Dolomitization and Silicification in Syn-Rift Lacustrine Carbonates: Evidence from the Late Oligocene–Early Miocene Duwi Basin, Red Sea, Egypt
Abstract
1. Introduction
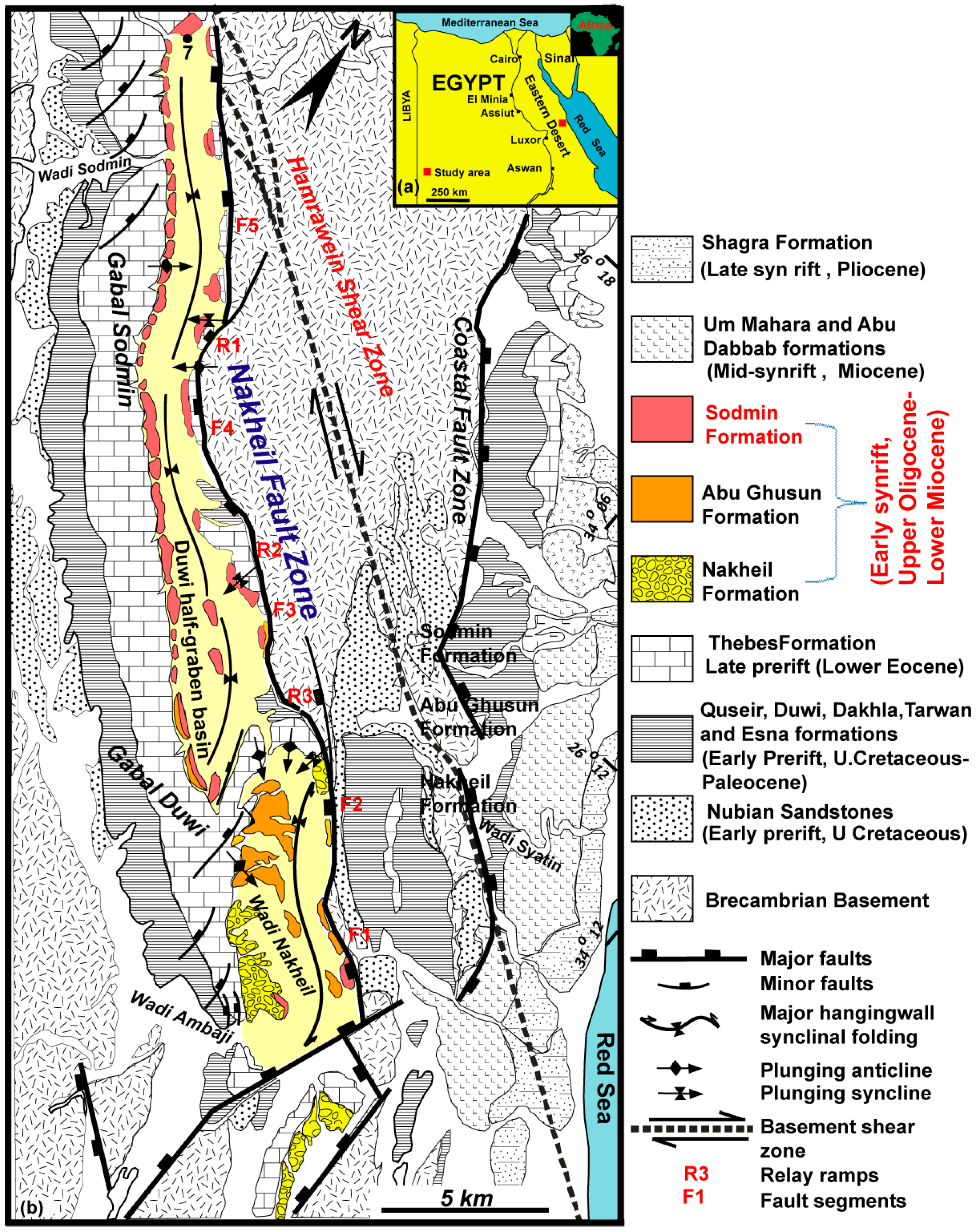
2. Geologic Setting
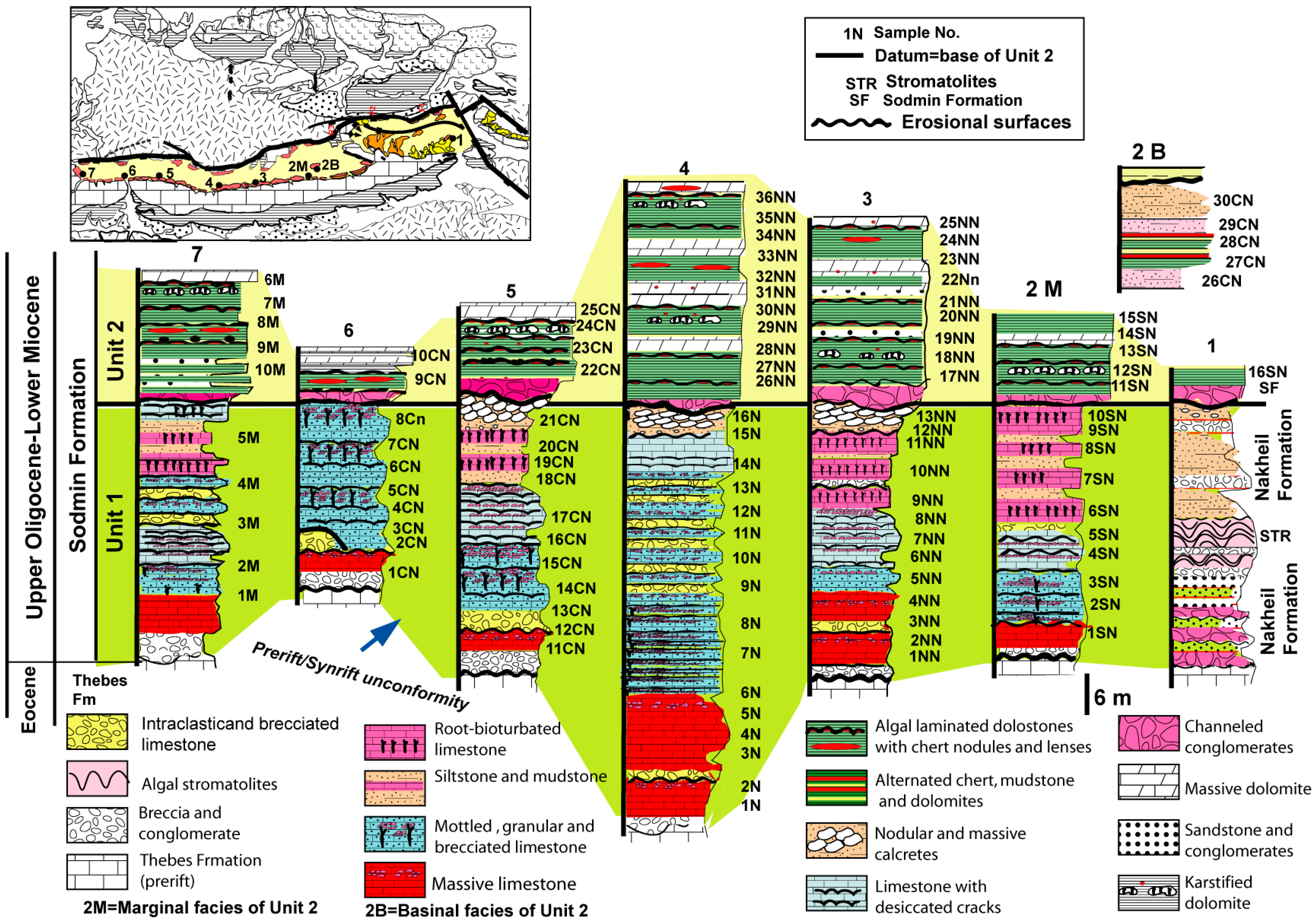
3. Stratigraphy
3.1. Unit 1
3.2. Unit 2
4. Material and Methods
5. Results
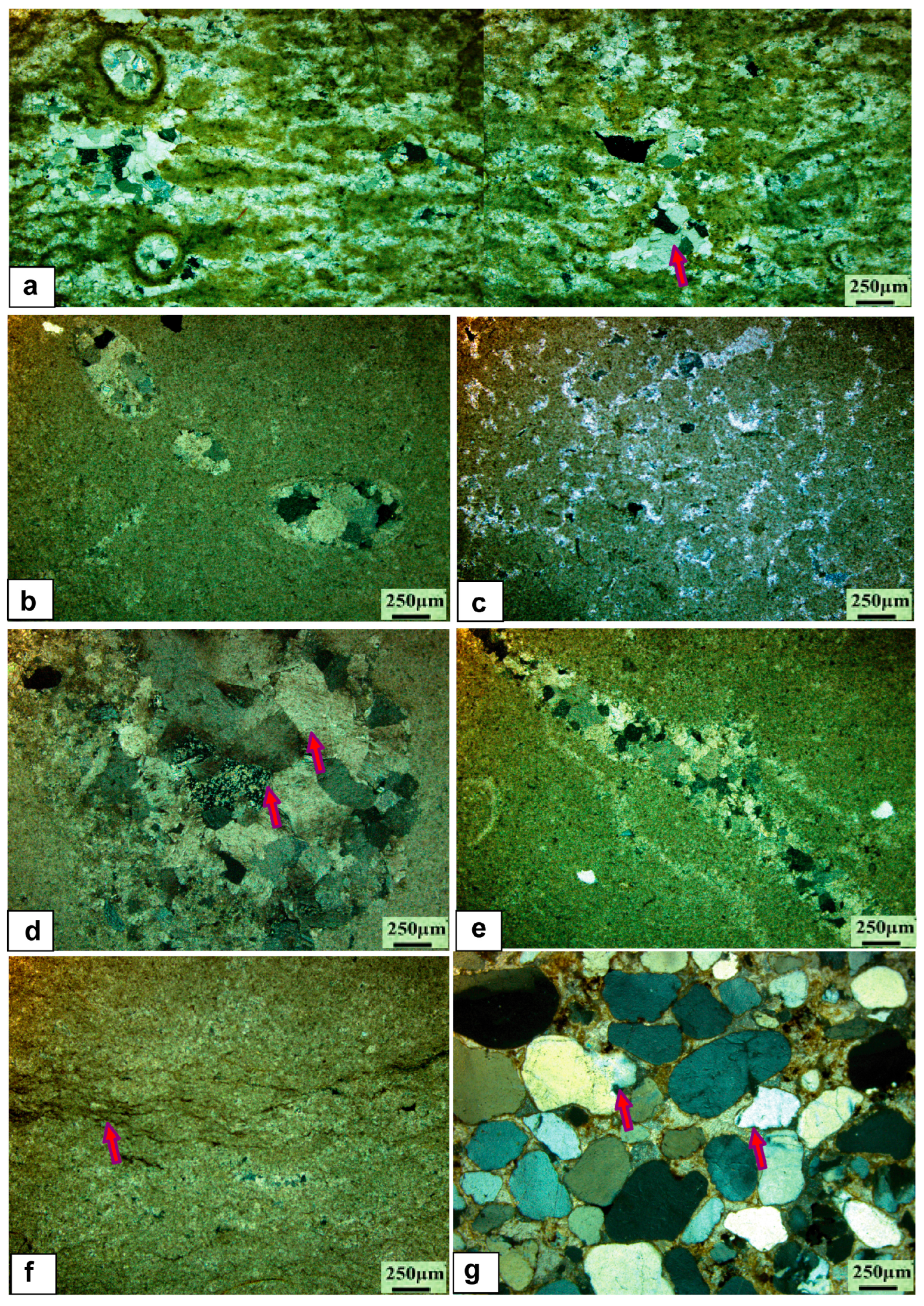
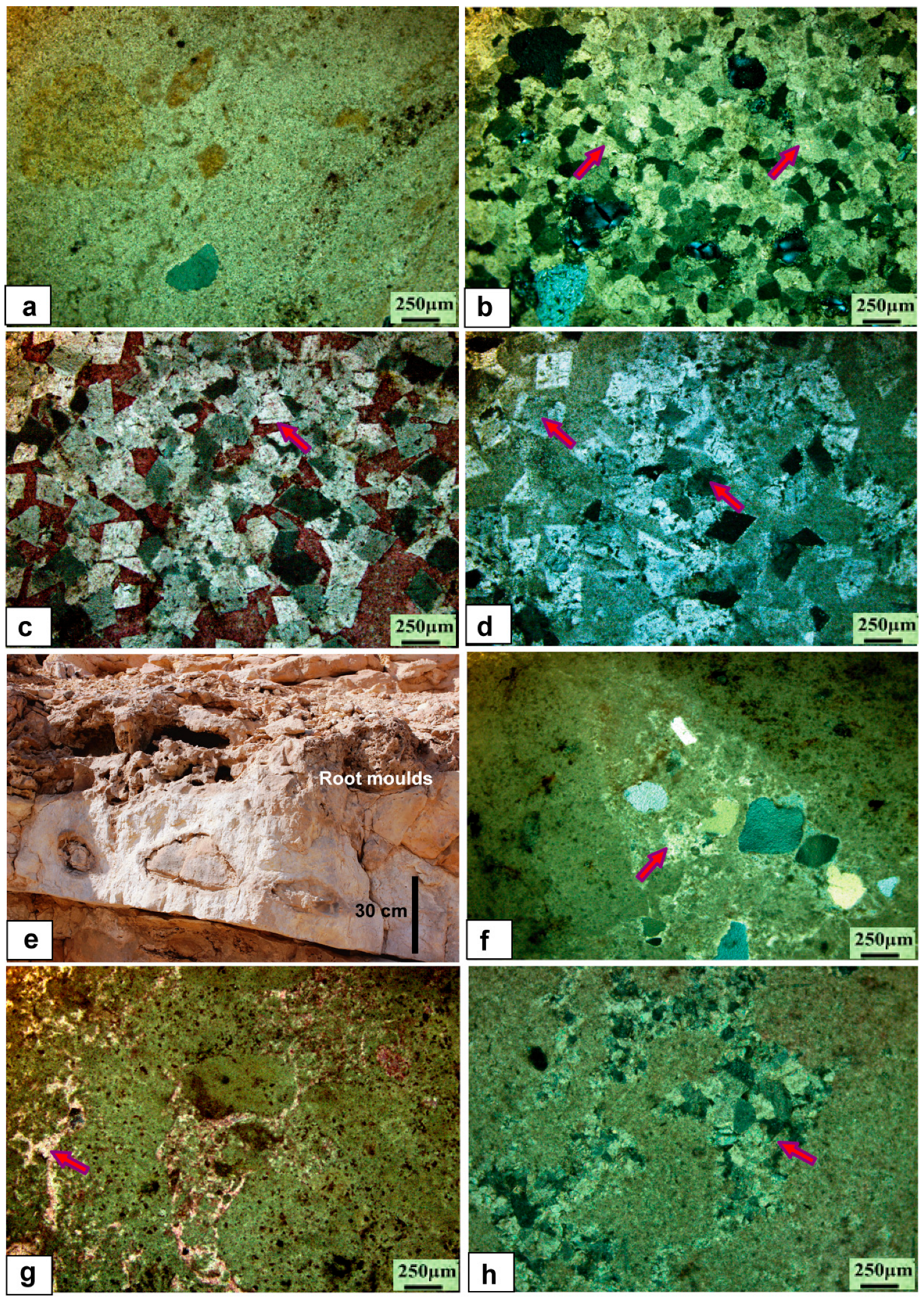
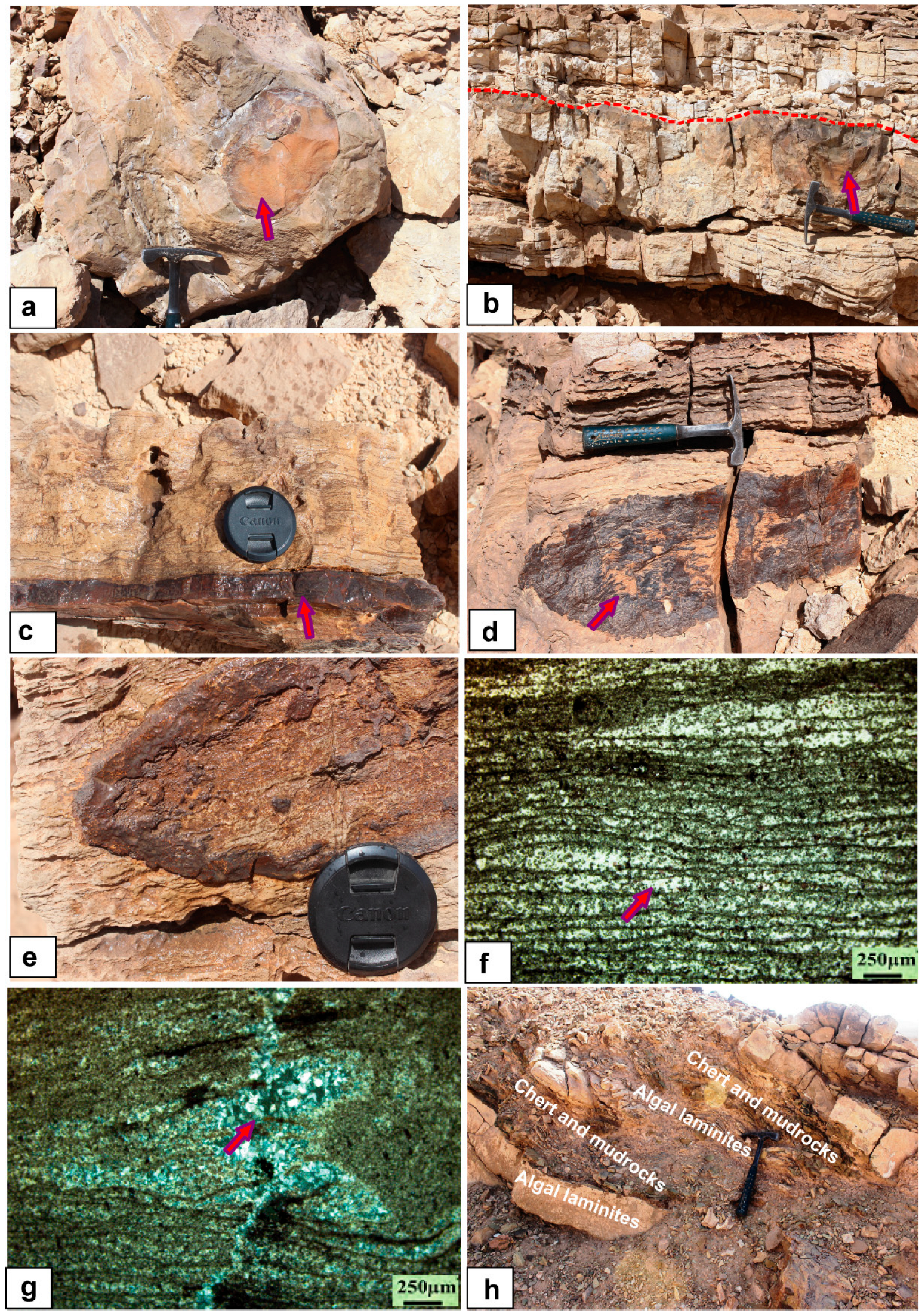
5.1. Petrology and Sedimentology
5.1.1. Limestone Facies
5.1.2. Calcrete Facies
5.1.3. Channeled to Distal Alluvial, Siliciclastic Facies
5.1.4. Dolostone Facies
5.1.5. Chert Facies
6. Mineralogy and Geochemistry
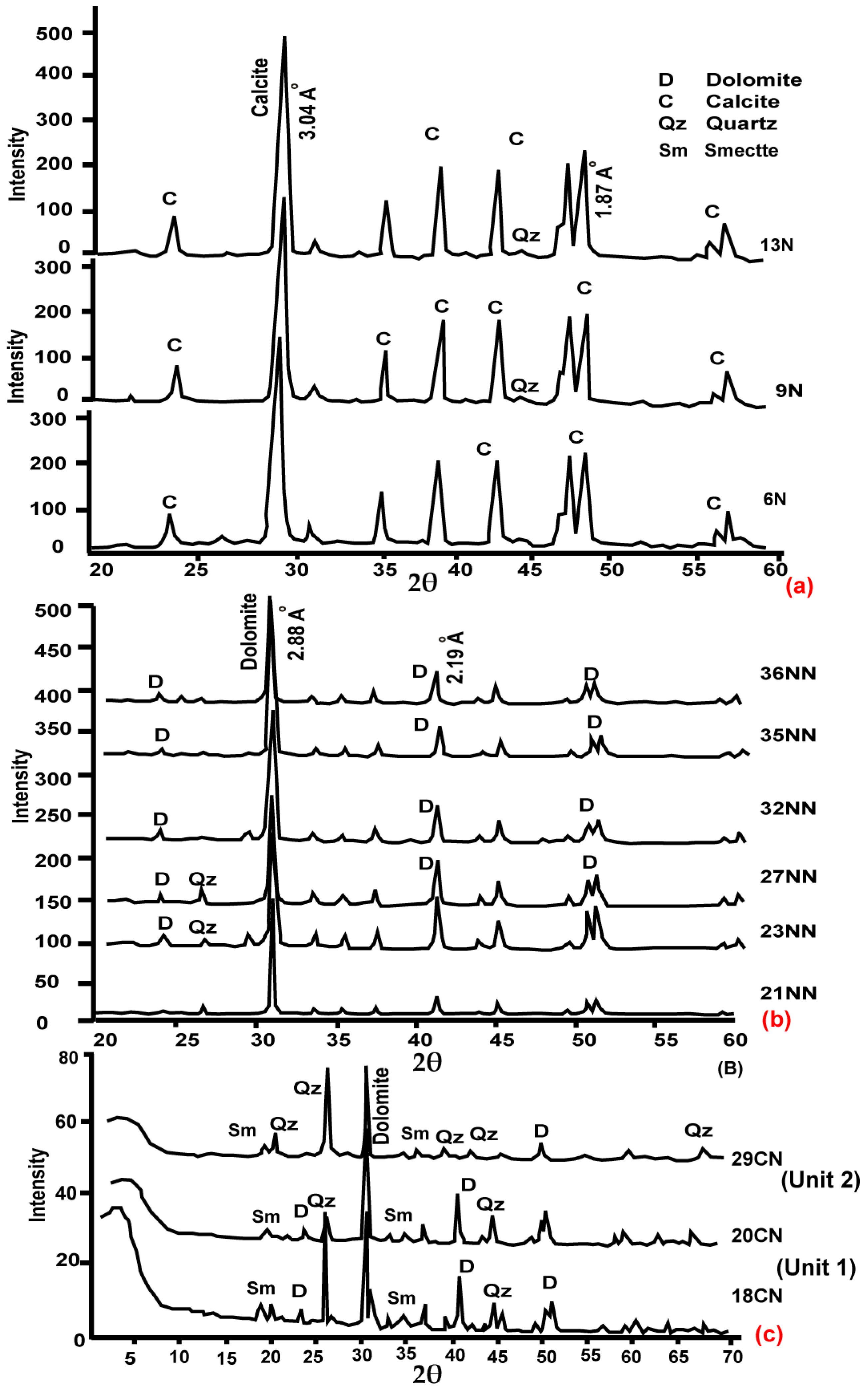
6.1. Mineralogy
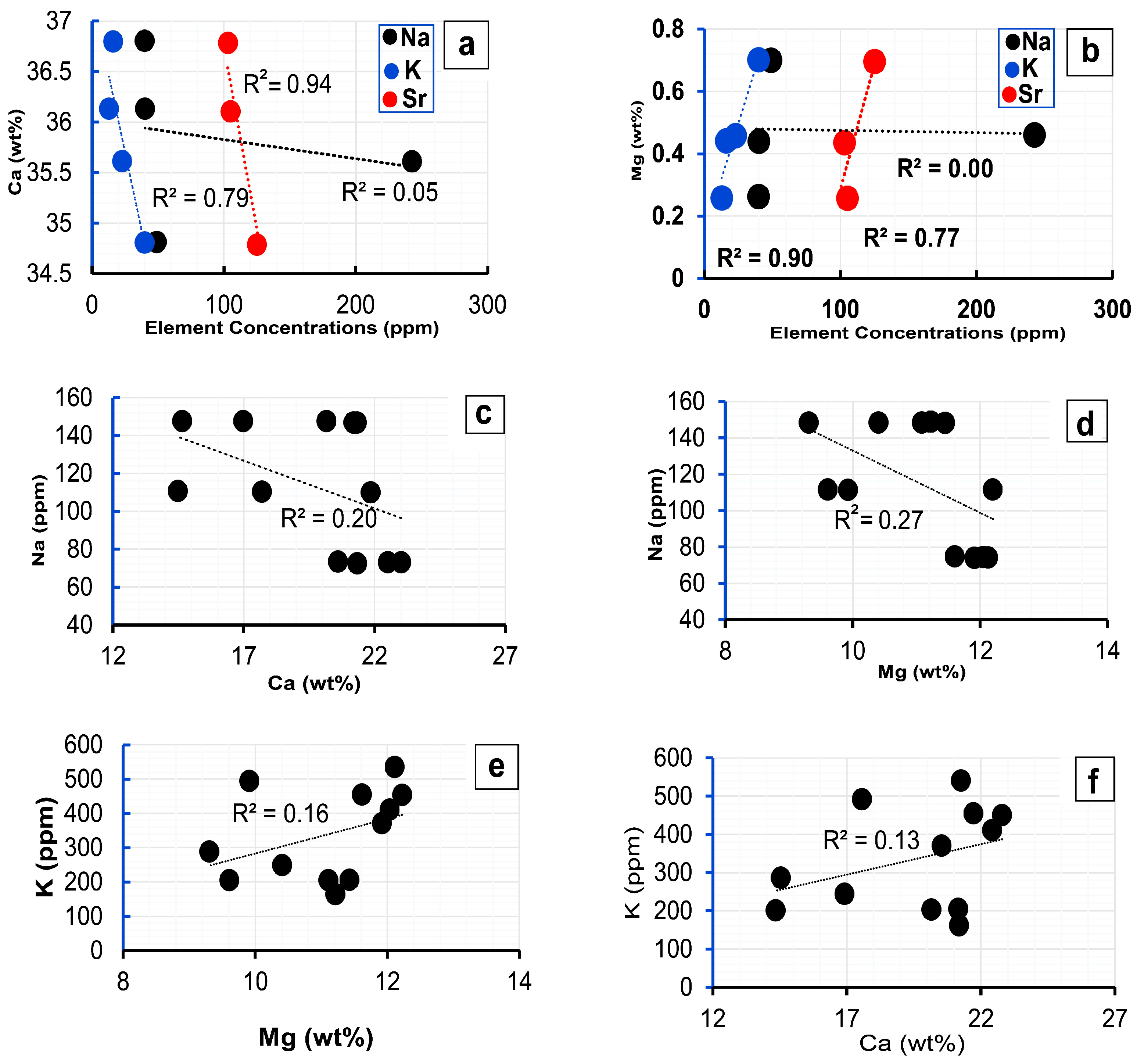
6.2. Geochemistry
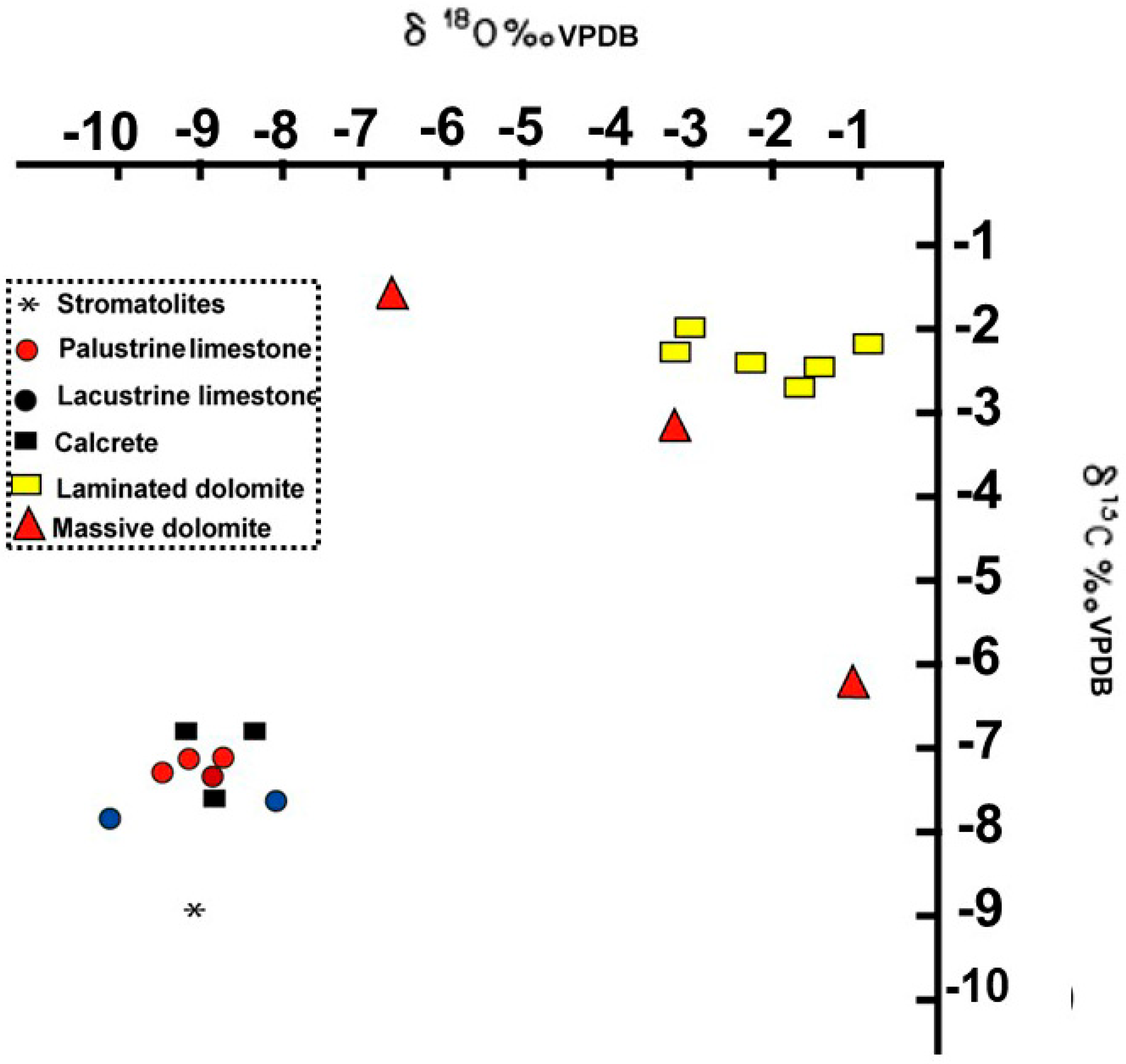
6.3. Isotopic Analysis
7. Discussion
7.1. Geochemical Discrimination of Depositional Environment
7.2. Sources and Mechanisms of Dolomite and Chert Formation
7.2.1. Biogenic Formation Mechanism
7.2.2. Abiogenic Formation Mechanism
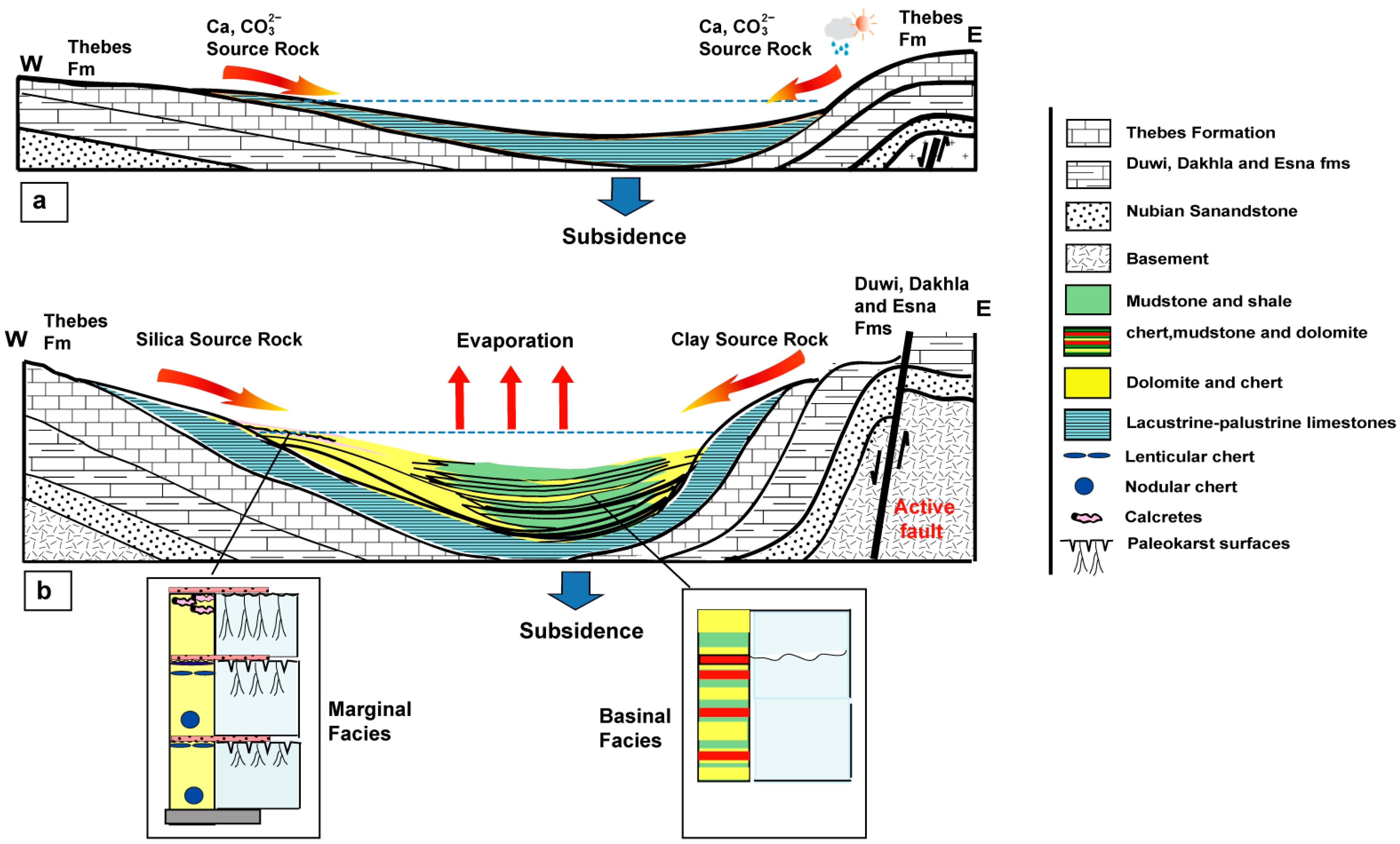
7.3. Major Controls: Tectonics, Climate, and Source Rocks
7.3.1. Tectonics
7.3.2. Climate
7.3.3. Source Rocks (Provenance)
8. Conclusions
- The early syn-rift continental deposits of the Sodmin Formation (Upper Oligocene–Lower Miocene) in the Duwi Basin comprise two units (Units 1 and 2). Unit 1 is dominated by mixed lacustrine and palustrine limestone deposits exhibiting superimposed regressive cycles. This unit is terminated by mixed fine siliciclastics and palustrine limestone. Unit 2 comprises cherts and dolomites interrupted by coarse siliciclastics.
- The studied Sodmin Formation exhibits variable facies that correspond to deposition in a fluvio-lacustrine environment. These facies are as follows: stromatlitic limestone, lacustrine–palustrine limestone, calcrete, channels and distal floodplains, dolomite, and chert.
- Analysis of the lacustrine carbonate facies using petrographical, geochemical, mineralogical, and stable carbon and oxygen isotopes clearly reflects the vertical changes in the paleoenvironments and the lake water chemistry. It demonstrates an abrupt shift from an open freshwater lake condition (Unit 1) to closed saline-alkaline waters (Unit 2) and is consistent with the presence of dolomites and cherts in Unit 2 and their rarity in Unit 1.
- The vertical variations developed in the lacustrine environment reveal the effects of tectonic events (growth faults) and provenance evolution, as well as a tendency towards aridity in the climate. Tectonic activity, particularly the development of hanging-wall syncline folds associated with the propagation of buried normal faults, followed by fault linkage and the establishment of a half-graben basin, influenced the overall architectural evolution of the lake basin. The climate changes indicate that the lower part of Unit 1 was deposited under predominantly humid climatic conditions, while the upper part of this unit suggests alternating periods of humidity and aridity. During Unit 2 formation, greater aridity favored the deposition of evaporitic facies. The provenance data show that Unit 1 was sourced from Eocene pre-rift carbonate sedimentary rocks, whereas Unit 2 was derived from pre-rift Mesozoic rocks with a mixed clastic-carbonate composition.
- Two proposed mechanisms exist for the dolomitization and silicification stages of Unit 2 in the Duwi Basin. The first is microbial (biogenic origin), produced by the dissolution of algal mortality, whereas the non-microbial (abiogenic origin) mechanism is formed by pH oscillations in an alkaline lake environment during the early diagenesis. This can be explained by the fact that alkaline environments provide suitable chemical conditions for the development of microbial mats and precipitation of magnesium carbonates, as well as the dissolution of silicate minerals, which leads to the creation of fluids that were extremely rich in silica.
Author Contributions
Funding
Conflicts of Interest
References
- Colson, J.; Cojan, I. Groundwater Dolocretes in a Lake-marginal Environment: An Alternative Model for Dolocrete Formation in Continental Settings (Danian of the Provence Basin, France). Sedimentology 1996, 43, 175–188. [Google Scholar] [CrossRef]
- Bustillo, M.A.; Arribas, M.E.; Bustillo, M. Dolomitization and Silicification in Low-Energy Lacustrine Carbonates (Paleogene, Madrid Basin, Spain). Sediment. Geol. 2002, 151, 107–126. [Google Scholar] [CrossRef]
- Martín Pérez, A.; Martín García, R.; Alonso Zarza, A.M. Diagenesis of a Drapery Speleothem from Castañar Cave: From Dissolution to Dolomitization. Int. J. Speleol. 2012, 41, 251–266. [Google Scholar] [CrossRef][Green Version]
- Guo, P.; Wen, H.; Li, C.; He, H.; Sánchez-Román, M. Lacustrine Dolomite in Deep Time: What Really Matters in Early Dolomite Formation and Accumulation? Earth Sci. Rev. 2023, 246, 104575. [Google Scholar] [CrossRef]
- Yao, T.; Zhu, H.; Fu, B.; Du, X.; Wang, Q.; Li, S.; Yang, X.; Sánchez-Román, M. Origin of Eocene Lacustrine Dolomite and Evolution of Multiple Diagenetic Fluids in the Huanghekou Sag, Bohai Bay Basin, China. Am. Assoc. Pet. Geol. Bull. 2024, 108, 1957–1984. [Google Scholar] [CrossRef]
- Casado, A.I.; Alonso-Zarza, A.M.; La Iglesia, Á. Morphology and Origin of Dolomite in Paleosols and Lacustrine Sequences. Examples from the Miocene of the Madrid Basin. Sediment. Geol. 2014, 312, 50–62. [Google Scholar]
- SANZ-MONTERO, M.E.; RODRÍGUEZ-ARANDA, J.P.; Garcia Del Cura, M.A. Dolomite–Silica Stromatolites in Miocene Lacustrine Deposits from the Duero Basin, Spain: The Role of Organotemplates in the Precipitation of Dolomite. Sedimentology 2008, 55, 729–750. [Google Scholar] [CrossRef]
- Bustillo, M.A. Silicification of Continental Carbonates. Dev. Sedimentol. 2010, 62, 153–178. [Google Scholar]
- Krainer, K.; Spötl, C. Abiogenic Silica Layers within a Fluvio-lacustrine Succession, Bolzano Volcanic Complex, Northern Italy: A Permian Analogue for Magadi-type Cherts? Sedimentology 1998, 45, 489–505. [Google Scholar] [CrossRef]
- Kuznetsov, V.G.; Skobeleva, N.M. Silicification of Riphean Carbonate Sediments (Yurubcha-Tokhomo Zone, Siberian Craton). Lithol. Miner. Resour. 2005, 40, 552–563. [Google Scholar] [CrossRef]
- Montenat, C.; d’Estevou, P.O.; Jarrige, J.-J.; Richert, J.-P. Rift Development in the Gulf of Suez and the North-Western Red Sea: Structural Aspects and Related Sedimentary Processes. In Sedimentation and Tectonics in Rift Basins Red Sea:-Gulf of Aden; Springer: Berlin/Heidelberg, Germany, 1998; pp. 97–116. [Google Scholar]
- Mahran, T.M. Late Oligocene Lacustrine Deposition of the Sodmin Formation, Abu Hammad Basin, Red Sea, Egypt: Sedimentology and Factors Controlling Palustrine Carbonates. J. Afr. Earth Sci. 1999, 29, 567–592. [Google Scholar] [CrossRef]
- Khalil, S.M.; McClay, K.R. Extensional Fault-Related Folding in the Northwestern Red Sea, Egypt: Segmented Fault Growth, Fault Linkages, Corner Folds and Basin Evolution. In Passive Margins: Tectonics, Sedimentology, and Magmatism; Geological Society Publishing House: London, UK, 2020. [Google Scholar]
- Yousuf, R.; Mahran, T.; Hassan, A.M. Palustrine Limestone in an Extensional Northern Duwi Basin, West of Quseir, Red Sea, Egypt. Sohag J. Sci. 2023, 8, 271–279. [Google Scholar] [CrossRef]
- Freytet, P.; JC, P.; BH, P. Continental Carbonate Sedimentation and Pedogenesis-Late Cretaceous and Early Tertiary of Southern France; Schweizerbart Science Publishers: Stuttgart, Germany, 1982. [Google Scholar]
- Platt, N.H. Continental Sedimentation in an Evolving Rift Basin: The Lower Cretaceous of the Western Cameros Basin (Northern Spain). Sediment. Geol. 1989, 64, 91–109. [Google Scholar] [CrossRef]
- Platt, N.H.; Wright, V.P. Palustrine Carbonates and the Florida Everglades; towards an Exposure Index for the Fresh-Water Environment? J. Sediment. Res. 1992, 62, 1058–1071. [Google Scholar] [CrossRef]
- Alonso-Zarza, A.M.; Calvo, J.P. Palustrine Sedimentation in an Episodically Subsiding Basin: The Miocene of the Northern Teruel Graben (Spain). Palaeogeogr. Palaeoclim. Palaeoecol. 2000, 160, 1–21. [Google Scholar] [CrossRef]
- Alonso-Zarza, A.M. Palaeoenvironmental Significance of Palustrine Carbonates and Calcretes in the Geological Record. Earth Sci. Rev. 2003, 60, 261–298. [Google Scholar] [CrossRef]
- Tucker, M.E.; Wright, V.P. Carbonate Sedimentology; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 1444314165. [Google Scholar]
- Khalil, S.M.; McClay, K.R. Extensional Fault-Related Folding, Northwestern Red Sea, Egypt. J. Struct. Geol. 2002, 24, 743–762. [Google Scholar] [CrossRef]
- El Akkad, S.; Dardir, A.A. Geology of the Red Sea Coast Between Ras Shagra and Mersa Alam; US Government Printing Office: Washington, DC, USA, 1966.
- Samuel, M.D.; Saleeb, R.G.S. Lithostratigraphy and petrographical analysis of the Neogene sediments at Abu Ghusun-Um Mahara area, Red Sea coast, Egypt, Freiburger Forschungsberichte. Earth Sci. 1977, 323, 47–56. [Google Scholar]
- El-Haddad, A.A. Sedimentological and Geological Studies on the Neogene Sediments of the Egyptian Part of the Red Sea. Ph.D. Thesis, Assiut University, Asyut, Egypt, 1984. [Google Scholar]
- Purser, B.H.; Philobbos, E.R. The Sedimentary Expressions of Rifting in the NW Red Sea, Egypt. Geodyn. Sediment. Red. Sea-Gulf Aden Rift. Syst. Spec. Publ. 1993, 1, 1–45. [Google Scholar]
- Purser, B.H.; Soliman, M.; M’Rabet, A. Carbonate, Evaporite, Siliciclastic Transitions in Quaternary Rift Sediments of the Northwestern Red Sea. Sediment. Geol. 1987, 53, 247–267. [Google Scholar] [CrossRef]
- Tucker, M.E. Triassic Lacustrine Sediments from South Wales: Shore-Zone, Evaporites and Carbonates. Mod. Anc. Lake Sediments 1978, 205–224. [Google Scholar]
- Machette, M.N. Calcic Soils of the Southwestern United States; Geological Society of America: Boulder, CO, USA, 1985; Volume 203, pp. 23–41. [Google Scholar]
- Goldsmith, J.R.; Graf, D.L. Structural and Compositional Variations in Some Natural Dolomites. J. Geol. 1958, 66, 678–693. [Google Scholar] [CrossRef]
- Morrow, D.W. Diagenesis 1. Dolomite-Part 1: The Chemistry of Dolomitization and Dolomite Precipitation. Geosci. Can. 1982, 9, 5–13. [Google Scholar]
- Hardy, R.; Tucker, M.E. Techniques in Sedimentology. Blackwell Sci. Publ. 1988, 484, 191–228. [Google Scholar]
- Manche, C.J.; Kaczmarek, S.E. Evaluating Reflux Dolomitization Using a Novel High-Resolution Record of Dolomite Stoichiometry: A Case Study from the Cretaceous of Central Texas, USA. Geology 2019, 47, 586–590. [Google Scholar] [CrossRef]
- Burns, S.J.; Baker, P.A. A Geochemical Study of Dolomite in the Monterey Formation, California. J. Sediment. Res. 1987, 57, 128–139. [Google Scholar] [CrossRef]
- Anan, T.; Wanas, H. Dolomitization in the Carbonate Rocks of the Upper Turonian Wata Formation, West Sinai, NE Egypt: Petrographic and Geochemical Constraints. J. Afr. Earth Sci. 2015, 111, 127–137. [Google Scholar] [CrossRef]
- Smoot, J.P. Origin of the Carbonate Sediments in the Wilkins Peak Member of the Lacustrine Green River Formation (Eocene), Wyoming, USA. In Modern and Ancient Lake Sediments; Wiley Online Library: Hoboken, NJ, USA, 1978; pp. 109–127. [Google Scholar]
- Usman, U.A.; Abdulkadir, A.B.; El-Nafaty, J.M.; Bukar, M.; Baba, S. Lithostratigraphy and Geochemical Characterization of Limestone Deposits around Kushimaga Area in Yobe of North-Eastern Nigeria. Niger. J. Technol. 2018, 37, 885–897. [Google Scholar] [CrossRef]
- Olatunji, J.A. Chemical Characterization of Isale-Osin Marble, Kwara State; Geoscience Consultancy Report for Ministry of Commerce and Industry: Ilorin, Nigeria, 1989.
- Müller, G.; Irion, G.; Förstner, U. Formation and Diagenesis of Inorganic Ca-Mg Carbonates. Naturwissenschaften 1972, 59, 158–164. [Google Scholar] [CrossRef]
- Chilingar, G. V Relationship between Ca/Mg Ratio and Geologic Age. Am. Assoc. Pet. Geol. Bull. 1956, 40, 2256–2266. [Google Scholar]
- Veizer, J.A.N. Chemical Diagenesis of Carbonates: Theory and Application of Trace Element Technique; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1983. [Google Scholar]
- Scholle, P.A. Chalk Diagenesis and Its Relation to Petroleum Exploration: Oil from Chalks, a Modern Miracle? Am. Assoc. Pet. Geol. Bull. 1977, 61, 982–1009. [Google Scholar]
- Anadón, P.; Ortí, E.; Rosell, L. AAPG Studies in Geology# 46, Chapter 46: Neogene Lacustrine Systems of the Southern Teruel Graben (Spain); Anadón, P., Ortí, E., Rosell, L., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2000; pp. 497–504. [Google Scholar]
- Yang, Y.-Q.; Qiu, L.-W.; Gregg, J.; Shi, Z.; Yu, K.-H. Formation of Fine Crystalline Dolomites in Lacustrine Carbonates of the Eocene Sikou Depression, Bohai Bay Basin, East China. Pet. Sci. 2016, 13, 642–656. [Google Scholar] [CrossRef]
- Hardie, L.A. Dolomitization; a Critical View of Some Current Views. J. Sediment. Res. 1987, 57, 166–183. [Google Scholar] [CrossRef]
- Sass, E.; Bein, A. Dolomites and Salinity: A Comparative Geochemical Study; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1988. [Google Scholar]
- Warren, J. Dolomite: Occurrence, Evolution and Economically Important Associations. Earth Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Shukla, V. Sedimentology and Geochemistry of a Regional Dolostone: Correlation of Trace Elements with Dolomite Fabrics; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1988. [Google Scholar]
- Friedman, G.M.; Amiel, A.J.; Braun, M.; Miller, D.S. Generation of Carbonate Particles and Laminites in Algal Mats—Example from Sea-Marginal Hypersaline Pool, Gulf of Aqaba, Red Sea. Am. Assoc. Pet. Geol. Bull. 1973, 57, 541–557. [Google Scholar]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of Sedimentary Carbonates; Elsevier: Amsterdam, The Netherlands, 1990; Volume 48, ISBN 0080869629. [Google Scholar]
- Lumsden, D.N.; Chimahusky, J.S. Relationship between Dolomite Nonstoichiometry and Carbonate Facies Parameters; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1980. [Google Scholar]
- Zhang, L.; Jiao, Y.; Rong, H.; Li, R.; Wang, R. Origins and Geochemistry of Oolitic Dolomite of the Feixianguan Formation from the Yudongzi Outcrop, Northwest Sichuan Basin, China. Minerals 2017, 7, 120. [Google Scholar] [CrossRef]
- Alçiçek, M.C.; Brogi, A.; Capezzuoli, E.; Liotta, D.; Meccheri, M. Superimposed Basin Formation during Neogene–Quaternary Extensional Tectonics in SW-Anatolia (Turkey): Insights from the Kinematics of the Dinar Fault Zone. Tectonophysics 2013, 608, 713–727. [Google Scholar] [CrossRef]
- Tandon, S.K.; Andrews, J.E. Lithofacies Associations and Stable Isotopes of Palustrine and Calcrete Carbonates: Examples from an Indian Maastrichtian Regolith. Sedimentology 2001, 48, 339–355. [Google Scholar] [CrossRef]
- Huerta, P.; Armenteros, I. Calcrete and Palustrine Assemblages on a Distal Alluvial-Floodplain: A Response to Local Subsidence (Miocene of the Duero Basin, Spain). Sediment. Geol. 2005, 177, 253–270. [Google Scholar] [CrossRef]
- Alçiçek, H.; Jiménez-Moreno, G. Late Miocene to Plio-Pleistocene Fluvio-Lacustrine System in the Karacasu Basin (SW Anatolia, Turkey): Depositional, Paleogeographic and Paleoclimatic Implications. Sediment. Geol. 2013, 291, 62–83. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Pang, Z.; Wang, X. Characteristics of Chemistry and Stable Isotopes in Groundwater of the Chaobai River Catchment, Beijing. Procedia Earth Planet. Sci. 2013, 7, 487–490. [Google Scholar] [CrossRef]
- Nehza, O.; Woo, K.S.; Lee, K.C. Combined Textural and Stable Isotopic Data as Proxies for the Mid-Cretaceous Paleoclimate: A Case Study of Lacustrine Stromatolites in the Gyeongsang Basin, SE Korea. Sediment. Geol. 2009, 214, 85–99. [Google Scholar] [CrossRef]
- Last, W.M. Lacustrine Dolomite—An Overview of Modern, Holocene, and Pleistocene Occurrences. Earth Sci. Rev. 1990, 27, 221–263. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.; Grujic, D.; Tiens, A.J. Microbial Mediation as a Possible Mechanism for Natural Dolomite Formation at Low Temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A. Microbial Mediation of Modern Dolomite Precipitation and Diagenesis under Anoxic Conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sediment. Res. 1997, 67, 378–390. [Google Scholar] [CrossRef]
- Wright, D.T. The Role of Sulphate-Reducing Bacteria and Cyanobacteria in Dolomite Formation in Distal Ephemeral Lakes of the Coorong Region, South Australia. Sediment. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Calvo, J.P.; Mckenzie, J.A.; Vasconcelos, C. Microbially Mediated Lacustrine Dolomite Formation: Evidence and Current. Limnogeologia España: Un. Tribut. Kerry Kelts 2003, 14, 229. [Google Scholar]
- Wright, D.T.; Wacey, D. Precipitation of Dolomite Using Sulphate-reducing Bacteria from the Coorong Region, South Australia: Significance and Implications. Sedimentology 2005, 52, 987–1008. [Google Scholar] [CrossRef]
- Wacey, D.; Wright, D.T.; Boyce, A.J. A Stable Isotope Study of Microbial Dolomite Formation in the Coorong Region, South Australia. Chem. Geol. 2007, 244, 155–174. [Google Scholar] [CrossRef]
- Krause, S.; Liebetrau, V.; Gorb, S.; Sánchez-Román, M.; McKenzie, J.A.; Treude, T. Microbial Nucleation of Mg-Rich Dolomite in Exopolymeric Substances under Anoxic Modern Seawater Salinity: New Insight into an Old Enigma. Geology 2012, 40, 587–590. [Google Scholar] [CrossRef]
- Mather, C.C.; Lampinen, H.M.; Tucker, M.; Leopold, M.; Dogramaci, S.; Raven, M.; Gilkes, R.J. Microbial Influence on Dolomite and Authigenic Clay Mineralisation in Dolocrete Profiles of NW Australia. Geobiology 2023, 21, 644–670. [Google Scholar] [CrossRef]
- Liu, D.; Chen, T.; Dai, Z.; Papineau, D.; Qiu, X.; Wang, H.; Benzerara, K. A Non-Classical Crystallization Mechanism of Microbially-Induced Disordered Dolomite. Geochim. Cosmochim. Acta 2024, 381, 198–209. [Google Scholar] [CrossRef]
- Curtis, C.D. Possible Links between Sandstone Diagenesis and Depth-Related Geochemical Reactions Occurring in Enclosing Mudstones. J. Geol. Soc. Lond. 1978, 135, 107–117. [Google Scholar] [CrossRef]
- Irwin, W.P.; Barnes, I. Tectonic Relations of Carbon Dioxide Discharges and Earthquakes. J. Geophys. Res. Solid. Earth 1980, 85, 3115–3121. [Google Scholar] [CrossRef]
- Duncan, W.I.; Buxton, N.W.K. New Evidence for Evaporitic Middle Devonian Lacustrine Sediments with Hydrocarbon Source Potential on the East Shetland Platform, North Sea. J. Geol. Soc. Lond. 1995, 152, 251–258. [Google Scholar] [CrossRef]
- Bustillo, M.A.; Bustillo, M. Miocene Silcretes in Argillaceous Playa Deposits, Madrid Basin, Spain: Petrological and Geochemical Features. Sedimentology 2000, 47, 1023–1037. [Google Scholar] [CrossRef]
- Montero, E.S.; del Cura, M.Á.G.; Aranda, J.P.R. Mediación Microbiana En La Formación de Barita En Sistemas Lacustres Miocenos de Las Cuencas Del Duero y de Madrid. Macla Rev. La Soc. Española Mineral. 2006, 449–451. [Google Scholar]
- Torres, N.A. Stratigraphy and Facies of the Pliocene Mayrán Lacustrine Basin System, Northeast México; The University of Manchester: Manchester, UK, 2012; ISBN 107327005X. [Google Scholar]
- Gunatilaka, A. Spheroidal Dolomites–Origin by Hydrocarbon Seepage? Sedimentology 1989, 36, 701–710. [Google Scholar] [CrossRef]
- Ramzan, M.; Ullah, A.; Ahmad, T. Sedimentological Attributes of the Middle Jurassic Samana Suk Formation Exposed in Village Rani-Wah Haripur, Southern Hazara Basin (NW Himalayan Fold and Thrust Belt, Pakistan). Lithol. Miner. Resour. 2023, 58, 501–519. [Google Scholar] [CrossRef]
- Weaver, C.E. Potassium, Illite and the Ocean. Geochim. Cosmochim. Acta 1967, 31, 2181–2196. [Google Scholar] [CrossRef]
- Malak, E.K.; Philobbos, E.R.; Abdou, I.K.; Ashry, M.M. Some Petrographical Mineralogical and Organic Geochemical Characteristics of Black Shales from Quseir and Safaga, Red Sea Area, (Egypt). Desert Inst. Bull. 1977, 27, 1–15. [Google Scholar]
- Peterson, A.R. Paleoenvironments of the Colton Formation, Colton, Utah. Earth Sci. 1976, 23, 3–35. [Google Scholar]
- Wells, N.A. Carbonate Deposition, Physical Limnology and Environmentally Controlled Chert Formation in Paleocene-Eocene Lake Flagstaff, Central Utah. Sediment. Geol. 1983, 35, 263–296. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the Mechanism of the Dissolution of Quartz and Silica in Aqueous Solutions. ACS Omega 2017, 2, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J. Dissolution and Formation of Quartz in Soil Environments: A Review. Soil. Sci. Annu. 2020, 71, 99–110. [Google Scholar] [CrossRef]
- Zhou, C.; Rosén, C.; Engvall, K. Fragmentation of Dolomite Bed Material at Pressurized Conditions in the Presence of H2O and CO2: Implications for Pressurized Fluidized Bed Gasification. Fuel 2021, 285, 119061. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Gong, S.; Tang, S.; Cheng, R.; Yang, Z.; Ren, H.; Yang, H. Research Progress of Clay Transformation in Drilling Fluids. In Proceedings of the E3S Web of Conferences; EDP Sciences: Paris, France, 2021; Volume 300, p. 02013. [Google Scholar]
- Chahi, A.; Duplay, J.; Lucas, J. Analyses of Palygorskites and Associated Clays from the Jbel Rhassoul (Morocco): Chemical Characteristics and Origin of Formation. Clays Clay Min. 1993, 41, 401–411. [Google Scholar] [CrossRef]
- Jones, B.F.; Mumpton, F.A. Clay Mineral Diagenesis in Lacustrine Sediments. United States Geol. Surv. Bull. 1986, 1578, 291–300. [Google Scholar]
- Darragi, F.; Tardy, Y. Authigenic Trioctahedral Smectites Controlling PH, Alkalinity, Silica and Magnesium Concentrations in Alkaline Lakes. Chem. Geol. 1987, 63, 59–72. [Google Scholar] [CrossRef]
- Calvo, J.P.; Blanc-Valleron, M.M.; Rodriguez-Arandia, J.P.; Rouchy, J.M.; Sanz, M.E. Authigenic Clay Minerals in Continental Evaporitic Environments. In Palaeoweathering, Palaeosurfaces and Related Continental Deposits; Wiley Online Library: Hoboken, NJ, USA, 1995; pp. 129–151. [Google Scholar]
- Polyak, V.J.; Güven, N. Authigenesis of Trioctahedral Smectite in Magnesium-Rich Carbonate Speleothems in Carlsbad Cavern and Other Caves of the Guadalupe Mountains, New Mexico. Clays Clay Min. 2000, 48, 317–321. [Google Scholar] [CrossRef]
- Deocampo, D.M. Evaporative Evolution of Surface Waters and the Role of Aqueous CO2 in Magnesium Silicate Precipitation: Lake Eyasi and Ngorongoro Crater, Northern Tanzania. S. Afr. J. Geol. 2005, 108, 493–504. [Google Scholar] [CrossRef]
- Deocampo, D.M. Authigenic Clay Minerals in Lacustrine Mudstones; Geological Society of America: Boulder, CO, USA, 2015. [Google Scholar]
- Furquim, S.A.C.; Graham, R.C.; Barbiero, L.; de Queiroz Neto, J.P.; Valles, V. Mineralogy and Genesis of Smectites in an Alkaline-Saline Environment of Pantanal Wetland, Brazil. Clays Clay Min. 2008, 56, 579–595. [Google Scholar] [CrossRef]
- Pozo, M.; Casas, J. Origin of Kerolite and Associated Mg Clays in Palustrine-Lacustrine Environments. The Esquivias Deposit (Neogene Madrid Basin, Spain). Clay Min. 1999, 34, 395–418. [Google Scholar] [CrossRef]
- Díaz-Hernández, J.L.; Sánchez-Navas, A.; Reyes, E. Isotopic Evidence for Dolomite Formation in Soils. Chem. Geol. 2013, 347, 20–33. [Google Scholar] [CrossRef]
- Tosca, N.J.; Masterson, A.L. Chemical Controls on Incipient Mg-Silicate Crystallization at 25 C: Implications for Early and Late Diagenesis. Clay Min. 2014, 49, 165–194. [Google Scholar] [CrossRef]
- Cuadros, J.; Diaz-Hernandez, J.L.; Sanchez-Navas, A.; Garcia-Casco, A.; Yepes, J. Chemical and Textural Controls on the Formation of Sepiolite, Palygorskite and Dolomite in Volcanic Soils. Geoderma 2016, 271, 99–114. [Google Scholar] [CrossRef]
- Ordóñez, S.; Calvo, J.P.; García del Cura, M.A.; Alonso-Zarza, A.M.; Hoyos, M. Sedimentology of Sodium Sulphate Deposits and Special Clays from the Tertiary Madrid Basin (Spain). In Lacustrine Facies Analysis; Wiley Online Library: Hoboken, NJ, USA, 1991; pp. 39–55. [Google Scholar]
- Botha, G.A.; Hughes, J.C. Pedogenic Palygorskite and Dolomite in a Late Neogene Sedimentary Succession, Northwestern Transvaal, South Africa. Geoderma 1992, 53, 139–154. [Google Scholar] [CrossRef]
- Bustillo, M.A.; Alonso-Zarza, A.M. Overlapping of Pedogenesis and Meteoric Diagenesis in Distal Alluvial and Shallow Lacustrine Deposits in the Madrid Miocene Basin, Spain. Sediment. Geol. 2007, 198, 255–271. [Google Scholar] [CrossRef]
- Wanas, H.A.; Sallam, E. Abiotically-Formed, Primary Dolomite in the Mid-Eocene Lacustrine Succession at Gebel El-Goza El-Hamra, NE Egypt: An Approach to the Role of Smectitic Clays. Sediment. Geol. 2016, 343, 132–140. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y.; Papineau, D.; Yu, N.; Fan, Q.; Qiu, X.; Wang, H. Experimental Evidence for Abiotic Formation of Low-Temperature Proto-Dolomite Facilitated by Clay Minerals. Geochim. Cosmochim. Acta 2019, 247, 83–95. [Google Scholar] [CrossRef]
- Robertson, H.; Corlett, H.; Hollis, C.; Kibblewhite, T.; Whitaker, F. Listwanitization as a Source of Mg for Dolomitization: Field Evaluation in Atlin. In Proceedings of the British Columbia. Goldschmidt Conference, Barcelona, Spain, 18–23 August 2019. [Google Scholar]
- De Wet, C.B.; Yocum, D.A.; Mora, C.I. Carbonate Lakes in Closed Basins: Sensitive Indicators of Climate and Tectonics: An Example from the Gettysburg Basin (Triassic), Pennsylvania, USA; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1998. [Google Scholar]
- Gierlowski-Kordesch, E.H. Carbonate Deposition in an Ephemeral Siliciclastic Alluvial System: Jurassic Shuttle Meadow Formation, Newark Supergroup, Hartford Basin, USA. Palaeogeogr. Palaeoclim. Palaeoecol. 1998, 140, 161–184. [Google Scholar] [CrossRef]
- Ahmad, M.; Tanoli, U.F.; Umar, M.; Ahmad, T.; Ahmed, A. Shallow-Marine Late Thanetian Lockhart Limestone from the Hazara Basin, Pakistan: Insights into Foraminiferal Biostratigraphy and Microfacies Analysis. Geosciences 2025, 15, 63. [Google Scholar] [CrossRef]
- Bohacs, K.M.; Carroll, A.R.; Neal, J.E.; Mankiewicz, P.J. Lake-Basin Type, Source Potential, and Hydrocarbon Character: An Integrated Sequence-Stratigraphic–Geochemical Framework; American Association of Petroleum Geologists: Tulsa, OK, USA, 2000. [Google Scholar]
- Kevin, M. Stratigraphic Classification of Ancient Lakes: Balancing Tectonic and Climatic Controls. Geology 1999, 27, 99–102. [Google Scholar] [CrossRef]
- Strecker, U.; Steidtmann, J.R.; Smithson, S.B. A Conceptual Tectonostratigraphic Model for Seismic Facies Migrations in a Fluvio-Lacustrine Extensional Basin. Am. Assoc. Pet. Geol. Bull. 1999, 83, 43–61. [Google Scholar]
- Reading, H.G. Sedimentary Environments: Processes, Facies and Stratigraphy; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 144431369X. [Google Scholar]
- Alonso-Zarza, A.M.; Wright, V.P. Calcretes. Dev. Sedimentol. 2010, 61, 225–267. [Google Scholar]
- Alonso-Zarza, A.M.; Meléndez, A.; Martín-García, R.; Herrero, M.J.; Martín-Pérez, A. Discriminating between Tectonism and Climate Signatures in Palustrine Deposits: Lessons from the Miocene of the Teruel Graben, NE Spain. Earth Sci. Rev. 2012, 113, 141–160. [Google Scholar] [CrossRef]
- Hanneman, D.L.; Wideman, C.J. Continental Sequence Stratigraphy and Continental Carbonates. Dev. Sedimentol. 2010, 62, 215–273. [Google Scholar]
- Lettéron, A.; Hamon, Y.; Fournier, F.; Séranne, M.; Pellenard, P.; Joseph, P. Reconstruction of a Saline, Lacustrine Carbonate System (Priabonian, St-Chaptes Basin, SE France): Depositional Models, Paleogeographic and Paleoclimatic Implications. Sediment. Geol. 2018, 367, 20–47. [Google Scholar] [CrossRef]
- Khalaf, F.I.; Gaber, A.S. Occurrence of Cyclic Palustrine and Calcrete Deposits within the Lower Pliocene Hagul Formation, East Cairo District, Egypt. J. Afr. Earth Sci. 2008, 51, 298–312. [Google Scholar] [CrossRef]
- Cabaleri, N.G.; Benavente, C.A. Sedimentology and Paleoenvironments of the Las Chacritas Carbonate Paleolake, Cañadón Asfalto Formation (Jurassic), Patagonia, Argentina. Sediment. Geol. 2013, 284, 91–105. [Google Scholar] [CrossRef]



| Facies | S. No. | Major Oxides and Elements (wt%) | Trace Elements (ppm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | MgO | SiO2 | Al2O3 | Fe2O3 | LOI | Ca% | Mg% | Na | K | Sr | Ba | Cao/MgO | Sr/Ca | Mg/Ca | ||
| Massive dolomites | 36 NN | 28.16 | 18.49 | 2.3 | 0.13 | 1.29 | 49.54 | 20.1 | 11.09 | 148.4 | 207.4 | 560 | 29 | 1.52 | 0.0013 | 0.92 |
| 35 NN | 30.51 | 20.27 | 0.88 | 0.15 | 1.06 | 46.99 | 21.8 | 12.2 | 111.3 | 456.4 | 171 | 27 | 1.51 | 0.00036 | 0.933 | |
| 32 NN | 32 | 19.37 | 0.95 | 0.13 | 0.3 | 47.12 | 22.9 | 11.6 | 74.2 | 456.4 | 800 | 30 | 1.65 | 0.0016 | 0.844 | |
| Laminated dolomites | 27 NN | 28.79 | 19.81 | 4.5 | 0.23 | 0.78 | 45.78 | 20.6 | 11.9 | 74.2 | 373.4 | 195 | 23 | 1.45 | 0.00043 | 0.96 |
| 21 NN | 31.5 | 20.05 | 1.04 | 0.09 | 0.25 | 46.95 | 22.5 | 12.03 | 74.2 | 414.9 | 107 | 26 | 1.54 | 0.00022 | 0.89 | |
| Lacustrine and palustrine limestones | 13 N | 49.84 | 0.762 | 4.16 | 4.16 | 0.66 | 40.35 | 35.6 | 0.457 | 254 | 23 | 122 | 23 | 65.41 | 0.00016 | 0.036 |
| 9 N | 48.72 | 1.16 | 3.96 | 4.02 | 0.16 | 41.96 | 34.8 | 0.696 | 50 | 40 | 125 | 40 | 42 | 0.00016 | 0.033 | |
| 8 N | 51.52 | 0.73 | 1.18 | 5.17 | 0.28 | 41.11 | 36.8 | 0.438 | 40 | 16 | 103 | 31 | 70.58 | 0.00013 | 0.02 | |
| 3 N | 50.57 | 0.43 | 2.2 | 3.3 | 0.3 | 43.19 | 36.1 | 0.258 | 40 | 13 | 105 | 24 | 117.6 | 0.00013 | 0.012 | |
| Sample No. | Stable Isotopes | |
|---|---|---|
| δ18 O | δ13 C | |
| 1N * | −7.59 | −8.01 |
| 3N * | −7.98 | −9.44 |
| 6N * | −7.89 | −10.04 |
| 9N ** | −7.14 | −9.13 |
| 8N ** | −7.24 | −8.89 |
| 11N ** | −6.77 | −8.95 |
| 13N ** | −7.14 | −8.9 |
| 15N *** | −6.6 | 9.21 |
| 16N *** | −6.8 | −8.4 |
| STR **** | −9 | −9.37 |
| 21NN + | −8.4 | −6.8 |
| 27NN + | −1.82 | −2.69 |
| 32NN + | −3.05 | −2.05 |
| 35NN + | −2.2 | −2.34 |
| 25CN + | −3 | −1.99 |
| 36NN + | −0.68 | −2.35 |
| 13SN ++ | −3.4 | −7.73 |
| 6M ++ | −3.06 | −3.55 |
| 10CN ++ | −6.74 | −1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahran, T.; Abu Elwafa, R.Y.; Ahmed, A.; Abdelghany, O.; Abdelfadil, K.M. Dolomitization and Silicification in Syn-Rift Lacustrine Carbonates: Evidence from the Late Oligocene–Early Miocene Duwi Basin, Red Sea, Egypt. Geosciences 2025, 15, 356. https://doi.org/10.3390/geosciences15090356
Mahran T, Abu Elwafa RY, Ahmed A, Abdelghany O, Abdelfadil KM. Dolomitization and Silicification in Syn-Rift Lacustrine Carbonates: Evidence from the Late Oligocene–Early Miocene Duwi Basin, Red Sea, Egypt. Geosciences. 2025; 15(9):356. https://doi.org/10.3390/geosciences15090356
Chicago/Turabian StyleMahran, Tawfiq, Reham Y. Abu Elwafa, Alaa Ahmed, Osman Abdelghany, and Khaled M. Abdelfadil. 2025. "Dolomitization and Silicification in Syn-Rift Lacustrine Carbonates: Evidence from the Late Oligocene–Early Miocene Duwi Basin, Red Sea, Egypt" Geosciences 15, no. 9: 356. https://doi.org/10.3390/geosciences15090356
APA StyleMahran, T., Abu Elwafa, R. Y., Ahmed, A., Abdelghany, O., & Abdelfadil, K. M. (2025). Dolomitization and Silicification in Syn-Rift Lacustrine Carbonates: Evidence from the Late Oligocene–Early Miocene Duwi Basin, Red Sea, Egypt. Geosciences, 15(9), 356. https://doi.org/10.3390/geosciences15090356








