Pułtusk H5 Chondrite—A Compilation of Chemical, Physical, and Thermophysical Data
Abstract
1. Introduction
2. Materials and Methods
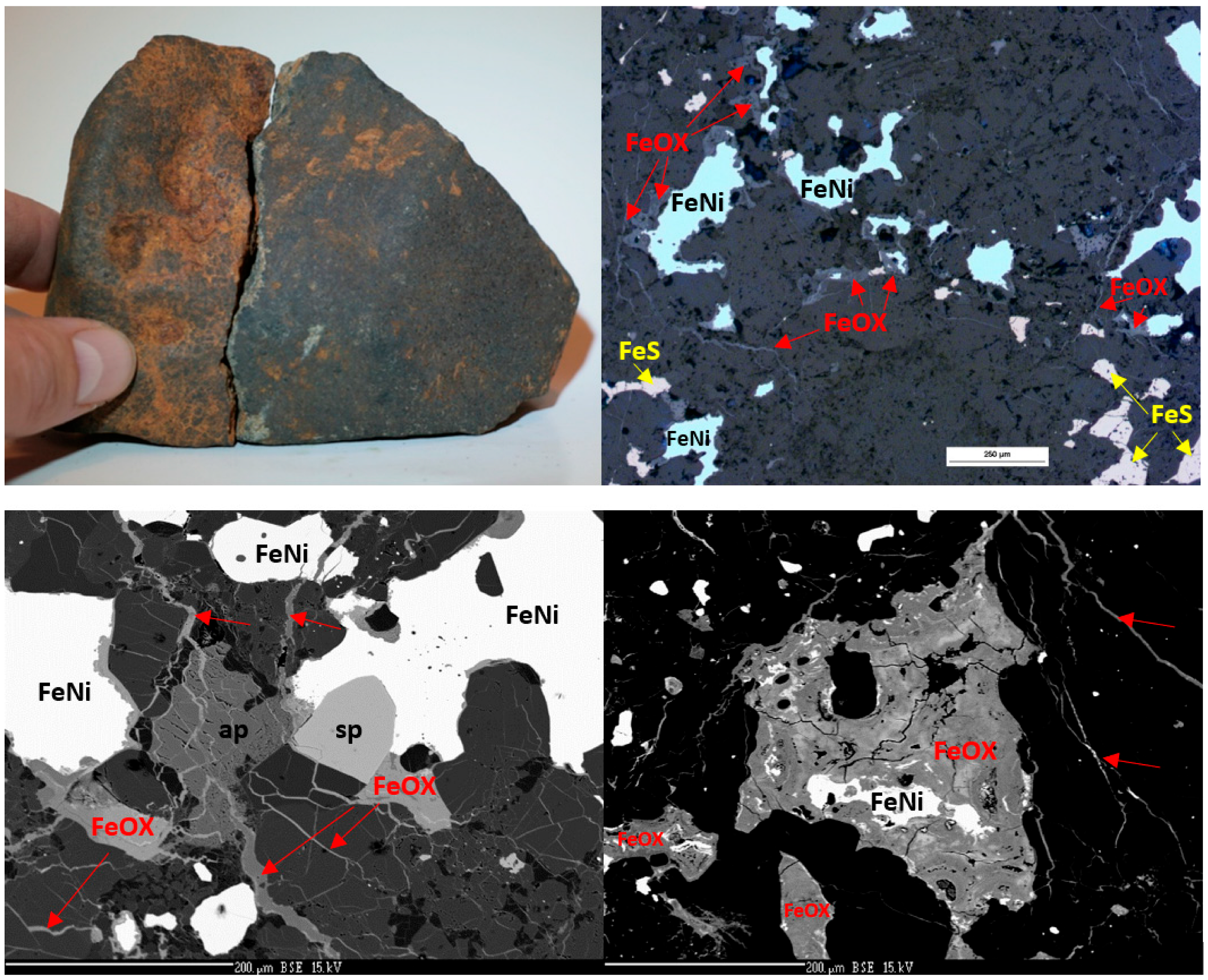
3. Results and Discussion
3.1. Bulk Chemical Composition
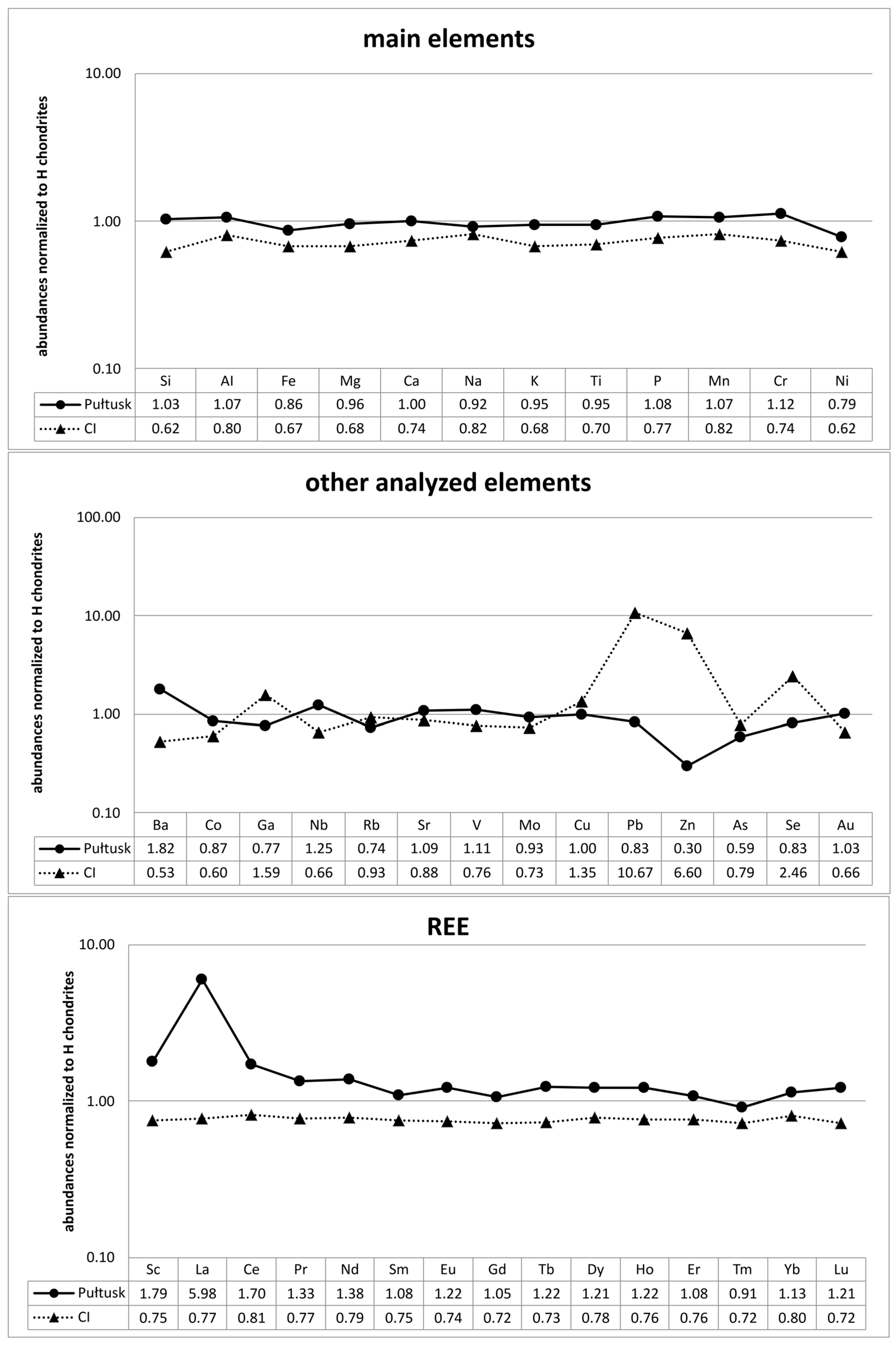
| Element | Pułtusk | Average H | Range H | Element | Pułtusk | Average H | Range H |
|---|---|---|---|---|---|---|---|
| Si | 175,400 | 171,000 | 82,000–235,000 | Pb | 0.2 | 0.24 | 0.008–2.28 |
| Al | 11,300 | 10,600 | 690–11,100 | Zn | 14 | 47 | 0.54–540 |
| Fe | 235,000 | 272,000 | 42,000–912,000 | As | 1.3 | 2.2 | 0.078–15.1 |
| Mg | 134,700 | 141,000 | 4600–213,000 | Cd | <0.1 | <0.01 | 0.000038–1.24 |
| Ca | 12,200 | 12,200 | 4100–127,000 | Sb | <0.1 | 0.066 | 0.0015–0.78 |
| Na | 5600 | 6110 | 40–29,400 | Bi | <0.1 | <0.01 | 0.00016–0.907 |
| K | 740 | 780 | 100–4700 | Ag | <0.1 | 0.045 | 0.0032–1.87 |
| Ti | 600 | 630 | 100–4700 | Hg | <0.01 | bd | 0.19–1.93 |
| P | 1300 | 1200 | 100–2400 | Tl | <0.1 | <0.001 | 0.00004–0.361 |
| Mn | 2500 | 2340 | 30–5800 | Se | 6.6 | 8 | 0.56–42.5 |
| Cr | 3920 | 3500 | 30–37,100 | Zr * | 215.7 | 7.3 | 3.09–10.00 |
| Ni | 13,440 | 17,100 | 100–130,000 | Au | 0.2262 | 0.22 | 0.00213–1.8 |
| Ba | 8 | 4.4 | 0.13–26.50 | Sc | 14 | 7.8 | 0.04–13.8 |
| Be | <1 | 0.03 | 0.03–0.39 | Y * | 15.1 | 2 | 0.74–6.80 |
| Co | 718.4 | 830 | 25.20–5000.00 | La | 1.8 | 0.301 | 0.087–7.68 |
| Cs | <0.1 | <0.2 | 0.001–2.16 | Ce | 1.3 | 0.763 | 0.45–13.8 |
| Ga | 4.6 | 6 | 0.58–37.30 | Pr | 0.16 | 0.12 | 0.05–0.4 |
| Hf * | 4.9 | 0.15 | 0.10–0.38 | Nd | 0.8 | 0.581 | 0.24–1.22 |
| Nb | 0.5 | 0.4 | 0.20–0.46 | Sm | 0.21 | 0.194 | 0.068–0.73 |
| Rb | 1.7 | 2.3 | 0.51–86.80 | Eu | 0.09 | 0.074 | 0.055–0.15 |
| Sn | <1 | 0.35 | 0.103–2.3 | Gd | 0.29 | 0.275 | 0.1–0.457 |
| Sr | 9.6 | 8.8 | 8.00–938.00 | Tb | 0.06 | 0.049 | 0.02–0.091 |
| Ta | <0.1 | 0.021 | 0.02–0.05 | Dy | 0.37 | 0.305 | 0.12–0.568 |
| Th | <0.2 | 0.038 | 0.02–0.285 | Ho | 0.09 | 0.074 | 0.03–0.12 |
| U | <0.1 | 0.013 | 0.01–2.44 | Er | 0.23 | 0.213 | 0.07–0.592 |
| V | 81 | 73 | 2.30–91.10 | Tm | 0.03 | 0.033 | 0.01–0.045 |
| W | <0.5 | 0.164 | 0.16–0.87 | Yb | 0.23 | 0.203 | 0.03–0.345 |
| Mo | 1.3 | 1.4 | 1.24–4.88 | Lu | 0.04 | 0.033 | 0.008–0.068 |
| Cu | 94.3 | 94 | 48–759 | Pb | 0.2 | 0.24 | 0.008–2.28 |


3.2. Physical Properties
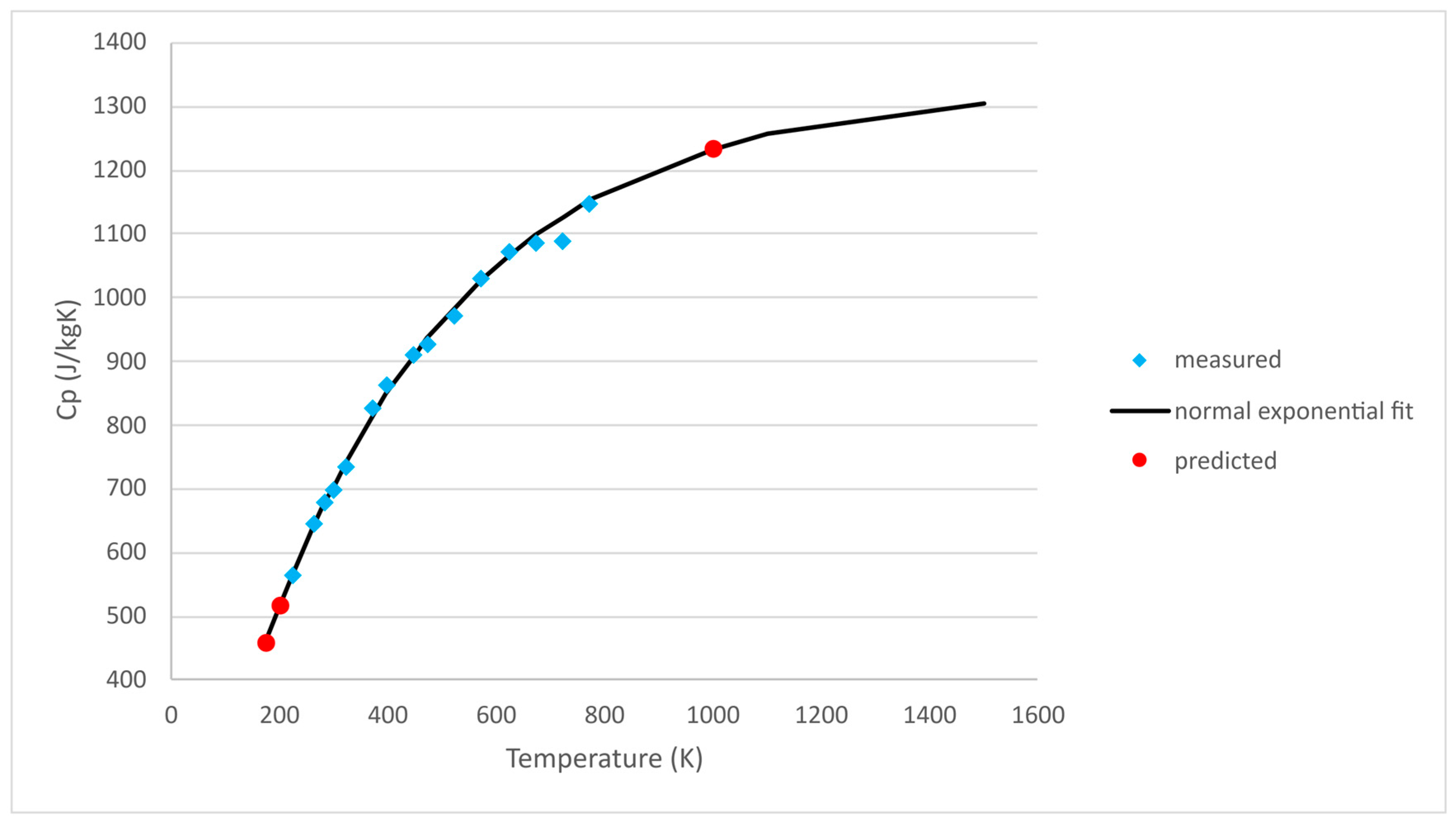
3.3. Thermophysical Properties
3.3.1. Specific Heat Capacity
3.3.2. Volumetric Heat Capacity
3.3.3. Mean Atom-Molar Heat Capacity
3.3.4. Thermal Diffusivity
3.3.5. Thermal Conductivity
3.3.6. Thermal Inertia
4. Conclusions
- -
- Volumetric heat capacity Cvol(300 K) = 2.3 MJ/(m3K) and Cvol(200 K) = 1.7 MJ/(m3K);
- -
- Mean atomic weight Amean = 23.39 g/mol;
- -
- Mean molar-atomic heat capacity Catom(200 K) = 12.1 J/(molK) and Catom(300 K) = 16.4 J/(molK);
- -
- Thermal diffusivity D(296 K, vacuum) = 0.81 × 10−6 m2/s, D(298 K, 1 atm) = 1.10 × 10−6 m2/s, D(200 K, 1 Pa) = 0.72 × 10−6 m2/s;
- -
- Thermal conductivity K(RT, vacuum) = 1.83 W/(m·K), K(RT, 1 atm) = 2.54 W/(m·K), K(200 K, vacuum) = 1.22 W/(m·K);
- -
- Thermal inertia Γ(300 K, vacuum) = 2.05 × 103 J/(s0.5K·m2), Γ(300 K, air 1 atm) = 2.42 × 103 J/(s0.5K·m2), Γ(200 K, vacuum) = 1.44 × 103 J/(s0.5K·m2).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.lpi.usra.edu/meteor/ (accessed on 9 May 2025).
- Pilski, A.S. Pułtuski deszcz meteorytów. Meteoryt 1992, 4, 11–14. [Google Scholar]
- Bus, S.J.; Binzel, R.P. Phase II of the small main-belt asteroid spectroscopic survey: A feature-based taxonomy. Icarus 2002, 158, 146–177. [Google Scholar] [CrossRef]
- Gaffey, M.J.; Gilbert, S.L. Asteroid 6 Hebe: The probable parent body of the H-type ordinary chondrites and the IIE iron meteorites. Meteorit. Planet. Sci. 1998, 33, 1281–1295. [Google Scholar] [CrossRef]
- Vernazza, P.; Zanda, B.; Binzel, R.P.; Hiroi, T.; DeMeo, F.E.; Birlan, M.; Hewins, R.; Ricci, L.; Barge, P.; Lockhart, M. Multiple and fast: The accretion of ordinary chondrite parent bodies. Astrophys. J. 2014, 791, 120. [Google Scholar] [CrossRef]
- Noonan, J.W.; Reddy, V.; Harris, W.M.; Bottke, W.F.; Sanchez, J.A.; Furfaro, R.; Brown, Z.; Fernandes, R.; Kareta, T.; Lejoly, C.; et al. Search for the H Chondrite Parent Body among the Three Largest S-type Asteroids: (3) Juno, (7) Iris, and (25) Phocaea. Astron. J. 2019, 158, 213. [Google Scholar] [CrossRef]
- Krzesińska, A.M. Contribution of early impact events to metal-silicate separation, thermal annealing, and volatile redistribution: Evidence in the Pułtusk H chondrite. Meteorit. Planet. Sci. 2017, 52, 2305–2321. [Google Scholar] [CrossRef]
- Krzesińska, A.M. Thermal metamorphic evolution of the Pułtusk H chondrite breccia–compositional and textural properties not included in petrological classification. Geol. Q. 2016, 60, 211–224. [Google Scholar] [CrossRef][Green Version]
- Krzesińska, A.; Fritz, J. Weakly shocked and deformed CM microxenoliths in the Pułtusk H chondrite. Meteorit. Planet. Sci. 2014, 49, 595–610. [Google Scholar] [CrossRef]
- Łuszczek, K.; Krzesińska, A.M. Copper in ordinary chondrites: Proxies for resource potential of asteroids and constraints for minimum-invasive and economically efficient exploitation. Planet. Space Sci. 2020, 194, 105092. [Google Scholar] [CrossRef]
- Krzesińska, A.; Gattacceca, J.; Friedrich, J.M.; Rochette, P. Impact-related noncoaxial deformation in the Pułtusk H chondrite inferred from petrofabric analysis. Meteorit. Planet. Sci. 2015, 50, 401–417. [Google Scholar] [CrossRef]
- Ganapathy, E.; Anders, E. Noble gases in eleven H-chondrites. Geochim. Cosmochim. Acta 1973, 37, 359–362. [Google Scholar] [CrossRef]
- Bogusz, P.; Gałązka-Friedman, J.; Brzózka, K.; Jakubowska, M.; Woźniak, M.; Karwowski, Ł.; Duda, P. Mössbauer spectroscopy as a useful method for distinguishing between real and false meteorites. Hyperfine Interact. 2019, 240, 126. [Google Scholar] [CrossRef]
- Burkhardt, C.; Borg, L.E.; Brennecka, G.A.; Shollenberger, Q.R.; Dauphas, N.; Kleine, T. A nucleosynthetic origin for the Earth’s anomalous 142Nd composition. Nature 2016, 537, 394. [Google Scholar] [CrossRef]
- Voropaev, S.A.; Nugmanov, I.I.; Dushenko, N.V.; Kuz’mina, T.G.; Korochantsev, A.V.; Senin, V.G.; Eliseev, A.A.; Jianguo, Y. Relationship Between the H5 Chondrite Composition, Structure and Mechanical Properties from the Example of NWA 12370 and Pultusk. Sol. Syst. Res. 2021, 55, 409–419. [Google Scholar] [CrossRef]
- Phelan, N.; Day, J.M.D.; Dhaliwal, J.K.; Liu, Y.; Corder, C.A.; Strom, C.; Pringle, E.; Assayag, N.; Cartigny, P.; Marti, K.; et al. A 187Re-187Os, 87Rb-87Sr, highly siderophile and incompatible trace element study of some carbonaceous, ordinary and enstatite chondrite meteorites. Geochim. Cosmochim. Acta 2022, 318, 19–54. [Google Scholar] [CrossRef]
- Medvedev, R.V.; Gorbatsevich, F.I.; Zotkin, I.T. Determination of the physical properties of stony meteorites applied to the study of their destruction processes. Meteoritika 1985, 44, 105–110. [Google Scholar]
- Rozitis, B.; Duddy, S.R.; Green, S.F.; Lowry, S.C. A thermophysical analysis of the (1862) Apollo Yarkovsky and YORP effects. Astron. Astrophys. 2013, 555, A20. [Google Scholar] [CrossRef]
- Rozitis, B.; Green, S.F. The influence of rough surface thermal-infrared beaming on the Yarkovsky and YORP effects. Mon. Not. R. Astron. Soc. 2012, 423, 367–388. [Google Scholar] [CrossRef]
- Farnocchia, D.; Chesley, S.R.; Vokrouhlický, D.; Milani, A.; Spoto, F.; Bottke, W.F. Near Earth Asteroids with measurable Yarkovsky effect. Icarus 2013, 224, 1–13. [Google Scholar] [CrossRef]
- Loehle, S.; Jenniskens, P.; Böhrk, H.; Bauer, T.; Elsäβer, H.; Sears, D.W.; Zolensky, M.E.; Shaddad, M.H. Thermophysical properties of Almahata Sitta meteorites (asteroid 2008 TC3) for high-fidelity entry modeling. Meteorit. Planet. Sci. 2017, 52, 197–205. [Google Scholar] [CrossRef]
- Limonta, S.; Trisolini, M.; Frey, S.; Colombo, C. Fragmentation model and strewn field estimation for meteoroids entry. Icarus 2021, 367, 114553. [Google Scholar] [CrossRef]
- Vida, D.; Brown, P.G.; Campbell-Brown, M.; Egal, A. First holistic modelling of meteoroid ablation and fragmentation: A case study of the Orionids recorded by the Canadian Automated Meteor Observatory. Icarus 2024, 408, 115842. [Google Scholar] [CrossRef]
- Gail, H.P.; Trieloff, M. Thermal history modelling of the L chondrite parent body. Astron. Astrophys. 2019, 628, A77. [Google Scholar] [CrossRef]
- Henke, S.; Gail, H.P.; Trieloff, M.; Schwarz, W.H.; Kleine, T. Thermal history modelling of the H chondrite parent body. Astron. Astrophys. 2012, 545, A135. [Google Scholar] [CrossRef]
- Henke, S.; Gail, H.P.; Trieloff, M.; Schwarz, W.H.; Kleine, T. Thermal evolution and sintering of chondritic planetesimals. Astron. Astrophys. 2012, 537, A45. [Google Scholar] [CrossRef]
- Consolmagno, S.J.G.J.; Britt, D.T. The density and porosity of meteorites from the Vatican collection. Meteorit. Planet. Sci. 1998, 33, 1231–1241. [Google Scholar] [CrossRef]
- Britt, D.T.; Consolmagno, S.J.G.J. Stony meteorite porosities and densities: A review of the data through 2001. Meteorit. Planet. Sci. 2003, 38, 1161–1180. [Google Scholar] [CrossRef]
- Consolmagno SJ, G.J.; Macke SJ, R.J.; Rochette, P.; Britt, D.T.; Gattacceca, J. Density, Magnetic Susceptibility, and the Characterization of Ordinary Chondrite Falls and Showers. Meteorit. Planet. Sci. 2006, 41, 331–342. [Google Scholar] [CrossRef]
- Wilkinson, S.L.; McCoy, T.J.; McCamant, J.E.; Robinson, M.S.; Britt, D.T. Porosity and density of ordinary chondrites: Clues to the formation of friable and porous ordinary chondrites. Meteorit. Planet. Sci. 2003, 38, 1533–1546. [Google Scholar] [CrossRef]
- Macke, R.J. Survey of Meteorite Physical Properties Density, Porosity and Magnetic Susceptibility. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2010. Available online: https://stars.library.ucf.edu/etd/1638 (accessed on 10 October 2025).
- Opeil, C.P.; Consolmagno, G.J.; Safarik, D.J.; Britt, D.T. Stony meteorite thermal properties and their relationship with meteorite chemical and physical states. Meteorit. Planet. Sci. 2012, 47, 319–329. [Google Scholar] [CrossRef]
- Smith, D.L.; Samson, C.; Herd, R.; Deslauriers, A.; Sink, J.; Christie, I.; Ernst, R.E. Measuring the bulk density of meteorites nondestructively using three-dimensional laser imaging. J. Geophys. Res. Planets 2006, 111. [Google Scholar] [CrossRef]
- Opeil, C.P.; Consolmagno, G.J.; Britt, D.T. The thermal conductivity of meteorites: New measurements and analysis. Icarus 2010, 208, 449–454. [Google Scholar] [CrossRef]
- Consolmagno, G.J.; Schaefer, M.W.; Schaefer, B.E.; Britt, D.T.; Macke, R.J.; Nolan, M.C.; Howell, E.S. The measurement of meteorite heat capacity at low temperatures using liquid nitrogen vaporization. Planet. Space Sci. 2013, 87, 146–156. [Google Scholar] [CrossRef]
- Macke, R.J.; Opeil, C.; Consolmagno, G.J. Heat capacities of ordinary chondrite falls below 300 K. Meteorit. Planet. Sci. 2019, 54, 2729–2743. [Google Scholar] [CrossRef]
- Kimberley, J.; Ramesh, K.T. The dynamic strength of an ordinary chondrite. Meteorit. Planet. Sci. 2011, 46, 1653–1669. [Google Scholar] [CrossRef]
- Svetsov, V.V.; Nemtchinov, I.V.; Teterev, A.V. Disintegration of Large Meteoroids in Earth’s Atmosphere: Theoretical Models. Icarus 1995, 116, 131–153. [Google Scholar] [CrossRef]
- Flynn, G.J.; Consolmagno, G.J.; Brown, P.; Macke, R.J. Physical properties of the stone meteorites: Implications for the properties of their parent bodies. Geochemistry 2018, 78, 269–298. [Google Scholar] [CrossRef]
- Szurgot, M.A. Specific heat and atomic heat of the Jezersko chondrite. Prz. Geol. 2020, 68, 54–59. [Google Scholar] [CrossRef]
- Szurgot, M. Mean atomic weight of Pułtusk meteorite and H chondrites. Meteorit. Planet. Sci. 2015, 50 (Suppl. S1), #5013.Pdf. [Google Scholar] [PubMed]
- Przylibski, T.A.; Łuszczek, K. Wyniki badań mineralogicznych i petrologicznych nowych okazów meteorytu Pułtusk w 150 rocznicę spadku. Przegląd Geol. 2018, 6, 368–378. [Google Scholar]
- Wlotzka, F. A Weathering Scale for the Ordinary Chondrites. Metic 1993, 28, 460. [Google Scholar]
- Szurgot, M.; Wach, R.A.; Przylibski, T.A. Thermophysical properties of Sołtmany meteorite. Meteorites 2012, 2, 53–65. [Google Scholar] [CrossRef]
- Łuszczek, K.; Wach, R.A. NWA 6255 meteorite-thermophysical properties of interior and the crust. Meteorites 2014, 3, 33–44. [Google Scholar] [CrossRef]
- Krzesińska, A. High resolution X-ray tomography as a tool for analysis of internal textures in meteorites. Meteorites 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Grady, M.M.; Pratesi, G.; Moggi Cecchi, V. Atlas of Meteorites; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Hutchison, R. Meteorites: A Petrologic, Chemical, and Isotopic Synthesis; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Mason, B.; Graham, A.L. Minor and trace elements in meteoritic minerals. Smithson. Contrib. Earth Sci. 1970, 1–17. [Google Scholar] [CrossRef]
- McSween, H., Jr.; Huss, G. Cosmochemistry; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Koblitz, J. MetBase®; Version 7.3; Meteorite Data Retrieval Software; Ritterhude, Germany, 2010. [Google Scholar]
- Jarosewich, E. Chemical analyses of meteorites at the Smithsonian Institution: An update. Meteorit. Planet. Sci. 2006, 41, 1381–1382. [Google Scholar] [CrossRef]
- Morgan, J.W.; Janssens, M.J.; Takahashi, H.; Hertogen, J.; Anders, E. H-chondrites: Trace element clues to their origin. Geochim. Cosmochim. Acta 1985, 49, 247–259. [Google Scholar] [CrossRef]
- Soini, A.J.; Kukkonen, I.T.; Kohout, T.; Luttinen, A. Thermal and porosity properties of meteorites: A compilation of published data and new measurements. Meteorit. Planet. Sci. 2020, 55, 402–425. [Google Scholar] [CrossRef]
- Consolmagno, G.J.; Britt, D.T.; Macke, R.J. The significance of meteorite density and porosity. Geochemistry 2008, 68, 1–29. [Google Scholar] [CrossRef]
- Kohout, T.; Havrila, K.; Tóth, J.; Husárik, M.; Gritsevich, M.; Britt, D.; Borovička, J.; Spurný, P.; Igaz, A.; Svoreň, J.; et al. Density, porosity and magnetic susceptibility of the Košice meteorite shower and homogeneity of its parent meteoroid. Planet. Space Sci. 2014, 93–94, 96–100. [Google Scholar] [CrossRef]
- Bland, P.A.; Zolensky, M.E.; Benedix, G.K.; Sephton, M.A. Weathering of Chondritic Meteorites. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 853–867. Available online: https://ui.adsabs.harvard.edu/abs/2006mess.book..853B/abstract (accessed on 13 October 2025).
- Altunayar-Unsalan, C.; Unsalan, O.; Wach, R.A.; Szurgot, M.A. Physical and thermal properties of Bursa L6 chondrite: A combination of density, porosity, specific heat, water content, thermal conductivity, and thermal diffusivity results. Astrophys. Space Sci. 2025, 370, 53. [Google Scholar] [CrossRef]
- Bartoschewitz, R.; Appel, P.; Barrat, J.A.; Bischoff, A.; Caffee, M.W.; Franchi, I.A.; Gabelica, Z.; Greenwood, R.C.; Harir, M.; Harries, D.; et al. The Braunschweig meteorite—A recent L6 chondrite fall in Germany. Geochemistry 2017, 77, 207–224. [Google Scholar] [CrossRef]
- Altunayar-Unsalan, C.; Unsalan, O.; Szurgot, M.A.; Wach, R.A. Specific heat and thermal history of the Sariçiçek howardite. Meteorit. Planet. Sci. 2021, 56, 2103–2117. [Google Scholar] [CrossRef]
- Beech, M.; Coulson, I.M.; Nie, W.; McCausland, P. The thermal and physical characteristics of the Gao-Guenie (H5) meteorite. Planet. Space Sci. 2009, 57, 764–770. [Google Scholar] [CrossRef]
- Yomogida, K.; Matsui, T. Physical properties of ordinary chondrites. J. Geophys. Res. 1983, 88, 9513–9533. [Google Scholar] [CrossRef]
- Matsui, T.; Osako, M. Thermal property measurement of Yamato meteorites. Mem. Natl. Inst. Polar Res. Spec. Issue 1979, 243–252. [Google Scholar]
- Szurgot, M. On the specific heat capacity and thermal capacity of meteorites. In Proceedings of the 42nd Lunar and Planetary Science Conference, The Woodlands, TX, USA, 7–11 March 2011. [Google Scholar]
- Szurgot, M.; Wojtatowicz, T.W. Thermal diffusivity of meteorites. Meteorit. Planet. Sci. 2011, 46, A230. [Google Scholar]
- Osako, M. Thermal diffusivity measurements of chondrites and iron meteorite. Bull. Natl. Sci. Mus. Tokyo 1981, 4, 1–8. [Google Scholar]
- Ashby, M.; Sherdiff, H.; Cebon, D. Materials Engineering, Science, Processing and Design; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Noyes, C.S.; Consolmagno, G.J.; Macke, R.J.; Britt, D.T.; Opeil, C.P. Low-temperature thermal properties of iron meteorites. Meteorit. Planet. Sci. 2022, 57, 1706–1721. [Google Scholar] [CrossRef]
- Okada, T.; Fukuhara, T.; Tanaka, S.; Taguchi, M.; Imamura, T.; Arai, T.; Senshu, H.; Ogawa, Y.; Demura, H.; Kitazato, K.; et al. Thermal Infrared Imaging Experiments of C-Type Asteroid 162173 Ryugu on Hayabusa2. Space Sci. Rev. 2017, 208, 255–286. [Google Scholar] [CrossRef]
- Piqueux, S.; Vu, T.H.; Bapst, J.; Garvie, L.A.J.; Choukroun, M.; Edwards, C.S. Specific Heat Capacity Measurements of Selected Meteorites for Planetary Surface Temperature Modeling. J. Geophys. Res. Planets 2021, 126, e2021JE007003. [Google Scholar] [CrossRef]

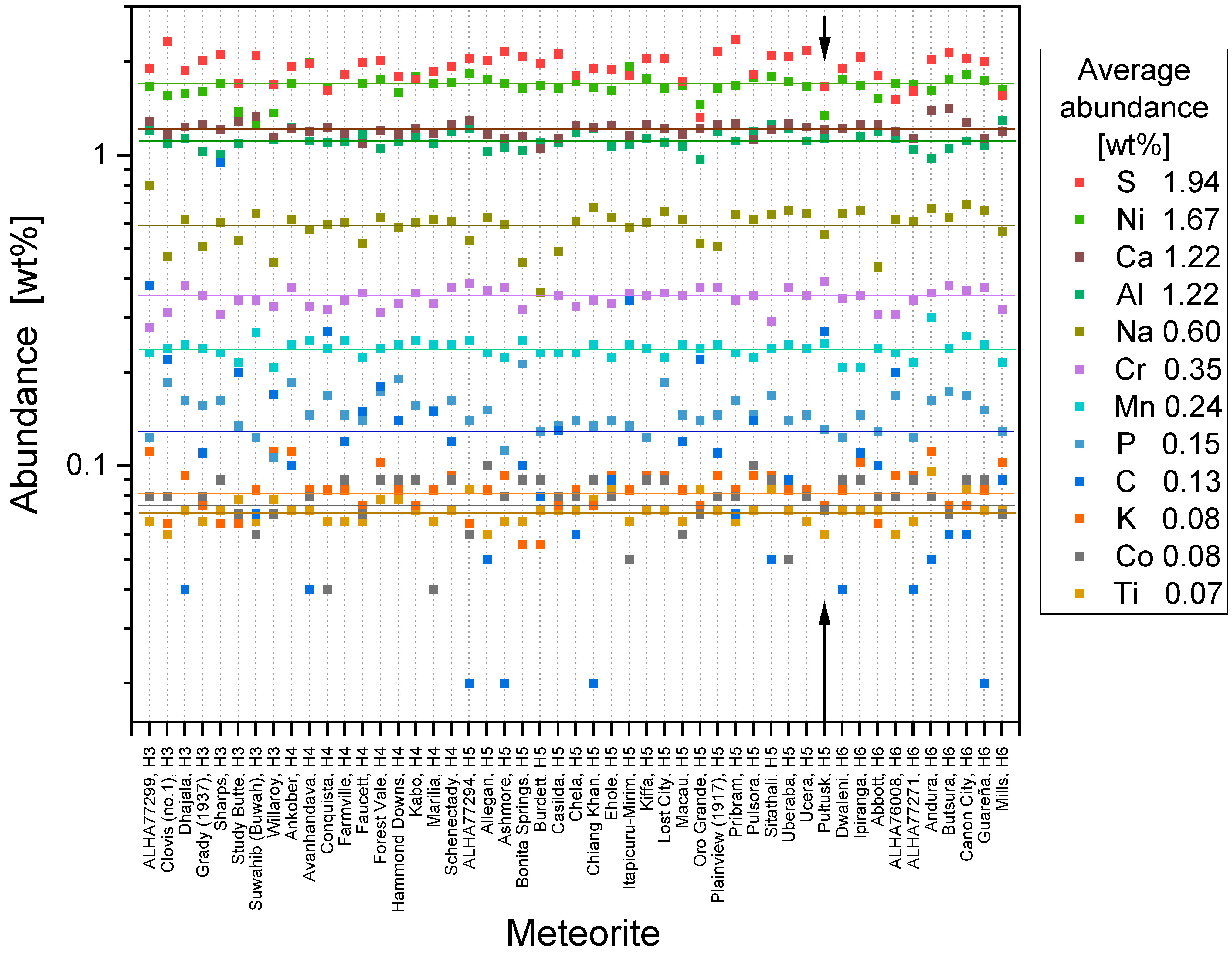
| Element | H | L | LL | Pułtusk | CI |
|---|---|---|---|---|---|
| Si | 16.9 | 18.5 | 18.9 | 17.5 | 10.5 |
| Ti | 0.06 | 0.063 | 0.062 | 0.06 | 0.042 |
| Al | 1.13 | 1.22 | 1.19 | 1.13 | 0.86 |
| Cr | 0.366 | 0.388 | 0.374 | 0.392 | 0.265 |
| Fe | 27.5 | 21.5 | 18.5 | 23.5 | 18.2 |
| Mn | 0.232 | 0.257 | 0.262 | 0.25 | 0.19 |
| Mg | 14 | 14.9 | 15.3 | 13.47 | 9.7 |
| Ca | 1.25 | 1.31 | 1.3 | 1.22 | 0.92 |
| Na | 0.64 | 0.7 | 0.7 | 0.56 | 0.49 |
| K | 0.078 | 0.083 | 0.079 | 0.07 | 0.056 |
| P | 0.108 | 0.095 | 0.085 | 0.13 | 0.102 |
| Ni | 1.6 | 1.2 | 1.02 | 1.344 | 1.07 |
| Co | 0.081 | 0.059 | 0.049 | 0.072 | 0.051 |
| S | 2 | 2.2 | 2.3 | 1.67 | 5.9 |
| C | 0.11 | 0.09 | 0.12 | 0.27 | 3.2 |
| Au | 215 | 162 | 140 | 226.2 | 144 |
| Atomic ratios | H | L | LL | Pułtusk | CI |
| Mg/Si | 0.957 | 0.931 | 0.935 | 0.887 | 1.068 |
| Al/Six104 | 696 | 686 | 655 | 672 | 853 |
| Ca/Six104 | 518 | 496 | 482 | 486 | 614 |
| Fe/Six104 | 8184 | 5845 | 4923 | 6740 | 8717 |
| Ca/Al | 0.74 | 0.72 | 0.74 | 0.72 | 0.72 |
| Ni/Six104 | 453 | 310 | 258 | 367 | 488 |
| CI-normalized atomic ratio | H | L | LL | Pułtusk | CI |
| Mg/Si | 0.90 | 0.87 | 0.88 | 0.83 | 1 |
| Al/Si | 0.82 | 0.81 | 0.77 | 0.79 | 1 |
| Fe/Si | 0.94 | 0.67 | 0.56 | 0.77 | 1 |
| Meteorite(s) | Bulk Density [g/cm3] | Grain Density [g/cm3] | Porosity [%] | n | Notes |
|---|---|---|---|---|---|
| Pułtusk (H5) | 3.30 | 3.41 | 3.22 | 2 | Current study, Archimedean method |
| Pułtusk (H5) | 3.44 | 3.72 | 7.4 | 23 | [31] |
| (3.22–3.77) | (3.54–3.89) | (0.3–12.1) | Helium pycnometry, glass bead method | ||
| Pułtusk (H5) | 3.57 | 1 | [33] | ||
| 3.60 | 1 | 3D laser scanning | |||
| Pułtusk (H5) | 3.47 | 2 | [28] Glass bead method, Archimedean method | ||
| Pułtusk (H5) | 3.60 | 1 | [30] | ||
| 3.56 | 1 | Glass bead method | |||
| Pułtusk (H5) | 3.36–3.8 | 3.55–3.82 | 0.17–11.97 | 11 | [29] Glass bead method, helium pycnometry |
| Košice (H5) | 3.0–3.6 | 3.7–4.3 | 4–20 | 67 | [56] Glass bead method, helium pycnometry |
| H falls | 3.35 ± 0.01 | 3.71 ± 0.01 | 9.5 ± 0.04 | 207 (116) a | [31] Helium pycnometry, glass bead method |
| (2.51–3.77) | (3.18–4.14) | (0–26.6) | |||
| H finds | 3.43 | 3.51 | 2.8 | 79 (63) b | |
| (2.86–4.21) | (3.19–3.79) | (0–10.2) | |||
| H | 3.42 ± 0.19 | 3.72 ± 0.12 | 7.0 c ± 4.9 | [55] | |
| L | 3.37 ± 0.18 | 3.56 ± 0.10 | 5.6 c ± 4.6 | Glass bead method, helium pycnometry |
| Sample | Petrographic Type | Mass [10−3 kg] | Specific Heat Capacity [J/(kg∙K)] | Temperature [K] | References |
|---|---|---|---|---|---|
| Pułtusk | H5 | 0.023 | 699 | 300 | Current study |
| H chondrites | - | - | 714 | [63] | |
| Y-7301 | H4 | 0.016 | 364 | ||
| Y-74647 | H4-5 | 0.0074 | 601 | ||
| Y-74371 | H5-6 | 0.0012 | 535 | ||
| Pułtusk | H5 | 0.023 | 779.5 ± 48.7 | 350 | Current study |
| Gao–Guenie (07C-TPRL) Gao–Guenie -(08) Jilin | H5 | 3.01 | 732.0 ± 7.5 | [61] | |
| H5 | 61.37 | 739.7 ± 27.5 | |||
| H5 | 62.35 | 725.8 ± 13.2 |
| Pułtusk (H5) | Gao–Guenie (H5) | ||||
|---|---|---|---|---|---|
| Temperature | Heat Capacity Cp | Heat Capacity Cp | Diffusivity D × 10−7 | Conductivity K * | |
| (K) | (J/(kg∙K)) | SD | (J/(kg∙K)) | (m2/s) | (W/(m∙K)) |
| 296 | 699.0 | 40.5 | 732.0 | 12.10 | 29.92 |
| 323 | 733.4 | 42.6 | 773.0 | 10.90 | 28.46 |
| 373 | 825.7 | 54.7 | 832.0 | 9.54 | 26.81 |
| 473 | 927.2 | 57.2 | 904.0 | 7.65 | 23.36 |
| 573 | 1031.0 | 75.8 | 950.0 | 6.50 | 20.86 |
| 673 | 1084.9 | 52.6 | 976.0 | 5.89 | 19.42 |
| 773 | 1147.0 | 57.1 | 989.0 | 5.51 | 18.41 |
| Thermophysical Properties | Pułtusk H5 (Current Study) | Pułtusk H5 [40] | Jezersko H4 [40] | Jilin H5 [40] | Gao–Guenie H5 [40] | Barbotan H5 [40] |
|---|---|---|---|---|---|---|
| Cvol(MJ/m3K) 200 K | 1.7 | 1.9 | 1.8 | 1.9 | 1.9 | 1.9 |
| Cvol(MJ/m3K) 300 K | 2.3 | 2.4 | 2.3 | 2.5 | 2.5–2.6 | 2.6 |
| Catom(J/molK) 200 K | 12.1 | 13.4 | 13.1 | - | - | - |
| Catom(J/molK) 300 K | 16.4 | 17.1–17.2 | 17.4 | 18.4 | 17.4–18.0 | - |
| Amean(g/mol) | 23.39 | 24.75 * | 24.68 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuszczek, K.; Wach, R.A. Pułtusk H5 Chondrite—A Compilation of Chemical, Physical, and Thermophysical Data. Geosciences 2025, 15, 438. https://doi.org/10.3390/geosciences15110438
Łuszczek K, Wach RA. Pułtusk H5 Chondrite—A Compilation of Chemical, Physical, and Thermophysical Data. Geosciences. 2025; 15(11):438. https://doi.org/10.3390/geosciences15110438
Chicago/Turabian StyleŁuszczek, Katarzyna, and Radosław A. Wach. 2025. "Pułtusk H5 Chondrite—A Compilation of Chemical, Physical, and Thermophysical Data" Geosciences 15, no. 11: 438. https://doi.org/10.3390/geosciences15110438
APA StyleŁuszczek, K., & Wach, R. A. (2025). Pułtusk H5 Chondrite—A Compilation of Chemical, Physical, and Thermophysical Data. Geosciences, 15(11), 438. https://doi.org/10.3390/geosciences15110438







