Abstract
Researchers are exploring eco-friendly alternatives to Portland cement, such as geopolymers, which require reactive aluminosilicate sources. This study evaluated the reactivity of six calcined clays (heated at 700 °C) in the presence of an alkaline solution. The calcined samples from kaolinite quarries in Kamboinsé, Kandarfa, Saaba, Sabcé, Selogo, and Tougou were subjected to chemical and mineralogical analyses. The results indicated a high aluminosilicate content (>50%), with kaolinite reaching up to 83.1%, and an amorphous fraction of up to 31.8%, a key factor influencing reactivity. Geopolymer pastes, prepared using a 12 M NaOH solution and each of these calcined clays, exhibited varying setting times: 24 h for the Saaba clay (the most reactive) compared with 48 h or even up to 7 days for the least reactive. The evaluation of the compressive strength of the geopolymer pastes revealed varying performances depending on the composition of clay. The Saaba clay showed the highest strength (14 MPa), attributed to its high kaolinite content (83.1%) and amorphous phase (31.8%), and thus reactivity. This was followed by Kamboinsé with 10.5 MPa (58.3% kaolinite; 24.3% amorphous phase), Selogo with 4.6 MPa (42.9%; 20.4%), Tougou with 1.4 MPa (44.1%; 20.4%), Kandarfa with only 0.7 MPa (31.3%; 19.2%), and Sabcé, which did not set with 0 MPa (24.1%; 13.7%). A discussion between the chemical and mineralogical compositions of the different clays and the mechanical characteristics of the synthesized pastes highlighted the importance of kaolinite content and its amorphous nature on the reactivity of the geopolymer binders. These findings highlight its potential for applications such as stabilized bricks or geopolymer concrete, offering a low-carbon alternative to traditional materials.
1. Introduction
Actors in the construction industry unanimously agree on the need to prioritize less polluting materials to limit greenhouse gas emissions. Annual emissions associated with Portland cement production amount to approximately 1.5 billion tons, accounting for 5–8% of global emissions on average [1]. To reduce the carbon footprint and ensure sustainable development in the construction industry, researchers have adopted various measures [2] that include the use of eco-friendly and cost-effective earth-based materials, achieved by enhancing the engineering properties of stabilized compressed earth bricks as an alternative to cement bricks [3]. The development of geopolymer binders for stabilization serves as a viable and promising substitute for ordinary Portland cement [4] due to their favorable properties [5,6].
Geopolymer binders represent a new generation of inorganic materials that have been the subject of extensive research in recent decades. They are formed through the consolidation of natural or synthetic aluminosilicates at room or low temperatures in alkaline environments, resulting in stable chemical structures that are resistant to external attacks including fire [7,8].
The geopolymerization reaction, a complex process involving multiple key steps, begins with the dissolution of aluminosilicates in an alkaline solution. This initial phase is followed by the formation of a gel composed of aluminate (AlO4) and silicate (SiO4) tetrahedra, which gradually evolves into a stable structure, ultimately leading to the formation of zeolites [9]. The efficiency of the geopolymerization mechanism is highly dependent on the nature and concentration of the activating alkaline solution. These parameters govern both the dissolution of aluminosilicate precursors and the formation of geopolymeric gels [10].
Davidovits [11] highlighted that alkaline activation using NaOH, KOH, or alkali-silicate solutions is crucial for dissolving the vitreous and amorphous phases of the precursors. The concentration of OH− ions plays a pivotal role. It not only governs the dissolution rate of silicates and aluminates, but also regulates the formation of N-A-S-H gels. A high concentration of alkaline hydroxide (typically 8–12 M for NaOH) promotes the cleavage of Si-O-Si and Al-O-Si bonds, thereby releasing reactive species [12]. However, excessive concentrations may lead to the uncontrolled precipitation of aluminosilicates, while insufficient concentrations slow the dissolution kinetics [13].
The curing temperature is another critical factor. A moderate increase (20–90 °C) accelerates the dissolution, polycondensation, and structural reorganization rates [14]. However, excessively high temperatures (beyond 100 °C) can have detrimental effects, such as premature water evaporation or microcrack formation, which compromise both reactivity and the final mechanical properties [15].
A wide variety of aluminosilicates can be used for their synthesis due to their amorphous nature and reactivity in alkaline solutions. They include fly ash [16], silica fume [17], volcanic slag [18], dehydrated waste [19], and calcined kaolinitic clays (metakaolin) [4,7,20,21], among others. Calcined kaolinitic clays appear to be the preferred precursors due to their chemical composition [22,23,24] and their widespread availability. According to Komnitsas and Zaharaki [25], the calcination of kaolin clays into metakaolin releases silicates and aluminates from their crystalline structure, transforming them into an amorphous state, thereby enhancing their reactivity in alkaline environments. This transformation occurs at an optimal temperature of 700 °C, following Equation (1) [11,26,27]. However, calcination beyond 850 °C leads to recrystallization into mullite, reducing the reactivity of metakaolin [26].
Al2O3·2SiO2·2H2O → Al2O3·2SiO2 + 2H2O
Although metakaolin-based geopolymers exhibit high mechanical strength (40–50 MPa) and low porosity [28], their performance depends on several physicochemical and mineralogical factors such as the particle size, specific surface area, amorphous silica and alumina content as well as the morphology of the precursors [29]. Additional studies [13,30] have revealed the significant influence of the mineralogical and chemical composition of aluminosilicates, particularly their degree of amorphousness.
This study addresses a gap in the literature regarding the precise influence of kaolinite content and the amorphous fraction of calcined clays on the properties of geopolymer binders. Therefore, it focused on characterizing six kaolinitic clays from three regions of Burkina Faso to assess their suitability for geopolymer synthesis. The clays, calcined at 700 °C to enhance reactivity, were analyzed for their chemical and mineralogical composition as well as their reaction with a 12 M NaOH solution. A key objective was to determine how variations in clay mineralogy (kaolinite and amorphous phase content) and chemical composition affect the reactivity and mechanical performance of geopolymer pastes for potential applications in construction. The findings could optimize the use of the underutilized clay resources, promoting a more efficient exploitation of local materials.
2. Materials and Methods
2.1. Materials: Clays and Activator
2.1.1. Identification, Preparation, and Physical Characteristics of Clays
Six clay quarries were identified as aluminosilicate sources due to their high kaolinite content, according to information collected at the Bureau of Mines and Geology of Burkina (BUMIGEB). These quarries are located in three regions of the country (Center, Center North, and North). The geographical coordinates of each sampling quarry are listed in Table 1.

Table 1.
Geographic coordinates of the clay quarries.
Six bags of raw clay were collected from each quarry, with each bag weighing approximately 100 kg. The samples were taken at a depth of 50 cm from 10 boreholes located at each sampling site. In the laboratory, the six bags from each quarry were mixed to obtain a single representative sample. These samples were then homogenized using a sampler, crushed, and dried at 105 °C in an oven for 24 h to facilitate sieving through a 100 μm mesh.
To enhance the clay’s reactivity in the presence of an alkaline solution, the raw clays were calcined at 700 °C, as recommended in previous studies [31,32]. The heat treatment was conducted in a vertical-loading muffle furnace, with calcination performed in three successive stages: (1) heating at a rate of 10 °C/min, (2) holding at a constant temperature of 700 °C for three hours (3 h), and (3) cooling inside the furnace. As shown in Figure 1, the calcination process altered the clay’s texture, resulting in a darker appearance compared with the raw material.

Figure 1.
Appearance of uncalcined and calcined clays from different quarries.
Figure 2 shows the particle size distributions of the six calcined clays based on the laser particle size test. The curves present the cumulative percentages of passing for each clay, which allowed for the evaluation of the diameter of particles corresponding to 50% (d50) and 90% (d90) of the cumulative passing. On average, 50% of the particles in the calcined clays of Tougou, Selogo, and Saaba had diameters less than or equal to 10 μm, whereas the other three calcined clays had 50% of their particles located between 11 and 16 μm. The particle size distribution of a material is a crucial parameter that influences its reactivity: the finer the particles, the higher their reactivity [33].
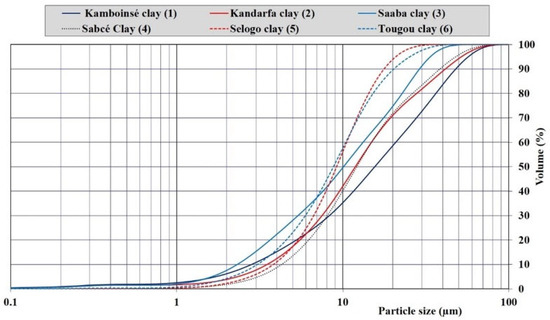
Figure 2.
Particle size distributions of the calcined clays.
Table 2 summarizes the physical characteristics of the calcined clays. The results indicate that the specific surface areas of the Kandarfa, Selogo, Tougou, Kamboinsé, and Saaba clays ranged between 5 and 12.74 m2/g, which is typical of kaolin [34]. In contrast, the Sabcé clay exhibited a slightly lower specific surface area (<5 m2/g), suggesting a lower kaolinite content. Additionally, the specific density values of the studied clays were nearly identical, averaging 2.65, except for the Kamboinsé clay, which had a higher density (2.78). This difference may be attributed to variations in the chemical composition of the materials.

Table 2.
Physical properties of the six calcined clays.
2.1.2. Activator
An alkaline solution of sodium hydroxide (NaOH) at a concentration of 12 mol/L (NaOH 12 M) was used to activate the calcined clays, assessing their reactivity in an alkaline environment and investigating the possibility of developing a geopolymer binder. This was obtained by the dissolution of NaOH crystals (99% purity) in distilled water, and was usable only after 24 h to ensure the complete dissolution of the crystals.
2.2. Experimental Methods
2.2.1. Study Protocol
The reactivity of the clays in the synthesis of the geopolymer binder was evaluated based on several aspects.
- Aspect 1: The chemical and mineralogical characterization of the clays was conducted to identify those with the highest kaolinite content and amorphous phase content, as these were more likely to react efficiently with an alkaline activator.
- Aspect 2: The pastes were prepared using 100 g of each calcined clay mixed with 80 g of a 12 M NaOH solution. Based on the criteria of this study, the clays were deemed reactive if the paste hardened within 48 h at laboratory room temperature (30 ± 5 °C). The hardening of the paste was evaluated through manual touch followed by a needle penetration test. A needle (2 mm in diameter, 50 mm in length) was dropped vertically from an average height of 15 cm onto the surface of the paste. A 150 g ball attached to the top of the needle ensured free fall. The test was performed every 24 h for up to 7 days. The paste was considered hardened if the needle penetration did not exceed 5 mm.
- Aspect 3: The mechanical characterization of the pastes after curing was conducted to determine the most reactive clay, which was expected to exhibit the highest compressive strength. This parameter is fundamental in selecting construction materials. The mechanical resistance was evaluated using prismatic specimens (4 × 4 × 16 cm3) prepared with 800 g of calcined clay and 640 g of 12 M NaOH (Figure 3). The pastes were poured into the molds in two layers, each compacted with 60 blows using a shock table. To prevent water evaporation during setting and hardening, the specimens were covered with a polyethylene film. These were then stored at laboratory room temperature (30 ± 5 °C) for 7 days, followed by heat treatment in an oven at 60 °C for an additional 7 days, following the procedure described by Ferone et al. [35]. The total curing period before mechanical testing was 14 days.
 Figure 3. Synthesis protocol of geopolymer samples (a) preparation of geopolymer paste, (b) molding and compaction using a shock table, (c) molded geopolymer samples, and (d) samples after treatment, ready for testing.
Figure 3. Synthesis protocol of geopolymer samples (a) preparation of geopolymer paste, (b) molding and compaction using a shock table, (c) molded geopolymer samples, and (d) samples after treatment, ready for testing. - Aspect 4: After identifying the most reactive clay, it was used to synthesize a geopolymer binder. Prismatic samples (4 × 4 × 16 cm3) were prepared and subjected to a 14-day curing process: 7 days at ambient laboratory temperature, followed by 7 days of heat treatment in an oven at 30, 60, and 90 °C.
2.2.2. Characterization Methods
The experimental methods used to characterize the clay and geopolymer binders are detailed in this section. These techniques included chemical and mineralogical composition analysis, thermal analysis, and compressive strength measurements
- Chemical analysis
The chemical composition of the calcined clay was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES), using an Optima 7000 DV, spectrometer (manufactured by PerkinElmer) equipped with a CCD sensor. The measurements were carried out at the LMDC laboratory of INSA Toulouse, France. The sample (50 mg) was ground, sieved through an 80 µm mesh, and fused with lithium tetraborate (Li2B4O7) to form a glass bead. The glass bead was then dissolved in a nitric acid solution to obtain a homogeneous solution, which was analyzed to quantify elemental oxides present in the clays.
- Thermal analysis (TG/DTA)
Thermogravimetric (TG) and differential thermal (DTA) analyses were performed on uncalcined clays using a Setaram Setsys Evolution device (manufactured by the NETZSCH group). The measurements were carried out at the LMDC laboratory of INSA Toulouse, France. The samples (50 mg) were heated up to 1050 °C at a rate of 10 °C/min under dry air (100 mL/min). The mass loss between 450 and 700 °C was used to estimate the kaolinite content using Equations (2)–(4).
where Mi is the initial mass of the sample (g); P is the relative mass loss of the sample under test (%); ME.K is the mass of chemical water from the dehydroxylated kaolinite (g); MK is the kaolinite mass (g); Mmol.K is the molar mass of kaolinite (258 g/mol); Mmol.H2O is the molar mass of water (18 g/mol); %K is the percentage of kaolinite.
- X-ray diffraction analysis (XRD)
The mineral phases in both the uncalcined and calcined clays as well as geopolymer binders were identified using X-ray diffraction (XRD) with two devices. The first diffractometer is a D5000 model (Co Kα, λ = 1.789 Å, 0.04° step from 4–70°, 2 s per step) manufactured by Siemens. The second is a D8 Advance model (Cu Kα, λ = 1.54056 Å, 0.04° step from 5–75°, graphite monochromator, 2 s per step) manufactured by Bruker. The analyses were carried out in two laboratories in France: the LMDC at INSA Toulouse (D8 Advance) and MATEIS at INSA Lyon (D5000). The relative quantities of minerals were estimated using the method by Yvon et al. [36] and used by Sore et al. [37], with Equation (5).
- Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were acquired using a Alpha-p spectrophotometer (manufactured by Bruker) on 80 µm powdered samples. The IR spectra were recorded in the range of 600–4000 cm−1 with a resolution of 4 cm−1. Data processing was performed using SPECTRUM software. The measurements were carried out at the LMDC laboratory of INSA Toulouse, France.
- Amorphous phase content
The amorphous content was determined using Keyser’s dissolution method [38]. A total of 1 g (M1) of calcined clay was dissolved in 200 mL of 1% HCl for 40 min, then filtered. The residue was dried at 105 °C for 24 h, yielding mass M2. The percentage of the amorphous phase was calculated using Equation (6).
- Reactivity evaluation
The reactivity was assessed by monitoring the setting behavior of the geopolymer pastes over a 7-day period. Pastes were prepared with calcined clays in a 12 M NaOH solution at a solution-to-powder mass ratio of 0.8.
- Compressive strength testing
The compressive strength was measured using a hydraulic press (manufactured by ETI-PROETI) with a 300 kN capacity and a loading rate of 0.25 kN/s. The tests were conducted on prismatic (4 × 4 × 16 cm3) geopolymer paste. For each formulation, three samples were tested, and the compressive strength was calculated using Equation (7). The measurements were carried out at the LEMHaD laboratory of 2iE, Burkina Faso.
3. Results
3.1. Chemical, Thermal, and Mineralogical Characteristics of Clays
3.1.1. Chemical Characteristics of Clays
The chemical compositions of the calcined clays, expressed as mass percentages of oxides (Table 3), revealed significant differences between the samples. Primarily composed of silica (SiO2) and alumina (Al2O3), the sum of which (an indicator of aluminosilicate richness) ranged from 79.9% (Kamboinsé, minimum value) to 96.2% (Saaba, maximum value), these clays exhibited SiO2 contents between 50.90% (Kamboinsé) and 67.20% (Kandarfa), with an average generally above 60%, except for the clays from Kamboinsé (50.9%) and Selogo (52.2%). The Al2O3 contents were higher for Saaba (38.30%) and Selogo (32.60%) compared with lower contents for Kandarfa (21.7%) and Tougou (23.4%). The Kamboinsé clay stood out due to its exceptionally high Fe2O3 content (16.5%), explaining its red color (Figure 1) and high specific density (2.78), while the other samples contained less than 5%. Alkali elements (K2O and Na2O) were particularly abundant in the Sabcé (K2O = 3.40%; Na2O = 2.74%) and Selogo (K2O = 4.36%) clays but were minimally present in Saaba (K2O = 0.11%; Na2O = 0.23%), reflecting the diversity of clay minerals. Finally, the loss on ignition, generally low (0.47–1.36%), indicates a low content of organic matter, with slightly higher values for Tougou (1.36%) and Selogo (1.19%). These compositional variations would significantly influence their properties and potential applications.

Table 3.
Chemical compositions of the calcined clays.
3.1.2. Thermal Characteristics of Clays
The six types of uncalcined clay, characterized by thermogravimetric analysis (TGA) and differential thermal analysis (DTA), are presented in Figure 4 and Figure 5. They were similar, with some divergences around the clay type. Generally, these thermograms revealed the presence of three common peaks: two endothermic peaks and one exothermic peak. The first endothermic peak, between 90 and 115 °C, was attributed to the loss of mass linked to the departure of hygroscopic water initially present in the clay. The second endothermic peak, between 563 and 586 °C, was related to the dehydroxylation of kaolinite and its transformation into metakaolinite. According to the literature, dehydroxylation temperatures range between 530 and 700 °C [32]. The relative mass losses observed between 450 and 700 °C are listed in Table 4 and varied from 11.6% (Saaba) to 3.36% (Sabcé). These values were lower than those expected for pure kaolinite (13.95%) [39], indicating the presence of other associated minerals. It is noteworthy that the double peak around 574 and around 631 °C, which showed the presence of minerals other than kaolinite in the Sabcé clay (4), has yet to be identified by XRD. Finally, the last common exothermic peak between 960 and 1000 °C was linked to the structural reorganization of metakaolinite into mullite [40].
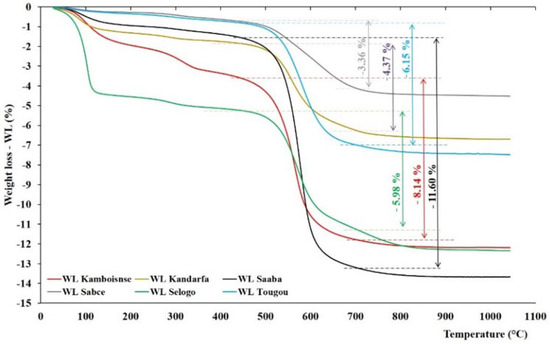
Figure 4.
Thermogravimetric analysis (TGA) of the different raw clays: horizontal dotted lines represent the beginning and ending of weight loss from the dihydroxylation of kaolinite.
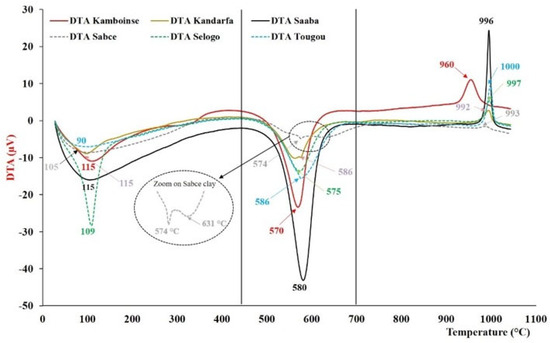
Figure 5.
Differential thermal analysis (DTA) of the different raw clays: vertical solid lines at 450 °C and 700°C represent the temperature interval for the dihydroxylation of kaolinite.

Table 4.
Estimation of the kaolinite content in the clay based on the thermal analysis.
However, the divergence of these thermal curves resides in certain endothermic peaks located around 300 °C (Kamboinsé, Kandarfa, and Selogo clay) and 705 °C (Kandarfa clay), which could respectively be attributed to the transformation of goethite into hematite and the dehydroxylation of paragonite [41]. The endothermic peak around 576 °C reflects the transformation of α-quartz into β-quartz [42]. The mass loss corresponding to the thermal incident between 450 and 700 °C made it possible to estimate the kaolinite composition, and the results are presented in Table 4. The clays of Saaba (3), Kamboinsé (1), Tougou (6), and Selogo (5) respectively had the highest contents of kaolinite of 83.1, 58.3, 44.1 and 42.9, and are supposedly more reactive than Kandarfa (2) and Sabcé (4), which contain low amounts of kaolinite (31.3 and 24.1%, respectively). Although the Kandarfa and Selogo clays originated from the same region, they displayed differences in both their physical and thermal properties.
It is accepted that the most reactive clays in an alkaline environment contain more metakaolinite after calcination at approximately 700 °C [29,32]. The endothermic peaks around 563–586 °C, related to the dehydroxylation of kaolinite, occurred below 700 °C, confirming the total transformation of kaolinite into metakaolinite in the different clays.
3.1.3. Mineral Characteristics of Clays
The mineralogical composition of the various raw and calcined clays was analyzed using X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). The XRD results are presented in Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11.
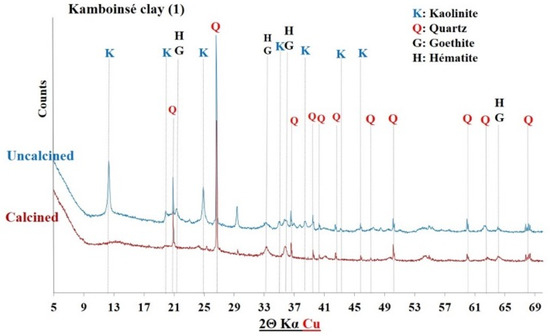
Figure 6.
XRD of Kamboinsé clay before and after calcination.
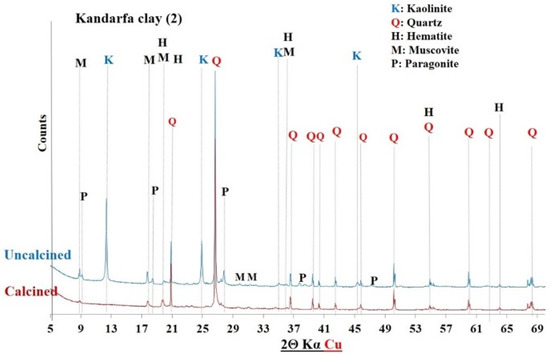
Figure 7.
XRD of Kandarfa clay before and after calcination.
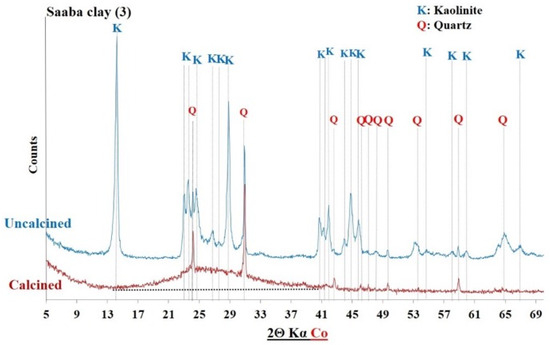
Figure 8.
XRD of Saaba clay before and after calcination.
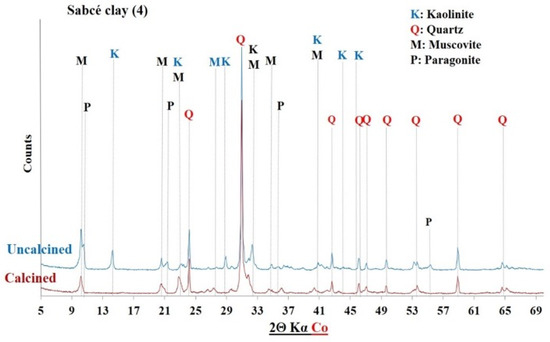
Figure 9.
XRD of Sabce clay before and after calcination.
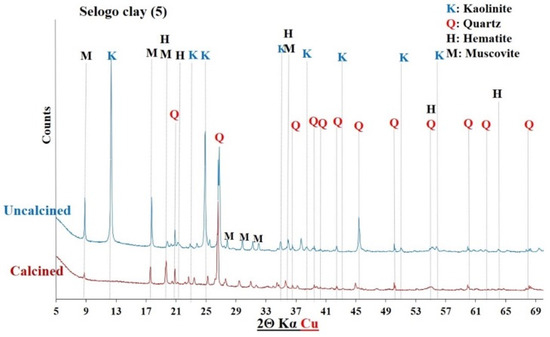
Figure 10.
XRD of Selogo clay before and after calcination.
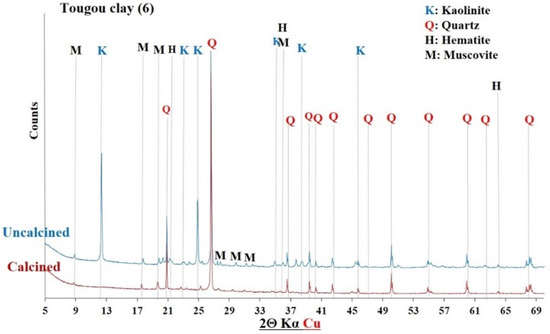
Figure 11.
XRD of Tougou clay before and after calcination.
The diffractograms of the uncalcined clays indicated the presence of common crystalline minerals including kaolinite (Al2Si2O5(OH)4) and quartz (SiO2). The raw clay from Saaba (3) contained only crystalline minerals. However, additional crystalline phases were detected depending on the sample’s origin, distinguishing each clay: hematite (Fe2O3) in the Kandarfa, Selogo, and Tougou samples; muscovite (KAl2(AlSi3O10)(OH, F)2) in the Kandarfa, Sabcé, Selogo, and Tougou samples; and paragonite (NaAl2(AlSi3O10)(OH)2) in the Sabcé and Kandarfa samples.
Following calcination, the disappearance of kaolinite peaks in the XRD patterns confirmed its complete dehydroxylation and transformation into metakaolinite, along with the amorphization of its structural network [39]. Similarly, goethite (FeO(OH)), initially detected in the raw clay from Kamboinsé, transformed into hematite under thermal treatment (at temperatures ≥ 250 °C) [43]. The persistence of crystalline peaks for other minerals suggests their limited reactivity to calcination. However, the disappearance of paragonite peaks after calcination demonstrated its thermal sensitivity, leading to amorphization at 700 °C, similarly to kaolinite.
The diffractograms of the calcined clays exhibited a halo (Figure 8), confirming the partially amorphous nature of the material. However, the absence of this halo in other diffractograms does not necessarily imply a lack of amorphous phases. Therefore, a more detailed analysis of the amorphous characteristics of the six calcined clay materials is presented in Section 3.1.4.
The mineralogical compositions of the clays were determined by combining chemical analysis and X-ray diffractometry. As shown in Table 5, the Saaba calcined clay had the highest kaolinite content (83%), followed by Kamboinsé (64%). The samples from Selogo, Tougou, and Kandarfa contained intermediate kaolinite levels (25–40%), which is consistent with the TGA results. However, a discrepancy was observed for Sabcé, where XRD detected only 0.7% kaolinite compared with the 27.4% estimated by TGA. This difference may be attributed to the coexistence of paragonite (32.20%) and kaolinite in Sabcé.

Table 5.
Mineralogical composition of the clays.
Figure 12, Figure 13 and Figure 14 display the FTIR spectra (4000–600 cm−1) of the uncalcined and calcined clays, while Table 6 summarizes the absorption bands and their corresponding bond assignments. In the uncalcined clays, the absorption bands observed at 3691–3616 cm−1 were characteristic of kaolinite’s OH bonds [44]. The disappearance of these bands in the calcined clays confirmed the transformation of kaolinite into metakaolinite, consistent with the XRD results (Figure 6, Figure 7 and Figure 8). Additionally, the bands near 1631–1621 cm−1 in the raw clays from Selogo (Figure 14a), Saaba (Figure 13a), Kamboinsé (Figure 12a), and Kandarfa (Figure 12b) arose from the H-O-H deformation vibrations of adsorbed water [45]. The Si-O bond bands identified in the raw clays (e.g., 1158, 1113, and 1017 cm−1 for Saaba clay) merged after calcination into a broad band around 1056 cm−1, indicative of amorphous silica [44]. The Al-OH and Si-O-T (T = Al or Si) bands listed in Table 6 were attributed to kaolinite if they vanished upon calcination, otherwise, they perhaps corresponded to other minerals, such as muscovite, which was also detected in the XRD patterns of the raw and calcined clays from Kandarfa, Sabcé, Selogo, and Kamboinsé. Finally, the presence of quartz was evidenced by the bands at approximately 824 and 782–769 cm−1.
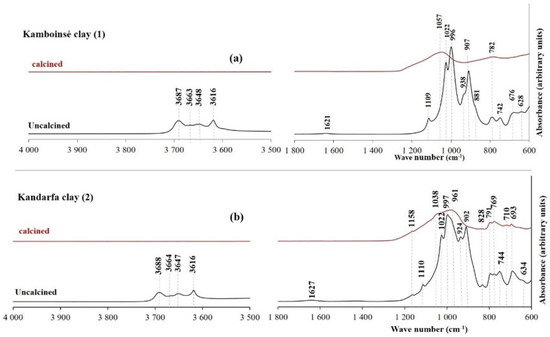
Figure 12.
FTIR of the uncalcined and calcined clays of Kamboinsé (a) and Kandarfa (b).
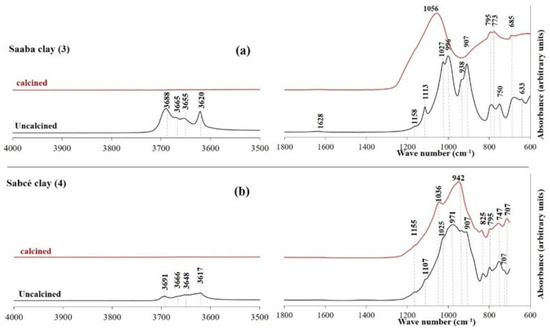
Figure 13.
FTIR of the uncalcined and calcined clays of Saaba (a) and Sabcé (b).
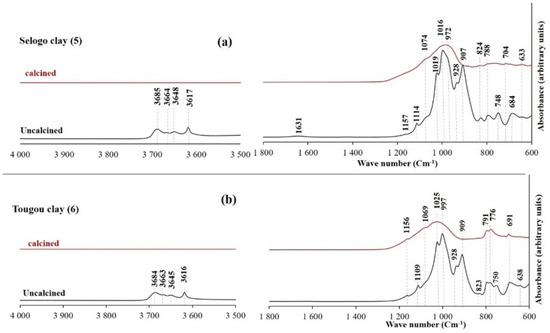
Figure 14.
FTIR of the uncalcined and calcined clays of Selogo (a) and Tougou (b).

Table 6.
Summary of the infrared bands of the samples.
3.1.4. Amorphous Characteristics of Calcined Clays
The XRD results (Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11) revealed that some calcined clays did not exhibit a halo, typically associated with the material’s partially amorphous nature, despite the disappearance of the characteristic kaolinite peak. This observation prompted the exploration of alternative methods to quantify the amorphous phase, a key criterion in selecting aluminosilicates for geopolymer binder synthesis [4].
As shown in Table 7, the amorphous content of the calcined clays, determined through dissolution tests, varied significantly. The Saaba clay had the highest amorphous proportion (32%), followed by Kamboinsé (24%). The remaining clays, Selogo, Tougou, and Kandarfa, exhibited similar amorphous content (~20%), while Sabcé had the lowest (13%). A potential correlation between the kaolinite content and amorphous content was observed (Figure 15). In all cases, the amorphous proportion was lower than the initial kaolinite content. Notably, Saaba clay, which had the highest kaolinite content (83%), also displayed the greatest amorphous character (32%). Conversely, Sabcé clay, with the lowest kaolinite content (24%), exhibited the least amorphous phase (13%).

Table 7.
Amorphous proportion by acid attack of the calcined clays.
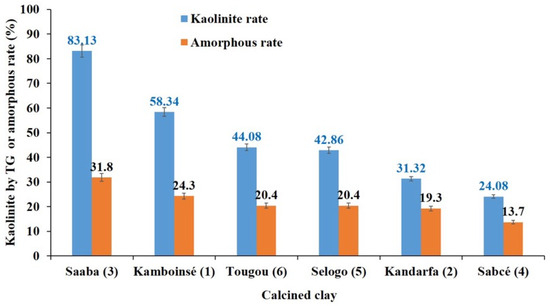
Figure 15.
Kaolinite in the clays and amorphous rate of the calcined clays.
3.2. Feasibility of the Synthesis of Geopolymer Binder Using Calcined Clays
The reactivity of the clays for geopolymer binder synthesis was evaluated based on the visual appearance, setting time, and compressive strength of the paste. For comparison purposes, clay was deemed reactive in the presence of the alkaline solution if the paste set within 48 h.
3.2.1. Reactivity of Calcined Clays in the Presence of an Alkaline Solution
Table 8 and Figure 16 illustrate three distinct scenarios regarding the feasibility of synthesizing geopolymer binders using calcined clays. In the first case (high reactivity), the pastes made from the Saaba (3) and Kamboinsé (1) calcined clays hardened within 24 and 48 h, respectively, demonstrating their strong reactivity in an alkaline medium. In the second case, the pastes derived from Selogo (5), Tougou (6), and Kandarfa (2) exhibited slower hardening, taking 72 h for the first two and 92 h for the latter, indicating limited reactivity. For the third case (no reactivity), the paste formulated with the Sabcé (4) calcined clay showed no hardening even after seven days of observation, suggesting a complete lack of reactivity in alkaline conditions.

Table 8.
Reactivity of calcined clays by setting time.

Figure 16.
Appearance of different geopolymer pastes during hardening depending on the origin of the clay: (a) Kamboinsé; (b) Kandarfa; (c) Saaba; (d) Sabcé; (e) Selogo; and (f) Tougou: green and red font colors respectively represent pastes that achieved setting and those which did not achieve setting.
Additionally, the reactivity of the calcined clays was further confirmed by assessing the mechanical resistance of the pastes, as detailed in Section 2.2.2.
3.2.2. Mechanical and Mineralogical Characteristics of the Geopolymer Binder
Figure 17 presents the 14-day compressive strength of the hardened pastes derived from the six clays. The results indicate that the pastes made from calcined Saaba (3) and Kamboinsé (1) clays exhibited higher compressive strengths, 14 MPa and 10 MPa, respectively, compared with those synthesized from the other four clays. This enhanced performance can be attributed to their higher metakaolinite content and amorphous structure, which promote more effective geopolymerization reactions. In contrast, the pastes produced from the Selogo (4), Tougou (6), and Kandarfa (2) clays achieved significantly lower compressive strengths of 4.6 MPa, 1.6 MPa, and 0.7 MPa, respectively. Additionally, the paste prepared with calcined Sabce (4) clay did not harden, preventing the measurement of its compressive strength. These findings align with the work of Xu and Van Deventer [46], who demonstrated that the superior compressive strength of geopolymers was linked to the enhanced dissolution capacity of amorphous aluminosilicates in alkaline solutions.
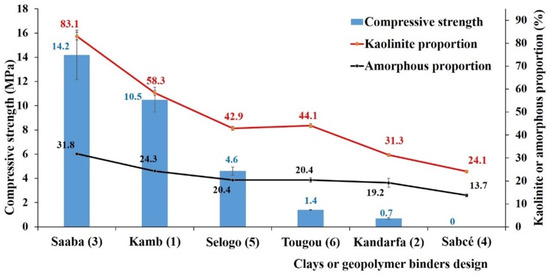
Figure 17.
Mechanical performance of the six hardened geopolymer pastes combined with the kaolinite content and amorphous character, depending on the clay types.
Figure 18 shows the mechanical and physical properties of the geopolymer binder made from calcined clay sourced from Saaba after 14 days of maturation, as a function of curing temperature. The results demonstrated an increase in compressive strength with higher curing temperatures, reaching a maximum value of 22.3 MPa after thermal curing at 90 °C. These findings align with studies by Yu et al. [47], who investigated the influence of temperature on the geopolymerization process. Their work confirmed that elevated temperatures enhanced precursor reactivity, thereby promoting effective geopolymerization. However, the mass loss of the binder due to drying also rose with the curing temperature, likely attributable to the evaporation of water from the alkaline solution.
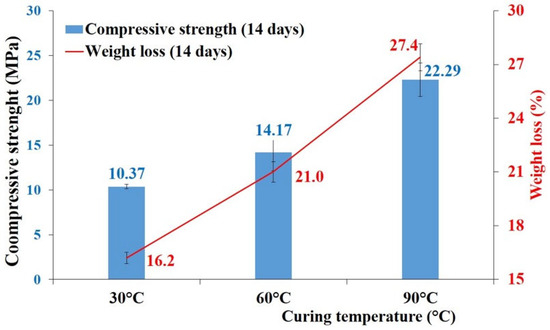
Figure 18.
Compressive strength and mass loss of the geopolymer binder made of Saaba calcined clay with the curing temperature.
Furthermore, Figure 19 presents the X-ray diffractogram of the geopolymer binder synthesized from Saaba clay after 14 days of curing at 60 °C. The diffractogram revealed the formation of new crystalline phases, including faujasite—F (Na2Al2Si2.4O8.8·6.7H2O), hydrosodalite (Na8Si6Al6O24(OH)2(H2O)2), zeolite A—Z (Na12Al12Si12O48), and residual quartz—Q (SiO2), which was initially present in the calcined clay (Figure 8). The emergence of these phases indicates the dissolution of the Saaba calcined clay and subsequent reorganization into a geopolymeric network [48]. Similar mineral formations have been reported in previous studies [23,49,50]. These newly formed phases contribute to the enhanced mechanical properties, as evidenced in Figure 18.
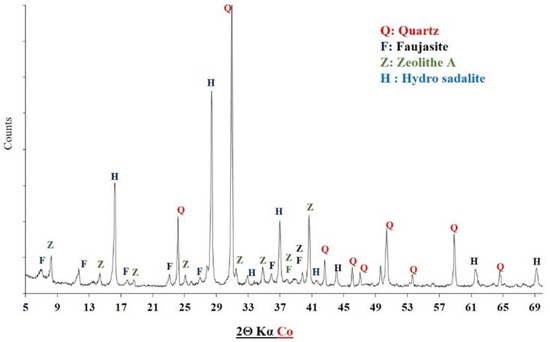
Figure 19.
XRD diffractogram of the geopolymer binder made of Saaba calcined clay.
4. Discussion
This study evaluated the suitability of various clays for geopolymer synthesis by examining their physical properties, chemical and mineralogical compositions, and reactivity.
- Physical characteristics of clays and their reactivity
Particle size and specific surface area were found to be critical factors directly influencing the geopolymerization kinetics, which initiates at the particle–liquid interface [33]. Materials with finer particle sizes and higher specific surface areas exhibited increased reactivity in alkaline environments [33]. In this context, the calcined clay from Saaba, with a median diameter (d50) of 10 µm and a high specific surface area (12.7 m2/g), demonstrated superior reactivity, confirming the significant impact of these physical parameters.
- Correlations between mineralogical composition and reactive behavior of clays
Mineral and chemical analyses (Table 5) revealed a strong consistency between the mineralogical composition and thermogravimetric results (TGA, Table 4), with the notable exception of the kaolinite content in Sabcé (0.7%). This discrepancy can be attributed to the simultaneous presence of paragonite (32.2%). Upon calcination at 700 °C, the amorphization of paragonite (evidenced by the disappearance of XRD peaks, Figure 9) contributed to the amorphous fraction (13.7%) without enhancing reactivity. A general correlation was established between the kaolinite content and amorphous character (Figure 15). A higher kaolinite content consistently corresponded to a more disordered structure.
- Impact of kaolinite content on geopolymerization and the performance of geopolymers
Figure 15, in conjunction with Figure 17, illustrates the relationship between kaolinite content, the proportion of amorphous phase, and mechanical strength. The Saaba clay, with 83% kaolinite and 32% amorphous phase, produced the strongest geopolymer (14.2 MPa). This phenomenon can be attributed to the fact that a high kaolinite content promotes, after calcination, the formation of a disordered and more reactive structure. In an alkaline environment, this structure is attacked, leading to the release of soluble silicate and aluminate species (Si(OH)4 and Al(OH)4−). These dissolved species (Si, Al) then recombine to form monomers, followed by oligomers via Si-O-Al bonds, gradually transforming the solution into a colloidal gel. This gel eventually evolves into an amorphous or semi-crystalline solid with a structure analogous to that of zeolites [51]. The condensation of this gel is enhanced by the curing temperature, promoting densification of the geopolymer matrix, which would explain its improved resistance to mechanical stress. Moreover, higher kaolinite and amorphous phase contents significantly reduce the paste’s setting time [52]. This is remarkable, as seen in Figure 20. Conversely, clays with low kaolinite content (Kandarfa, Sabcé) exhibited poor performance, including a lack of hardening in some cases.
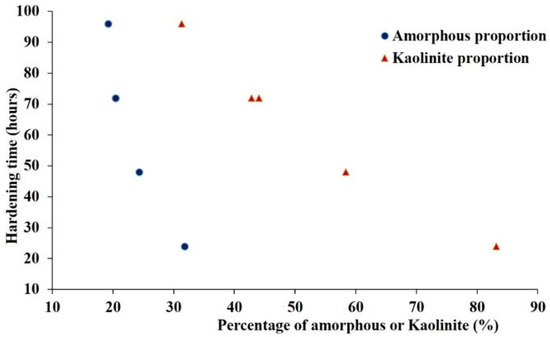
Figure 20.
Evolution of the setting time depending on the content of kaolinite and amorphous character.
- Inhibitory role of paragonite on geopolymerization
Unlike kaolinite, paragonite, even when rendered amorphous by calcination, did not participate in the geopolymerization reaction. The literature supports this observation: studies such as that by Mirković et al. [53] have demonstrated that paragonite and quartz remain inert in geopolymer matrices. Thus, despite a high aluminosilicate content (90.6% in Sabcé), the dominant presence of paragonite (32.2%) inhibited reactivity.
- Implications for precursor selection
This study highlights the need for a multi-approach characterization (physical, chemical, mineralogical, and thermal analyses) to identify clays suitable for geopolymers. The case of Sabcé demonstrates that a high aluminosilicate content or a moderate proportion of amorphous material is not sufficient to guarantee reactivity. Key parameters include a kaolinite content of greater than 80% (ideal), amorphous nature derived from kaolinite (as opposed to other phases such as paragonite), a short curing time (24 h for Saaba compared with 96 h for Kandarfa), and mechanical strength correlated with these factors.
The Saaba clay emerged as the most promising material, while those from Kandarfa and Sabcé, despite their partially amorphous properties, proved unsuitable due to their unfavorable mineralogical composition.
5. Conclusions
This study aimed to evaluate the chemical and mineralogical characteristics of six (06) clays (Kamboinsé, Kandarfa, Saaba, Sabcé, Selogo, and Tougou) from Burkina Faso and to study their reactivity in the presence of a solution of sodium hydroxide for the synthesis of a geopolymer binder. At the end of the investigation, the following conclusions were drawn.
- Aluminosilicate composition: All clays were confirmed as aluminosilicates based on their alumina and silica content. Mineralogical analysis revealed significant variations in the initial kaolinite content and amorphous proportions. The Saaba and Kamboinsé clays exhibited the highest kaolinite content (83.1% and 58.3%, respectively) and amorphous proportions (31.8% and 24.3%, respectively), surpassing the other samples (Selogo, Tougou, Kandarfa, and Sabcé).
- The reactivity of these clays through the setting/hardening times of their pastes made it possible to highlight the hardening of five (05) pastes out of six (06) at variable time intervals: 24 h for the paste from Saaba clay, 48 h for that with Kamboinsé clay, and beyond 48 h for pastes from clays from Selogo, Tougou, and Kandarfa. The paste made with Sabcé clay did not harden after seven days due to its low reactivity.
- Compressive strength of geopolymers: The geopolymer samples derived from the Saaba and Kamboinsé clays exhibited the highest compressive strengths (14 MPa and 10 MPa, respectively), which were attributed to their elevated amorphous kaolinite contents. These results suggest that Saaba clay is particularly well-suited for the synthesis of geopolymer binders.
- The increase in compressive strength, combined with a weight loss of the Saaba clay geopolymer binders, was observed as a function of the temperature variation (from 30 to 90 °C). Its mineralogical characterization revealed the formation of new crystalline phases (faujasite, zeolite A, and hydrosodalite), indicating a favorable geopolymerization reaction, particularly due to the dissolution of aluminosilicates from the Saaba sample and the subsequent formation of the geopolymer gel.
Further investigations should focus on identifying the factors that hinder the reactivity of calcined clay containing paragonite minerals in alkaline solutions.
Author Contributions
Conceptualization, A.M., G.E. and F.T.; Methodology, A.M., E.P. and G.E.; Formal analysis, S.O.S. and P.N.; Validation, A.M., E.P. and G.E.; Investigation, S.O.S.; Data curation, S.O.S.; Writing—original draft preparation, S.O.S. and P.N.; Writing—review and editing, A.M., E.P. and G.E.; Visualization, G.E. and F.T.; Supervision, G.E. and F.T.; Project administration, F.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive any financial support for the writing and publication of this article.
Data Availability Statement
Data will be available from the corresponding author, S.S.O., upon request.
Acknowledgments
The authors sincerely acknowledge the management of Institut 2iE, INSA Toulouse and INSA Lyon for granting permission to conduct these studies in their respective laboratories (LEMHaD, LMDC, and MATEIS). They also extend their gratitude to the Swiss DDC (Directorate for Development and Cooperation) and the Rhône-Alpes Region (Lyon) for their support of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Symbols | Definitions |
| °C | Degree centigrade |
| M | Mole |
| OPC | Ordinary Portland cement |
| Al2O3·2SiO2·2H2O | Chemical formula of kaolinite |
| Al2O3·2SiO2 | Chemical formula of metakaolinite |
| 2H2O | Chemical formula of water |
| MK | Metakaolin |
| D90 | Size of the particles for which 90% of the sample is smaller |
| RC | Compressive strength |
| S | Cross-sectional area of a sample |
| SiO2 | Silicon oxide |
| Fe2O3 | Iron oxide |
| MgO | Magnesium oxide |
| MnO2 | Manganese oxide |
| P2O5 | Phosphorus oxide |
| NaOH | Sodium hydroxide |
| MPa | Mega Pascal |
| CaO | Calcium oxide |
| D50 | Size of the particles for which 50% of the sample is smaller |
| BET | Brunauer, Emmett, and Teller (specific surface area) |
| H2SO4 | Sulfuric acid |
| Mair | Mass of a saturated sample measured in air |
| Mwater | Mass of a saturated sample measured in water |
| Al2O3 | Aluminum oxide |
| K2O | Potassium oxide |
| Na2O | Sodium oxide |
| TiO2 | Titanium oxide |
| LOI | Loss on ignition |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| DTA | Differential thermal analyses |
| DG | Thermogravimetric and differential |
| Mi | Initial mass of the sample |
| P | Relative mass loss of the sample |
| ME.K | Mass of chemical water from the dehydroxylated kaolinite |
| Mmol.H2O | Molar mass of water |
| % K | Percentage of kaolinite |
References
- Castel, A. Bond between steel reinforcement and geopolymer concrete. Handb. Low Carbon Concr. 2016, 375–387. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geopolymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Nshimiyimana, P.; Omar, S.; Zoungrana, O. A discussion of “optimisation of compressed earth blocks (CEBs) using natural origin materials: A systematic literature review”. Constr. Build. Mater. 2022, 325, 126887. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Z.; Tian, X.; Wu, J.; Wang, L. Review on the impact of metakaolin-based geopolymer’s reaction chemistry, nanostructure and factors on its properties. Constr. Build. Mater. 2024, 412, 134760. [Google Scholar] [CrossRef]
- Sore, O.S.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Synthesis and characterization of geopolymer binders based on local materials from Burkina Faso-Metakaolin and rice husk ash. Constr. Build. Mater. 2016, 124, 301–311. [Google Scholar] [CrossRef]
- Camarini, G.C.; Abdullah, H.H.; Shahin, M.A. One-Part Geopolymer for Stabilising Crushed Rock Road Base Material. Geosciences 2025, 15, 122. [Google Scholar] [CrossRef]
- Balde, M.Y.; Sidibe, D.; Bakam, É.S.S.; Njiomou, C.D.; Blanchart, P. Formulation of Geopolymer Cements from Two Clays Containing Kaolinite and Muscovite: Effect of Temperature on the Physicomechanical Properties of the Products. J. Mater. Sci. Chem. Eng. 2023, 11, 34–45. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Skibsted, J.; Palomo, A. Clay reactivity: Production of alkali activated cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- Xia, M.; Nematollahi, B.; Sanjayan, J. Printability, accuracy and strength of geopolymer made using powder-based 3D printing for construction applications. Autom. Constr. 2019, 101, 179–189. [Google Scholar] [CrossRef]
- Davidovits, J. Properties of geopolymer cements. In Proceedings of the First International Conference on Alkaline Cements and Concretes; Institute on Binders and Materials, Kiev State Technical University: Kiev, Ukraine, 1994; Volume 1, pp. 131–149. [Google Scholar]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Yuan, C.; Wang, Q.; Sarker, P.K.; Shi, X. Deterioration of ambient-cured and heat-cured fly ash geopolymer concrete by high temperature exposure and prediction of its residual compressive strength. Constr. Build. Mater. 2020, 262, 120924. [Google Scholar] [CrossRef]
- Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties. Sustain. 2022, 14, 15062. [Google Scholar] [CrossRef]
- Hossein, H.A.; Hamzawy, E.M.A.; El-Bassyouni, G.T.; Nabawy, B.S. Mechanical and physical properties of synthetic sustainable geopolymer binders manufactured using rockwool, granulated slag, and silica fume. Constr. Build. Mater. 2023, 367, 130143. [Google Scholar] [CrossRef]
- Játiva, A.; Ruales, E.; Etxeberria, M. Volcanic ash as a sustainable binder material: An extensive review. Materials 2021, 14, 1302. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, J.; Dai, X.; Zheng, Y.; Zheng, M. Mechanical properties of geopolymer concrete solidified with waste materials. Constr. Build. Mater. 2025, 460, 139835. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Sahmaran, M. Extensive evaluation on the effect of glass powder on the rheology, strength, and microstructure of metakaolin-based geopolymer binders. Constr. Build. Mater. 2021, 268, 121168. [Google Scholar] [CrossRef]
- Kaze, C.R.; Nana, A.; Lecomte-Nana, G.L.; Deutou, J.G.N.; Kamseu, E.; Melo, U.C.; Andreola, F.; Leonelli, C. Thermal behaviour and microstructural evolution of metakaolin and meta-halloysite-based geopolymer binders: A comparative study. J. Therm. Anal. Calorim. 2022, 147, 2055–2071. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Ofer-Rozovsky, E.; Arbel Haddad, M.; Bar Nes, G.; Katz, A. The formation of crystalline phases in metakaolin-based geopolymers in the presence of sodium nitrate. J. Mater. Sci. 2016, 51, 4795–4814. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejía De Gutiérrez, R.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Souri, A.; Golestani-Fard, F.; Naghizadeh, R.; Veiseh, S. An investigation on pozzolanic activity of Iranian kaolins obtained by thermal treatment. Appl. Clay Sci. 2015, 103, 34–39. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-varela, M.T.; Palomo, A. Alkaline activation of metakaolin: Effect of calcium hydroxide in the products of reaction. J. Am. Ceram. Soc. 2002, 31, 225–231. [Google Scholar] [CrossRef]
- Kuenzel, C.; Neville, T.P.; Donatello, S.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83, 308–314. [Google Scholar] [CrossRef]
- Taborda-barraza, M.; Tambara, L.U.D.; Vieira, C.M.; De Azevedo, A.R.G.; Gleize, P.J.P. Parametrization of Geopolymer Compressive Strength Obtained from Metakaolin Properties. Minerals 2024, 14, 974. [Google Scholar] [CrossRef]
- Kenne Diffo, B.B.; Elimbi, A.; Cyr, M.; Dika Manga, J.; Tchakoute Kouamo, H. Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. J. Asian Ceram. Soc. 2015, 3, 130–138. [Google Scholar] [CrossRef]
- Essaidi, N.; Samet, B.; Baklouti, S.; Rossignol, S. Feasibility of producing geopolymers from two different Tunisian clays before and after calcination at various temperatures. Appl. Clay Sci. 2014, 88–89, 221–227. [Google Scholar] [CrossRef]
- Nmiri, A.; Hamdi, N.; Yazoghli-Marzouk, O.; Duc, M.; Srasra, E. Synthesis and characterization of kaolinite-based geopolymer: Alkaline activation effect on calcined kaolinitic clay at different temperatures. J. Mater. Environ. Sci. 2017, 8, 276–290. [Google Scholar]
- Diaz, E.I.; Allouche, E.N.; Eklund, S. Factors affecting the suitability of fly ash as source material for geopolymers. Fuel 2010, 89, 992–996. [Google Scholar] [CrossRef]
- Du, H.; Dixit, A.; Pang, S. Calcined Clays for Sustainable Concrete; Scrivener, K., Favier, A., Eds.; Rilem Book; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9789401799386. [Google Scholar]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F. Engineering Mechanical performances of weathered coal fly ash based geopolymer bricks. Procedia Eng. 2011, 21, 745–752. [Google Scholar] [CrossRef]
- Yvon, J.; Garin, P.; Delon, J.F.; Cases, J.M. Valorisation des argiles kaoliniques des Charentes dans le caoutchouc naturel. Bull. Mineral. 1982, 105, 535–541. [Google Scholar] [CrossRef]
- Omar Sore, S.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Stabilization of compressed earth blocks (CEBs) by geopolymer binder based on local materials from Burkina Faso. Constr. Build. Mater. 2018, 165, 333–345. [Google Scholar] [CrossRef]
- Yameogo, A.; Sorgho, B.; Zerbo, L.; Seynou, M.; Millogo, Y.; Ouedraogo, R. Preparation D’Une Pouzzolane a Base D’Une Matiere Premiere Argileuse Du Burkina Fas. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 16, 371–383. [Google Scholar]
- Bich, C. Contribution à L’étude de L’activation Thermique du Kaolin: Évolution de la Structure Cristallographique et Activité Pouzzolanique; INSA: Lyon, France, 2005. [Google Scholar]
- Millogo, Y.; Traoré, K.; Ouedraogo, R.; Kaboré, K.; Blanchart, P.; Thomassin, J.H. Geotechnical, mechanical, chemical and mineralogical characterization of a lateritic gravels of Sapouy (Burkina Faso) used in road construction. Constr. Build. Mater. 2008, 22, 70–76. [Google Scholar] [CrossRef]
- Comodi, P.; Zanazzi, P.F. Structural thermal behavior of paragonite and its dehydroxylate: A high-temperature single-crystal study. Phys. Chem. Miner. 2000, 27, 377–385. [Google Scholar] [CrossRef]
- Prinsloo, L.C.; van der Merwe, E.M.; Wadley, L. The thermal behaviour of silica varieties used for tool making in the Stone Age. J. Therm. Anal. Calorim. 2018, 131, 1135–1145. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R.L. Thermal treatment of natural goethite: Thermal transformation andphysical properties. Thermochim. Acta 2013, 568, 115–121. [Google Scholar] [CrossRef]
- Bich, C.; Ambroise, J.; Péra, J. Influence of degree of dehydroxylation on the pozzolanic activity of metakaolin. Appl. Clay Sci. 2009, 44, 194–200. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Elimbi, A.; Mbey, J.A.; Ngally Sabouang, C.J.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, F.; Wang, S.; Fan, L.; Zhang, J.; Li, P.; Yu, L. Influence mechanism of curing temperature on geopolymerization reaction: A comprehensive review. J. Build. Eng. 2025, 103, 112195. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. the activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Tome, S.; Nana, A.; Tchakouté, H.K.; Temuujin, J.; Rüscher, C.H. Mineralogical evolution of raw materials transformed to geopolymer materials: A review. Ceram. Int. 2024, 50, 35855–35868. [Google Scholar] [CrossRef]
- Mirković, M.; Yilmaz, M.S.; Kljajević, L.; Pavlović, V.; Ivanović, M.; Djukić, D.; Eren, T. Design of PEI and Amine Modified Metakaolin-Brushite Hybrid Polymeric Composite Materials for CO2 Capturing. Polymers 2023, 15, 1669. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).