H2 Transport in Sedimentary Basin
Abstract
1. Introduction
2. H2 System
2.1. Hydrogen Transport Mode
2.1.1. Role of Faults
2.1.2. H2 Solubility
2.1.3. Diffusion
2.1.4. Advection
2.1.5. Free-Phase H2
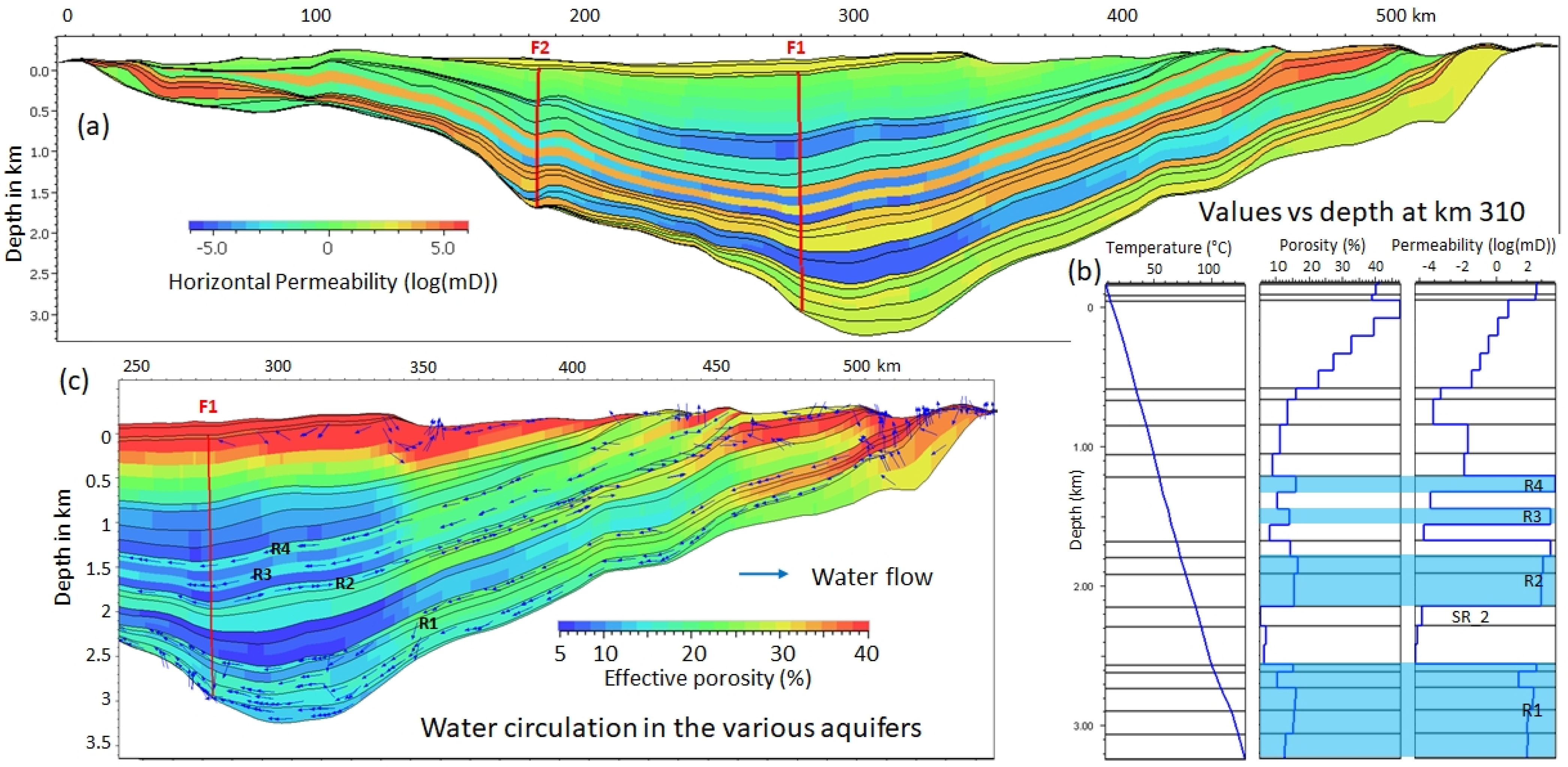
2.2. Methodology Description
2.3. Hypotheses
- 1‑
- Fresh water only. The impact of the initial formation water on the salinity of the water circulating during the last Myr is not taken into account.
- 2‑
- Gas phase is solely H2. The numerical approaches are still limited regarding H2 in the subsurface, and the solubility of a blend, such as CH4 and H2 for instance, is rather unknown, although data show that H2 is not expected to be alone in the gas phase.
- 3‑
- Static geometry. The evolution of the section versus time is not considered. Previous back-stripping allowed for realistic porosity and permeability profiles in the basin. The H2 transport is modeled over 1.5 Myr to 10 Myr. The influx of H2 generated in a deepest zone is constant during a given period ranging from 10 Kyr to 1.5 Myr.
- 4‑
- No biotic H2 generation nor consumption.
- 5‑
- No consumption of H2 by chemical reactions with surrounding rocks.
2.4. Boundary Conditions
2.5. Tested Contexts
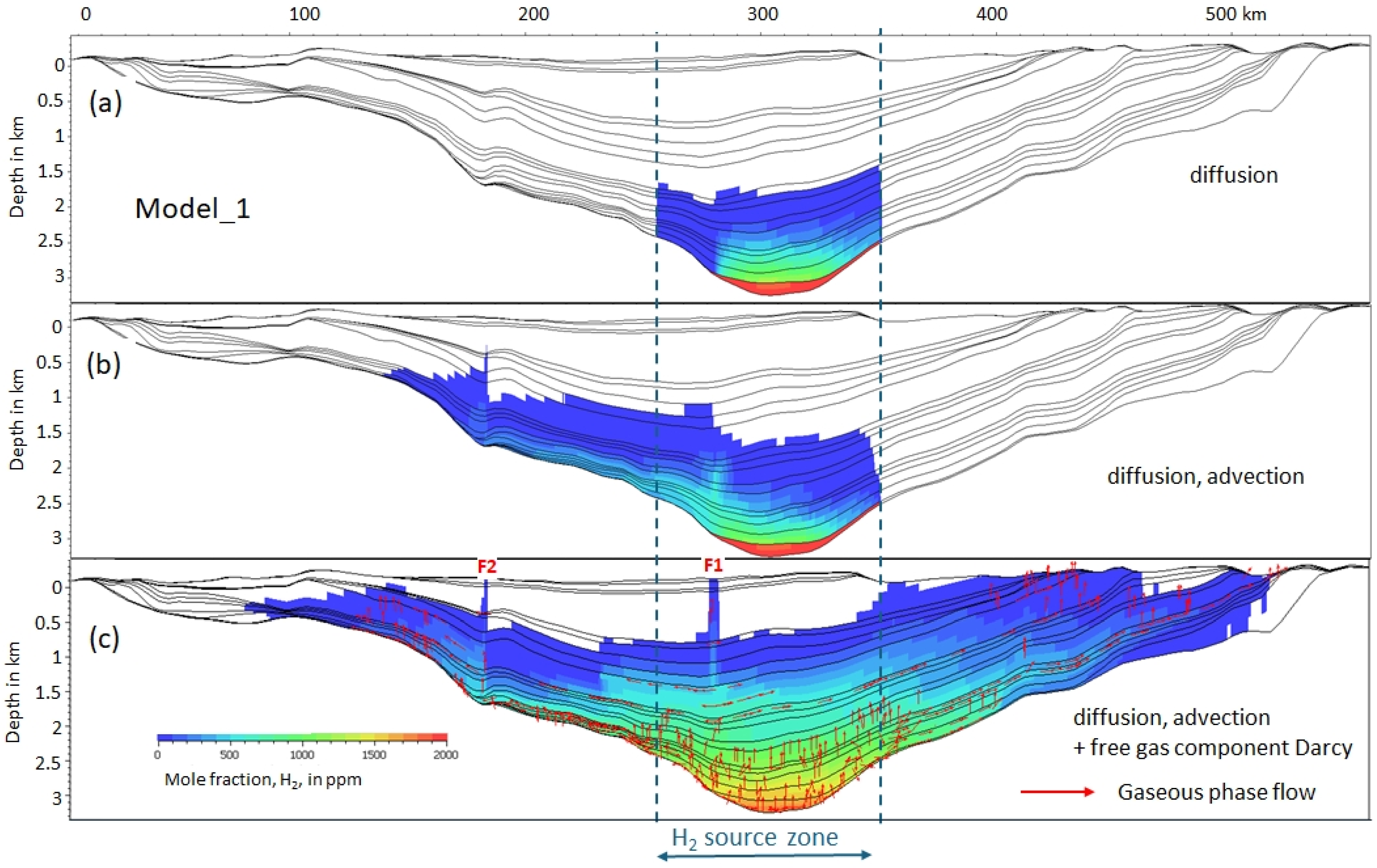
- Various transport modes: the H2 gaseous phase Darcy flow model was compared to the case with only diffusion and the case with diffusion and advection.
- H2 kitchen positions: As mentioned, the generation of H2 is not modeled, but the influence of the H2 concentration, duration, and position resulting from this generation is tested through an “injection” of H2 within the model. The source is always in the lowest layer in contact with the basement and placed in three different positions to challenge the aquifer’s importance versus invasion/percolation and to compare the free gas and dissolved H2 paths.
- The overall quantity of H2 that reaches the bottom of the basin.
3. Results
3.1. Transport Mode
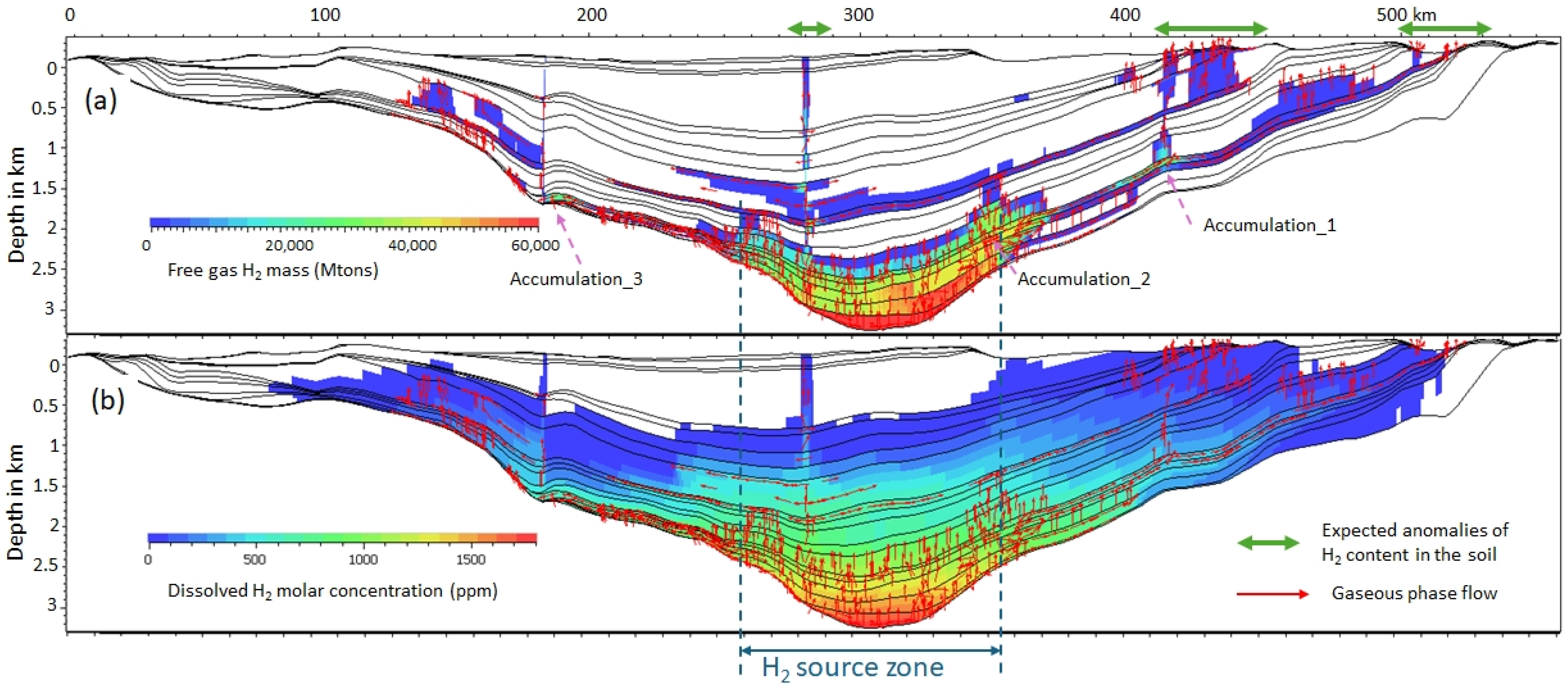
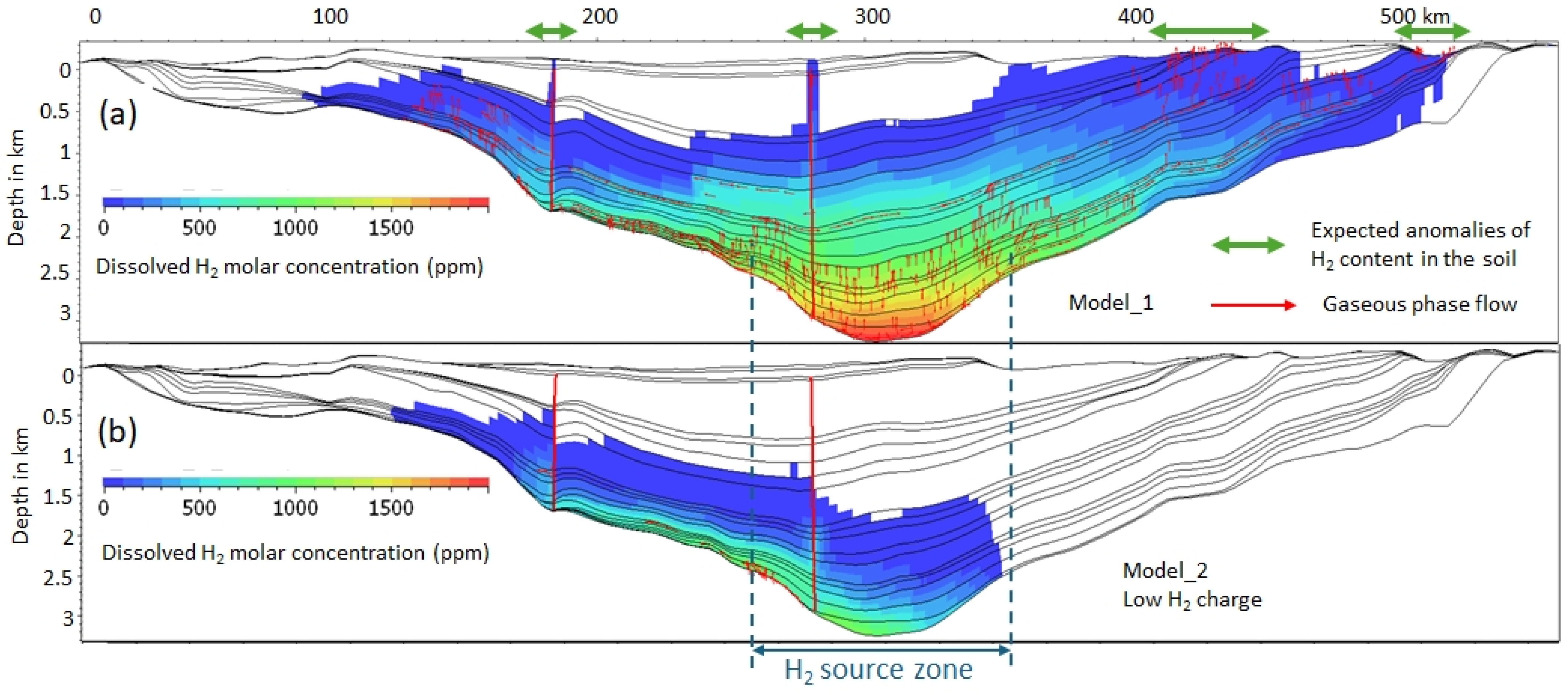
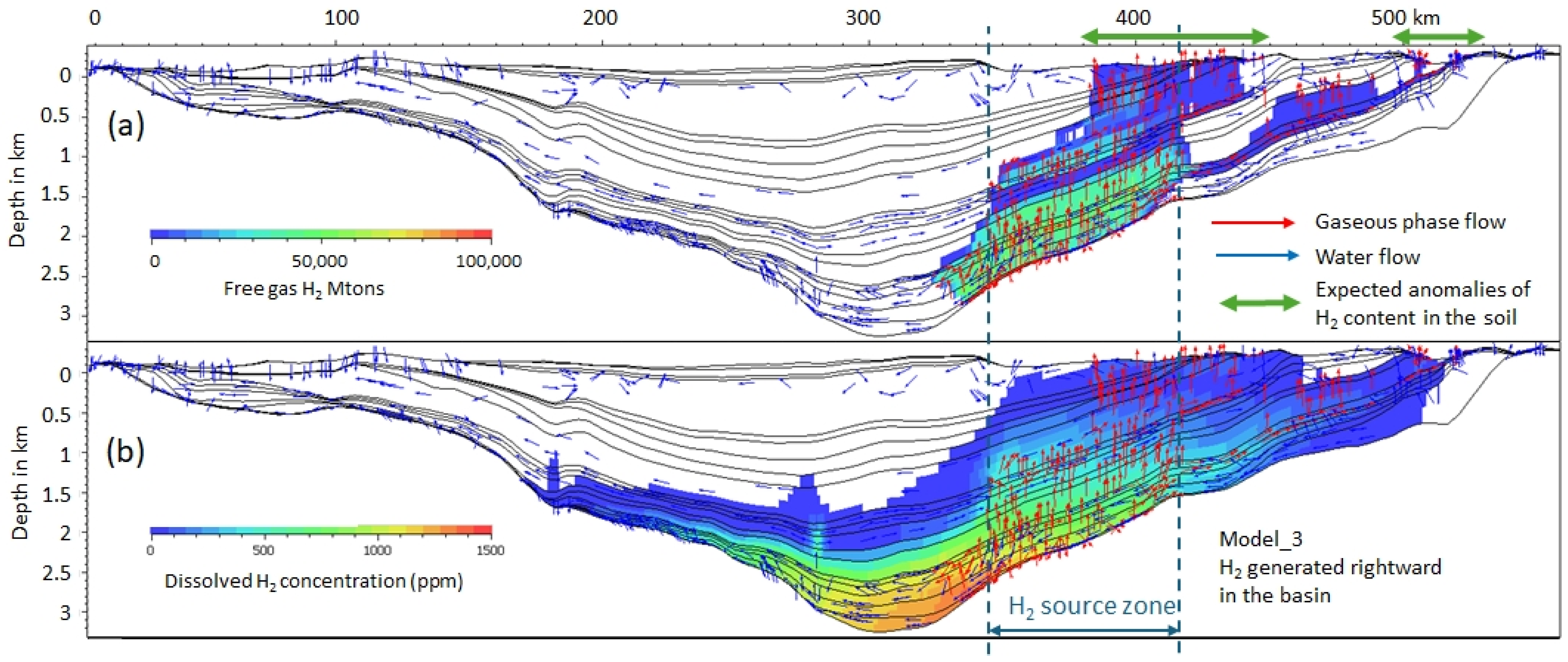
3.2. Dissolved and Free Gas
3.3. Near-Surface Gas and Exsolution
3.4. Gas Flow Versus Water Flow
3.5. H2 Pathway
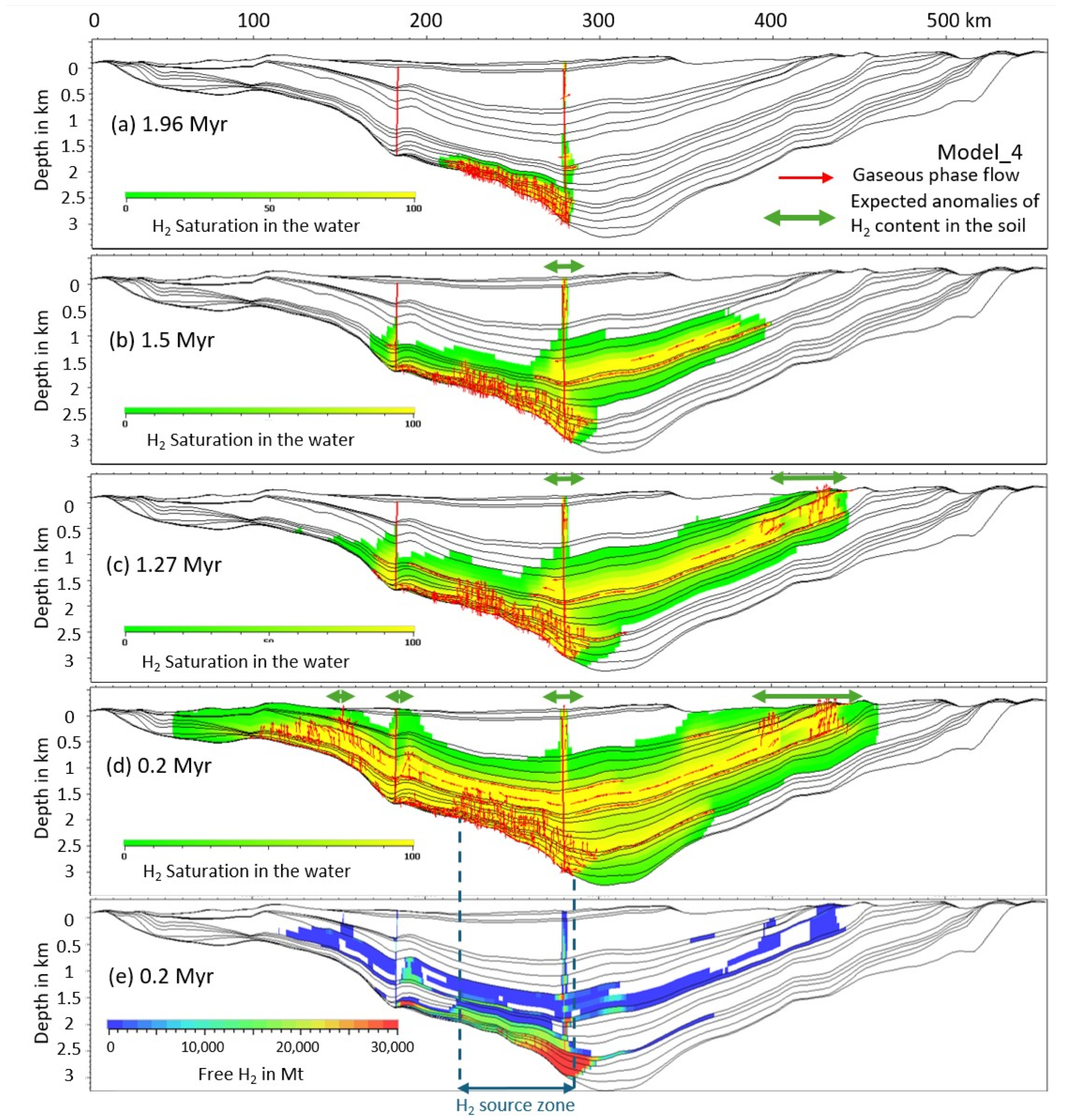
3.6. H2 in the Near-Surface Soils and Beds
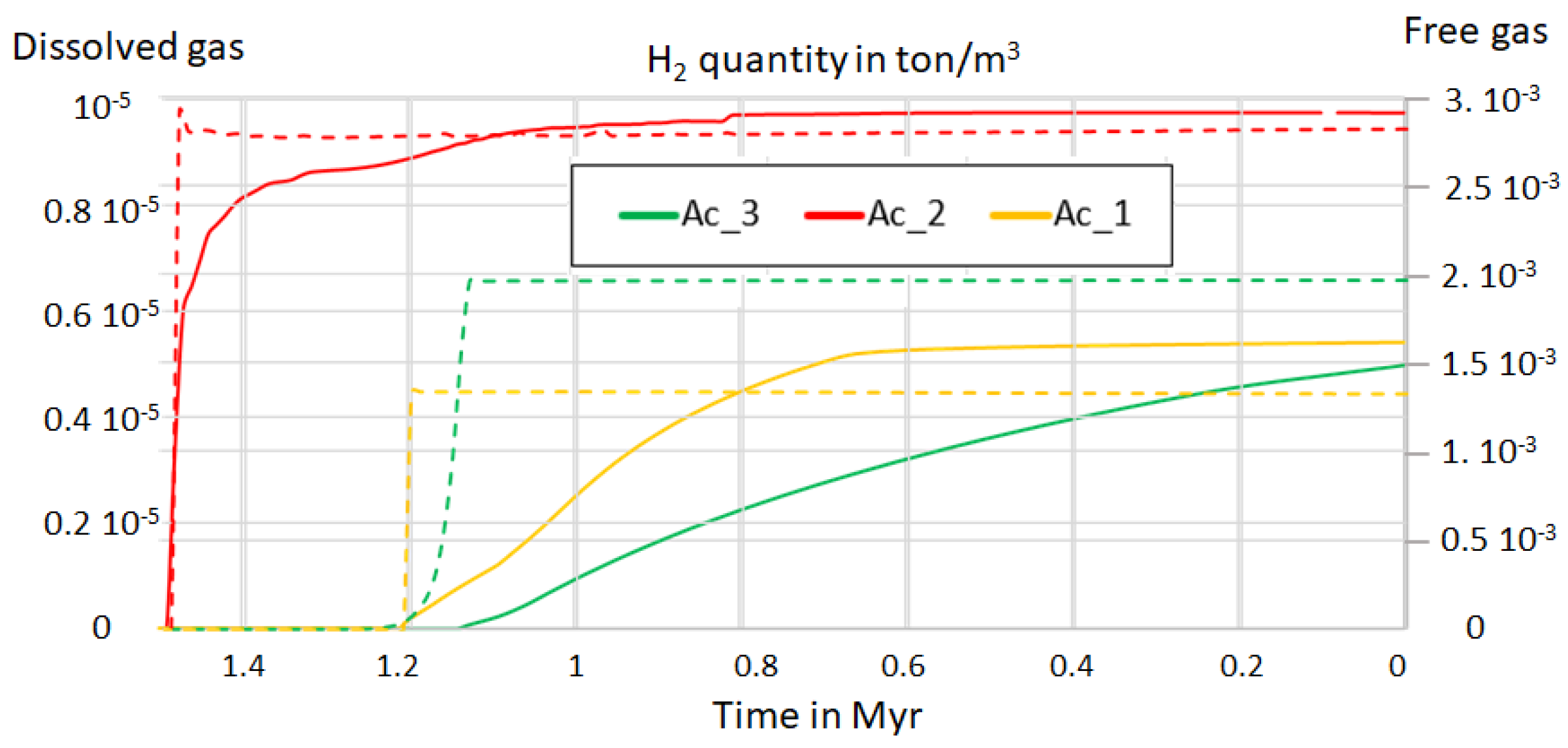
3.7. Expected Distance Between the H2 Kitchen, the H2 Accumulation, and the H2 Seeps
3.8. Depth of Exsolution
4. Conclusions/Perspectives
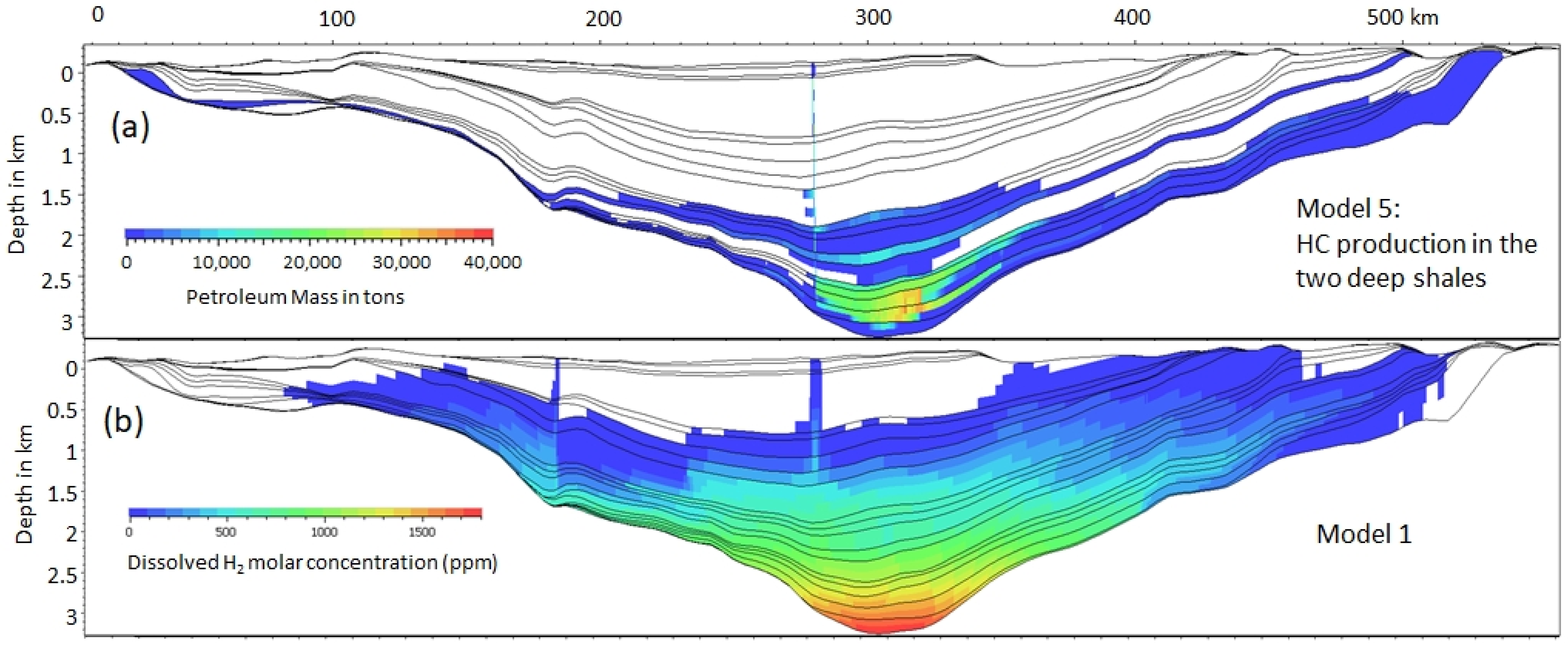
4.1. Fossil vs. Dynamic Accumulations
4.2. Transport Model Scheme
4.3. Accounting for H2 Consumption in the Subsurface
4.4. Exploration Strategy
- ‑
- Existence of H2 in the soil is a relevant proxy to prove the existence of an active H2 system.
- ‑
- The size of the region with emanations is related to the importance of the deep H2 charge.
- ‑
- The SCD density map should be compared with the aquifer map, but the SCDs’ positions are not particularly relevant to locate an accumulation.
- ‑
- The measurement of dissolved H2 in the aquifer should be performed more systematically to confirm the presence of a H2 deep flow. Measurements at different depths to verify whether or not the water is systematically saturated with H2 would be useful.
- ‑
- If degassing occurs above an aquifer, geophysical acquisitions should be targeted to find a play in the part of the basin where the aquifer is deeper. The flow of the water must be between the expected H2-generating zone and the SCDs.
- ‑
- The water flow must be directed from the generation zone to the target, but the POS is better if the free gas flow follows the same path.
- ‑
- An active H2-charged aquifer may continuously recharge the reservoirs.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCD | Subcircular Depression |
| H2_GR | Generating Rock in H2 System |
| HC | Hydrocarbon |
| SR | Source Rock in HC System |
| TOC | Total Organic Carbon |
| POS | Probability of Success |
References
- Truche, L.; McCollom, T.M.; Martinez, I. Hydrogen and Abiotic Hydrocarbons: Molecules That Change the World. Elements 2020, 16, 13–18. [Google Scholar] [CrossRef]
- Lévy, D.; Roche, V.; Pasquet, G.; Combaudon, V.; Geymond, U.; Loiseau, K.; Moretti, I. Natural H2 Exploration: Tools and Workflows to Characterize a Play. Sci. Technol. Energy Transit. 2023, 78, 27. [Google Scholar] [CrossRef]
- Hand, E. Hidden Hydrogen. Science 2023, 379, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, E.C.; Moretti, I.; Pélissier, N.; Burridge, G.; Gonthier, N. The Place of Natural Hydrogen in the Energy Transition: A Position Paper. Eur. Geol. 2023, 55, 5–9. [Google Scholar] [CrossRef]
- Brandt, A.R. Greenhouse Gas Intensity of Natural Hydrogen Produced from Subsurface Geologic Accumulations. Joule 2023, 7, 1818–1831. [Google Scholar] [CrossRef]
- Mathur, Y.; Moise, H.; Aydin, Y.; Mukerji, T. Techno-Economic Analysis of Natural and Stimulated Geological Hydrogen. Nat. Rev. Earth Environ. 2025, 6, 342–356. [Google Scholar] [CrossRef]
- Boreham, C.J.; Edwards, D.S.; Czado, K.; Rollet, N.; Wang, L.; van der Wielen, S.; Champion, D.; Blewett, R.; Feitz, A.; Henson, P.A. Hydrogen in Australian Natural Gas: Occurrences, Sources and Resources. APPEA J. 2021, 61, 163. [Google Scholar] [CrossRef]
- Ellis, G.S.; Gelman, S.E. Model Predictions of Global Geologic Hydrogen Resources. Sci. Adv. 2024, 10, eado0955. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Tahara Cissé, C.S.; Diallo, A.B. Discovery of a Large Accumulation of Natural Hydrogen in Bourakebougou (Mali). Int. J. Hydrogen Energy 2018, 43, 19315–19326. [Google Scholar] [CrossRef]
- Maiga, O.; Deville, E.; Laval, J.; Prinzhofer, A.; Diallo, A.B. Trapping Processes of Large Volumes of Natural Hydrogen in the Subsurface: The Emblematic Case of the Bourakebougou H2 Field in Mali. Int. J. Hydrogen Energy 2024, 50, 640–647. [Google Scholar] [CrossRef]
- Jackson, O.; Lawrence, S.R.; Hutchinson, I.P.; Stocks, A.E.; Barnicoat, A.C.; Powney, M. Natural Hydrogen: Sources, Systems and Exploration Plays. Geoenergy 2024, 2, geoenergy2024-002. [Google Scholar] [CrossRef]
- Klein, F.; Tarnas, J.D.; Bach, W. Abiotic Sources of Molecular Hydrogen on Earth. Elements 2020, 16, 19–24. [Google Scholar] [CrossRef]
- Zgonnik, V. The Occurrence and Geoscience of Natural Hydrogen: A Comprehensive Review. Earth Sci. Rev. 2020, 203, 103140. [Google Scholar] [CrossRef]
- Leila, M.; Loiseau, K.; Moretti, I. Controls on Generation and Accumulation of Blended Gases (CH4/H2/He) in the Neoproterozoic Amadeus Basin, Australia. Mar. Pet. Geol. 2022, 140, 105643. [Google Scholar] [CrossRef]
- Coveney, R.M., Jr.; Goebel, E.D.; Zeller, E.; Dreschhoff, G. Serpentinization and the Origin of Hydrogen Gas in Kansas. Bulletin 1987, 71, 39–48. [Google Scholar] [CrossRef]
- De Freitas, V.A.; Prinzhofer, A.; Françolin, J.B.; Ferreira, F.J.F.; Moretti, I. Natural Hydrogen System Evaluation in the São Francisco Basin (Brazil). Sci. Technol. Energy Transit. 2024, 79, 95. [Google Scholar] [CrossRef]
- Murray, J.; Clément, A.; Fritz, B.; Schmittbuhl, J.; Bordmann, V.; Fleury, J.M. Abiotic Hydrogen Generation from Biotite-Rich Granite: A Case Study of the Soultz-Sous-Forêts Geothermal Site, France. Appl. Geochem. 2020, 119, 104631. [Google Scholar] [CrossRef]
- Horsfield, B.; Mahlstedt, N.; Weniger, P.; Misch, D.; Vranjes-Wessely, S.; Han, S.; Wang, C. Molecular Hydrogen from Organic Sources in the Deep Songliao Basin, P.R. China. Int. J. Hydrogen Energy 2022, 47, 16750–16774. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Z.; Chen, X.; Su, Y.; Huang, X. The Origin and Occurrence of Natural Hydrogen. Energies 2023, 16, 2400. [Google Scholar] [CrossRef]
- Boreham, C.J.; Edwards, D.S.; Feitz, A.J.; Murray, A.P.; Mahlstedt, N.; Horsfield, B. Modelling of Hydrogen Gas Generation from Overmature Organic Matter in the Cooper Basin, Australia. APPEA J. 2023, 63, S351–S356. [Google Scholar] [CrossRef]
- Lollar, B.S.; Onstott, T.C.; Lacrampe-Couloume, G.; Ballentine, C.J. The Contribution of the Precambrian Continental Lithosphere to Global H2 Production. Nature 2014, 516, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Deville, E.; Prinzhofer, A. The Origin of N2-H2-CH4-Rich Natural Gas Seepages in Ophiolitic Context: A Major and Noble Gases Study of Fluid Seepages in New Caledonia. Chem. Geol. 2016, 440, 139–147. [Google Scholar] [CrossRef]
- Vacquand, C.; Deville, E.; Beaumont, V.; Guyot, F.; Sissmann, O.; Pillot, D.; Arcilla, C.; Prinzhofer, A. Reduced Gas Seepages in Ophiolitic Complexes: Evidences for Multiple Origins of the H2-CH4-N2 Gas Mixtures. Geochim. Cosmochim. Acta 2018, 223, 437–461. [Google Scholar] [CrossRef]
- Leong, J.A.; Nielsen, M.; McQueen, N.; Karolytė, R.; Hillegonds, D.J.; Ballentine, C.; Darrah, T.; McGillis, W.; Kelemen, P. H2 and CH4 Outgassing Rates in the Samail Ophiolite, Oman: Implications for Low-Temperature, Continental Serpentinization Rates. Geochim. Cosmochim. Acta 2023, 347, 1–15. [Google Scholar] [CrossRef]
- Palmowski, D.; Fernandez, N.; Kleine, A.; Lefeuvre, N.; Tari, G. Modelling Natural Hydrogen Systems. Extending the Basin Modeler’s Comfort Zone. In Proceedings of the 85th EAGE Annual Conference & Exhibition (including the Workshop Programme), Oslo, Norway, 10–13 June 2024; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2024. [Google Scholar]
- Zgonnik, V.; Beaumont, V.; Deville, E.; Larin, N.; Pillot, D.; Farrell, K.M. Evidence for Natural Molecular Hydrogen Seepage Associated with Carolina Bays (Surficial, Ovoid Depressions on the Atlantic Coastal Plain, Province of the USA). Prog. Earth Planet. Sci. 2015, 2, 31. [Google Scholar] [CrossRef]
- Moretti, I.; Brouilly, E.; Loiseau, K.; Prinzhofer, A.; Deville, E. Hydrogen Emanations in Intracratonic Areas: New Guide Lines for Early Exploration Basin Screening. Geosciences 2021, 11, 145. [Google Scholar] [CrossRef]
- Roche, V.; Geymond, U.; Boka-Mene, M.; Delcourt, N.; Portier, E.; Revillon, S.; Moretti, I. A New Continental Hydrogen Play in Damara Belt (Namibia). Sci. Rep. 2024, 14, 11655. [Google Scholar] [CrossRef]
- Ginzburg, N.; Daynac, J.; Hesni, S.; Geymond, U.; Roche, V. Identification of Natural Hydrogen Seeps: Leveraging AI for Automated Classification of Sub-Circular Depressions. Earth Space Sci. 2025, 12, e2025EA004227. [Google Scholar] [CrossRef]
- Pasquet, G.; Houssein Hassan, R.; Sissmann, O.; Varet, J.; Moretti, I. An Attempt to Study Natural H2 Resources across an Oceanic Ridge Penetrating a Continent: The Asal–Ghoubbet Rift (Republic of Djibouti). Geosciences 2021, 12, 16. [Google Scholar] [CrossRef]
- Truche, L.; Donzé, F.-V.; Goskolli, E.; Muceku, B.; Loisy, C.; Monnin, C.; Dutoit, H.; Cerepi, A. A Deep Reservoir for Hydrogen Drives Intense Degassing in the Bulqizë Ophiolite. Science 2024, 383, 618–621. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Rigollet, C.; Lefeuvre, N.; Françolin, J.; Valadão De Miranda, P.E. Maricá (Brazil), the New Natural Hydrogen Play Which Changes the Paradigm of Hydrogen Exploration. Int. J. Hydrogen Energy 2024, 62, 91–98. [Google Scholar] [CrossRef]
- Frery, E.; Langhi, L.; Maison, M.; Moretti, I. Natural Hydrogen Seeps Identified in the North Perth Basin, Western Australia. Int. J. Hydrogen Energy 2021, 46, 31158–31173. [Google Scholar] [CrossRef]
- Vrolijk, P.J.; Urai, J.L.; Kettermann, M. Clay Smear: Review of Mechanisms and Applications. J. Struct. Geol. 2016, 86, 95–152. [Google Scholar] [CrossRef]
- Moretti, I.; Labaume, P.; Sheppard, S.M.F.; Boulègue, J. Compartmentalisation of Fluid Migration Pathways in the Sub-Andean Zone, Bolivia. Tectonophysics 2002, 348, 5–24. [Google Scholar] [CrossRef]
- Faulkner, D.R.; Jackson, C.A.L.; Lunn, R.J.; Schlische, R.W.; Shipton, Z.K.; Wibberley, C.A.J.; Withjack, M.O. A Review of Recent Developments Concerning the Structure, Mechanics and Fluid Flow Properties of Fault Zones. J. Struct. Geol. 2010, 32, 1557–1575. [Google Scholar] [CrossRef]
- Akinfiev, N.N.; Diamond, L.W. Thermodynamic Description of Aqueous Nonelectrolytes at Infinite Dilution over a Wide Range of State Parameters. Geochim. Cosmochim. Acta 2003, 67, 613–629. [Google Scholar] [CrossRef]
- Lopez-Lazaro, C.; Bachaud, P.; Moretti, I.; Ferrando, N. Predicting the Phase Behavior of Hydrogen in NaCl Brines by Molecular Simulation for Geological Applications. BSGF Earth Sci. Bull. 2019, 190, 7. [Google Scholar] [CrossRef]
- Strauch, B.; Pilz, P.; Hierold, J.; Zimmer, M. Experimental Simulations of Hydrogen Migration through Potential Storage Rocks. Int. J. Hydrogen Energy 2023, 48, 25808–25820. [Google Scholar] [CrossRef]
- Ehhalt, D.H.; Rohrer, F. The Tropospheric Cycle of H2: A Critical Review. Tellus B Chem. Phys. Meteorol. 2009, 61, 500. [Google Scholar] [CrossRef]
- Cheng, A.; Sherwood Lollar, B.; Gluyas, J.G.; Ballentine, C.J. Primary N2–He Gas Field Formation in Intracratonic Sedimentary Basins. Nature 2023, 615, 94–99. [Google Scholar] [CrossRef]
- Bossennec, C.; Géraud, Y.; Böcker, J.; Klug, B.; Mattioni, L.; Bertrand, L.; Moretti, I. Characterisation of Fluid Flow Conditions and Paths in the Buntsandstein Gp. Sandstones Reservoirs, Upper Rhine Graben. BSGF Earth Sci. Bull. 2021, 192, 35. [Google Scholar] [CrossRef]
- Hantschel, T.; Kauerauf, A. Fundamentals of Basin and Petroleum Systems Modeling; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-72317-2. [Google Scholar]
- Mas, P.; Calcagno, P.; Caritg-Monnot, S.; Beccaletto, L.; Capar, L.; Hamm, V. A 3D Geomodel of the Deep Aquifers in the Orléans Area of the Southern Paris Basin (France). Sci. Data 2022, 9, 781. [Google Scholar] [CrossRef]
- Lefeuvre, N.; Thomas, E.; Truche, L.; Donzé, F.; Cros, T.; Dupuy, J.; Pinzon-Rincon, L.; Rigollet, C. Characterizing Natural Hydrogen Occurrences in the Paris Basin From Historical Drilling Records. Geochem. Geophys. Geosyst 2024, 25, e2024GC011501. [Google Scholar] [CrossRef]
- De Hoyos, A.; Viennot, P.; Ledoux, E.; Matray, J.-M.; Rocher, M.; Certes, C. Influence of Thermohaline Effects on Groundwater Modelling–Application to the Paris Sedimentary Basin. J. Hydrol. 2012, 464–465, 12–26. [Google Scholar] [CrossRef]
- McCollom, T.M.; Bach, W. Thermodynamic Constraints on Hydrogen Generation during Serpentinization of Ultramafic Rocks. Geochim. Cosmochim. Acta 2009, 73, 856–875. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional Controls on Hydrogen Generation during Serpentinization of Ultramafic Rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- Geymond, U.; Briolet, T.; Combaudon, V.; Sissmann, O.; Martinez, I.; Duttine, M.; Moretti, I. Reassessing the Role of Magnetite during Natural Hydrogen Generation. Front. Earth Sci. 2023, 11, 1169356. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Cacas-Stentz, M.-C. Natural Hydrogen and Blend Gas: A Dynamic Model of Accumulation. Int. J. Hydrogen Energy 2023, 48, 21610–21623. [Google Scholar] [CrossRef]
- Brunet, F.; Malvoisin, B. Can Natural H2 Be Considered Renewable? The Reference Case of a Deep Aquifer in an Intracratonic Sedimentary Basin. Adv. Geochem. Cosmochem. 2025, 1, 738. [Google Scholar] [CrossRef]
- Moretti, I.; Bouton, N.; Ammouial, J.; Carrillo Ramirez, A. The H2 Potential of the Colombian Coals in Natural Conditions. Int. J. Hydrogen Energy 2024, 77, 1443–1456. [Google Scholar] [CrossRef]
- Myagkiy, A.; Brunet, F.; Popov, C.; Krüger, R.; Guimarães, H.; Sousa, R.S.; Charlet, L.; Moretti, I. H2 Dynamics in the Soil of a H2-Emitting Zone (São Francisco Basin, Brazil): Microbial Uptake Quantification and Reactive Transport Modelling. Appl. Geochem. 2020, 112, 104474. [Google Scholar] [CrossRef]
- Ménez, B. Abiotic Hydrogen and Methane: Fuels for Life. Elements 2020, 16, 39–46. [Google Scholar] [CrossRef]
- Cascone, M.; Climent Gargallo, G.; Migliaccio, F.; Corso, D.; Tonietti, L.; Cordone, A.; Pietrini, I.; Franchi, E.; Carpani, G.; Di Benedetto, A.; et al. Hydrogenotrophic Metabolisms in the Subsurface and Their Implications for Underground Hydrogen Storage and Natural Hydrogen Prospecting. Preprint 2025. [Google Scholar] [CrossRef]
- Etiope, G.; Ciotoli, G.; Benà, E.; Mazzoli, C.; Röckmann, T.; Sivan, M.; Squartini, A.; Laemmel, T.; Szidat, S.; Haghipour, N.; et al. Surprising Concentrations of Hydrogen and Non-Geological Methane and Carbon Dioxide in the Soil. Sci. Total Environ. 2024, 948, 174890. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Fatah, A. Impact of H2-CH4 Mixture on Pore Structure of Sandstone and Limestone Formations Relevant to Subsurface Hydrogen Storage. Fuel 2024, 358, 130192. [Google Scholar] [CrossRef]
- Yekta, A.E.; Pichavant, M.; Audigane, P. Evaluation of Geochemical Reactivity of Hydrogen in Sandstone: Application to Geological Storage. Appl. Geochem. 2018, 95, 182–194. [Google Scholar] [CrossRef]
- Jähne, B.; Heinz, G.; Dietrich, W. Measurement of the Diffusion Coefficients of Sparingly Soluble Gases in Water. J. Geophys. Res. 1987, 92, 10767–10776. [Google Scholar] [CrossRef]
- Wygrala, B.P.; Yalcin, M.N.; Dohmen, L. Thermal Histories and Overthrusting—Application of Numerical Simulation Technique. Org. Geochem. 1990, 16, 267–285. [Google Scholar] [CrossRef]
- Aquino, K.A.; Perez, A.D.C.; Juego, C.M.M.; Tagle, Y.G.M.; Leong, J.A.M.; Codillo, E.A. High Hydrogen Outgassing from an Ophiolite-Hosted Seep in Zambales, Philippines. Int. J. Hydrogen Energy 2025, 105, 360–366. [Google Scholar] [CrossRef]
- Moretti, I.; Prinzhofer, A.; Françolin, J.; Pacheco, C.; Rosanne, M.; Rupin, F.; Mertens, J. Long-Term Monitoring of Natural Hydrogen Superficial Emissions in a Brazilian Cratonic Environment. Sporadic Large Pulses versus Daily Periodic Emissions. Int. J. Hydrogen Energy 2021, 46, 3615–3628. [Google Scholar] [CrossRef]
- Schumacher, D. Hydrocarbon-Induced Alteration of Soils and Sediments. In Hydrocarbon Migration and Its Near-Surface Expression; American Association of Petroleum Geologists: Tulsa, OK, USA, 1996; ISBN 978-1-62981-081-2. [Google Scholar]
- Larin, N.; Zgonnik, V.; Rodina, S.; Deville, E.; Prinzhofer, A.; Larin, V.N. Natural Molecular Hydrogen Seepage Associated with Surficial, Rounded Depressions on the European Craton in Russia. Nat. Resour. Res. 2015, 24, 369–383. [Google Scholar] [CrossRef]
- Lefeuvre, N.; Truche, L.; Donzé, F.-V.; Gal, F.; Tremosa, J.; Fakoury, R.-A.; Calassou, S.; Gaucher, E.C. Natural Hydrogen Migration along Thrust Faults in Foothill Basins: The North Pyrenean Frontal Thrust Case Study. Appl. Geochem. 2022, 145, 105396. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, S.; Huang, X.; Qi, S.; Lin, J.; Han, Y.; Wang, Y.; Wu, X.; Zheng, G. Enrichment Mechanisms of Natural Hydrogen and Predictions for Favorable Exploration Areas in China. Appl. Geochem. 2025, 182, 106316. [Google Scholar] [CrossRef]
- Schneider, F.; Dubille, M.; Patiño, C.; Medellin, F. Natural Hydrogen System Assessment with Basin Modeling Applied to the Colombian Foreland Basins. In Proceedings of the AAPG Cartagena, Cartagena, Colombia, 19–22 April 2022. [Google Scholar]
- Gonzalez-Penagos, F.; Moretti, I.; Guichet, X. Fluid Flow Modeling in the Llanos Basin, Colombia. AAPG Mem. 2017, 191–217. [Google Scholar]
- Maiga, O.; Deville, E.; Laval, J.; Prinzhofer, A.; Diallo, A.B. Characterization of the Spontaneously Recharging Natural Hydrogen Reservoirs of Bourakebougou in Mali. Sci. Rep. 2023, 13, 11876. [Google Scholar] [CrossRef] [PubMed]
- Prinzhofer, A. Fossil Noble Gas Isotopes: How Geological Hydrogen Systems Differ from Hydrocarbon Systems. Int. J. Hydrogen Energy 2025, 131, 318–324. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Karolytė, R.; Cheng, A.; Sherwood Lollar, B.; Gluyas, J.G.; Daly, M.C. Natural Hydrogen Resource Accumulation in the Continental Crust. Nat. Rev. Earth Env. 2025, 6, 342–356. [Google Scholar] [CrossRef]
- Arrouvel, C.; Prinzhofer, A. Genesis of Natural Hydrogen: New Insights from Thermodynamic Simulations. Int. J. Hydrogen Energy 2021, 46, 18780–18794. [Google Scholar] [CrossRef]
- Thaysen, E.M.; McMahon, S.; Strobel, G.J.; Butler, I.B.; Ngwenya, B.T.; Heinemann, N.; Wilkinson, M.; Hassanpouryouzband, A.; McDermott, C.I.; Edlmann, K. Estimating Microbial Growth and Hydrogen Consumption in Hydrogen Storage in Porous Media. Renew. Sustain. Energy Rev. 2021, 151, 111481. [Google Scholar] [CrossRef]
| Parameters | Values | Units | Ref |
| H2 diffusion coefficient in water | 3338 × 10−5 | cm2/s | [59] |
| Activation energy | 3.84 | kcal/mol | [59] |
| Solubility | function T and Z | [38] | |
| Fault capillarity pressure (FCP) | 0.5 | MPa | |
| Advection max speed | 0.2 | m/yr | |
| Heat flow | 60 | mW/m2 | |
| Surface temperature | around 10 °C (49° N maps) | °C | [60] |
| Lateral borders | adiabatic | ||
| Waterhead | relief | ||
| Water salinity | 0 |
| Model Number | Location and Size of the H2 Source | Start of H2 Input Flow Myr Ago | End of H2 Flow Myr | Total H2 Released (Mtons) | Flow ton/km2/yr |
|---|---|---|---|---|---|
| 1 | Center; 471 cells; 94 km | 1.5 | 0 | 471 | 41.8 |
| 2 | Center; 471 cells; 94 km | 1.5 | 0 | 0.471 | 6 |
| 3 | Right; 301 cells; 60 km | 1.5 | 0 | 301 | 116 |
| 4 | Left; 310 cells; 62 km | 2 | 1.5 | 471 | 1438 |
| HC SR | Run duration | TOC% | HI | ||
| 5 | Upper HC SR | none | 300 Myr | 5 | 600 |
| Lower HC SR | none | 300 Myr | 30 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, L.; Hidalgo, J.C.; Strąpoć, D.; Moretti, I. H2 Transport in Sedimentary Basin. Geosciences 2025, 15, 298. https://doi.org/10.3390/geosciences15080298
Nicoletti L, Hidalgo JC, Strąpoć D, Moretti I. H2 Transport in Sedimentary Basin. Geosciences. 2025; 15(8):298. https://doi.org/10.3390/geosciences15080298
Chicago/Turabian StyleNicoletti, Luisa, Juan Carlos Hidalgo, Dariusz Strąpoć, and Isabelle Moretti. 2025. "H2 Transport in Sedimentary Basin" Geosciences 15, no. 8: 298. https://doi.org/10.3390/geosciences15080298
APA StyleNicoletti, L., Hidalgo, J. C., Strąpoć, D., & Moretti, I. (2025). H2 Transport in Sedimentary Basin. Geosciences, 15(8), 298. https://doi.org/10.3390/geosciences15080298








