Abstract
The Kaiparowits Formation preserves one of the best fossil records of Cretaceous North America, which provides great insight into the paleoecology. In an effort to investigate the paleohydrology of the Kaiparowits Formation, stable isotope compositions (δ13C, δ18O-carbonate, δ18O-phosphate) of 41 hadrosaur teeth, 27 crocodile teeth, and 35 turtle shell fragments were analyzed. The mean O-isotope compositions of drinking water (δ18Ow) calculated from the O-isotope of bioapatite (phosphate-δ18Op) are −13.76 ± 2.08‰ (SMOW) for hadrosaur, −8.88 ± 2.76‰ (SMOW) for crocodile, and −10.14 ± 2.62‰ (SMOW) for turtle, which strongly reflect niche partitioning. The Kaiparowits formation does not fit the global trend in isotopic compositions of vertebrate skeletal remains from previous studies, which suggests a unique hydrological setting of the Kaiparowits basin. High-elevation runoff from the Mogollon Highlands and sea level fluctuation may have contributed to such a unique paleohydrology.
1. Introduction
As the stable isotope composition of skeletal tissues reflects both the physiologies of the animal and their living environment, stable isotope geochemistry is a practical technique for investigating paleobiology and paleoecology. The stable isotope compositions of vertebrate skeletal remains have been used to interpret diet (e.g., Kohn et al., 2005 [1]); Zazzo et al., 2010 [2]), thermophysiology of extinct animals (e.g., Barrick, 1998 [3]); Amiot et al., 2006 [4]) and paleoclimate (e.g., Fricke et al., 2010 [5]; Suarez et al., 2013 [6]). The isotopic composition of drinking water primarily controls the oxygen isotopic composition of vertebrate bioapatite, and dietary isotopic composition and local climate (aridity, seasonality, and temperatures) have smaller contributions (Kohn, 1996 [7]). In contrast, the carbon isotope composition of the vertebrate bioapatite is controlled by the carbon isotope composition of their food (Kohn et al., 2005 [1]; Zazzo et al., 2010 [2]). Ultimately, local hydrology and flora are controlled by climate (Kohn and Cerling, 2002 [8]). Hence, stable isotope analyses of skeletal remains are critical in paleoclimate investigations. Under arid conditions, both oxygen and carbon become enriched in the heavy isotopes (i.e., 18O and 13C, respectively) due to evaporative enrichment, while rainout effects deplete the heavy isotopes in wet climates (Suarez et al., 2013 [6]; 2017 [9]). Standard materials, including Pee Dee Belemnite (PDB) for carbon and Standard Mean Ocean Water (SMOW), have been used to compare isotope ratios and ensure consistent measurements across studies. These were later refined to VPDB (Vienna Pee Dee Belemnite) and VSMOW (Vienna Standard Mean Ocean Water) to cope with depleted original standard materials. Additionally, large seasonal swings in precipitation result in large isotopic shifts, and such a change is recorded in fossilized biological tissues, including teeth (e.g., Goedert et al., 2016 [10]; Suarez et al., 2017 [9]; Yamamura et al., 2021 [11]) and fish scales (e.g., During et al., 2022 [12]). As such, serial sampling of isotope compositions from fossils reveals seasonal 18O changes in precipitation. If high-elevation precipitation drains through an ecosystem, the δ18O value of skeletal tissue will reflect low δ18Ow of high elevation precipitation (Dutton et al., 2015 [13]) as a result of greater fractionation at lower temperatures and increased Rayleigh distillation as moisture systems become more and more depleted in 18O. Similarly, the correlation between latitudinal temperature change and δ18O of precipitation water has been well-established (Dansgaard, 1964 [14]) as a result of increased fractionation at colder temperatures of higher latitudes and Rayleigh distillation (continental effect). Amiot et al. (2004 [15]) reported global trends in δ18O values of apatite: (1) there is a negative correlation between paleolatitude and fossil δ18Op, and (2) δ18Op values of endothermic dinosaurs have a higher correlation with paleolatitude than ectothermic reptiles due to higher variability in ectothermic reptile fossils. Additionally, Amiot et al. (2006 [4]) show a negative correlation between the paleolatitude and δ18O difference between dinosaurs and ectothermic reptiles (Δ18O), which resulted from their metabolic difference. A deviation from these trends likely implies a unique paleohydrologic setting, such as monsoonal conditions, significant orographic effects, and differences in drinking water sources between animals.
Diagenesis during fossilization comprises the physical, chemical, and biological changes to vertebrate skeletal remains through time, during corpse decay and burial. Because diagenesis can alter the isotopic composition of skeletal remains, separating diagenetic signals from the authentic biogenic signal is essential for stable isotope studies. Although fossilized bones preserve histological characteristics, the chemical composition of bioapatite can be altered via chemical substitution and overgrowth of crystallites (e.g., Hubert et al., 1996 [16]; Trueman and Tuross, 2002 [17]; Wings, 2004 [18]). Therefore, careful analyses of bioapatite’s phosphatic and carbonate components are necessary to assess the authenticity of the isotope composition. In modern mammals, oxygen isotopic compositions (δ18O) of coexisting carbonate and phosphate in bioapatite have a strong correlation (R2 = 0.98) due to the kinetic difference in carbonate–metabolic water and phosphate–metabolic water isotopic exchange at the time of precipitation of bioapatite (Iacumin et al., 1996 [19]). Although it may not be identical, such a correlation between phosphate and carbonate should exist in dinosaurs with endothermic metabolism and also in ectothermic reptiles to a lesser extent, as reptiles regulate their body temperature through external sources. Thus, the lack of correlation between the oxygen isotope compositions of carbonate and phosphate indicates potential diagenetic alteration of one or both isotope compositions. Owing to a stronger binding of oxygen atom in PO43− ion group, the oxygen isotope composition in phosphate (δ18Op) is more resistant to diagenetic alteration than the oxygen isotope composition of carbonate (δ18Oc) under abiotic conditions, yet microbially mediated diagenesis may preferentially alter phosphate (Zazzo et al., 2004 [20]). Additionally, tooth enamel contains larger bioapatite crystallites and less organic content compared to bone (Trueman and Tuross, 2002 [17]) and is more resistant to diagenetic alteration (Kohn and Cerling, 2002 [8]). The investigation of diagenetic alteration is particularly important for fossilized skeletal remains of Mesozoic age and older because physicochemical alteration of bioapatite is more evident (e.g., crystallite size and incorporation of cations from pore-fluid) in older fossils (e.g., Kohn and Cerling, 2002 [8]; Trueman and Tuross, 2002 [17]; Piga et al., 2009 [21]). Diagenetic alteration of skeletal remains would converge their isotopic compositions to that of the diagenetic fluid; hence, taxonomic differences in isotopic composition would be reduced or absent among altered fossils. However, many studies, including Suarez et al. (2013 [6]) and Fricke and Pearson (2008 [22]), demonstrate that isotopic compositions of coexisting vertebrate remains reflect different habitat use in the fossil record.
Fricke et al. (2009 [23]) demonstrated that the isotopic compositions (δ13C and δ18O of structural carbonate) of the dinosaur fossils in the Kaiparowits Formation are lower than those of the coastal plain Fruitland Formation, suggesting that the dinosaur populations in these two formations were segregated during the Campanian. Foreman et al. (2015 [24]) utilized the unionid (bivalve) freshwater gastropods (snails) and paleosol, which suggest a humid environment and potential change in aggradation and hydrology related to the Laramide uplift. Crystal et al. (2019 [25]) focused on gar scales, bivalves, and hadrosaur (structural carbonate) to suggest that there are at least three fluvial components preserved within the Kaiparowits Formation. One component is characterized by low δ18Ow (ca. −14‰, VSMOW) derived from high-elevation runoff and represented by material preserved in larger sandstone bodies. The second component is characterized by well-drained seasonally dry paleosols with high (ca. −4‰) δ18O. The third component is represented by perennial wetlands (micritic pond deposits) that represent a combination of both components. Bergner et al. (2019 [26]) used clumped isotope paleothermomentry of pedogenic and carbonates to suggest (1) the mean annual range in temperature (MART) for the Kaiparowits Formation is large between 27 and 35 °C, and (2) the MART is not much different between the Two Medicine Formation of Montana and the Kaiparowits Formation of southern Utah. Thus, Burgener et al. (2019 [26]) conclude that the dinosaur provincialism within the western interior basin was influenced by factors other than temperature, such as precipitation. The serially sampled δ18Op shows that the oxygen isotope composition of drinking water (δ18Ow) ranged from −21.0 to −14.4‰, which supports the monsoonal condition (Yamamura et al., 2021 [11]). This study adds δ18Op of hadrosaur teeth, crocodile teeth, and turtle shells to the ongoing effort of the Kaiparowits Formation paleoclimate study.
Our objective is to contribute to the effort of investigating the Cretaceous paleoclimate using stable isotope geochemistry. The focus of this study is the paleohydrologic investigation of the Kaiparowits Formation using stable isotope geochemistry of fossilized skeletal remains. The Kaiparowits Formation was selected because of (1) well-constrained age and stratigraphic studies (Roberts, 2007 [27]; Roberts et al., 2013 [28]; Beveridge et al., 2020 [29]) and (2) the highly fossiliferous nature of the Kaiparowits Formation (Titus et al., 2005 [30] and 2013 [31]), which allows high sample size across the formation.
2. Geologic Setting
The Kaiparowits Formation was deposited between 76.6 and 74.5 Ma as a proximal prograding clastic wedge derived from thrust sheets of the Sevier orogenic belt in southeastern Nevada and southern California, and the Mogollon Slope in southwestern Arizona (Figure 1; Goldstrand et al., 1993 [32]; Roberts, 2007 [27]; Roberts et al., 2013 [28]). The majority of the Kaiparowits Formation was deposited in a fluvial setting, yet fluvial style and sediment accumulation rate vary throughout the formation (Roberts, 2007 [27]; Figure 1). Owing to the rich fossil record, the Kaiparowits Formation has been studied in great detail over the last three decades. These expeditions studies have yielded remarkable turtle diversity (at least 14 distinct taxa; Hutchison et al., 2013 [33]), at least three taxa of crocodyliform (Irmis et al., 2013 [34]), 16 dinosaur taxa (Sampson et al., 2013 [35]), and other vertebrates and invertebrates (Sampson et al., 2013 [36]).
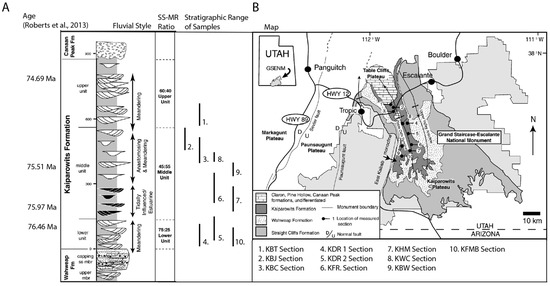
Figure 1.
The map and stratigraphic summary of the Kaiparowits Formation. Modified from Roberts (2007 [27]). (A) Informal subdivision (upper, middle, and lower units) by Roberts (2007) is based on the sandstone: mudrock ratio. The middle unit was further subdivided into lower-middle and upper-middle units in this study based on the change in fluvial style. (B) The reference sections (from Roberts et al., 2013 [28]) are listed below the map. The stratigraphic range of the samples from this study is shown in the stratigraphic summary with associated reference section numbers.
The Kaiparowits Formation is an unusually thick (860 m) and rapidly accumulated fluvial sedimentary sequence that crops out near and within the Grand Staircase Escalante National Monument (GSENM; Figure 1; Roberts, 2007 [27]). The Kaiparowits Formation is informally subdivided into three units based on the sandstone to mudstone ratio (Roberts, 2007 [27]). These units include the (1) lower unit (0–110 m; 75% sandstone and 25% mudstone), (2) middle unit (110–530 m, 45% sandstone and 55 mudstone), and (3) upper unit (530–860 m, 60% sandstone and 40% mudstone). The lower portion of the Middle Unit (110–300 m) exhibits evidence of tidal influence in sedimentation, leading Roberts (2007 [27]) and Foreman et al. (2015 [24]) to suggest transgression of the nearby western interior seaway (WIS) in the middle unit. Based on the Roberts (2007 [27]) observation, mixing with the ocean water is expected to shift the isotope compositions of groundwater closer to ocean water; hence, the middle unit is subdivided into lower-middle (110–300 m) with tidal influence and upper-middle (300–530 m) without tidal influence. Beveridge et al. (2020 [29]) added a new volcaniclastic member, which is 255 m thick and partially overlaps with the upper unit.
The Kaiparowits Formation is one of the thickest and most fossiliferous units in the Upper Cretaceous succession (Titus et al., 2005 [30] and 2013 [31]). The unique faunal composition includes the diverse ceratopsians and one of the highest diversities of turtles (Hutchison et al., 2013 [33]; Sampson et al., 2013 [35]). Despite the stratigraphic difference, paleontological and paleobotanical studies noted the similarity in the faunal/floral composition of the Kaiparowits Formation and the Maastrichtian Hell Creek Formation (Sampson et al., 2013 [35]; Miller et al., 2013 [37]). The Kaiparowits Formation contains many concretions, and alignment with the Table Cliff syncline axis suggests that these concretions formed later during diagenesis (Roberts and Chan, 2010 [38]).
3. Materials and Methods
3.1. Materials
Fossil specimens were loaned from the Natural History Museum of Utah (UMNH), including 41 hadrosaur teeth, 27 crocodile teeth, and 35 turtle shell fragments. The Kaiparowits Formation preserves three hadrosaur species, four crocodile taxa, and fourteen turtle taxa (Sampson et al., 2013 [35]). However, further taxonomic distinction is beyond the scope of this study, and we grouped samples based on familial affinity (i.e., Hadrosauridae and Crocodylidae). Turtles (Order: Testudines) in this study include families Baenidae, Trionychidae, Adocidae, and Compsemydidae that are considered semi-aquatic, and Basilemys (Family Nanhsiungchelyidae), which is considered a terrestrial turtle, was omitted. Based on the locality data provided by UMNH and field observations, the sampling range covers the majority of the Kaiparowits Formation except for the uppermost section of the Upper Member.
3.2. Stable Isotope Analysis
One to two milligrams of powdered samples for structural carbonate and phosphate isotope analyses were prepared using a dental drill. Preservation of the tooth enamel was variable among the specimens, and the sampling position on the tooth was not uniform. The structural carbonate samples were treated with acetic acid in order to remove diagenetic carbonate minerals (Koch et al., 1997 [39]). The powdered samples for phosphate analysis were dissolved in 0.1 N nitric acid. Samples were then re-precipitated as silver phosphate crystals following Vennemann et al. (2002 [40]).
Structural carbonate isotopic compositions of the powdered samples were analyzed using a Thermo Scientific (Bremen, Germany) Gas Bench II coupled to the Thermo Scientific (Bremen, Germany) Delta Plus XP Isotopic Ratio Mass Spectrometer (IRMS) at the University of Arkansas Stable Isotope Laboratory (UASIL). Instrumental stabilities were monitored using NBS-19, which produced a value of δ13C relative to Vienna Pee Dee Belemnite (VPDB) = 1.93 ± 0.05‰ 1σ standard deviation and δ18O relative to Vienna Standard Mean Ocean Water (VSMOW) = 28.65 ± 0.11‰ 1σ standard deviation. The reported true values of NBS-19 are δ13C = 1.95‰ VPDB and δ18O = 28.65‰ VSMOW. Two in-house standards, UASIL-22 and UASIL-23, were also used along with NBS-19 for calibration. True values of UASIL-22 are δ13C = −35.60‰ and δ18O = −17.07‰. True values of UASIL-23 are δ13C = −0.60‰ VPDB and δ18O = −14.71‰ VPDB. The precision was monitored via analysis of NIST 120c Florida phosphate rock (Ca5(PO4)2.5(CO3)0.5F), producing an average value of δ13C = −6.28 ± 0.12‰ VPDB and δ18O of 29.09 ± 0.30‰ VSMOW.
Silver phosphate crystals were analyzed using a Thermo Scientific (Bremen, Germany) TC/EA high-temperature conversion elemental analyzer coupled to Thermo Scientific (Bremen, Gerany) Delta Plus XP IRMS at UASIL. The δ18Op was calculated using laboratory standards, including ANU Sucrose, USGS 34 (KNO3), USGS 35 (NaNO3), and Alpha Aesar (Ag3PO4), and is reported in parts per thousand (‰) relative to VSMOW. ANU Sucrose returned values of δ18O = 36.37 ± 0.65‰; reported the true δ18O value of the sucrose standard is 36.20 ± 0.10‰. USGS 34 returned values of δ18O = −28.01 ± 0.63‰; reported true δ18O value of USGS 34 is −27.9 ± 0.3‰. USGS 35 returned 55.48 ± 0.87‰; reported true 18O value of USGS 35 is 21.73 ± 0.87‰. Alpha Aesar returned 4.98 ± 3.37‰. The precision was monitored via analysis of silver phosphate crystals prepared from NIST 120c (phosphate rock) using the Vennemann et al. (2003 [40]) method as a quality control standard, producing an average value of 21.73 ± 0.87‰.
The carbon isotope compositions of fossil structural carbonate are referred to as δ13C, and values are reported relative to VPDB. The oxygen isotope compositions of structural carbonate of fossils are referred to as δ18Oc. The oxygen isotope compositions of the fossil phosphate are referred to as δ18Op. Both δ18Oc and δ18Op are reported relative to VSMOW; VSMOW is the internationally accepted reference for isotopic composition of water prepared from distilled seawater by the International Atomic Energy Agency (IAEA).
The isotope composition of water consumed by animals was calculated by the following formulas:
for hadrosaur teeth, where h is relative humidity (Suarez et al., 2012 [41]). Humidity was estimated at 70% based on the mean annual temperature (20.2 °C) and precipitation rate (1700 mm) from Miller et al. (2013) [37].
for crocodile teeth (Amiot et al., 2007 [42]).
for turtle shells (Coulson et al., 2008 [43]).
δ18Ow = 1.41 (δ18Op) + 20.1 h − 49.69
δ18Ow = 0.82 (δ18Op) − 19.13
δ18Ow = (1.08 ± 0.09)δ18Op − (23.2 ± 1.8)
3.3. Statistical Analyses
The normality of the sample distribution was analyzed using a quantile-quantile (QQ) plot. For univariate comparison, two-sample t-tests were used to investigate the taxonomic differences in isotope compositions when data were normally distributed. The Wilcoxon Rank-Sum test is a non-parametric method that does not assume normality. Hence, it was used as an alternative test when the data were not normally distributed. Bivariate plots were used to analyze the taxonomic difference in δ13C and δ18Oc values. A simple linear regression was performed to analyze the correlation between δ18Oc and δ18Op values.
4. Results
A total of 9 samples (2 hadrosaurs, 1 crocodile, and 6 turtles) failed to produce silver phosphate crystals, and δ18Op were not analyzed. The statistical summary of isotope compositions is presented in Table 1; parameters include mean, range, standard deviation (SD), and variance (σ2). The complete data set is also presented in the Supplemental Material. The QQ plots demonstrate that the distribution of isotopic compositions for most of the taxa/elements is normal with few outliers. The oxygen isotope (δ18Oc and δ18Op) of crocodile teeth is not distributed normally; hence, Wilcoxon Rank-Sum tests were used instead of a t-test for statistical comparison with crocodile δ18Oc and δ18Op.

Table 1.
Summary of the isotope compositions.
The results from the statistical tests for taxonomic differences are summarized in Table 2. Crocodile teeth have the highest variability for all three isotope compositions (Table 1). There was no discernible relationship between isotope compositions and other factors, for example, size, hardness, and type of teeth (conical vs. bulbous/globidont); however, the higher variability is associated with the stratigraphic position (see the discussion).

Table 2.
Summary of statistical analysis.
4.1. Carbon Isotope (δ13C)
Mean δ13C values are −6.32 ± 1.75‰ (PDB) for hadrosaur, −7.84 ± 2.16‰ (PDB) for crocodile, and −7.58 ± 1.35‰ (PDB) for turtle. Statistically significant differences in δ13C were seen between 1) hadrosaur teeth and crocodile teeth (p-value = 0.0007 from a Wilcoxon Rank-Sum test), and 2) hadrosaur teeth and turtle shell (two-sided p-value is 0.0017 from a two-sample t-test). Crocodile and turtle δ13C did not have statistically significant differences (p-value = 0.25).
4.2. Oxygen Isotope (δ18Oc, δ18Op, and δ18Ow)
Mean δ18Oc are 21.93 ± 0.87‰ (SMOW) for hadrosaur, 21.37 ± 1.59‰ (SMOW) for crocodile, and 21.00 ± 1.18‰ (SMOW) for turtle. Mean δ18Op values are 13.77 ± 1.85‰ (SMOW) for hadrosaur, 12.50 ± 3.37‰ (SMOW) for crocodile, and 11.7 ± 2.55‰ (SMOW) for turtle (Table 2). Mean δ18Ow values calculated from δ18Op are −13.76 ± 2.08‰ (SMOW) for hadrosaur, −8.88 ± 2.76‰ (SMOW) for crocodile, and −10.4 ± 2.78‰ (SMOW) for turtle.
The δ18Oc values have low variability (σ2 = 0.76 to 2.53) compared to the δ18Op (σ2 = 3.43 to 11.3). The statistical tests fail to differentiate δ18Oc and δ18Op of crocodiles from those of hadrosaurs or turtles because of high variability in crocodiles (σ2 = 2.53 for δ18Oc and 11.32 for δ18Op); two to three times higher than hadrosaurs and turtles (Table 2). There is strong evidence for a difference between hadrosaur teeth and turtle shell δ18Oc (p-value = 0.0018 from a two-sample t-test) and δ18Op (p-value = 0.003 from a two-sample t-test; Table 2). Although the mean δ18Op of hadrosaur teeth is 1.25‰ higher than the mean δ18Op of crocodile teeth, statistical significance is not strong (p-value = 0.672 from Wilcoxon Rank-Sum test).
There is a weak correlation between δ18Oc and δ18Op in crocodile teeth and turtle shells (Figure 2C,D; r = 0.660, p-value < 0.001, n = 22 for crocodile, and r = 0.548, p-value < 0.001, n = 24 for turtle shell). Such a correlation was not observed in hadrosaur teeth (Figure 2B; r = 0.186, p-value = 0.285, n = 36).
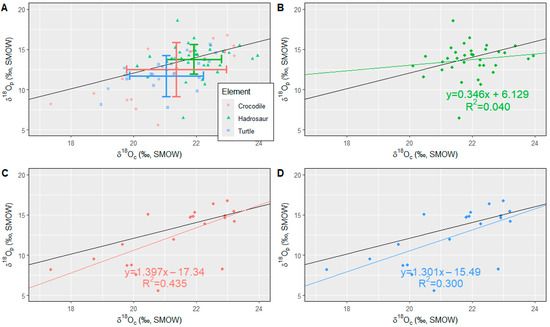
Figure 2.
Bivariate plots (δ18Oc vs. δ18Op) compared with the theoretical equilibrium line (Iacumin line, shown as black plain line). (A) Bivariate plot with all fossils with mean and standard deviation. Error bars indicate 1σ and mean δ18Oc and δ18Op. (B) Bivariate plot for the hadrosaur teeth. The correlation between δ18Oc and δ18Op is not evident, and isotopic values do not show affinity to the Iacumin line. (C) Bivariate plot for the crocodile teeth. There is a weak correlation between δ18Oc and δ18Op. (D) Bivariate plot for turtle shell. There is a weak correlation between δ18Oc and δ18Op, and the best-fit line was most similar to the Iacumin line among the three taxa.
The bivariate plot of the δ13C and δ18Oc indicates that there is no correlation between the δ13C and δ18Oc values for any taxa (Figure 3). The bivariate plot also shows similarities in isotopic compositions between crocodiles and turtles (Figure 3). Although there are overlaps in data, hadrosaur teeth tend to have higher δ13C and δ18Oc values.
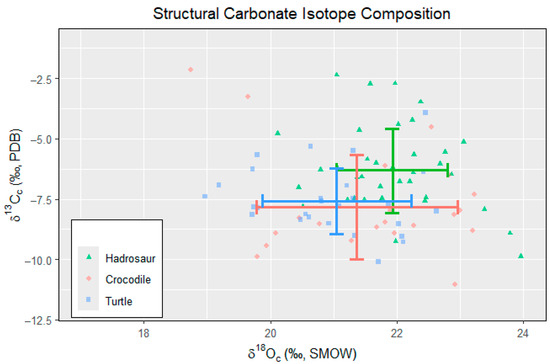
Figure 3.
Bivariate plot of δ13C and δ18Oc of all fossils. Error bars indicate 1σ and mean δ13C and δ18Oc of each taxon.
5. Discussion
5.1. Bone/Tooth Diagenesis
Powdered samples were prepared following the Koch et al. (1997 [39]) method for structural carbonate, which removes diagenetic carbonate. Phosphate samples were prepared using the Vennemann et al. (2002 [40]) method, and only oxygen in phosphate ions was analyzed. Thus, contamination from diagenetic carbonate is less likely to affect δ18Op. However, isotopic fractionation between bioapatite and pore fluid is not fully ruled out (Trueman and Tuross, 2002 [17], Lecuyer et al., 2003 [44]); hence, the isotopic composition difference between taxa was used to investigate the preservation of biogenic signals. Diagenetically altered fossils often show more consistent isotopic composition than unaltered skeletal remains, and isotopic values of altered fossils typically converge on the value of diagenetic fluids (Lecuyer et al., 2003 [44]). Our data shows isotope values are not uniform (variance ranges from 0.760 to 11.3; Table 1) for each taxon, and isotopic compositions in fossils do not show a trend similar to the meteoric calcite line; invariant δ18O coupled with variant δ13C (Lohmann, 1988 [45]). Among δ13C, δ18Oc, and δ18Op, δ18Oc has the smallest variance (0.760 for hadrosaur and 2.53 for crocodile,) whereas δ18Op has the highest variance (3.43 in hadrosaur and 11.3 in crocodile.) Wang and Cerling (1994 [46]) also reported that the δ13C compositions of structural carbonate in bioapatite are more resistant to diagenetic alteration than the δ18O. Thus, the use of δ18Oc data requires extra caution. For example, estimating carbonate content in tooth specimens could assess the effectiveness of the preparation method by Koch et al. (1997 [39]).
Iacumin et al. (1996 [19]) demonstrate equilibrium between the isotopic composition of structural carbonate and phosphate oxygen (δ18Oc and δ18Op, respectively) in mammal bone, suggesting the two should show a linear ~1:1 relationship, and this relationship should hold for dinosaurs that were thermoregulating (Eagle et al., 2011 [47]). Such a linear relationship should also hold for turtles and crocodiles since they precipitate their bioapatite at an optimal temperature: 27 ± 4° for turtles and 29 ± 3 °C for crocodiles (Barrick et al., 1999 [48]; Amiot et al., 2007 [42]). The teeth and bone from this study show a poor correlation between δ18Oc and δ18Op (R2 = 0.040 for hadrosaurs, R2 = 0.435 for crocodiles, and 0.318 for turtles; Figure 2). Barrick and Showers (1994 [49] and 1995 [50]), Barrick (1998 [3]), and Yamamura et al. (2021 [11]) also show a poor correlation between dinosaur δ18Oc and δ18Op, suggesting an alteration of oxygen isotope composition in structural carbonate.
5.2. The δ13C as Diet Proxy
The δ13C difference between crocodiles and turtles was statistically insignificant (p-value = 0.25 from a Wilcoxon Rank-Sum Test). Such a similarity likely reflects the shared ecological niches. Hadrosaur δ13C, on the other hand, shows significant differences with those of crocodiles (1.57‰, p-value = 0.0007 from a Wilcoxon Rank-Sum Test) and turtles (1.43‰, p-value = 0.0005 from a t-test). As such, the dietary differences between semiaquatic reptiles and hadrosaurs are reflected in the δ13C of fossilized skeletal remains. Tyrannosaurs from the Kaiparowits Formation are likely predators for the hadrosaurs, and reported δ13C (Yamamura et al., 2021 [11]) of Kaiparowits tyrannosaurs is −9.40‰ (n = 3 after averaging micro-sampling), which is 3.08‰ lower than hadrosaurs from this study. Although sampling methods and localities differ, the δ13C relationship between tyrannosaurs and hadrosaurs is similar in other studies (Bocherens, 2000 [51]; Cullen et al., 2020 [52]). Although this study can provide limited insight into the Kaiparowits trophic structure, further investigation may provide clear trophic relationships in the Kaiparowits Formation. For example, this study did not distinguish different taxa within hadrosaurs, crocodiles, and turtles. More precise taxonomic designations (for example, distinguishing crocodile taxa with different tooth morphologies) and analyzing more taxa are necessary.
5.3. The Kaiparowits Paleoclimate
The Kaiparowits Formation was deposited in the Sevier foreland basin with a wide variety of hydrologic sources, such as a river, pond/lake, and even estuarine water (Roberts, 2007 [27]). Animals in the Kaiparowits floodplain may consume water from different sources and can have a wide range of δ18O values in their biominerals. Our δ18Op data are consistent with such observations; variance ranges from 3.43 (hadrosaur) to 11.32 (crocodile). Our crocodile tooth specimens include various sizes and shapes, 5 to 20 mm in length, and conical and globidont shapes. Including multiple taxa of crocodiles into a single crocodile category likely contributed to a high variability in isotopic compositions. Additionally, a wide range in crocodile δ18Op may be reflecting a broader range of their habitat, including estuarine environments during the period of higher sea level.
Assuming a minimal diagenetic effect, the δ18Op value is expected to reflect different habitat uses between hadrosaurs and semi-aquatic reptiles (i.e., crocodiles and turtles). First, crocodiles and turtles share similar habitats and are expected to share similar δ18Op values. Our data confirms such a similarity in δ18Op (difference in δ18Op of 0.06‰ with p-value = 0.295 from the Wilcoxon Rank-Sum test). Second, the mean δ18Op value of hadrosaurs was 1.21‰ higher than that of turtles (p-values = 0.003 from a t-test), highlighting that hadrosaurs and turtles occupied different habitats. Third, the δ18Op difference between hadrosaur and crocodile teeth (1.27‰) is not statistically significant (p-value = 0.672 from a Wilcoxon Rank-Sum test), primarily due to the high variability in crocodile teeth (ranges from 5.6 to 16.8‰ with s2 = 11.32). Such a result likely highlights crocodiles’ ability to occupy various habitats, and some crocodiles shared a terrestrial habitat with hadrosaurs. Thus, our isotopic data show the taxonomic difference in their habitat use.
As hadrosaur, turtle, and crocodile fossils were used as proxies for the isotopic composition of meteoric water in previous studies (e.g., Barrick et al., 1999 [48], Amiot et al., 2007 [42], Suarez et al., 2013 [6]), the δ18O value of the meteoric water δ18Ow) of the Kaiparowits Formation was estimated using δ18Op. The mean δ18Ow values calculated from δ18Op are −13.76 ± 2.08‰ for hadrosaurs, −8.88 ± 2.76‰ for crocodiles, and −10.14 ± 2.62‰ for turtles (Figure 4). Assuming hadrosaurs are endothermic, the δ18Op of hadrosaur skeletal tissue records the δ18Ow throughout the year. Compared to the unionids data from Foreman et al. (2015 [24]), the δ18Ow calculated from hadrosaur teeth has a higher affinity to the δ18Ow from fluvial unionids (−16.3‰, Figure 4). In contrast, δ18Ow calculated from turtles and crocodiles has a higher affinity to δ18Ow pond unionid (−9.5‰, Figure 4). Such a result suggests that hadrosaurs preferred to drink water from rivers, while turtles and crocodiles lived in a ponded body of water on the floodplain. As many crocodile and turtle taxa described from the Kaiparowits Formation (Hutchson et al., 2013 [33]; Irmis et al., 2013 [37]) are interpreted to inhabit rivers, an alternative control on isotopic bias should exist.
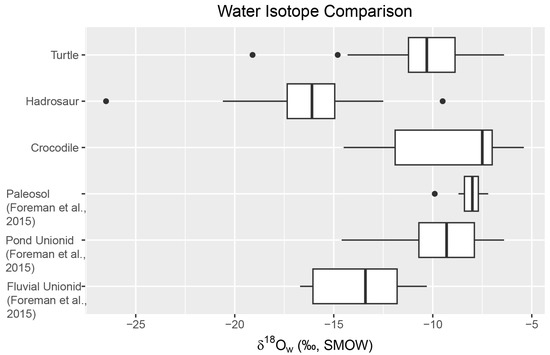
Figure 4.
Water isotope interpreted from fossils in comparison to paleosol and unionids from Foreman et al. (2015 [24]). Hadrosaur water δ18Ow is lower and has some overlap with river unionids. The δ18Ow from turtles and crocodiles overlaps are closer to pond unionids.
Reptile optimal growth temperature and mineralization of skeletal tissues occur at a temperature range of 26 to 36 °C with an average of 29 ± 3 °C for crocodiles (Amiot et al., 2007 [42]), and 20 to 35 °C with an average of 27 ± 4 °C for turtle (Barrick et al., 1999 [48]). Thus, the δ18Ow estimated from the δ18Op of turtles and crocodiles is biased toward a warmer time of year. The δ18Ow estimated from turtles and crocodiles (−8.88 and −10.14‰, respectively) is higher than that of hadrosaurs (−13.76‰), and this may be due to evaporative enrichment during warm seasons. Such a seasonal enrichment of 18O is observed in the paleosol carbonate data of Foreman et al. (2015 [24]). As such, a comparison of isotopic data derived from different materials reveals potential seasonal bias in paleohydrology, which is potentially important in paleoclimatic reconstruction.
5.4. Campanian Paleoclimate Model
A wide range in δ18Op within taxon and the range in calculated δ18Ow indicates high seasonal fluctuation in meteoric water, which is similar to the modern isotopic data of the monsoonal climate (Yamamura et al., 2021 [11], originally from IAEA). The mean δ18Op values of dinosaurs (hadrosaurs; 13.77 ± 1.85‰, SMOW) and crocodiles (12.56 ± 2.47‰, SMOW) from this study do not coincide with the global δ18Op trend in Amiot et al. (2004 [15]). The paleolatitude of southern Utah (Kaipaworits plateau) during the Campanian is estimated to be 48.8° N (PBDB), and both dinosaurs and crocodiles from the Kaiparowits Formation have lower δ18Op values than expected from the Amiot et al. (2004 [15]) models: 15.78‰ and 15.73‰, respectively (Figure 5A). The δ18Op values from the Kaiparowits Formation coincide with the δ18Op values of higher latitudes for both dinosaurs and crocodiles: ca. 55° N for dinosaurs and ca. 58.8° N for crocodiles.
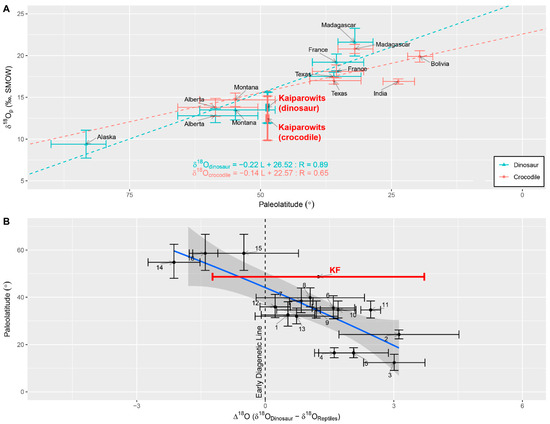
Figure 5.
Comparison of dinosaur and reptile δ18Op values with previous studies. Red dot and line represent the mean δ18Op (with 1σ) of the hadrosaur, and blue dot and line represent the δ18Op (with 1σ) of the crocodile. (A) The Kaiparowits dinosaur and reptile samples deviate from the previously interpreted latitudinal gradient of δ18Op. Adapted from Amiot et al. (2004 [15]). (B) Latitudinal gradient of the δ18Op difference between endothermic dinosaurs and ectothermic reptiles (Δ18Odinosaur-reptile) from the Cretaceous. (1) Berriasian, France; (2) Aptian-Albian, Thailand; (3) early Albian, Tunisia; (4) early Cenomanian, Morrocco; (5) early Cenomanian, Morrocco; (6) late Campanian, France; (7) Campanian-Maastrichtian, Slovenia; (8) late Maastrichtian, Romania; (9) late Maastrichtian, Ausseing, France; (10) late Maastrichtian, Cassagnau 1, France; (11) late Maastrichtian, Cassagnau 2, France; (12) late Campanian, Texas, (13) early Maastrichtian, Madagascar; (14) Campanian, Montana; (15) late Campanian, Alberta; (16) late Campanian, Alberta; and results from this study in the Campanian Utah (Kaiparowits). The Kaiparowits samples deviate from the previously interpreted relationship. Adapted from Amiot et al. (2006 [4]).
Several explanations can account for this discrepancy. First, the majority of the dinosaurs included in the Amiot et al. (2004 [15]) model are theropods. The deviation from the model is potentially due to physiological differences in the dinosaurs analyzed in this study. For example, the dataset in Amiot et al. (2006 [4]) shows herbivorous dinosaurs have ~3‰ lower δ18Op compared to that of Albertosaurus (a large carnivorous dinosaur) δ18Op. Although sample size and methods differ, mean δ18Op (14.1‰, n = 3 after averaging micro-sampling) from Yamamura et al. (2021 [11]) may partially support such a possibility. This study also shows a ~3‰ lower value for herbivores than carnivores. Second, Sewall and Fricke (2013 [53]) suggest that the rise of the Sevier Mountains during the Late Campanian caused a significant amount of orographic runoff and monsoonal conditions in southern Utah. A similar case is reported in other parts of the Western Interior Basin during the Cretaceous (e.g., Barrick et al., 1999 [48]; Suarez et al., 2012 [41]). This results in increased Raleigh distillation due to orographic and amount effects and a lower δ18Op than expected at the paleolatitude of the Kaiparowits fauna.
Amiot et al. (2006 [4]) developed another model for δ18Op and paleolatitude using the modern-day isotopic data from meteoric water δ18Ow, and δ18Op of (endothermic) mammal and (ectothermic) fish. Amiot et al. (2006 [4]) extended the relationship to the Campanian fauna using an extensive collection of vertebrate fossil data from the literature. The δ18Op offset between endotherms and ectotherms is derived from their metabolism and meteoric water δ18Ow; hence, deviation from the model should indicate a unique setting for meteoric water δ18Ow or a difference in the climate gradient. The δ18Op difference between dinosaur and reptile (δ18Odinosaur − δ18Oreptile, Δ18O) in this study is 1.25‰ at 48.8°N, whereas the model of Amiot et al. (2006 [4]) indicates expected Δ18O close to −1‰ (Figure 5B). The Δ18O data of the Kaiparowits Formation does not align with the trend and has a higher affinity to the lower latitude Δ18O data. The comparison of the Kaiparowits Formation data to the global trends in the Amiot et al. (2004 [15] and 2006 [4]) models seems to suggest that reptiles from the Kaiparowits Formation have unusually low δ18Op for their paleolatitude. Fossils from Montana and Alberta in the Amiot et al. (2004 [15] and 2006 [4] dataset are originally from Fricke and Rogers, 2000 [23]) and are from the Judith River and Dinosaur Park formations, respectively. While the Judith River and Dinosaur Park formations were deposited by stream systems from the Sevier Thrust Belt, the Kaiparowits Formation was deposited by the stream system from the Sevier and Mogollon Highlands (Jinnah et al., 2009 [54]). The Kaiparowits Formation’s disagreement with the global isotope model (Amiot et al., 2004 [15] and 2006 [4]) may be the result of high-elevation runoffs from the Mogollon highlands. Although hadrosaurs did not migrate between the Kaiparowits basin and the near-shore Fruitland Formation in Colorado and New Mexico (Fricke and Pearson, 2008 [22]), this disagreement with the global isotopic trend may be due to hadrosaur migration to another location. Alternatively, unlike other Campanian faunas, hadrosaurs did not share the same water source with turtles and crocodiles. Thus, the stable isotope composition of the fossils from the Kaiparowits Formation highlights the unique hydrologic setting of the Kaiparowits Formation.
6. Conclusions
Taxonomic differences in isotopic composition were observed in the vertebrates analyzed from the Kaiparowits Formation. These vertebrates are useful proxies for paleoclimate. This study also highlights the importance of multiple proxies for paleoclimate investigation; analyzing multiple taxa allowed for comparisons to other Campanian formations and deciphering the impact of evaporative enrichment/seasonal bias and high elevation runoff. The Kaiparowits specimens have relatively low δ18O values, indicating high-elevation runoff influenced local paleohydrology. Hadrosaur teeth had a particularly low δ18O value, which may be a result of (1) their capacity to grow teeth throughout the year, reflecting cold months precipitation, and (2) potential migration to the upstream region or seasonal drainage of upland water into the lowlands. Because of this unique hydrologic setting, the Kaiparowits Formation does not fit with the global trend by Amiot et al. (2004 [15], 2006 [4]). Such a result supports the presence of various hydrologic settings across the Campanian of Laramidia. The finding is important for future studies of the Laramidian ecosystem, as there is no consensus on the potential cause for dinosaur endemism. Similar investigations into other Campanian formations in Laramidia may reveal the paleohydrologic contribution to such a unique biogeography.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geosciences15070262/s1, Table S1: Complete Dataset.
Author Contributions
Conceptualization, D.Y. and C.S.; methodology, D.Y. and C.S.; validation, D.Y. and C.S.; formal analysis, D.Y. and C.S.; investigation, D.Y. and C.S.; resources, C.S.; data curation, D.Y.; writing—original draft preparation, D.Y.; writing—review and editing, C.S.; visualization, D.Y.; supervision, C.S.; project administration, C.S.; funding acquisition, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated by this study are available in this manuscript and the Supplemental Material.
Acknowledgments
We would like to thank Erik Pollock and Lindsay Conaway of the University of Arkansas Stable Isotope Laboratory for isotope analysis, Randall Irmis and Carrie Levitt-Bussian of the Natural History Museum of Utah for sample loan, and Allan Titus and Scott Richardson for field advice.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UMNH | Natural History Museum of Utah |
| UASIL | University of Arkansas Stable Isotope Laboratory |
| VPDB | Vienna Pee Dee Belemnite |
| VSMOW | Vienna Standard Mean Ocean Water |
References
- Kohn, M.J.; McKay, M.P.; Knight, J.L. Dining in the Pleistocene—Who’s on the Menu? Geology 2005, 33, 649–652. [Google Scholar] [CrossRef]
- Zazzo, A.; Balasse, M.; Passey, B.; Moloney, A.; Monahan, F.; Schmidt, O. The Isotope Record of Short-and Long-Term Dietary Changes in Sheep Tooth Enamel: Implications for Quantitative Reconstruction of Paleodiets. Geochim. Cosmochim. Acta 2010, 74, 3571–3586. [Google Scholar] [CrossRef]
- Barrick, R.E. Isotope Paleobiology of the Vertebrates: Ecology, Physiology, and Diagenesis. Paleontol. Soc. Pap. 1998, 4, 101–137. [Google Scholar] [CrossRef]
- Amiot, C.E.; Terry, D.J.; Jimmieson, N.L.; Callan, V.J. A Longitudinal Investigation of Coping Processes during a Merger: Implications for Job Satisfaction and Organizational Identification. J. Manag. 2006, 32, 552–574. [Google Scholar] [CrossRef]
- Fricke, H.C.; Foreman, B.Z.; Sewall, J.O. Integrated Climate Model-Oxygen Isotope Evidence for a North American Monsoon during the Late Cretaceous. Earth Planet. Sci. Lett. 2010, 289, 11–21. [Google Scholar] [CrossRef]
- Suarez, C.A.; Ludvigson, G.A.; Gonzalez, L.A.; Fiorillo, A.R.; Flaig, P.P.; McCarthy, P.J. Use of Multiple Oxygen Isotope Proxies for Elucidating Arctic Cretaceous Palaeo-Hydrology. Geol. Soc. 2013, 382, 185–202. [Google Scholar] [CrossRef]
- Kohn, M.J.; Schoeninger, M.J.; Valley, J.W. Herbivore Tooth Oxygen Isotope Compositions: Effects of Diet and Physiology. Geochim. Cosmochim. Acta 1996, 60, 3889–3896. [Google Scholar] [CrossRef]
- Kohn, M.J.; Cerling, T.E. Stable Isotope Compositions of Biological Apatite. Rev. Miner. Geochem. 2002, 48, 455–488. [Google Scholar] [CrossRef]
- Suarez, C.A.; You, H.-L.; Suarez, M.B.; Li, D.-Q.; Trieschmann, J. Stable Isotopes Reveal Rapid Enamel Elongation (Amelogenesis) Rates for the Early Cretaceous Iguanodontian Dinosaur Lanzhousaurus magnidens. Sci. Rep. 2017, 7, 15319. Available online: https://www.nature.com/articles/s41598-017-15653-6 (accessed on 30 April 2025). [CrossRef]
- Goedert, J.; Amiot, R.; Boudad, L.; Buffetaut, E.; Fourel, F.; Godefroit, P.; Kusuhashi, N.; Suteethorn, V.; Tong, H.; Watabe, M. Preliminary Investigation of Seasonal Patterns Recorded in the Oxygen Isotope Compositions of Theropod Dinosaur Tooth Enamel. Palaios 2016, 31, 10–19. [Google Scholar] [CrossRef]
- Yamamura, D.; Suarez, C.A.; Titus, A.L.; Manlove, H.M.; Jackson, T. Multiproxy Approaches to Investigating Palaeoecology and Palaeohydrology in the Upper Cretaceous Kaiparowits Formation, USA. Geol. Soc. 2021, 507, 293–311. [Google Scholar] [CrossRef]
- During, M.A.; Smit, J.; Voeten, D.F.; Berruyer, C.; Tafforeau, P.; Sanchez, S.; Stein, K.H.; Verdegaal-Warmerdam, S.J.; van der Lubbe, J.H. The Mesozoic Terminated in Boreal Spring. Nature 2022, 603, 91–94. Available online: https://www.nature.com/articles/s41586-022-04446-1 (accessed on 30 April 2025). [CrossRef] [PubMed]
- Dutton, A.; Carlson, A.E.; Long, A.J.; Milne, G.A.; Clark, P.U.; DeConto, R.; Horton, B.P.; Rahmstorf, S.; Raymo, M.E. Sea-Level Rise Due to Polar Ice-Sheet Mass Loss during Past Warm Periods. Science 2015, 349, aaa4019. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable Isotopes in Precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Amiot, R.; Lécuyer, C.; Buffetaut, E.; Fluteau, F.; Legendre, S.; Martineau, F. Latitudinal Temperature Gradient during the Cretaceous Upper Campanian–Middle Maastrichtian: Δ18O Record of Continental Vertebrates. Earth Planet. Sci. Lett. 2004, 226, 255–272. [Google Scholar] [CrossRef]
- Hubert, J.; Panish, P.; Chure, D.; Prostak, K. Chemistry, Microstructure, Petrology, and Diagenetic Model of Jurassic Dinosaur Bones, Dinosaur National Monument, Utah. J. Sediment. Res. 1996, 66, 531–547. [Google Scholar] [CrossRef]
- Trueman, C.N.; Tuross, N. Trace Elements in Recent and Fossil Bone Apatite. Rev. Miner. Geochem. 2002, 48, 489–521. [Google Scholar] [CrossRef]
- Wings, O. Authigenic Minerals in Fossil Bones from the Mesozoic of England: Poor Correlation with Depositional Environments. Palaeogeogr. Palaeoclim. Palaeoecol. 2004, 204, 15–32. [Google Scholar] [CrossRef]
- Iacumin, P.; Bocherens, H.; Mariotti, A.; Longinelli, A. Oxygen Isotope Analyses of Co-Existing Carbonate and Phosphate in Biogenic Apatite: A Way to Monitor Diagenetic Alteration of Bone Phosphate? Earth Planet. Sci. Lett. 1996, 142, 1–6. [Google Scholar] [CrossRef]
- Zazzo, A.; Lécuyer, C.; Mariotti, A. Experimentally-Controlled Carbon and Oxygen Isotope Exchange between Bioapatites and Water under Inorganic and Microbially-Mediated Conditions. Geochim. Cosmochim. Acta 2004, 68, 1–12. [Google Scholar] [CrossRef]
- Piga, G.; Santos-Cubedo, A.; Solà, S.M.; Brunetti, A.; Malgosa, A.; Enzo, S. An X-Ray Diffraction (XRD) and X-Ray Fluorescence (XRF) Investigation in Human and Animal Fossil Bones from Holocene to Middle Triassic. J. Archaeol. Sci. 2009, 36, 1857–1868. [Google Scholar] [CrossRef]
- Fricke, H.C.; Pearson, D.A. Stable Isotope Evidence for Changes in Dietary Niche Partitioning among Hadrosaurian and Ceratopsian Dinosaurs of the Hell Creek Formation, North Dakota. Paleobiology 2008, 34, 534–552. [Google Scholar] [CrossRef]
- Fricke, H.C.; Rogers, R.R.; Gates, T.A. Hadrosaurid Migration: Inferences Based on Stable Isotope Comparisons among Late Cretaceous Dinosaur Localities. Paleobiology 2009, 35, 270–288. [Google Scholar] [CrossRef]
- Foreman, B.Z.; Roberts, E.M.; Tapanila, L.; Ratigan, D.; Sullivan, P. Stable Isotopic Insights into Paleoclimatic Conditions and Alluvial Depositional Processes in the Kaiparowits Formation (Campanian, South-Central Utah, USA). Cretac. Res. 2015, 56, 180–192. [Google Scholar] [CrossRef]
- Crystal, V.F.; Evans, E.S.; Fricke, H.; Miller, I.M.; Sertich, J.J. Late Cretaceous Fluvial Hydrology and Dinosaur Behavior in Southern Utah, USA: Insights from Stable Isotopes of Biogenic Carbonate. Palaeogeogr. Palaeoclim. Palaeoecol. 2019, 516, 152–165. [Google Scholar] [CrossRef]
- Burgener, L.; Hyland, E.; Huntington, K.W.; Kelson, J.R.; Sewall, J.O. Revisiting the Equable Climate Problem during the Late Cretaceous Greenhouse Using Paleosol Carbonate Clumped Isotope Temperatures from the Campanian of the Western Interior Basin, USA. Palaeogeogr. Palaeoclim. Palaeoecol. 2019, 516, 244–267. [Google Scholar] [CrossRef]
- Roberts, E.M. Facies Architecture and Depositional Environments of the Upper Cretaceous Kaiparowits Formation, Southern Utah. Sediment. Geol. 2007, 197, 207–233. [Google Scholar] [CrossRef]
- Roberts, E.M.; Sampson, S.D.; Deino, A.L.; Bowring, S.A.; Buchwaldt, R. The Kaiparowits Formation: A Remarkable Record of Late Cretaceous Terrestrial Environments, Ecosystems, and Evolution in Western North America; Indiana University Press: Bloomington, Indiana, 2013; ISBN 0253008832. [Google Scholar]
- Beveridge, T.L.; Roberts, E.M.; Titus, A.L. Volcaniclastic Member of the Richly Fossiliferous Kaiparowits Formation Reveals New Insights for Regional Correlation and Tectonics in Southern Utah during the Latest Campanian. Cretac. Res. 2020, 114, 104527. [Google Scholar] [CrossRef]
- Titus, A.L.; Powell, J.D.; Roberts, E.M.; Sampson, S.D.; Pollock, S.L.; Kirkland, J.I.; Albright, L.B. Late Cretaceous Stratigraphy, Depositional Environments, and Macrovertebrate Paleontology of the Kaiparowits Plateau, Grand Staircase–Escalante National Monument, Utah. Field Guides 2005, 6, 101–128. [Google Scholar] [CrossRef]
- Titus, A.L.; Roberts, E.M.; Albright, L.B., III. Geologic Overview. In At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah; Indiana University Press: Bloomington, Indiana, 2013; pp. 13–41. [Google Scholar]
- Goldstrand, P.M.; Trexler, J.; Kowallis, B.J.; Eaton, J.G.; Morales, M. Late Cretaceous to Early Tertiary Tectonostratigraphy of Southwestern Utah. In Aspects of Mesozoic Geology and Paleontology of the Colorado Plateau: Flagstaff, Arizona, Museum of Northern Arizona, Bulletin; Museum of Northern Arizona: Flagstaff, AZ, USA, 1993; Volume 59, pp. 181–188. [Google Scholar]
- Hutchison, J.H.; Knell, M.J.; Brinkman, D.B.; Titus, A.; Loewen, M. Turtles from the Kaiparowits Formation, Utah. In At the Top of the Grand Staircase, The Late Cretaceous of Southern Utah; Indiana University Press: Bloomington, Indiana, 2013; pp. 295–318. [Google Scholar]
- Irmis, R.B.; Hutchison, J.H.; Sertich, J.J.; Titus, A.L. Crocodyliforms from the Late Cretaceous of Grand Staircase-Escalante National Monument and Vicinity, Southern Utah, USA. In At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah; Indiana University Press: Bloomington, Indiana, 2013; pp. 424–444. [Google Scholar]
- Sampson, S.D.; Loewen, M.A.; Roberts, E.M.; Getty, M.A. A New Macrovertebrate Assemblage from the Late Cretaceous (Campanian) of Southern Utah; Indiana University Press: Bloomington, Indiana, 2013; ISBN 0253008832. [Google Scholar]
- Sampson, S.D.; Lund, E.K.; Loewen, M.A.; Farke, A.A.; Clayton, K.E. A Remarkable Short-Snouted Horned Dinosaur from the Late Cretaceous (Late Campanian) of Southern Laramidia. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131186. [Google Scholar] [CrossRef]
- Miller, I.; Johnson, K.; Kline, D.; Nichols, D.; Barclay, R. A Late Campanian Flora from the Kaiparowits Formation, Southern Utah, and a Brief Overview of the Widely Sampled but Little-Known Campanian Vegetation of the Western Interior of North America. In At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah; Indiana University Press: Bloomington, Indiana, 2013; pp. 107–131. [Google Scholar]
- Roberts, E.M.; Chan, M.A. Variations in Iron Oxide, Iron Sulfide, and Carbonate Concretions and Their Distributions in Fluvio-Deltaic and Nearshore Sandstones: Cretaceous Examples from the Kaiparowits Plateau, Utah and San Juan Basin, New Mexico; Utah Geological Association: Salt Lake City, UT, USA, 2010. [Google Scholar]
- Koch, P.L.; Tuross, N.; Fogel, M.L. The Effects of Sample Treatment and Diagenesis on the Isotopic Integrity of Carbonate in Biogenic Hydroxylapatite. J. Archaeol. Sci. 1997, 24, 417–429. [Google Scholar] [CrossRef]
- Vennemann, T.W.; Fricke, H.C.; Blake, R.E.; O’Neil, J.R.; Colman, A. Oxygen Isotope Analysis of Phosphates: A Comparison of Techniques for Analysis of Ag3PO4. Chem. Geol. 2002, 185, 321–336. [Google Scholar] [CrossRef]
- Suarez, C.A.; González, L.A.; Ludvigson, G.A.; Cifelli, R.L.; Tremain, E. Water Utilization of the Cretaceous Mussentuchit Member Local Vertebrate Fauna, Cedar Mountain Formation, Utah, USA: Using Oxygen Isotopic Composition of Phosphate. Palaeogeogr. Palaeoclim. Palaeoecol. 2012, 313, 78–92. [Google Scholar] [CrossRef]
- Amiot, R.; Lécuyer, C.; Escarguel, G.; Billon-Bruyat, J.-P.; Buffetaut, E.; Langlois, C.; Martin, S.; Martineau, F.; Mazin, J.-M. Oxygen Isotope Fractionation between Crocodilian Phosphate and Water. Palaeogeogr. Palaeoclim. Palaeoecol. 2007, 243, 412–420. [Google Scholar] [CrossRef]
- Coulson, A.B.; Kohn, M.J.; Shirley, M.H.; Joyce, W.G.; Barrick, R.E. Phosphate–Oxygen Isotopes from Marine Turtle Bones: Ecologic and Paleoclimatic Applications. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 264, 78–84. [Google Scholar] [CrossRef]
- Lécuyer, C.; Bogey, C.; Garcia, J.-P.; Grandjean, P.; Barrat, J.-A.; Floquet, M.; Bardet, N.; Pereda-Superbiola, X. Stable Isotope Composition and Rare Earth Element Content of Vertebrate Remains from the Late Cretaceous of Northern Spain (Laño): Did the Environmental Record Survive? Palaeogeogr. Palaeoclim. Palaeoecol. 2003, 193, 457–471. [Google Scholar] [CrossRef]
- Lohmann, K.C. Geochemical Patterns of Meteoric Diagenetic Systems and Their Application to Studies of Paleokarst. In Paleokarst; Springer: Berlin/Heidelberg, Germany, 1988; pp. 58–80. [Google Scholar]
- Wang, Y.; Cerling, T.E. A Model of Fossil Tooth and Bone Diagenesis: Implications for Paleodiet Reconstruction from Stable Isotopes. Palaeogeogr. Palaeoclim. Palaeoecol. 1994, 107, 281–289. [Google Scholar] [CrossRef]
- Eagle, R.A.; Tütken, T.; Martin, T.S.; Tripati, A.K.; Fricke, H.C.; Connely, M.; Cifelli, R.L.; Eiler, J.M. Dinosaur Body Temperatures Determined from Isotopic (13C-18O) Ordering in Fossil Biominerals. Science 2011, 333, 443–445. [Google Scholar] [CrossRef]
- Barrick, R.E.; Fischer, A.G.; Showers, W.J. Oxygen Isotopes from Turtle Bone: Applications for Terrestrial Paleoclimates? Palaios 1999, 14, 186–191. [Google Scholar] [CrossRef]
- Barrick, R.E.; Showers, W.J. Thermophysiology of Tyrannosaurus Rex: Evidence from Oxygen Isotopes. Science 1994, 265, 222–224. [Google Scholar] [CrossRef]
- Barrick, R.E.; Showers, W.J. Oxygen Isotope Variability in Juvenile Dinosaurs (Hypacrosaurus): Evidence for Thermoregulation. Paleobiology 1995, 21, 552–560. [Google Scholar] [CrossRef]
- Bocherens, H. Preservation of Isotopic Signals (13C, 15N) _in Pleistocene Mammals. In Biogeochemical Approaches to Paleodietary Analysis; Springer: Berlin/Heidelberg, Germany, 2000; pp. 65–88. [Google Scholar]
- Cullen, T.M.; Longstaffe, F.J.; Wortmann, U.G.; Huang, L.; Fanti, F.; Goodwin, M.B.; Ryan, M.J.; Evans, D.C. Large-Scale Stable Isotope Characterization of a Late Cretaceous Dinosaur-Dominated Ecosystem. Geology 2020, 48, 546–551. [Google Scholar] [CrossRef]
- Sewall, J.O.; Fricke, H.C. Andean-Scale Highlands in the Late Cretaceous Cordillera of the North American Western Margin. Earth Planet. Sci. Lett. 2013, 362, 88–98. [Google Scholar] [CrossRef]
- Jinnah, Z.A.; Roberts, E.M.; Deino, A.L.; Larsen, J.S.; Link, P.K.; Fanning, C.M. New 40Ar-39Ar and Detrital Zircon U-Pb Ages for the Upper Cretaceous Wahweap and Kaiparowits Formations on the Kaiparowits Plateau, Utah: Implications for Regional Correlation, Provenance, and Biostratigraphy. Cretac. Res. 2009, 30, 287–299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).