The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal–Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese
Abstract
1. Introduction
2. Materials and Methods
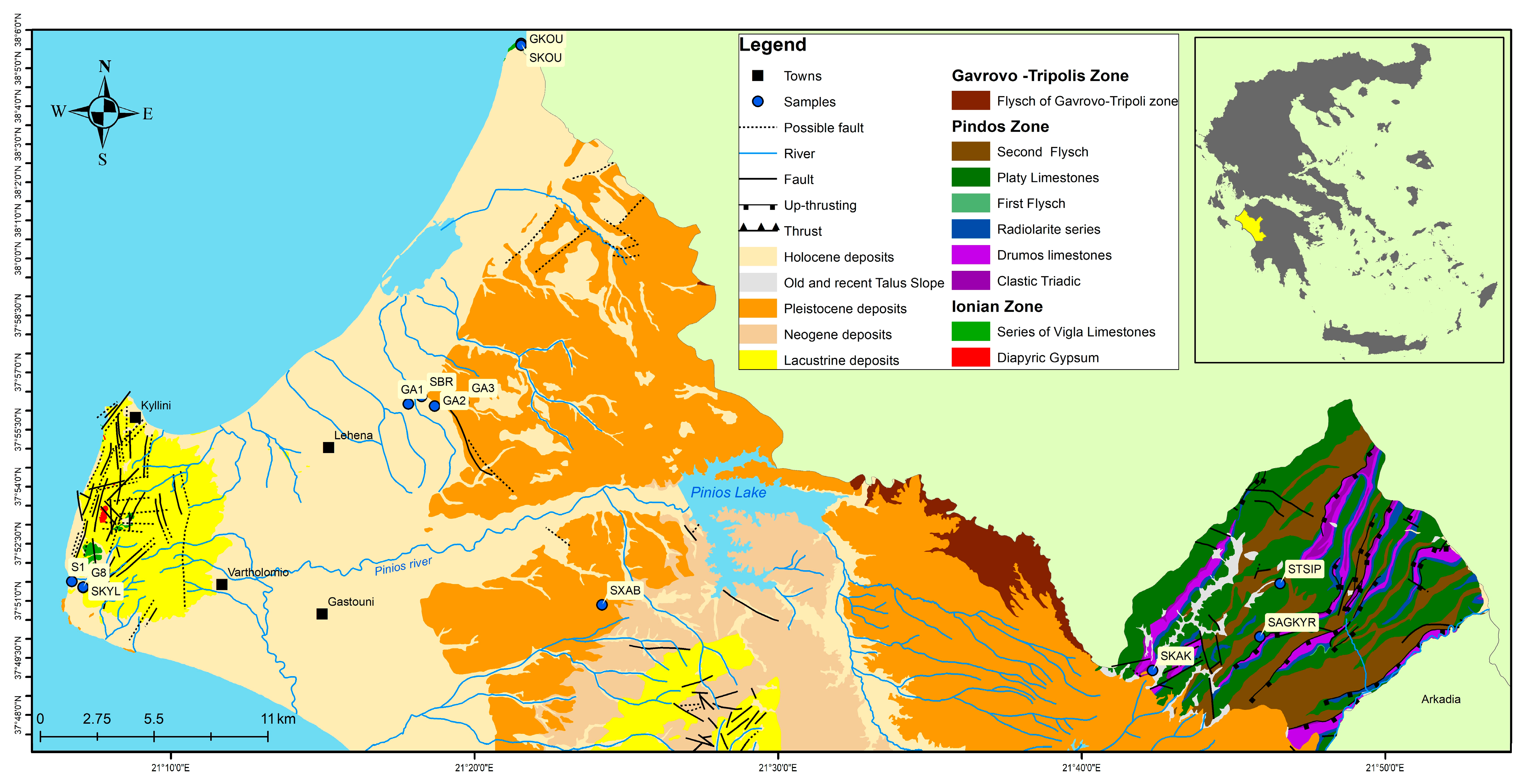
2.1. Study Area
2.2. Methodology
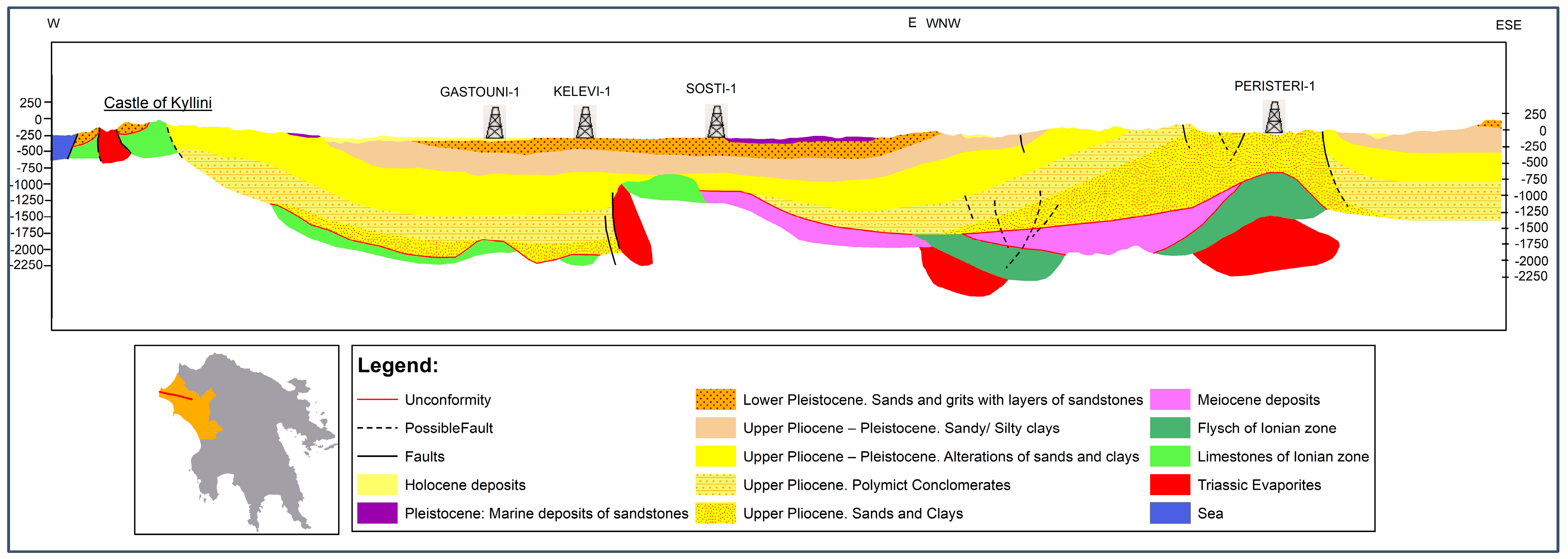
3. Results and Discussion
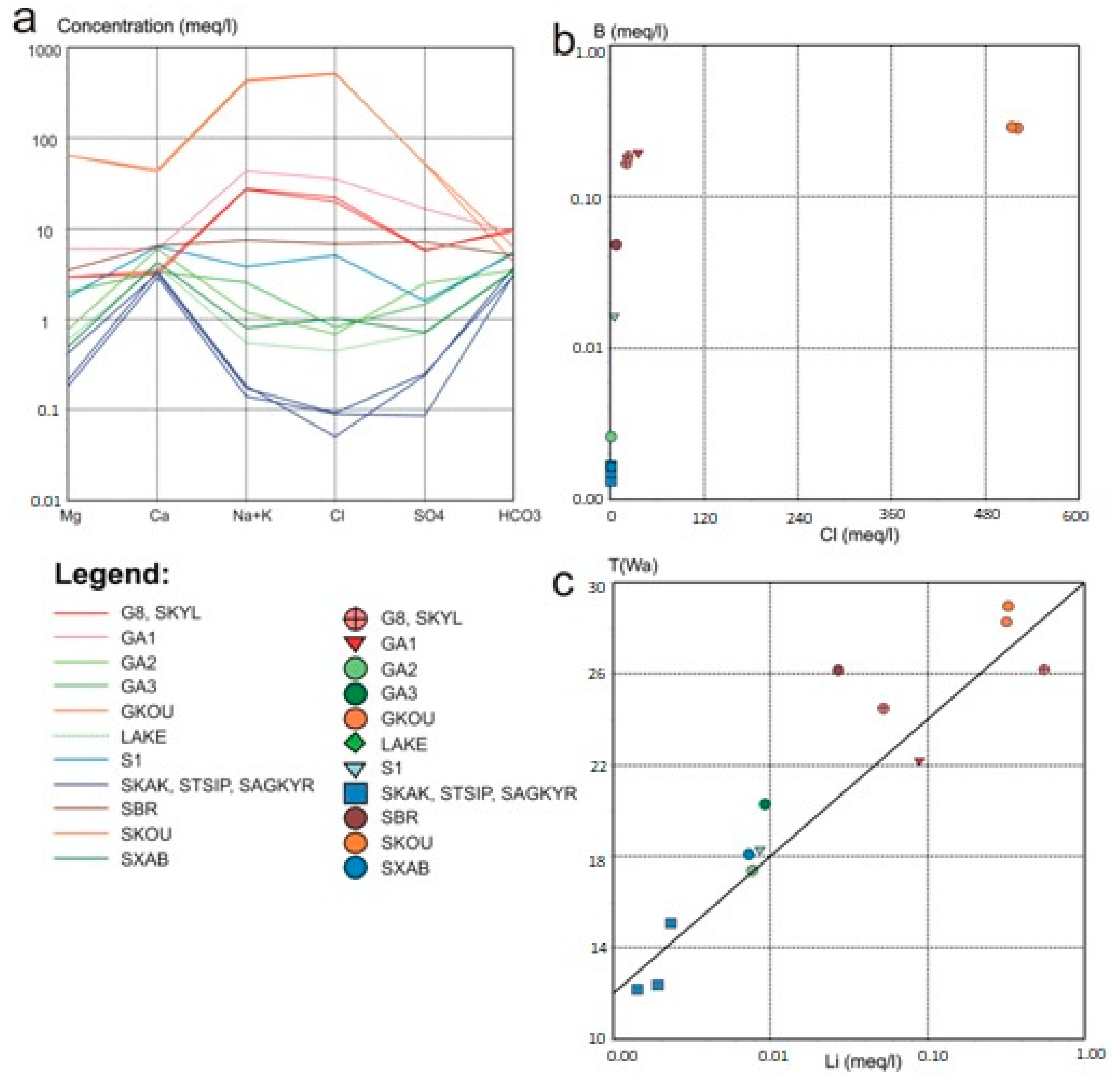
3.1. Hydrochemistry Characteristics
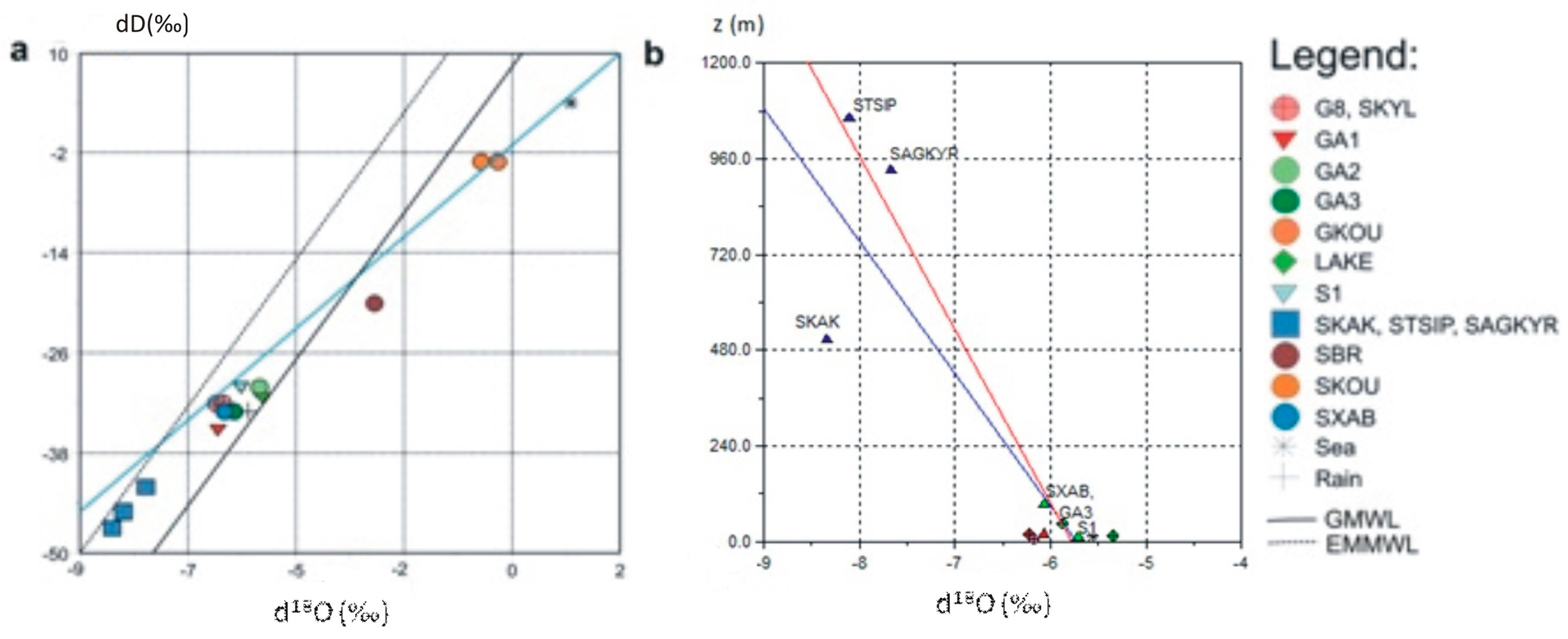
3.2. Isotopic Analysis
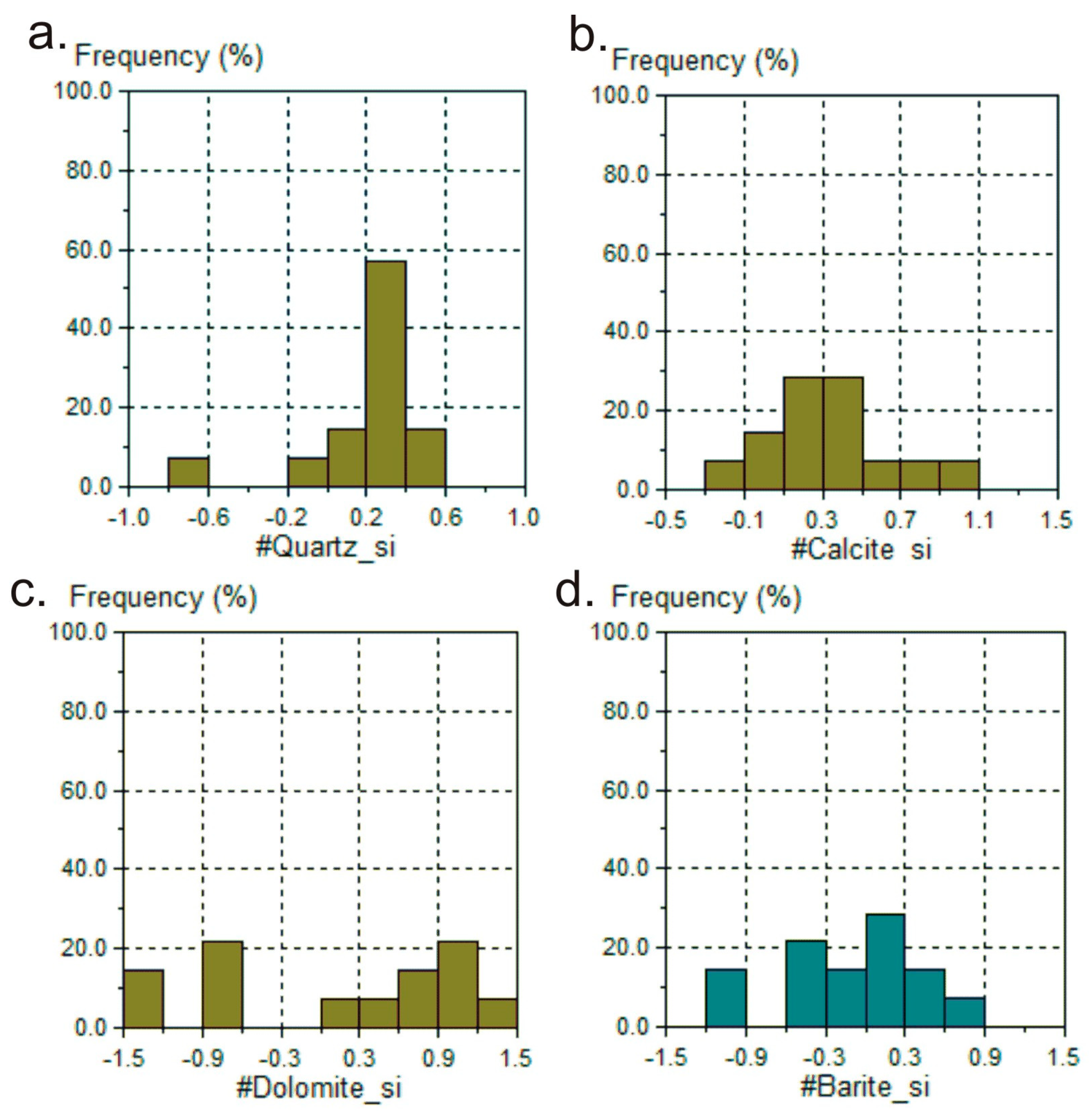
3.3. Index Saturation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakóbczyk-Karpierz, S.; Ślósarczyk, K. Isotopic Signature of Anthropogenic Sources of Groundwater Contamination with Sulfate and Its Application to Groundwater in a Heavily Urbanized and Industrialized Area (Upper Silesia, Poland). J. Hydrol. 2022, 612, 128255. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Agbasi, J.C.; Ayejoto, D.A.; Khan, M.I.; Khan, M.Y.A. Extent of Anthropogenic Influence on Groundwater Quality and Human Health-Related Risks: An Integrated Assessment Based on Selected Physicochemical Characteristics. Geocarto Int. 2023, 38, 2210100. [Google Scholar] [CrossRef]
- Luque-Espinar, J.A.; Chica-Olmo, M. Impacts of Anthropogenic Activities on Groundwater Quality in a Detritic Aquifer in SE Spain. Expo. Health 2020, 12, 681–698. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, Y.; Xia, X.; Li, X.; Lu, H.; Teng, Y.; Wang, J. Anthropogenic Organic Pollutants in Groundwater Increase Releases of Fe and Mn from Aquifer Sediments: Impacts of Pollution Degree, Mineral Content, and pH. Water 2021, 13, 1920. [Google Scholar] [CrossRef]
- Skar, T.; Berg, S.S.; Braathen, A.; Gabrielsen, R.H.; Garrido, I.; Øian, E. Fluid Flow in Faults-Modelling with the Athena Reservoir Simulator; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2003. [Google Scholar]
- Soden, A.M.; Shipton, Z.K.; Lunn, R.J.; Pytharouli, S.I.; Kirkpatrick, J.D.; Do Nascimento, A.F.; Bezerra, F.H.R. Brittle Structures Focused on Subtle Crustal Heterogeneities: Implications for Flow in Fractured Rocks. J. Geol. Soc. 2014, 171, 509–524. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.; Megna, M.; Annunziata, M.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef] [PubMed]
- Voudouris, K.; Yapijakis, C.; Georgaki, Μ.-Ν.; Angelakis, A.N. Historical Issues of Hydrotherapy in Thermal–Mineral Springs of the Hellenic World. Sustain. Water Resour. Manag. 2023, 9, 24. [Google Scholar] [CrossRef]
- Bäumle, R.; Himmelsbach, T.; Noell, U. Hydrogeology and Geochemistry of a Tectonically Controlled, Deep-Seated and Semi-Fossil Aquifer in the Zambezi Region (Namibia). Hydrogeol. J. 2019, 27, 885–914. [Google Scholar] [CrossRef]
- Fronzi, D.; Mirabella, F.; Cardellini, C.; Caliro, S.; Palpacelli, S.; Cambi, C.; Valigi, D.; Tazioli, A. The Role of Faults in Groundwater Circulation before and after Seismic Events: Insights from Tracers, Water Isotopes and Geochemistry. Water 2021, 13, 1499. [Google Scholar] [CrossRef]
- Kim, J.; Ryan, P.; Klepeis, K.; Gleeson, T.; North, K.; Bean, J.; Davis, L.; Filoon, J. Tectonic Evolution of a Paleozoic Thrust Fault Influences the Hydrogeology of a Fractured Rock Aquifer, Northeastern Appalachian Foreland. Geofluids 2014, 14, 266–290. [Google Scholar] [CrossRef]
- Glotov, V.E.; Glotova, L.P. Terrane Tectonics in the Formation of the Groundwater Runoff in the Active Water-Exchange Zone of Mountainous River Valleys in the Cryolithozone. Russ. J. Pac. Geol. 2011, 5, 458–468. [Google Scholar] [CrossRef]
- Das, P.P.; Sahoo, H.K.; Mohapatra, P.P. Assessing the Effects of Regional Tectonic Activity on Groundwater Flow in a Coastal Aquifer in India. Environ. Earth Sci. 2016, 75, 1331. [Google Scholar] [CrossRef]
- Tillner, E.; Langer, M.; Kempka, T.; Kühn, M. Fault Damage Zone Volume and Initial Salinity Distribution Determine Intensity of Shallow Aquifer Salinisation in Subsurface Storage. Hydrol. Earth Syst. Sci. 2016, 20, 1049–1067. [Google Scholar] [CrossRef]
- El Fartati, M.; Hinaje, S.; Yaagoub, D.; El Fellah Idrissi, B.; Amrani, S.; Gharmane, Y.; Laiche, M.; Drissi, Y.; Tagma, T. Influence of the Meso-Cenozoic Tectonics on Groundwater and Surface Water Flows in the Skoura Hydrogeological Basin (Folded Middle Atlas, Morocco). J. Afr. Earth Sci. 2023, 205, 104996. [Google Scholar] [CrossRef]
- Malkovsky, V.; Liebscher, A.; Nagel, T.; Magri, F. Influence of Tectonic Perturbations on the Migration of Long-Lived Radionuclides from an Underground Repository of Radioactive Waste. Environ. Earth Sci. 2022, 81, 537. [Google Scholar] [CrossRef]
- Olaka, L.A.; Kasemann, S.A.; Sültenfuß, J.; Wilke, F.D.H.; Olago, D.O.; Mulch, A.; Musolff, A. Tectonic Control of Groundwater Recharge and Flow in Faulted Volcanic Aquifers. Water Resour. Res. 2022, 58, e2022WR032016. [Google Scholar] [CrossRef]
- Cambi, C.; Mirabella, F.; Petitta, M.; Banzato, F.; Beddini, G.; Cardellini, C.; Fronzi, D.; Mastrorillo, L.; Tazioli, A.; Valigi, D. Groundwater Flow Changes in Response to Extensional Earthquakes: A Case Study from the 2016–2017 Seismic Sequence in Central Italy; EGU: Vienna, Austria, 2023. [Google Scholar]
- Martínez, M.; Hornero, J.; Trujillo, C. Physico-Chemical Evolution of Groundwater in Tectonically Active Areas. Application to the Leana Hot Spring (Murcia Region, SE Spain). J. Seism. 2017, 21, 349–366. [Google Scholar] [CrossRef]
- Sandiford, M.; Lawrie, K.; Brodie, R.S. Hydrogeological Implications of Active Tectonics in the Great Artesian Basin, Australia. Hydrogeol. J. 2020, 28, 57–73. [Google Scholar] [CrossRef]
- Christodoulou, G. About the Age despite the Village of Kastro (NW Peloponnese) of Limestones. Bull. Geol. Soc. Greece 1968, 2, 121–134. [Google Scholar]
- Dimopoulos, G. Patra D Hydrogeological and Hydrochemical Research in Region of Kyllini’s Bath. Bull. Geol. Soc. Greece Athens 1988, 3, 37–47. [Google Scholar]
- Kallergis, G.; Lambrakis, N. Contribution à l’étude Des Sources Thermominerales de Grece- Les Sources Thermominerals de Kyllini Par Raport Au Regime Hydrothermale Du Peloponnes Occidental. Steir. Beitr. Z. Hydrogeol. 1991, 44, 207–220. [Google Scholar]
- Yapijakis, C. Hippocrates of Kos, the Father of Clinical Medicine, and Asclepiades of Bithynia, the Father of Molecular Medicine. In Vivo 2009, 23, 507–514. [Google Scholar]
- Angelakis, A.N.; Antoniou, G.P.; Yapijakis, C.; Tchobanoglous, G. History of Hygiene Focusing on the Crucial Role of Water in the Hellenic Asclepieia (i.e., Ancient Hospitals). Water 2020, 12, 754. [Google Scholar] [CrossRef]
- Kelletat, D.; Kowalczyk, G.; Schröder, B.; Winter, K.-P. A Synoptic View on the Neotectonic Development of the Peloponnesian Coastal Regions. Z. Der Dtsch. Geol. Ges. 1976, 127, 447–465. [Google Scholar] [CrossRef]
- Saravanavel, J.; Ramasamy, S.M. Active Tectonics and Its Impacts over Groundwater Systems in the Parts of Tamil Nadu, India. Arab. J. Geosci. 2016, 9, 429. [Google Scholar] [CrossRef]
- Povara, I.; Simion, G.; Marin, C. Thermo-Mineral Waters from the Cerna Valley Basin (Romania). Stud. UBB Geol. 2008, 53, 41–54. [Google Scholar] [CrossRef]
- Kifouche, R.; Bouaıcha, F.; Bouteraa, O. Impact of Thermal Water on Environment Case Study of Mila and Guelma Region, Algeria. Bull. Min. Res. Exp. 2023, 171, 143–157. [Google Scholar] [CrossRef]
- Pavić, M.; Briški, M.; Pola, M.; Borović, S. Hydrogeochemical Research of Thermal Waters from Topusko, Croatia; Copernicus Meetings: Vienna, Austria, 2023. [Google Scholar]
- Mahala, S.C. Geochemistry of Thermal Water. In Geology, Chemistry and Genesis of Thermal Springs of Odisha, India; Mahala, S.C., Ed.; Springer International Publishing: Cham, Germany, 2019; pp. 49–73. ISBN 978-3-319-90002-5. [Google Scholar]
- Kazakis, N. Groundwater Pollution Risk Assessment in Anthemountas Basin. Ph.D. Thesis, Department of Geology, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2013. [Google Scholar]
- Kumar, C.P. Hydrological Studies Using Isotopes. Int. J. Innov. Res. Dev. 2013, 2, 8–15. [Google Scholar]
- Liu, F.; Song, X.; Yang, L.; Zhang, Y.; Han, D.; Ma, Y.; Bu, H. Identifying the Origin and Geochemical Evolution of Groundwater Using Hydrochemistry and Stable Isotopes in the Subei Lake Basin, Ordos Energy Base, Northwestern China. Hydrol. Earth Syst. Sci. 2015, 19, 551–565. [Google Scholar] [CrossRef]
- Aubouin, J. Geosyclines, Developments in Geotectonic; Elsevier: Amsterdam, The Netherlands, 1965; Volume 1, pp. 43–65. [Google Scholar]
- Kokinou, E.; Kamberis, E.; Vafidis, A.; Monopolis, D.; Ananiadis, G.; Zelilidis, A. Deep seismic reflection data from offshore western greece: A new crustal model for the ionian sea. J. Pet. Geol. 2005, 28, 185–202. [Google Scholar] [CrossRef]
- Kamberis, E. Geological and Oil Study of the NW Peloponnese. Ph.D. Thesis, University of Patras, Patra, Greece, 1987. [Google Scholar]
- Maroukian, H.; Gaki-Papanastassiou, K.; Papanastassioi, D.; Palyvos, N. Geomorphological Observations in the Coastal Zone of Kyllini Peninsula, NW Peloponnesus-Greece, and Their Relation to the Seismotectonic Regime of the Area. J. Coast. Res. 2000, 16, 853–863. [Google Scholar]
- Aubouin, J. Contribution a l’ Etude Geologique de La Grece Septentrionale: Les Confins de l’ Epire et de La Thessalie. Ann. Geol. Des. Pays Hell. 1959, 10, 1–525. [Google Scholar]
- Mariolakos, I.; Papanikolaou, D. The Neogene Basins of the Aegean Arc from the Paleogeographic and the Geodynamic Point of View. Proc. Int. Symp. Hell. Arc Trench (HEAT) Athens 1981, 1, 383–399. [Google Scholar]
- Rusch, K.; Stümpel, H.; Gauß, W.; Müth, S.; Sokolicek, A.; Kissas, K.; Rabbel, W. Geological Challenges of Archaeological Prospecting: The Northern Peloponnese as a Type Location of Populated Syn-Rift Settings. Remote Sens. 2020, 12, 2450. [Google Scholar] [CrossRef]
- Athanassas, C.; Fountoulis, I. Quaternary Neotectonic Configuration of the Southwestern Peloponnese, Greece, Based on Luminescence Ages of Marine Terraces. J. Earth Sci. 2013, 24, 410–427. [Google Scholar] [CrossRef]
- Degnan, P.J.; Robertson, A.H.F. Synthesis of the Tectonic–Sedimentary Evolution of the Mesozoic–Early Cenozoic Pindos Ocean: Evidence from the NW Peloponnese, Greece. Geol. Soc. Lond. Spec. Publ. 2006, 260, 467–491. [Google Scholar] [CrossRef]
- Konstantinou, K.I.; Evangelidis, C.P.; Melis, N.S. The 8 June 2008 Mw 6.4 Earthquake in Northwest Peloponnese, Western Greece: A Case of Fault Reactivation in an Overpressured Lower Crust? Bull. Seismol. Soc. Am. 2011, 101, 438–445. [Google Scholar] [CrossRef]
- Wardell, N. The Structural Framework of the Peloponnese Continental Margin from Zakynthos to Pylos from Seismic Reflection and Morpho-Bathymetric Data. Boll. Geof. Teor. Appl. 2014, 55, 343–367. [Google Scholar] [CrossRef]
- Kowalczyk, G.; Winter, K.P.; Reisch, L. Die Geologische Entwicklung Der Kyllini-Halbinsel Im Neogen Und Quartär (West-Peloponnes, Griechenland). Z. Der Dtsch. Geol. Ges. 1979, 130, 323–346. [Google Scholar]
- Mariolakos, I. Neotectonic Evolutation of the Kyllini Peninsula (NW. Peloponnesus). Bull. Geol. Soc. Greece Athens 1991, 3, 163–176. [Google Scholar]
- Piper, D.J.W. Sedimentology and Tectonic Setting of the Pindos Flysch of the Peloponnese, Greece. Geol. Soc. Lond. Spec. Publ. 2006, 260, 493–505. [Google Scholar] [CrossRef]
- Kamberis, E.; Sotiropouos, S.; Aximniotou, O.; Tsaila-Monopolis, S.; Ioakim, C. Late Cenozoic Deformation of the Gavrovo and Ionian Zones in NW Peloponnesos (Western Greece). Ann. Di Geofis. 2000, 3, 905–919. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Sunrise Valley Dr, VA, USA, 1999.
- Underhill, J.R. Triassic Evaporites and Plio-Quaternary Diapirism in Western Greece. J. Geol. Soc. 1988, 145, 269–282. [Google Scholar] [CrossRef]
- Stamatakis, M. Τhe Distribution of Βoron in Fumes-Thermometallic Springs-Marine Evaporites and in Volcanic Sedimentary Cenozoic Formations of Greece. Ph.D. Thesis, University of Athens, Athens, Greece, 1986. [Google Scholar]
- Fidelibus, M.D.; Tulipano, L. Major and Minor Ions as Natural Tracers in Mixing Phenomena in Coastal Carbonate Aquifers of the Apulia. In Proceedings of the 11th Salt Water Intrusion Meeting, Gdansk, Poland, 17–22 June 2018. [Google Scholar]
- Edmunds, W.M.; Smedley, P.L. Residence Time Indicators in Groundwater: The East Midlands Triassic Sandstone Aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Uhlman, K. The Geochemistry of Boron in a Landfill Monitoring Program. Groundw. Monit. Remediat. 1991, 11, 139–143. [Google Scholar] [CrossRef]
- Li Vigni, L.; Daskalopoulou, K.; Calabrese, S.; Kyriakopoulos, K.; Bellomo, S.; Brusca, L.; Brugnone, F.; D’Alessandro, W. Characterization of Trace Elements in Thermal and Mineral Waters of Greece. Env. Sci. Pollut. Res. 2023, 30, 78376–78393. [Google Scholar] [CrossRef]
- Li Vigni, L.; Daskalopoulou, K.; Calabrese, S.; Kyriakopoulos, K.; Parello, F.; Brugnone, F.; D’Alessandro, W. Geochemical Characterisation of the Thermo-Mineral Waters of Greece. Env. Geochem. Health 2022, 44, 2111–2133. [Google Scholar] [CrossRef]
- Li, J.; Sagoe, G.; Wang, X.; Yang, Z. Assessing the Suitability of Lithium-Related Geothermometers for Estimating the Temperature of Felsic Rock Reservoirs. Geothermics 2021, 89, 101950. [Google Scholar] [CrossRef]
- Lambrakis, N.; Kallergis, G. Contribution to the Study of Greek Thermal Springs: Hydrogeological and Hydrochemical Characteristics and Origin of Thermal Waters. Hydrogeol. J. 2005, 13, 506–521. [Google Scholar] [CrossRef]
- Dotsika, E.; Poutoukis, D.; Michelot, J.L.; Kloppmann, W. Stable Isotope and Chloride, Boron Study for Tracing Sources of Boron Contamination in Groundwater: Boron Contents in Fresh and Thermal Water in Different Areas in Greece. Water Air Soil. Pollut. 2006, 174, 19–32. [Google Scholar] [CrossRef]
- Sekuła, K.; Rusiniak, P.; Wątor, K.; Kmiecik, E. Hydrogeochemistry and Related Processes Controlling the Formation of the Chemical Composition of Thermal Water in Podhale Trough, Poland. Energies 2020, 13, 5584. [Google Scholar] [CrossRef]
- Oudeika, M.S.; İlkimen, E.M.; Taşdelen, S.; Aydin, A. Distinguishing Groundwater Flow Paths in Fractured Rock Aquifers Formed under Tectonic Stress Using Geophysical Techniques: Cankurtaran Basin, Denizli, Turkey. Int. J. Environ. Res. 2020, 14, 567–581. [Google Scholar] [CrossRef]
- Millar, C.; Janzen, K.; Nehemy, M.F.; Koehler, G.; Hervé-Fernández, P.; Wang, H.; Orlowski, N.; Barbeta, A.; McDonnell, J.J. On the Urgent Need for Standardization in Isotope-Based Ecohydrological Investigations. Hydrol. Process. 2022, 36, e14698. [Google Scholar] [CrossRef]
- Zang, C.; Dame, J.; Nüsser, M. Hydrochemical and Environmental Isotope Analysis of Groundwater and Surface Water in a Dry Mountain Region in Northern Chile. Environ. Monit. Assess. 2018, 190, 334. [Google Scholar] [CrossRef]


| STSIP | SAGKYR | SKAK | SXAB | GA2 | S1 | GA3 | LAKE | GA1 | SBR | SKYL | G8 | SKOU | GKOU | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tw | 12.4 | 15.1 | 12.2 | 18.1 | 17.4 | 18.3 | 20.3 | 28.7 | 22.2 | 26.2 | 24.5 | 26.2 | 29 | 28.3 |
| Eh | 69 | n.d. | 64 | 67 | 1 | −26 | −34 | n.d. | −42 | −48 | −26 | −37 | −9 | −11 |
| pH | 7.69 | 7.38 | 7.84 | 7.32 | 7.04 | 7.42 | 7.58 | 8.18 | 7.78 | 7.57 | 7.38 | 7.58 | 6.88 | 6.93 |
| EC | 347.1 | 460.0 | 264.1 | 717.0 | 796.0 | 1490.0 | 763.0 | 431.5 | 6720.0 | 2120.0 | 4175.0 | 4275.0 | 51,600 | 47,400 |
| CO2 | n.d. | n.d. | 98 | n.d. | 105 | 93 | 91 | 165 | 263 | n.d. | 104 | 178 | 218 | 168 |
| HCO3− | 185.4 | 225.7 | 189.1 | 212.3 | 211.6 | 324.5 | 340.4 | 206.2 | 559.9 | 311.1 | 612.4 | 568.5 | 381.9 | 263.5 |
| K+ | 0.84 | 1.4 | 0.39 | 0.68 | 2.59 | 3.92 | 5.3 | 2.16 | 14.46 | 5.95 | 13.08 | 13.32 | 322 | 322 |
| Na+ | 3.43 | 3.44 | 3.05 | 18.17 | 26.06 | 84.65 | 56.30 | 11.28 | 986.00 | 168.80 | 622.20 | 636.20 | 9570.0 | 9870.0 |
| Mg2+ | 5.11 | 2.54 | 2.18 | 5.94 | 9.28 | 21.5 | 24.5 | 7.16 | 72.1 | 42.45 | 35.25 | 35.45 | 780.50 | 781.50 |
| Ca2+ | 64.00 | 68.50 | 58.00 | 85.00 | 119.50 | 128.00 | 66.50 | 77.50 | 119.50 | 131.00 | 62.00 | 69.00 | 852.00 | 905.00 |
| NH4+ | 0.093 | 0.008 | 0.014 | 0 | 0.008 | 0.042 | 1.866 | 0.059 | 13.215 | 0.685 | 2.373 | 2.606 | 3.415 | 3.35 |
| NO3− | 3.00 | 4.00 | 8.00 | 4.00 | 127.00 | 1.00 | 5.00 | 4.00 | 4.40 | 2.40 | 13.00 | 18.00 | 5.20 | 12.20 |
| NO2− | 0.019 | 0.047 | 0.01 | 0.009 | 0.058 | 0.003 | 0.047 | 0.055 | 0.0106 | 0.00 | 0.002 | 0.004 | 0.059 | 0.172 |
| SO42− | 12.10 | 11.70 | 4.20 | 34.70 | 121.00 | 77.30 | 70.2 | 34.10 | 790.00 | 345.00 | 277.00 | 282.00 | 2560.0 | 2490.0 |
| PO4− | 0.034 | 0.084 | 0.094 | 0.289 | 0.08 | 0.061 | 0.077 | 0.027 | 0.743 | 0.072 | 0.215 | 0.077 | 0.095 | 0.06 |
| SiO2 | 7.50 | 5.90 | 4.70 | 18.20 | 20.50 | 15.40 | 16.40 | 2.00 | 13.20 | 16.80 | 17.10 | 15.70 | 13.10 | 13.10 |
| F− | 0.24 | 0.06 | 0.05 | 0.08 | 0.16 | 0.19 | 0.35 | 1.25 | 0.74 | 1.37 | 1.32 | 2.36 | 1.73 | |
| Cl− | 3.3 | 1.8 | 3.2 | 36.0 | 24.4 | 182.0 | 29.2 | 16.0 | 1260.0 | 244.0 | 710.0 | 790.0 | 18,200 | 18,460 |
| B (*) | 18.0 | 14.4 | 16.3 | 17.9 | 28.3 | 175.4 | 152.2 | 33.4 | 2115.0 | 522.8 | 1805.9 | 1996.6 | 3143.0 | 3095.1 |
| Ba (*) | 11.3 | 125.7 | 27.4 | 30.1 | 11.8 | 59.1 | 40.7 | 33.6 | 105.0 | 78.7 | 69.0 | 56.3 | 57.7 | 65.1 |
| Fe (*) | 74.0 | 84.0 | 72.6 | 110.1 | 99.3 | 168.5 | 99.3 | 93.1 | 486.1 | 176.2 | 237.3 | 244.0 | 0.0 | 0.0 |
| Mn (*) | 0.9 | 0.4 | 0.4 | 551.9 | 0.2 | 69.2 | 182.7 | 36.2 | 14.4 | 220.3 | 13.1 | 10.5 | 378.4 | 363.2 |
| Li (*) | 1.9 | 2.3 | 1.4 | 7.2 | 7.6 | 8.5 | 9.1 | 4.1 | 88.0 | 26.9 | 52.0 | 54.7 | 325.9 | 317.5 |
| Sr (*) | 81.0 | 261.1 | 123.3 | 320.7 | 354.5 | 773.5 | 573.5 | 244.4 | 3610.9 | 1377.4 | 2002.5 | 2110.4 | 11,321 | 11,232 |
| Min | Max | Average | StDev | |
|---|---|---|---|---|
| S2− * | 19,200 | 33,100 | 25,840 | 3813.19 |
| Ph | 7.18 | 7.44 | 7.267 | 0.08 |
| Tw | 24.1 | 28.7 | 26.19 | 1.38 |
| EC | 3869 | 4495 | 4245.9 | 220.05 |
| CO2 | 98.4 | 395 | 213.04 | 112.93 |
| Alk | 480 | 680 | 558.3 | 70.19 |
| HCO3− | 585.6 | 829.6 | 681.126 | 85.63 |
| NH4+ | 7.825 | 9.85 | 8.485 | 0.62 |
| NO3− | 1 | 5 | 2.6 | 1.51 |
| NO2− | 0.008 | 0.087 | 0.0295 | 0.02 |
| SO42− | 177 | 262 | 219.5 | 26.38 |
| PO42− | 0.04 | 1.63 | 0.571 | 0.46 |
| Cl− | 890 | 1100 | 968 | 78.29 |
| K+ | 14.1 | 14.55 | 14.325 | 0.16 |
| Na+ | 820 | 900 | 860 | 29.81 |
| Mg2+ | 16.3 | 41.4 | 29.8 | 7.60 |
| Ca2+ | 56 | 100.4 | 74.12 | 13.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulou, V.; Pyrgaki, A.; Zagana, E.; Pouliaris, C.; Kazakis, N. The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal–Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese. Geosciences 2024, 14, 205. https://doi.org/10.3390/geosciences14080205
Stavropoulou V, Pyrgaki A, Zagana E, Pouliaris C, Kazakis N. The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal–Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese. Geosciences. 2024; 14(8):205. https://doi.org/10.3390/geosciences14080205
Chicago/Turabian StyleStavropoulou, Vasiliki, Anastasia Pyrgaki, Eleni Zagana, Christos Pouliaris, and Nerantzis Kazakis. 2024. "The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal–Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese" Geosciences 14, no. 8: 205. https://doi.org/10.3390/geosciences14080205
APA StyleStavropoulou, V., Pyrgaki, A., Zagana, E., Pouliaris, C., & Kazakis, N. (2024). The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal–Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese. Geosciences, 14(8), 205. https://doi.org/10.3390/geosciences14080205







