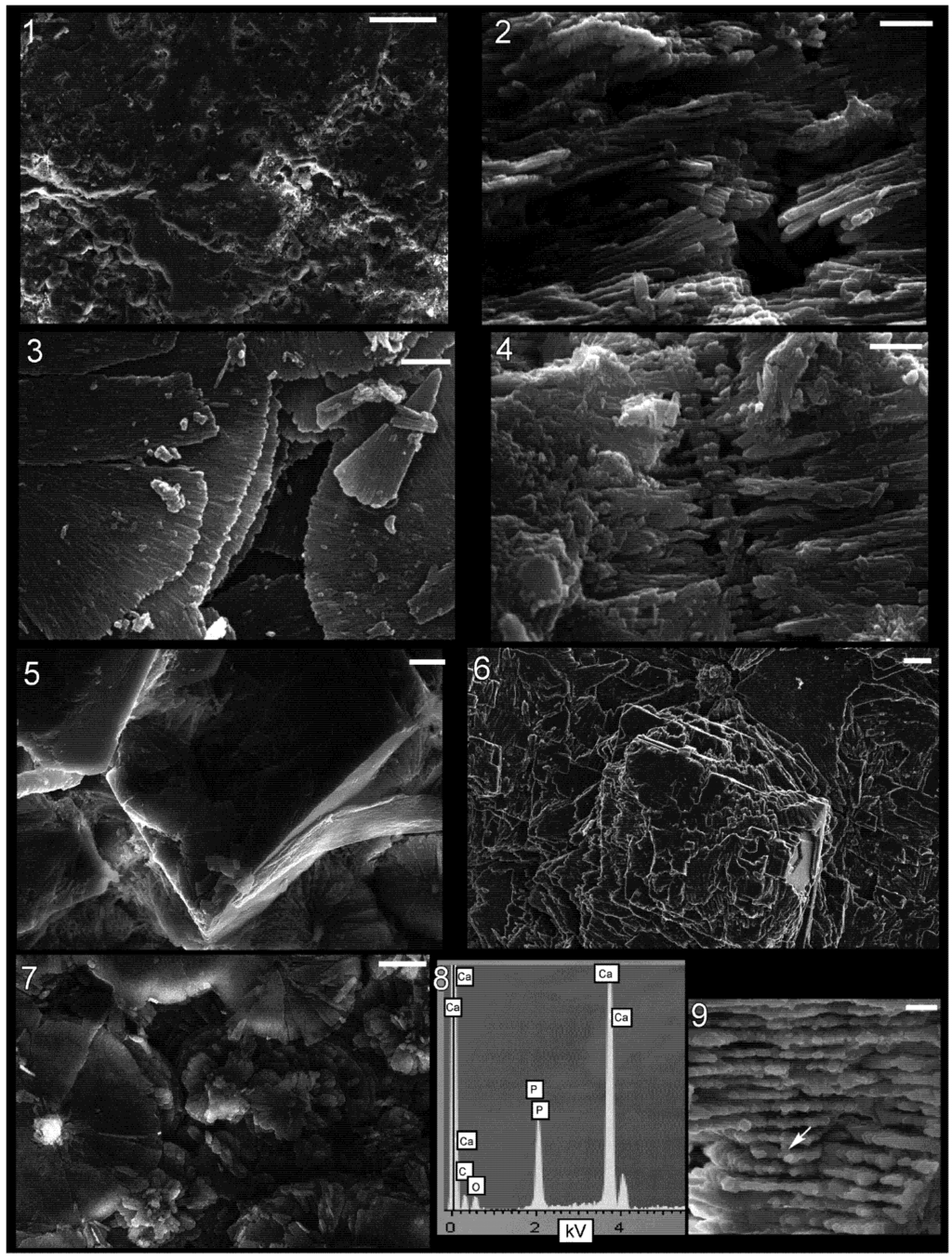
Figure 9.
Shell microstructures in a specimen of the gastropod Cyclora (Cyclonema?) (1) and (2); the bivalves Palaeoconcha sp. (3)–(7); and the bellerophont Cyrtolites sp. (8) from Ordovician localities around Cincinatti, Ohio. (1) and (2) LACMIP 14444, from the Fairview Formation near Dearborn/Lawrenceburg, Indiana, arrow in (2) shows location of (1); (3)–(6) LACMIP 14445, from the Fairview Formation near Dearborn/Lawrenceburg, Indiana: (3) horizontal view of surface of internal mould near midpoint of valve; (4) horizontal view of surface of internal mould near aperture (broken aperture mould at top of photo); (5) horizontal view of surface of internal mould near umbo; (6) horizontal view of surface of internal mould near midpoint of valve; (7) LACMIP 14446, from the “Arnheim Formation” along Kemper Road, Cincinnati, Ohio , horizontal view of surface of internal mould in region between midpoint of valve and umbo; and (8) LACMIP 14447, from the “Arnheim Formation” in Middletown, Ohio, horizontal view of internal mould or thin shell replacement near columellar side of aperture, imaginary line normal to plane of aperture would extend diagonally down/leftward on the photo. Scale bars: 10 µm for (1), (6) and (7); 200 µm for (2); and 20 µm for (3)–(5) and (8).
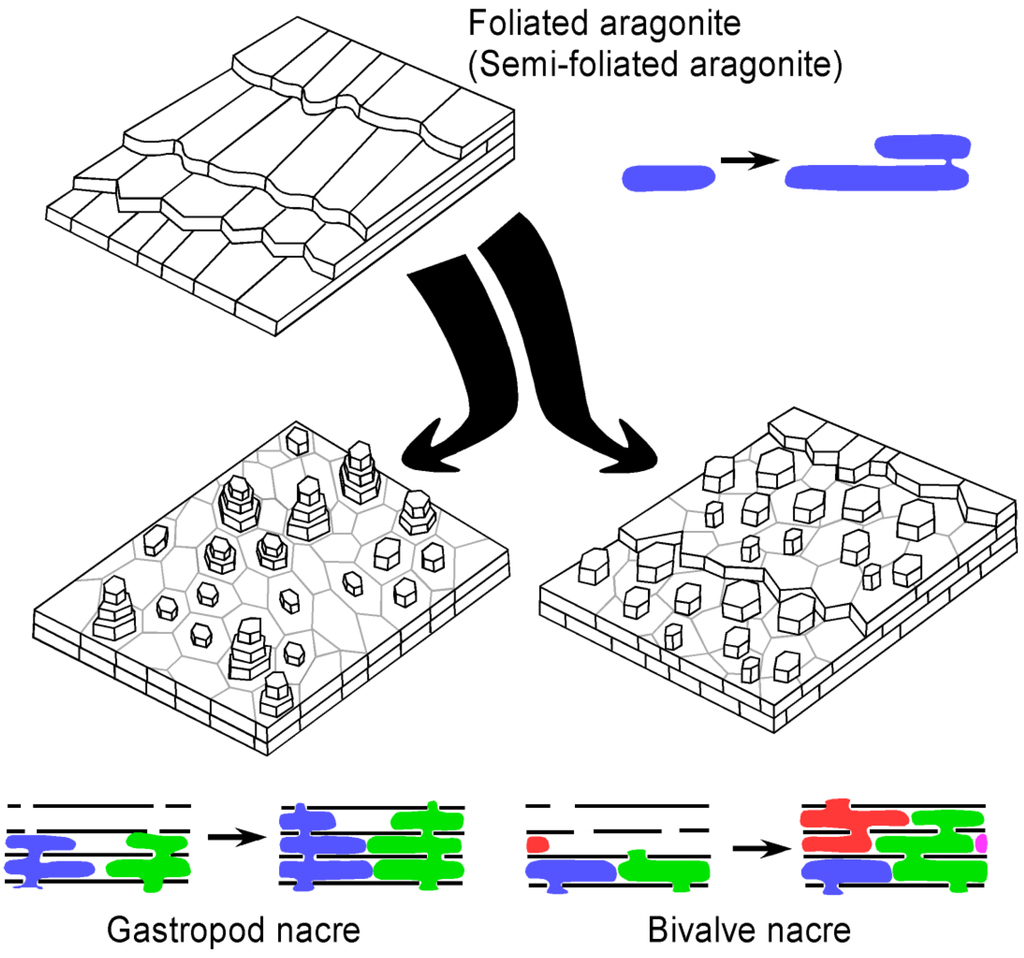
Figure 10.
3-D reconstruction block diagrams and inferred mode of growth of foliated (or semi-foliated) aragonite as well as bivalve and gastropod nacre. The colours represent different aragonite crystals growing through interlamellar membranes. The transformation of foliated (or semi-foliated) aragonite to nacre could have been achieved by addition of a porous interlamellar membrane.
Some bellerophonts from the Cyclora beds likewise reveal a laminar inner shell microstructure (Figure 9.8). One specimen shows slightly raised polygonal sectors. This may represent a replication of nacre tablets, but it has thus far been seen in only one bellerophont, and the outlines of these sectors, while consistent with the range of nacre, show no characteristic that would allow the exclusion of the hypothesis that they are artefacts of diagenesis.
Such cases of equivocal shell microstructure in fossils suggest some principles in doing work on fossil shell microstructures: (1) the same texture should be seen on multiple specimens of the same species to provide certainty that the texture is characteristic of that species; and (2) if the same texture is seen in multiple types of distantly related fossils from the same bed, then some or all of the texture was likely produced by diagenesis.
3.4. Diversification of Shell Microstructures in Early Palaeozoic Molluscs
An overview of the distribution of shell microstructures in molluscs from the Cambrian and Ordovician reveal some basic patterns (Table 1). First, shell microstructures rapidly diversified during the early stages of molluscan evolution, and early molluscs had a composite shell that was composed of multiple types of shell microstructure. In the early Cambrian alone, at least 7 types of shell microstructure occurred in molluscs.

Table 1.
Shell microstructure data for Cambrian molluscs.
| Age (Ma) | Series | Siberia | Australia | China | W. Avalonia/Laurentia |
|---|---|---|---|---|---|
| 514 | Series 3 | Mellopegma | Pseudomyona | Tuarangia | |
| Ribeiria | |||||
| Pseudomyona | |||||
| Corystos | |||||
| Figurina | |||||
| Eurekapegma | |||||
| Pelagiella | |||||
| Protowenella | |||||
| Yochelcionella | |||||
| Costipelagiella | |||||
| Eotebenna | |||||
| 509 | Anabarella | Parailsanella | |||
| Mackinnonia | Yuwenia | ||||
| Obtusoconus | Anabarella | ||||
| Pelagiella | Bemella | ||||
| Mellopegma | Anhuiconus | ||||
| Beshtashiella | |||||
| Series 2 | Aequiconus | ||||
| Calyptroconus | |||||
| Figurina | |||||
| First mass extinction | |||||
| Pojetaia | |||||
| Ilsanella | Pararaconus | ||||
| Yochelcionella | Leptostega | ||||
| Fordilla | Mackinnonia | ||||
| Adrossania | |||||
| Pelagiella | |||||
| 521 | Bemella | Watsonella | |||
| Aldanella | Latouchella | ||||
| Anabarella | Ilsanella | ||||
| Terre- | Ceratoconus | Archaeospira | |||
| neuvian | Securiconus | Ocruranus | |||
| Watsonella | |||||
| Mellopegma | |||||
| Transition to calcite seas | |||||
| Oelandiella | |||||
| 542 |
Moreover, there is a clear correlation between shell microstructure configuration and phylogenetic relationships among Cambrian molluscs: for example, highly coiled molluscs tended to have lamello-fibrillar []; stenothecids had an internal shell layer of calcitic semi-nacre []; the Anabarella Vostokova, 1962–Watsonella Grabau 1900 lineage had the same complex three-component shell microstructure consisting of fibrous, foliated (or semi-foliated) aragonite, and prismatic textures []; bivalves had foliated (or semi-foliated) aragonite []; and Pseudomyona Pojeta & Runnegar, 1976 and Tuarangia Mackinnon, 1982 had foliated calcite []. Shell microstructures appear to be fairly conserved in some lineages through the Cambrian Period, although it is also clear that modifications continued to take place in various mollusc lineages.
Prismatic was probably the first type of shell microstructure to originate in molluscs, although it may have been accompanied by some type of laminar inner shell microstructure. Prismatic shell microstructure is among the most common during the early Cambrian, and it is the typical type of outer shell microstructure in modern molluscs. Feng and Sun [] noted the commonality of lamello-fibrillar microstructure (a configuration similar to what occurs in plywood) in Cambrian molluscs, and this is seen in Table 1 as well. This type of shell microstructure also occurs in the problematic mollusc Ocruranus Liu, 1979 [], and it may represent the ancestral type of inner shell microstructure in molluscs. Thus the archetypal mollusc may have had a bi-layer shell with crystals in a prismatic configuration in the outer layer and lamello-fibrillar texture in the inner one.
Also, it appears that aragonitic shell mineralogy was common in newly originated metazoan taxa during the early Cambrian whereas calcitic microstructures became more common in those originating during the middle Cambrian []. This pattern reflects the change from “aragonite seas” to “calcite seas” around this time [], although within molluscs the record does not show a tight correlation with this aspect of seawater chemistry (Table 1). Transitions from aragonite to calcite have been shown to occur in abundance during a switch to calcite seas [], but calcite can also be favoured for other reasons like temperature and in bivalves switches to calcite often occurred during times of aragonite seas [,]. Thus it is still unclear to what extent these changes in seawater chemistry controlled the transitions in the mineralogy of mollusc shells at this time.
Foliated (or semi-foliated) aragonite was recently discovered in modern monoplacophorans [,], and this type of microstructure characterized the earliest known bivalves []. A similar shell microstructure can also be seen in other molluscs from the early and middle Cambrian []. This shell microstructure appears to have been modified into nacre in bivalves, and possibly also other molluscs like monoplacophorans and cephalopods.
3.5. The Origin of Nacre
Shell microstructures are now known from about forty genera of some of the earliest (early to middle Cambrian) molluscs. This information has come mostly from phosphatic internal moulds, a type of preservation that was common in the early to middle Cambrian but became much less frequent since then. These data reveal a remarkable diversity of different types of shell microstructure, but no nacre (Table 1). Vendrasco et al. [,] demonstrated that nacre previously described from the Cambrian mollusc Mellopegma [] was calcitic semi-nacre instead.
Nacre had previously been thought to be homologous within the Conchifera, primitive in this molluscan clade [,] because: (1) it only occurs in molluscs; (2) it occurs in four different molluscan classes: Gastropoda, Cephalopoda, Bivalvia, and Monoplacophora; (3) it was thought to occur widely in the most primitive conchiferan group, the Monoplacophora; and (4) it was thought to occur in Cambrian molluscs, in part because of its inferred presence in the middle Cambrian mollusc Mellopegma.
However, Vendrasco et al. [] concluded that nacre instead convergently evolved in early molluscs because: (1) there are no clear cases of nacre in molluscs from the early or middle Cambrian, in spite of much data on their shell microstructures; (2) there are differences in the microscopic form and growth method of nacre between modern molluscan classes; (3) the constituent proteins differ markedly between nacre-bearing molluscs []; (4) the genes associated with nacre vary among modern classes of mollusc []; and (5) many modern monoplacophorans have foliated (or semi-foliated) aragonite instead of or together with nacre in their inner shell.
The earliest nacre appears to be from Ordovician molluscs. Drushchits et al. [] argued that nacre was present in an Ordovician monoplacophoran, but their photos of vertical sections merely show a laminar shell microstructure that might be nacre. Our results herein provide support for the hypothesis that nacre occurred in cephalopods from the Maquoketa Formation, and additional evidence presented here suggests nacre also occurred in bivalves and possibly gastropods from this time.
3.6. Patterns in the Evolution of Shell Microstructures through the Phanerozoic
The fossil record reveals that through the Phanerozoic the mineralogy and constituent types of shell microstructures varied through time within specific mollusc lineages. While some aspects of shell microstructure remained consistent in most or all groups (e.g., high proportion of organic matter; prismatic outer layer; mineral composition of calcium carbonate), other features are more mutable (e.g., thickness of shell layers, microstructure and mineralogy of the middle and inner shell layers). The adaptability in the construction of the shell allowed molluscs to keep pace with intense shell-crushing pressure during the Marine Mesozoic Revolution.
During the Cambrian, shell microstructure was largely conserved in most mollusc lineages. However, some aspects of the shell changed in mollusc lineages, producing better defences that reflected increasing predatory pressure. Subsequent changes in shell microstructure in various mollusc lineages likely reflect complex interplay between biotic factors like predation and parasitism and abiotic factors like seawater composition and temperature. The type of microstructure within the shell is expected to be under strong selective pressure because it influences: (1) energy demand of the animal; (2) resistance to different types and intensity of predatory attack; (3) speed of formation of shell (and hence how much time the juvenile animal is without significant armour); (4) rate of dissolution in seawater; and (5) mass of the animal. The most popular hypothesis about the control of shell microstructure is that predation is the primary influencing force, whereas for shell mineralogy arguments about seawater chemistry and temperature are common. There is evidence for a strong influence on shell composition from both of these environmental factors. Thus, while West and Cohen [] documented changes in the number of crossed-lamellar layers in snails from Lake Tanganyika correlated with intensity of predation, others documented changes in shell mineralogy correlated with changes in seawater chemistry [,] and temperature [,].
In spite of this preliminary evidence, we are still somewhat in the dark both about the typical adaptability of molluscs with respect to aspects of shell microstructure as well as the conceptual heights of adaptive peaks of various shell microstructures. Additional work is needed to determine more complete sets of costs and benefits for each type of shell microstructure in each environmental milieu.
3.7. Evolution and Escalation during the Great Ordovician Biodiversification Event
Predation is a strong selective pressure. Evidence from the fossil record reveals that as predators became increasingly efficient at capturing prey through geologic time, ever-improving defensive co-adaptations evolved in their prey. This evolutionary escalation or arms race between predator and prey produced many of the notable characteristics of different groups of modern animals such as thick shells, mineral-reinforced jaws and teeth, powerful claws, sensory systems, and mobility.
Escalation is documented in molluscs through the Mesozoic and Cainozoic Eras (from 250 million years ago until now) []. However, the earliest history of the arms race between predators and mollusc prey is poorly known, and most studies of escalation with molluscs focus only on macroscopic changes in the shell or on signs of predation like shell breakage. In addition, factors besides predation can also strongly influence mollusc shell mineralogy and microstructure, and the comparative importance of defence in controlling shell microstructure is still unclear. It is also uncertain to what extent nacre is ideal under intense predation, as many bivalves and gastropods appear to have lost it since the early Mesozoic while predation intensity increased [].
The arms race between predators and their molluscan prey began about 540 million years ago with the earliest diversification of animals during the “Cambrian Explosion” []. This arms race appears to have accelerated during the continuation of this early major adaptive radiation: the Great Ordovician Biodiversification Event (GOBE, beginning about 490 million years ago). The compilation of key evolutionary events related to predation (Table 2) shows a prominent increase in both predatory and defensive abilities during both the Cambrian and Ordovician. Specific evidence for predatory attacks on shelled prey from the Ordovician of the Midwestern United States include shell breakage and repair in brachiopod shells [], a fossil of an asteroid wrapped around a bivalve [], damage and repair to crinoid skeletons [,,], and damage to trilobite skeletons []. Alexander [] hypothesized that nautiloids were the likely shell-breaking culprits in these cases; Babcock [] agreed and added eurypterids as an additional suspect.
Increasing intensity of predation beginning in the early Palaeozoic put pressure on early molluscs to defend themselves. One of the primary ways that molluscs avoid being eaten is to strengthen the shell via thickening and through specific shell microstructures. Early tests indicated that nacre is the strongest shell microstructure (Figure 11) [,]. Thus nacre might be expected to be selectively favoured under conditions of high shell-crushing intensity, when the benefits of forming a costly structure outweigh the disadvantages. However previous studies on strength of shell microstructure were not comprehensive in testing. Additional work is needed to test the strength of all major types of shell microstructures in molluscs, to demonstrate whether nacre is in fact the ideal shell microstructure under most scenarios of high predation. Nacre is thought to possess its strength largely through the high abundance of organic matter within the shell, but not all other types of shell microstructure with high organic content have been tested for strength. For example, calcitic semi-nacre as well as calcitic prismatic and aragonitic granular prismatic shell microstructures have a high proportion of organic matrix in the shell but were never previously subjected to biomechanical tests against nacre. Nacre has been lost in many molluscan lineages, which might indicate it is not ideal under all types of predation pressure, or it may reflect the success of other predator-avoidance strategies.
One possible reason why nacre has been lost repeatedly in molluscs is that it is an energetically expensive type of shell microstructure to form [,]. This hypothesis is supported by the high proportion of organic matrix within nacre compared to other types of shell microstructure. The organic component should entail a greater cost than the mineral component of a shell, but the hypothesis that nacre is the most expensive type of shell microstructure has likewise not been rigorously tested. For example, the energetic cost of formation has not been estimated for the aforementioned calcitic semi-nacre as well as calcitic prismatic and aragonitic granular prismatic shell microstructures.

Table 2.
Estimated time of first appearance of key features in the early arms race between predators and their molluscan prey. Innovations listed in red are predatory, those in black are defence, and the one in blue indicates evidence for shell crushing predation. Data derived from [,,,,,,,,,] and herein.
| Predator/prey innovation | Terreneuvian Cambrian | Series 2 Cambrian | Series 3 Cambrian | Furongian Cambrian | Lower Ordovician | Middle Ordovician | Upper Ordovician |
|---|---|---|---|---|---|---|---|
| 542 Ma | 521 Ma | 509 Ma | 497 Ma | 542 Ma | 542 Ma | 542 Ma | |
| Shell drilling | | ||||||
| Whole animal ingestion | | ||||||
| Grasping appendages | | ||||||
| >500 cm long predators | | ||||||
| Arthropod predators | | ||||||
| Durophagy | | ||||||
| Crossed-lamellar shell | | ||||||
| Type 1 driller (Brett) | | ||||||
| Cephalopod predators | | ||||||
| Large, thick mollusc shells | | ||||||
| Diversification of cephalopods | | ||||||
| Type 2 driller (Brett) | | ||||||
| Very large (>1.5 m) predators | | ||||||
| Echinoderm predators | | ||||||
| Hard annelid jaws | | ||||||
| Diversification of conodonts | | ||||||
| Eurypterids | | ||||||
| Shell lip peeling | | ||||||
| Brachiopod spines | | ||||||
| Large (>60 mm) shell scars | | ||||||
| Durophagous eurypterids | | ||||||
| Crinoid spines | | ||||||
| Thick crinoid calyx | | ||||||
| Brachiopod geniculation | | ||||||
| Brachiopod commissure ridge | | ||||||
| Nacre | |
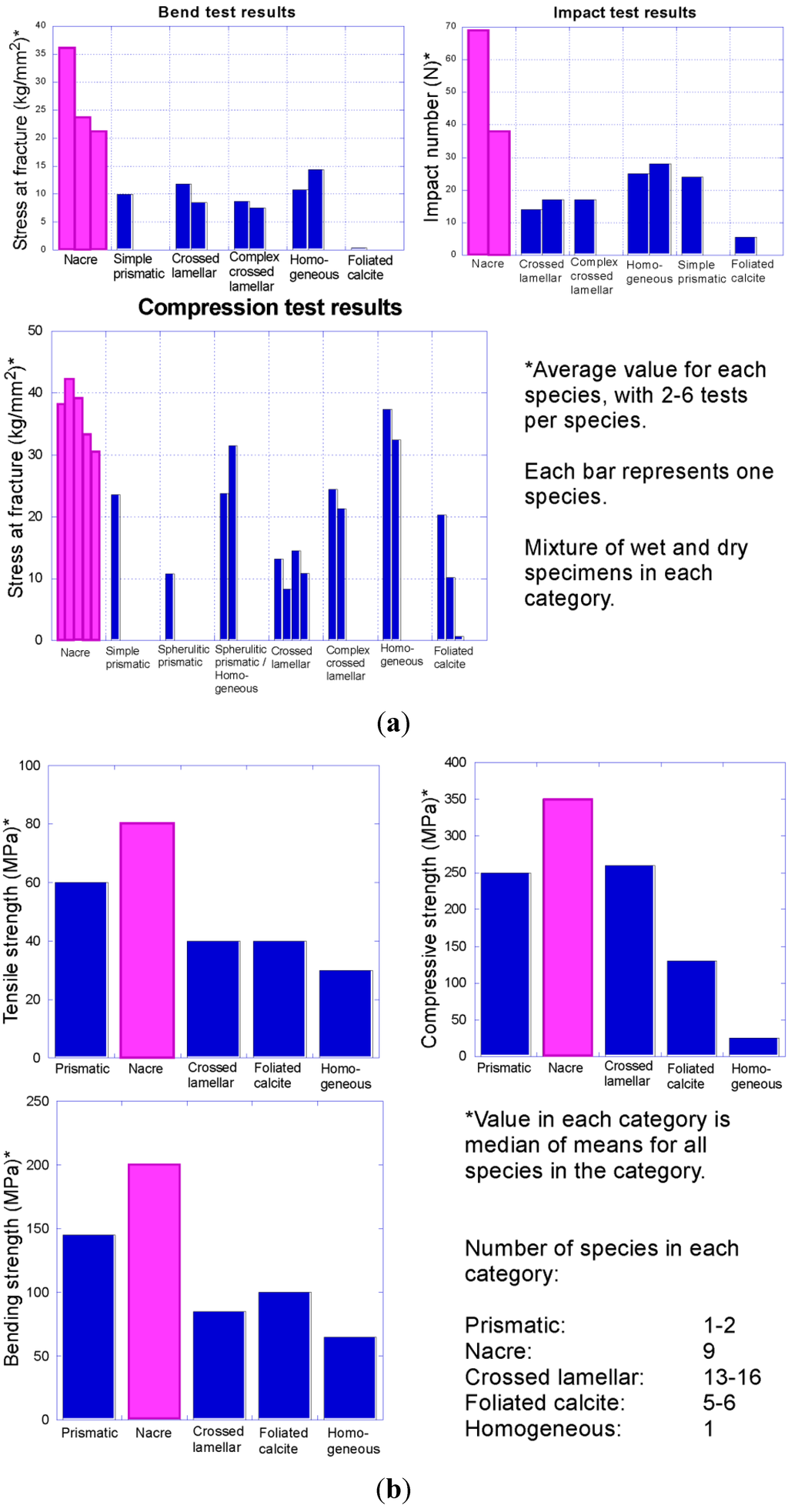
Figure 11.
Compilation of data on comparative strength of molluscan shell microstructures. Note that nacre (pink) consistently outperformed all other shell microstructures in tests of toughness in these two separate studies: (a) Fracture resistance of different bivalve shell microstructures (data from Taylor and Layman []); (b) strength of different mollusc shell microstructures (data from Currey []).
4. Conclusions
Contrary to previous beliefs, nacre does not appear to have been primitive in conchinferans but instead evolved multiple times later in molluscan evolution. Our preliminary work indicates that nacre originated sometime during the interval between the middle Cambrian and late Ordovician. The earliest records of nacre are from the Upper Ordovician, but the lack of deposits with suitable preservation from the late Cambrian through middle Ordovician may hide an even earlier origin of nacre. This type of shell microstructure may have evolved as a response to increasing predation pressure during the Great Ordovician Biodiversification Event.
Acknowledgments
We thank Steve Felton and Tom Bantel for their donations of Ordovician fossils from Ohio. In addition, Tom Bantel provided assistance in the field and Steve Felton provided important locality details. Steve, Tom, and many other members of the Dry Dredgers continue to contribute fossil specimens and locality information to advance research on the Cincinnatian Series. We thank Carl Brett for on-site stratigraphic information and help. We thank Mary Stecheson, Los Angeles Museum of Natural History, and Mark Florence, United States National Museum, for their assistance and loans of fossils. We also thank the Miami University library for loaning masters theses. Joseph Carter, John Pojeta, Jr., Susannah Porter, and Christian Skovsted each provided insightful and detailed suggestions for improving our manuscript. This work was funded by projects CGL2010-20748-CO2-01 of the Spanish Ministerio de Ciencia e Innovación and RNM6433 of the Andalusian Consejería de Innovación Ciencia y Tecnología.
References
- Runnegar, B. Shell microstructures of Cambrian molluscs replicated by phosphate. Alcheringa 1985, 9, 245–257. [Google Scholar] [CrossRef]
- Kouchinsky, A. Shell microstructures in Early Cambrian molluscs. Acta Palaeontol. Pol. 2000, 45, 119–150. [Google Scholar]
- Vendrasco, M.J.; Porter, S.M.; Kouchinsky, A.; Li, G.; Fernandez, C.Z. New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 2010, 53, 97–135. [Google Scholar] [CrossRef]
- Vendrasco, M.J.; Kouchinsky, A.V.; Porter, S.M.; Fernandez, C.Z. Phylogeny and escalation in Mellopegma and other Cambrian molluscs. Palaeontol. Electron. 2011, 14, 11A:1–11A:44. [Google Scholar]
- Taylor, J.D.; Layman, M. The mechanical properties of bivalve (Mollusca) shell structures. Palaeontology 1972, 15, 73–87. [Google Scholar]
- Currey, J.D. Shell form and strength. In The Mollusca, Volume 11: Form and Function; Wilbur, K.M., Trueman, E.R., Clarke, M.R., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 183–210. [Google Scholar]
- Palmer, A.R. Relative cost of producing skeletal organic matrix versus calcification: Evidence from marine gastropods. Mar. Biol. 1983, 75, 287–292. [Google Scholar] [CrossRef]
- Palmer, A.R. Calcification in marine molluscs: How costly is it? Proc. Natl. Acad. Sci. USA 1992, 89, 1379–1382. [Google Scholar] [CrossRef]
- Vendrasco, M.J.; Checa, A.G.; Kouchinsky, A.V. Shell microstructure of the early bivalve Pojetaia and the independent origin of nacre within the Mollusca. Palaeontology 2011, 54, 825–850. [Google Scholar] [CrossRef]
- Grégoire, C. On organic remains in shells of Paleozoic and Mesozoic cephalopods (Nautiloids and Ammonoids). Bull. Inst. R. Sci. Nat. Belg. 1966, 42, 1–36. [Google Scholar]
- Mutvei, H. Flexible nacre in the nautiloid Isorthoceras, with remarks on the evolution of cephalopod nacre. Lethaia 1983, 16, 233–240. [Google Scholar] [CrossRef]
- Mutvei, H. Ultrastructural evolution of molluscan nacre. In Biomineralization and Biological Metal Accumulation; Westbroek, P., de Jong, E.W., Eds.; D. Reidel Publishing Company: Dordrecht, the Netherlands, 1983; pp. 267–271. [Google Scholar]
- Carter, J.G.; Lawrence, D.R.; Sanders, H. Shell microstructural data for the Bivalvia. Part II. Orders Nuculoida and Solemyoida. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, pp. 303–319. [Google Scholar]
- Baptista, B.M. Petrology of the Limestones of the Arnheim Formation (Cincinnatian Series) from Western Butler County, Ohio. Master’s Thesis, Miami University, Oxford, OH, USA, 1969. [Google Scholar]
- Martin, A.J. A Paleoenvironmental Interpretation of the “Arnheim” Micromorph Fossil Assemblage from the Cincinnatian Series (Upper Ordovician), Southeastern Indiana and Southwestern Ohio. Master’s Thesis, Miami University, Oxford, Ohio, USA, 1986. [Google Scholar]
- Meyer, D.L.; Davis, R.A. A Sea Without Fish; Indiana University Press: Bloomington, IN, USA, 2009. [Google Scholar]
- Caster, K.E.; Dalve, E.A.; Pope, J.K. Elementary Guide to the Fossils and Strata of the Ordovician in the Vicinity of Cincinnati, Ohio; Cincinnati Museum of Natural History: Cincinnati, OH, USA, 1961. [Google Scholar]
- Davis, R.A. Cincinnati Fossils, an Elementary Guide to the Ordovician Rocks and Fossils of the Cincinnati, Ohio, Region; Cincinnati Museum Center: Cincinnati, OH, USA, 1998. [Google Scholar]
- Martin, A.J.; Martin, W.D.; Pope, J.K. Genesis of phosphatic sediments in Cincinnatian Series (Upper Ordovician), southeastern Indiana and southwestern Ohio. AAPG Bull. 1985, 69, 1441. [Google Scholar]
- Martin, A.J.; Pope, J.K. Paleoenvironmental interpretation of a micromorph fossil assemblage from the Cincinnatian Series (Upper Ordovician), Indiana and Ohio. GSA Abstr. Programs 1985, 17, 653–654. [Google Scholar]
- Holland, S.M. The type Cincinnatian Series: An overview. In Stratigraphic Renaissance in the Cincinnati Arch; McLaughlin, P.I., Brett, C.E., Holland, S.M., Storrs, G.W., Eds.; Cincinnati Museum Center: Cincinnati, OH, USA, 2008; pp. 173–184. [Google Scholar]
- Holland, S.M. Department of Geology, The University of Georgia, Athens, GA, USA. Personal Communication.
- Baumann, S.D.J. Rock Outcrop of the Maquoketa Graf Section and Highway D-17 Section, Iowa, Lower Scales and Neda Formations; No. G-032009-1A; Midwest Institute of Geosciences and Engineering: Chicago, IL, USA, 2009. Available online: http://www.migeweb.org/ (accessed on 2 September 2012).
- Miller, A.K.; Youngquist, W. The Maquoketa coquina of cephalopods. J. Paleontol. 1949, 23, 199–204. [Google Scholar]
- Tasch, P. Paleoecologic observations on the orthoceratid coquina beds of the Maquoketa at Graf, Iowa. J. Paleontol. 1955, 29, 510–518. [Google Scholar]
- Hay, H.B.; Cuffey, R.J. The “Brookville Formation” (“Excello,” Waynesville, and Liberty Members) at Bon Well Hill near Brookville (Upper Ordovician, southeastern Indiana). In Sampling the Layer Cake That Isn’t: The Stratigraphy and Paleontology of the Type-Cincinnatian; Ohio Division of Geological Survey Guidebook No. 13; Davis, R.A., Cuffey, R.J., Eds.; Ohio Division of Geological Survey: Columbus, OH, USA, 1998; pp. 79–83. [Google Scholar]
- Hay, H.B.; Kirchner, B.; Cuffey, R.J. “Excello” (Arnheim) to basal Saluda strata on Indiana Route 1 at South Gate Hill (Upper Ordovician, southeastern Indiana). In Sampling the Layer Cake That Isn’t: The Stratigraphy and Paleontology of the Type-Cincinnatian; Ohio Division of Geological Survey Guidebook No. 13; Davis, R.A., Cuffey, R.J., Eds.; Ohio Division of Geological Survey: Columbus, OH, USA, 1998; pp. 89–94. [Google Scholar]
- Grandjean, J.; Grégoire, Ch.; Lutts, A. On the mineral components and the remnants of organic structures in shells of fossil molluscs. Bull. Acad. R. Sci. Nat. Belg. 1964, 50, 562–595. [Google Scholar]
- Prévôt, L.; Lucas, J. Microstructure of apatite-replacing carbonate in synthesized and natural samples. J. Sediment. Pet. 1986, 56, 153–159. [Google Scholar]
- Balthasar, U. Mummpikia gen. nov. and the origin of Calcitic-shelled Brachiopods. Palaeontology 2008, 51, 263–279. [Google Scholar] [CrossRef]
- Carter, J.G.; Bandel, K.; de Buffrénil, V.; Carlson, S.J.; Castanet, J.; Crenshaw, M.A.; Dalingwater, J.E.; Francillon-Vieillot, H.; Géraudie, J.; Meunier, F.J.; et al. Glossary of skeletal biomineralization. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, pp. 609–671. [Google Scholar]
- Carter, J.G. Evolutionary significance of shell microstructure in the Palaeotaxodonta, Pteriomorphia and Isofilibranchia (Bivalvia: Mollusca). In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, pp. 135–296. [Google Scholar]
- Carter, J.G. Shell and ligament microstructure of selected Silurian and Recent palaeotaxodonts (Mollusca: Bivalvia). Am. Malacol. Bull. 2001, 16, 217–238. [Google Scholar]
- Checa, A.; Ramírez, J.; González-Segura, A.; Sánchez-Navas, A. Nacre and false nacre (foliated aragonite) in extant monoplacophorans (=Triblidiida: Mollusca). Naturwissenschaften 2009, 96, 111–122. [Google Scholar] [CrossRef]
- Checa, A.; Sánchez-Navas, A.; Rodríguez-Navarro, A. Crystal growth in the foliated aragonite of monoplacophorans (Mollusca). Cryst. Growth Des. 2009, 9, 4574–4580. [Google Scholar] [CrossRef]
- Carter, J.G.; Harries, P.J.; Malchus, N.; Sartori, A.F.; Anderson, L.C.; Bieler, R.; Bogan, A.E.; Coan, E.V.; Cope, J.C.W.; Cragg, S.M.; et al. Illustrated glossary of the Bivalvia. Treatise Online 2012, 48, 1–209. [Google Scholar]
- Feng, W.; Sun, W. Phosphate replicated and replaced microstructure of molluscan shells from the earliest Cambrian of China. Acta Palaeontol. Pol. 2003, 48, 21–30. [Google Scholar]
- Qian, Y.; Bengtson, S. Palaeontology and Biostratigraphy of the Early Cambrian Meishucunian Stage in Yunnan Province, South China; Fossils and strata series; Universitetsforlaget: Oslo, Norway, 1989; pp. 1–156. [Google Scholar]
- Kouchinsky, A. Shell microstructures of the Early Cambrian Anabarella and Watsonella as new evidence on the origin of the Rostroconchia. Lethaia 1999, 32, 173–180. [Google Scholar] [CrossRef]
- Runnegar, B.; Pojeta, J., Jr. The earliest bivalves and their Ordovician descendents. Am. Malacol. Bull. 1992, 9, 117–122. [Google Scholar]
- Vendrasco, M.J.; Li, G.; Porter, S.M.; Fernandez, C.Z. New data on the enigmatic Ocruranus-Eohalobia group of early Cambrian small skeletal fossils. Palaeontology 2009, 52, 1373–1396. [Google Scholar] [CrossRef]
- Porter, S.M. Seawater chemistry and early carbonate biomineralization. Science 2007, 316, 1302. [Google Scholar] [CrossRef]
- Harper, E.M.; Palmer, T.J.; Alphey, J.R. Evolutionary response by bivalves to changing Phanerozoic sea-water chemistry. Geol. Mag. 1997, 134, 403–407. [Google Scholar] [CrossRef]
- Carter, J.G.; Barrera, E.; Tevesz, M.J.S. Thermal potentiation and mineralogical evolution in the Bivalvia (Mollusca). J. Paleontol. 1998, 72, 991–1010. [Google Scholar]
- Carter, J.G.; Seed, R. Thermal potentiation and mineralogical evolution in Mytilus (Mollusca: Bivalvia). In Bivalves: An Eon of Evolution—Paleobiological Studies Honouring Norman D. Newell; Johnston, P.A., Haggart, J.W., Eds.; University of Calgary Press: Calgary, Canada, 1998; pp. 87–117. [Google Scholar]
- Carter, J.G.; Clark, G.R., II. Classification and phylogenetic significance of molluscan shell microstructure. In Mollusks, Notes for a Short Course; Broadhead, T.W., Ed.; University of Tennessee: Knoxville, TN, USA, 1985; pp. 50–71. [Google Scholar]
- Marie, B.; Marin, F.; Marie, A.; Bédouet, L.; Dubost, L.; Alcaraz, G.; Milet, C.; Luquet, G. Evolution of nacre: Biochemistry and proteomics of the shell organic matrix of the cephalopod Nautilus macromphalus. ChemBioChem 2009, 10, 1495–1506. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Woodcroft, B.; Moase, P.; Rose, R.A.; Kube, M.; Reinhardt, R.; Rokhsar, D.S.; Montagnani, C.; Joubert, C.; et al. Marallel evolution of nacre building gene sets in molluscs. Mol. Biol. Evol. 2010, 27, 591–608. [Google Scholar] [CrossRef]
- Druschchits, V.V.; Doguzhayeva, L.A.; Korinevskiy, V.G. Shell microstructure of the Ordovician monoplacophoran Romaniella Doguzhaeva, 1972. Dokl. Akad. Nauk SSR 1979, 245, 458–461. [Google Scholar]
- West, K.; Cohen, A. Shell microstructure of gastropods from Lake Tanganyika. Evolution 1996, 50, 672–681. [Google Scholar] [CrossRef]
- Stanley, S.M. Influence of seawater chemistry on biomineralization throughout Phanerozoic time: Paleontological and experimental evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 214–236. [Google Scholar] [CrossRef]
- Vermeij, G.J. Evolution and Escalation; Princeton University Press: Princeton, NJ, USA, 1987. [Google Scholar]
- Bengtson, S. Origins and early evolution of predation. In The Fossil Record of Predation, Paleontological Society Papers; Kowalewski, M., Kelley, P.H., Eds.; The Paleontological Society: Boulder, CO, USA, 2002; Volume 8, pp. 289–317. [Google Scholar]
- Alexander, R.R. Resistance to and repair of shell breakage induced by durophages in Late Ordovician brachiopods. J. Paleontol. 1986, 60, 273–285. [Google Scholar]
- Blake, D.B.; Guensburg, T.E. Predation by the Ordovician asteroid Promopalaeaster on a pelecypod. Lethaia 1994, 27, 235–239. [Google Scholar]
- Ausich, W.I.; Baumiller, T.K. Column regeneration in an Ordovician crinoid (Echinodermata): paleobiologic implications. J. Paleontol. 1993, 67, 1068–1070. [Google Scholar]
- Donovan, S.K.; Schmidt, D.A. Survival of crinoid stems following decapitation: Evidence from the Ordovician and palaeobiological implications. Lethaia 2001, 34, 263–270. [Google Scholar] [CrossRef]
- Baumiller, T.K.; Gahn, F.J. Fossil record of parasitism on marine invertebrates with special emphasis on the platyceratid-crinoid interaction. In The Fossil Record of Predation, Paleontological Society Papers; Kowalewski, M., Kelley, P.H., Eds.; The Paleontological Society: Boulder, CO, USA, 2002; Volume 8, pp. 195–209. [Google Scholar]
- Babcock, L.E. Trilobites in Paleozoic predator-prey systems, and their role in reorganization of Early Paleozoic ecosystems. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Kowalewski, M., Eds.; Kluwer/Plenum: New York, NY, USA, 2003; pp. 55–92. [Google Scholar]
- Bruton, D.L. The arthropod Sidneyia inexpectans, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 1981, 295, 619–653. [Google Scholar] [CrossRef]
- Signor, P.W.; Vermeij, G.J. The plankton and the benthos: Origins and early history of an evolving relationship. Paleobiology 1994, 20, 297–319. [Google Scholar]
- Ebbestad, J.O.; Peel, J.S. Attempted predation and shell repair in Middle and Upper Ordovician gastropods from Sweden. J. Paleont. 1997, 71, 1007–1019. [Google Scholar]
- Brett, C.E. Durophagous predation in Paleozoic marine benthic assemblages. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Kowalewski, M., Eds.; Kluwer/Plenum: New York, NY, USA, 2003; pp. 401–432. [Google Scholar]
- Kröger, B. Large shell injuries in Middle Ordovician Orthocerida (Nautiloidea, Cephalopoda). GFF 2004, 126, 311–316. [Google Scholar] [CrossRef]
- Skovsted, C.B.; Brock, G.A.; Lindström, A.; Peel, J.S.; Paterson, J.R.; Fuller, M.K. Early Cambrian record of failed durophage and shell repair in an epibenthic mollusc. Biol. Lett. 2007, 3, 314–317. [Google Scholar] [CrossRef]
- Kröger, B.; Landing, E. Cephalopods and paleoenvironments of the Fort Cassin Formation (Upper Lower Ordovician), eastern New York and adjacent Vermont. J. Paleont. 2009, 83, 664–693. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).