Abstract
The permanence of a buried body in soil always induces the formation of a decomposition island that can serve as a significant recording location to understand how the persistence of a clandestine grave affects soil. This study aims to analyze the elemental exchange from buried bodies to soil, with a focus on phosphorus content, and to determine the effects of environmental factors on its persistency. The experiment was carried out using eleven swine carcasses buried in an open site (northern Italy). The analyses were performed using the Olsen P method, which allowed for a recognition of the trend of the amount of phosphorus over time, due to the decomposition of phospholipids, followed by the transfer of the element from bone to soil. Additionally, microanalyses performed using a scanning electron microscope (SEM-EDS) on two different soil sample specimens (i.e., “dust” and “plug”) allowed for the identification of numerous phosphatic features (i.e., coatings, infillings, impregnations, and organo-mineral associations), which are the result of the interaction between soil and body fluids and can thus be used as indicators of the former presence of decomposing body (even in its absence). The ultramicroscopic analysis also shows increasing and decreasing amounts of P2O5 over time in the soil, which could be related to environmental conditions (i.e., soil moisture), due to the leaching of phosphorus induced by the percolation of natural rainwater. The study underlines the potential use of these methods to evaluate the possibility of a cadaver–soil linkage and of assessing the burial in the soil for a variable period. Moreover, the study may aid in analyzing the dynamics of phosphorus migration from buried bodies to soil during and after the decomposition process.
1. Introduction
Many homicide victims are buried after death. Therefore, in forensic applications, the research of clandestine graves is not an infrequent event [1,2], nor is the need to identify a trench in the ground as an actual burial site from which the skeleton, after decomposition, has been removed (common in organized crime). The discovery and exhumation of a decomposing corpse presents to forensic scientists many questions to solve. In order both to precisely analyze every proof and provide the methods for studying and understanding the decomposition of human remains in soils, a multidisciplinary approach may aid the “environmental profiling” of a crime site [3].
The approaches used in forensic investigation usually deal primarily with the cadaver and associated items rather than the grave itself, whereas geoforensic and archaeoforensic approaches not only provide useful guides to the localization of clandestine graves but also allow for the analysis of different environmental situations and every aspect of the inhumation sites. Indeed, soils have complex biological, chemical, physical, and mineralogical properties that change with time [4]. Thus, geopedology not only contributes to identifying the current or previous location of a clandestine grave and to helping to link a suspect to a crime site, but it can also help clarify events and changes imposed by the burial of the corpse to the pedo-environment [5]. Hence, soil can play an active role in exchanging material with the corpse during decomposition, along with acting as an archive of evidence and illustrate the environmental conditions that could have concerned a criminal event [6]. Factors such as soil moisture [7], soil chemical and physical properties [8], microbial activity [9], and pedoclimatic conditions [10] can affect the interaction between buried remains and soil and may also have been recorded [11]. In particular, different studies of experimental burials have provided a relevant quantity of data about the migration of chemical elements from the corpse to the surrounding area, recognizing in phosphorus content one of the most suggestive clues of the presence of a dead body in soil, even after a consistent postmortem interval [12,13,14,15].
The present study focused on ten experimental burials of pigs in a uniform area [8], in order to obtain new data on the environmental responses to the inhumation over different time intervals. Through this work, some relevant data regarding the bone structure changes during time have been collected and published [11]. The study was part of a wider project, in which an interdisciplinary Italian team, composed of Earth scientists, biologists, anthropologists, and legal medical doctors was involved. Therefore, in the study area, the botanical data were periodically recorded to verify possible modifications induced by the inhumation [16,17] and periodic analyses of trenches were performed using ground penetrating radar and geo-electrical measurements [18,19]. In addition, geopedological analyses (i.e., physical, chemical, mineralogical, and micropedological) were carried out before the inhumations and after each exhumation to evaluate the soil characteristics at different burial intervals [14,20,21,22]. Decomposition processes were also analyzed by performing an autopsy for each specimen [9,23,24] and entomological samples were extracted from corpses for the postmortem interval reconstruction [25]. As shown in the literature [9,10,11,12,13,14], the analysis of chemical elements related to decomposition processes are an extremely valid tool to reconstruct a criminal act, especially when it concerns a body buried in soil. Nevertheless, only a few published works mention phosphorus (in the form of available phosphorus and phosphates) as a key or, if they do, they conduct experiments lasting a too short period of time [13].
Therefore, the present work is strictly focused on the chemical (Olsen P method) and ultramicroscopic (SEM-EDS) characterization of the phosphorus attributable to the impregnation of body fluids on soils sampled under the eleven swine carcasses, buried for increasing intervals of time (until 924 days (132 weeks)) in two adjacent areas, in order to reveal the environmental control on their persistency and also to evaluate the potential of this approach to the forensic subject of clandestine burials. Moreover, it has been considered a promising starting point for the design of a more recent project, aimed at the observation of the complex dynamic of the decomposition processes of a body buried in soil. The results here collected showed the interesting evolution of the concentration and persistence of phosphorus, attributable to the biochemical disturbance of a decomposing body in soil and, therefore, they have been considered a valid tool to investigate it.
2. Materials and Methods
2.1. Experimental Design
Eleven swine carcasses (Sus domesticus) were used to reproduce the decomposition of a buried human body, considering their similarity to human in weight, fat-to-muscle ratio, and hair coverage [26].
Their inhumation was carried out at the end of May 2009 in two areas located in the same site, named site A and B. Ten trenches, five for each area, were excavated by means of an excavator, at different depths: 90 cm for site A and 80 cm for site B. The sampling depths represent the average profundity of clandestine graves encountered in forensic scenarios (e.g., intermediate between “shallow” depths of 50–60 cm and “deep” depths of 100–110 cm [5]).
Each trench was filled with one specimen weighing between 70 and 90 kg, except for subjects 4 and 5, which were inhumed in the same grave to simulate a twin burial (Table 1), for a total of ten graves. Finally, the soil and sediments removed for the digging of the trenches were reinstated, in order to completely cover the corpses.

Table 1.
Summary of the weight of each swine corpse at the moment of the inhumations. The * indicates the two samples that were inhumed together to simulate a twin burial.
The exhumation phases provided for the opening of a single couple of graves (one from site A and one from site B) at each PBI, for a total of five increasing time intervals (so five couples of graves). After each exhumation, the soil was sampled and the grave was definitively abandoned. Therefore, even the last soil sampling was carried out in a grave that was not affected by disturbance after the inhumation of the pig.
The experiment was conducted for a total of 924 days (132 weeks, from May 2009 to December 2011) and the exhumation of the specimens was carried out at five time intervals (47, 207, 396, 711, and 923 days for site A and 42, 235, 383, 726, and 924 days for site B) to provide a range of varying burial times for the groups of subjects.
Macroscopic analyses were also performed for each specimen at different decomposition stages [23] according to Megyesi et al. [27,28].
2.2. Experimental Area
The experimental site was located in the Ticino River Regional Park in northern Italy (45°23′ N 8°50′ E) at 95 m above sea level. The climate is sub-oceanic with the highest rainfall in spring and autumn and with an average rainfall of 1050 mm/year [20,29,30].
Ten trenches were excavated at different depths in two areas, both located in the Park, with distinct vegetation covers: site A, where the graves were 90 cm deep, was an open area characterized by dry grasslands, dominated by grasses and sedges (Bromus sterilis, Aira caryophyllea, Koeleria pyramidata, Carex caryophyllea, and Vulpia myuros) [16]; site B, where the graves were 80 cm deep, had vegetation cover that was represented by acidophilic peduncolate oak (Quercus robur) woodlands, sometimes replaced by degraded communities of Robinia pseudoacacia and by highly invasive Prunus serotina [16].
The sampling depths represent the average profundity of clandestine graves encountered in forensic scenarios (e.g., intermediate between “shallow” depths of 50–60 cm and “deep” depths of 100–110 cm [5]).
The exhumation phases provided for the opening of a single couple of graves (one from site A and one from site B) at each PBI, for a total of five increasing time intervals (so five couples of graves). After each exhumation, the soil was sampled and the grave was definitively abandoned. Therefore, even the last soil sampling was carried out in a grave that was not affected by disturbance after the inhumation of the pig.
2.3. Soil Description
The site is characterized by poorly developed soils on coarse fluvial deposits composed by crystalline rocks [30], currently classified as Typic Udorthents sandy–skeletal, mixed, and mesic [31]. They have high permeable horizons with rapid drainage and sandy texture in the first meter; they are non-calcareous soils, with a pH of about 4.5–5.5 at the surface and 5.6–6.6 at 50 cm depth. The base–cation saturation ratio is very low, as is the AWC (Available Water Capacity) [30].
Moreover, the field activities of the present work included the descriptions of the soil profiles, along with the collection of samples from their horizons, before the inhumations [20,29]. The soil profile description for the soils in the experimental area is:
- O
- Organic material lying under herbaceous vegetation.
- A1
- Dark brown (7.5 YR 3/2); loamy and sandy-loam, weak fine granular structure; very porous; clear wavy boundary.
- A2
- Dark brown (7.5 YR 4/2); loamy and sandy-loam, weak fine granular structure; very porous; clear wavy boundary.
- C1
- Dark brown (7.5 YR 4/2); sandy loamy, frequent centimetric clasts (2–5 cm), weak structure; very porous, frequent roots; clear linear boundary.
- C2
- Brown (7.5 YR 5/2); sand, frequent centimetric clasts (5–10 cm), loose; clear linear boundary.
- C3
- Brown (7.5 YR 5/2); sand, overlapping levels of decimeter and centimeter clasts, loose; clear linear boundary.
- C4
- Brown (7.5 YR 5/2); sand, dominant decimeter and centimeter clasts, fining upward, loose; lower boundary not exposed.
2.4. Analytical Procedures
The soils were sampled, in the form of bulk samples, under the carcasses of pigs during the exhumation activities in both areas (A site and B site), approximately between 100 and 125 cm depth. A preliminary analysis consisted of the application of the “Olsen P” or “sodium bicarbonate soil test phosphorus (P)” method [32] to estimate the available phosphorus in soils by extraction with sodium bicarbonate [33].
In addition, SEM analyses were used to measure the mean phosphate concentration. For this purpose, a seven-point analysis was carried out on the surface of each sample, in correspondence of some features detected using the scanning electron microscope. Exclusively for these analyses, each soil specimen was extracted as a bulk sample, in the form of:
- −
- A “dust” specimen (Figure 1a), collected using the particular adhesive stripe for SEM analyses—the resulting specimens are easy to prepare but the EDS microanalyses are less accurate, due to the variable incidence angle of the electron beam, as described in [32];
 Figure 1. (a) Soil samples collected as “dust” specimens. (b) Soil samples extracted as “plug” specimens. The soil samples have been collected below each carcass, approximately between 100 and 125 cm depth.
Figure 1. (a) Soil samples collected as “dust” specimens. (b) Soil samples extracted as “plug” specimens. The soil samples have been collected below each carcass, approximately between 100 and 125 cm depth. - −
- A “plug” specimen (Figure 1b), subsampled in a plastic box, then impregnated with epoxy resin and finally dry polished by hand in order to obtain a flat surface, flawlessly suitable for EDS analyses—in this case the specimen preparation is longer (i.e., weeks), but the EDS microanalyses are extremely precise.
All the samples (“dust” and “plug”) were analyzed with a Cambridge 360 scanning electron microscope (SEM), imaging both secondary and back-scattered electrons to return a 3D compositional image of the objects detected on the specimen’s surface. The elemental analyses were performed using an energy dispersive X-ray analysis (EDS Link Isis 300) requiring carbon-coated samples [34]: energy dispersive X-ray spectroscopy with an accelerating voltage of 20 kV, filament intensity 1.70 A, and probe intensity of 280 pA. The analyzed elements have been standardized using several single-element standards (Micro-Analysis Consultants Ltd.); the phosphate concentrations measured using EDS were reported as oxide weights (P2O5) normalized to 100%.
2.5. Data Analysis
The bioavailable phosphorus concentration and phosphates mean concentrations are calculated separately for the samples collected from each grave. The concentrations of the bioavailable phosphorus and average concentrations phosphates (expressed as Pav and P2O5 %, respectively) in the soil are represented in different graphs, considering both the sites of sampling (A and B) and, exclusively for the ultramicroscopic analysis, the type of sample (“dust” or “plug”).
In this study, the rainfall data were also considered. The daily rainfall data at the Vigevano-Ponte Ticino SS494 (24 m a.s.l.) ARPA Lombardia weather station, derived for the period from 2009 to 2011 were used.
3. Results
3.1. Bioavailable Phosphorus Content
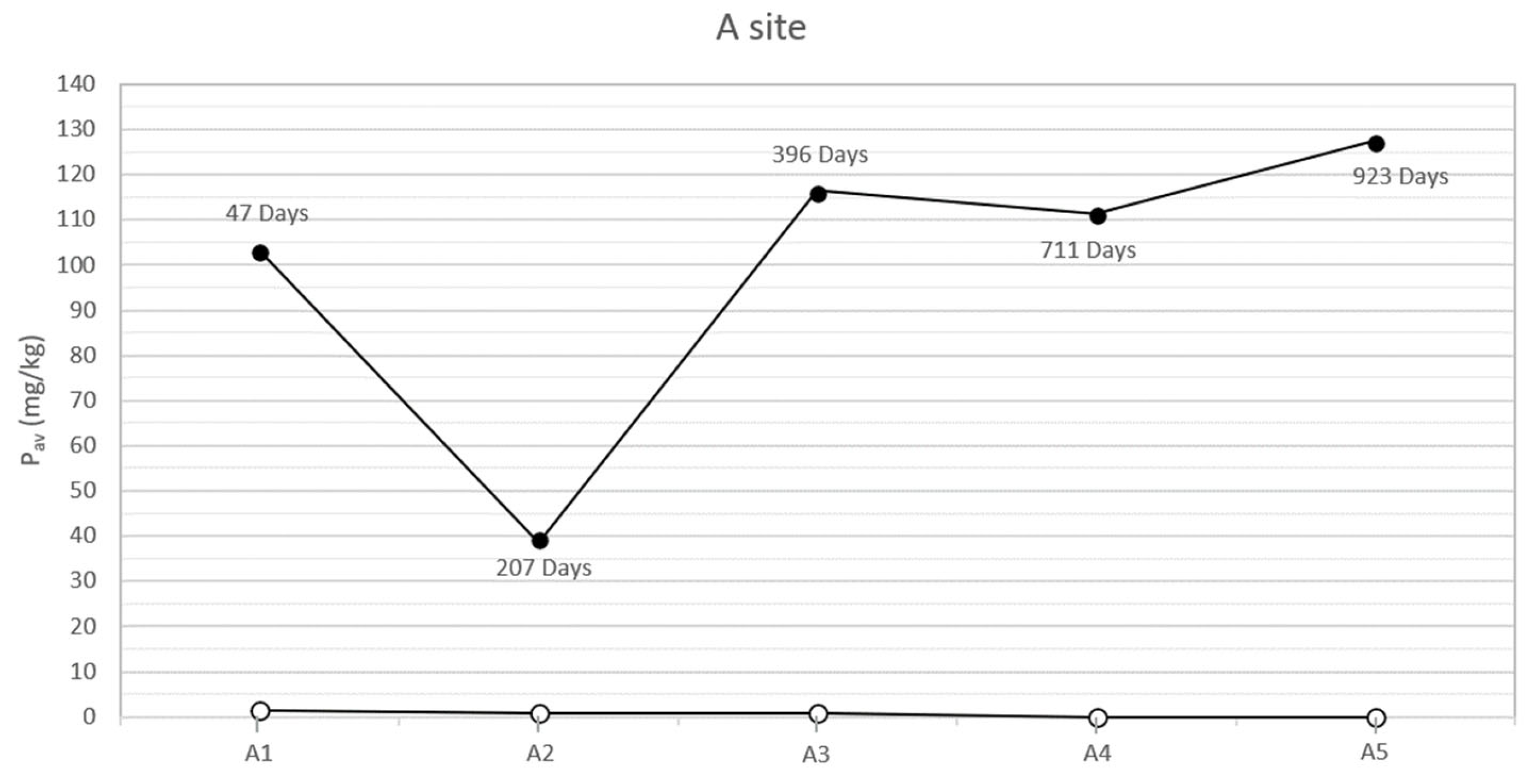
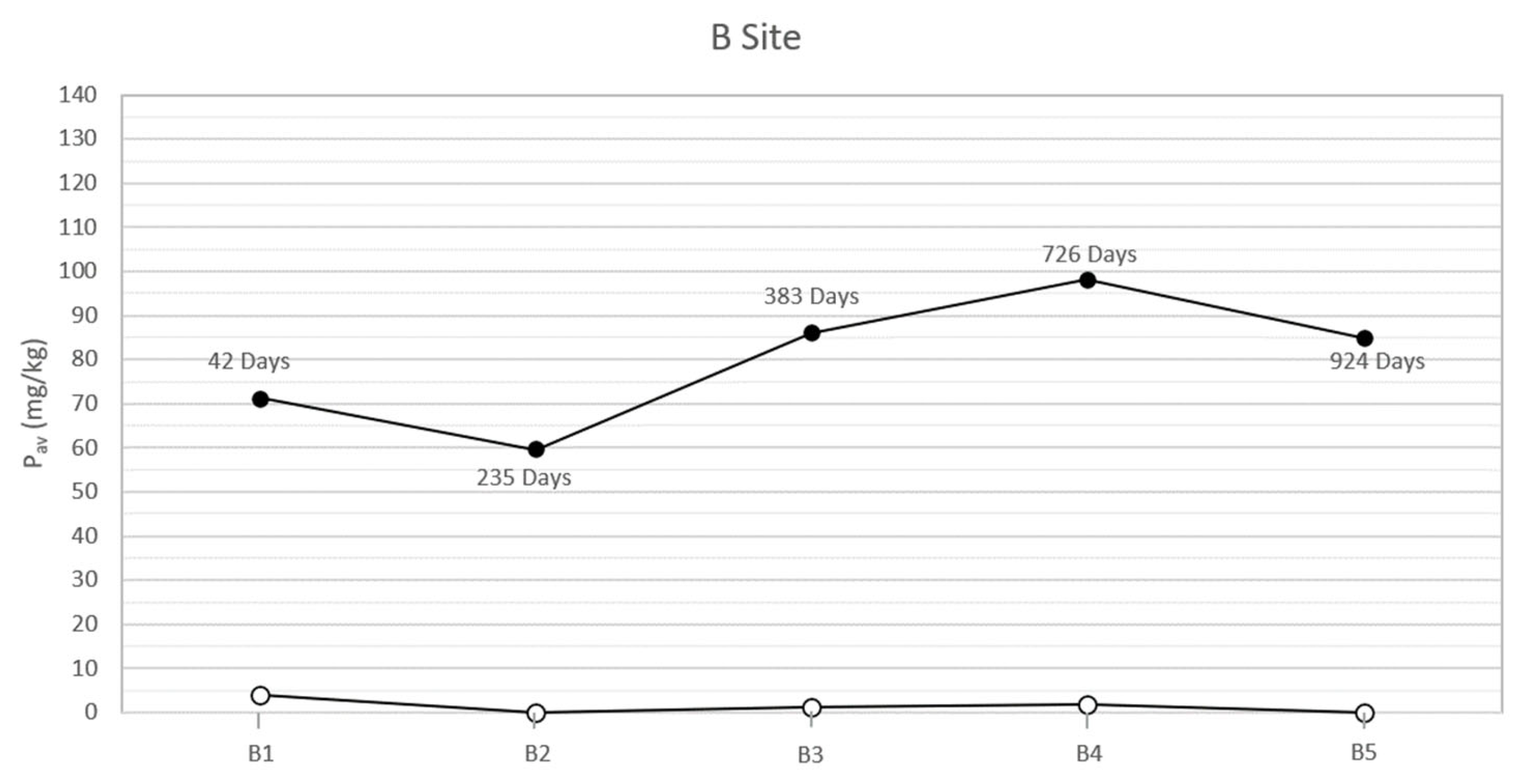
In the study area, the natural soils at the two sites, both sampled on the bottom of the trench and analyzed before each inhumation, showed low concentrations of bioavailable phosphorus (between 0 and 4 mg/kg). On the contrary, the analysis of the specimens collected below the corpses, concurrently with the exhumations, showed anomalous concentrations of bioavailable phosphorus. The results (Table 2 and Figure 2 and Figure 3) highlight two peaks of available phosphorus concentration at 47 and 396 days Post-Burial Interval (PBI) for site A (102 and 115 mg/kg, respectively) and at 42 and 726 days PBI for site B (71 and 98 mg/kg, respectively). Although the bioavailable phosphorus decreased again after the second peaks, the concentration stayed significantly higher until the end of the experiment (924 days), assuming a plateau-like end of the curve.

Table 2.
Summary of the bioavailable P (Pav) concentration found in each sample site at different Post-Burial Intervals (PBIs), obtained using the Olsen P method application. The soil samples have been collected below each carcass, approximately between 100 and 125 cm depth.
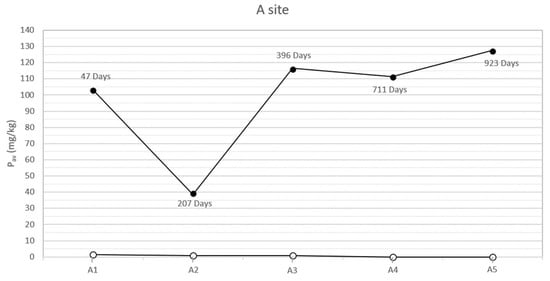
Figure 2.
Soil available phosphorus of grave soil sampled for each burial (A1–A5) before the inhumation (○) and concurrently each exhumation (●) in the site A at different PBIs. The soil samples have been collected below each carcass, approximately between 100 and 125 cm depth.
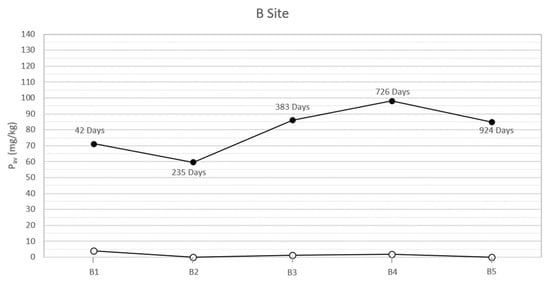
Figure 3.
Soil available phosphorus of grave soil sampled for each burial (B1–B5) before the inhumation (○) and concurrently each exhumation (●) in the site B at different PBI. The soil samples have been collected below each carcass, approximately between 100 and 125 cm depth.
3.2. Phosphate Mean Concentration
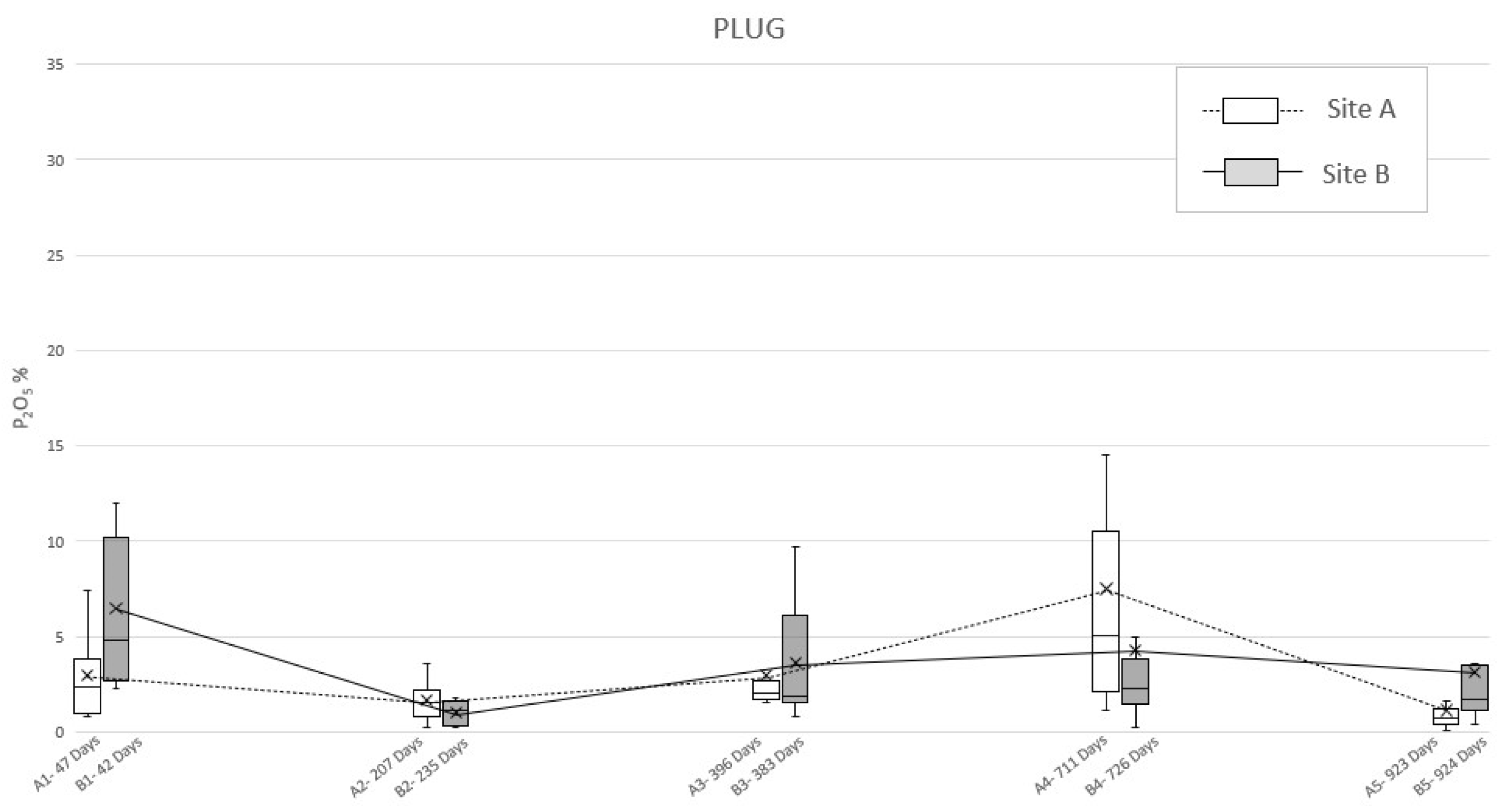
The application of ultramicroscopic analysis (SEM-EDS) allowed for identifying, in all the specimens of the soil sampled below the swine carcasses after each exhumation (A1–A5 and B1–B5 burials), anomalous mean concentrations of phosphates, referred to as P2O5. First of all, the plug samples (Table 3) reported a pattern rather similar to the one identified in the bioavailable phosphorus analysis, characterized by two peaks (Figure 4). In site A, it was possible to detect a first peak concentration of phosphate at 47 days PBI (2.9%) followed by a second one at 711 days PBI (7.5%); after that, the graph showed a decreased P2O5 concentration, but without reaching the background concentration, even at 923 days PBI (1.1%). Meanwhile, the plug specimen from site B (Table 3) showed a double-peaked curve at 42 days PBI (6.5%) and 726 days PBI (4.3%), but with this latter being smoother than in the site A case (Figure 4).

Table 3.
Summary of the mean phosphate concentration (P2O5) found in each “plug” sample from site A and B at different Post Burial Intervals (PBIs) obtained using SEM-EDS analysis.
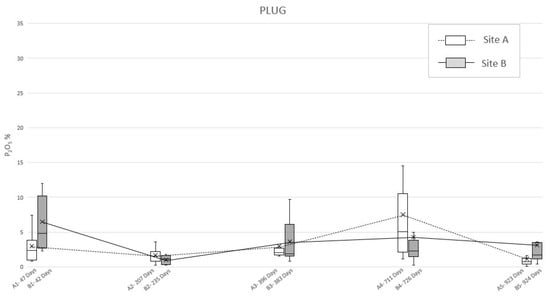
Figure 4.
Mean phosphate concentration (P2O5) detected in “plug” soil specimens sampled from site A and B at different PBI. The box plots show the minimum value, the maximum value, the sample median, and the first and third quartiles. Data obtained from SEM-EDS analysis.
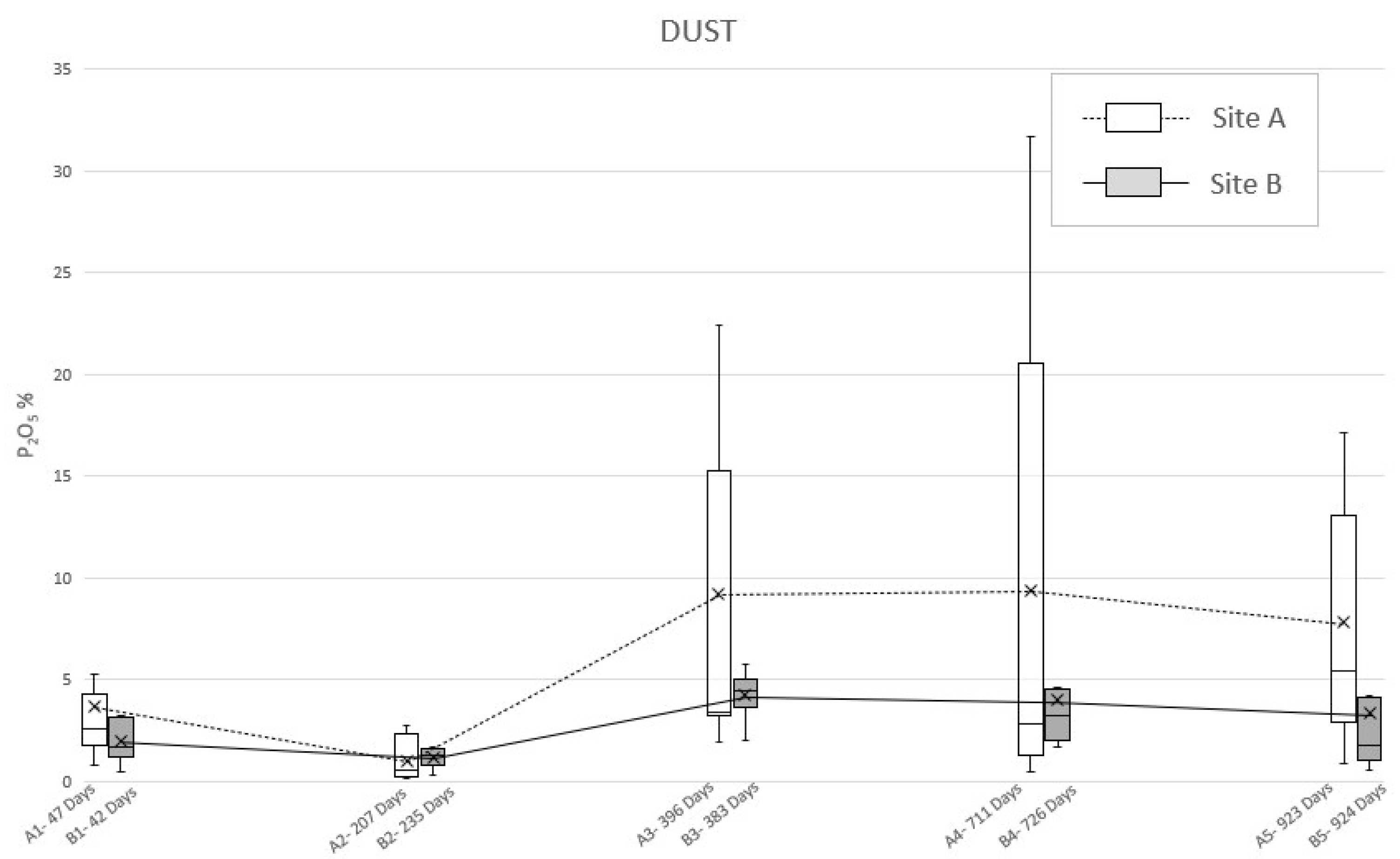
The analysis of the dust specimens showed a double-peaked curve comparable to the previous samples (Figure 5), even if the values showed a different behavior (Table 4). Specifically, in site A, the first peak (47 days PBI) is due to a mean P2O5 concentration equal to 3.7% while the second one is smoother, being expressed by the little mean concentration values’ variations, respectively, at 396 days PBI (9.2%), 711 days PBI (9.3%), and 923 days PBI (7.8%). However, the analysis of the dust samples from site B highlight the lower first peak, in correspondence of 42 days PBI (2.0%) and a smooth second peak at 383 days PBI (4.2%), due to a little within-test variability.
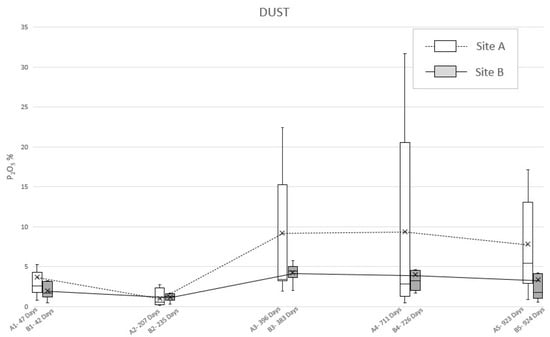
Figure 5.
Mean phosphate concentration (P2O5) detected in “dust” soil specimens sampled from site A and B at different PBIs. The box plots show the minimum value, the maximum value, the sample median, and the first and third quartiles. Data obtained from SEM-EDS analysis.

Table 4.
Summary of the mean phosphate concentration (P2O5) found in each “dust” sample from site A and B at different Post Burial Intervals (PBIs) obtained using SEM-EDS analysis.
The permanence of phosphorus in the soil samples was investigated from an ultramicroscopic point of view (SEM-EDS) by producing 3D compositional images of some peculiar objects detected in all the specimens of soil sampled (as “plug” and “dust”) below the swine carcasses, in both sites, after each exhumation. These have been identified as phosphatic features (Figure 6a–d) present in the form of:

Figure 6.
Phosphatic features investigated using SEM-EDS analysis. (a) Phosphate bearing coatings and infillings on a sand grain (300× SEM image taken in backscattered electron imaging); (b) Phosphate bearing coatings on a vegetal remain (24× SEM taken in secondary imaging); (c) Phosphate bearing impregnations on a crumby microaggregate (1400× SEM image taken in secondary image on the left and backscattered electron imaging on the right); (d) Organo-minerals associations (3000× SEM image taken in secondary image on the left and backscattered electron imaging on the right).
4. Discussion
4.1. Phosphorus Persistency through Phosphates
The application of the Olsen P method, together with the ultramicroscopic analysis of the phosphatic features’ P2O5 concentration, showed the evolution of bioavailable phosphorus and phosphates’ soil content due to corpse decomposition through time.
The results show how the soils of the study area, sampled and analyzed before the pigs’ inhumation, were almost completely devoid of available phosphorus, probably due to the acidic soil parent material [30]. On the contrary, in all the specimens sampled below the swine carcasses after each exhumation, the anomalous concentrations of phosphorus and phosphates have been identified. In particular, the presence of a first peak of the concentration of both the analytes at a PBI of 47 days for site A and 42 days for site B (Figure 2 and Figure 3) confirmed what has been affirmed in previous studies, which recognized an analogous peak concentration of the element after 36 days [12] and 40 days [13]. This soil condition can be attributed to an input of the chemical element from cadavers because the tissues store proteins, sugar phosphates, phospholipids, and other many compounds [35,36] and can be released to the environment during the decomposition processes.
In our study cases, a second peak of bioavailable phosphorus is also observable about one year post burial (396 days PBI, concentration of Pav equal to 115.79 mg/kg) for site A and with a PBI of two years for site B (726 days PBI, concentration of 98.03 mg/kg). The results from [12] showed an increase in the concentration of bioavailable phosphorus at 331 days PBI, suggesting that the pattern in the phosphorus concentration was very similar to the pattern in the present study. However, the authors concluded the experiment after only one year, hence a complete comparison of the results through a longer PBI is not possible.
According to [37], a second peak in the concentration of bioavailable phosphorus, after a PBI of a significant amount of time, could be attributed to a migration of phosphorus from bone to soil occurring during or immediately after complete skeletonization. A similar diagenetic dynamic has been described for sites where archaeological remains have been discovered [38] or simply where complete skeletonization has been observed [39]. Moreover, Fiedler et al. [37] affirm that acidic soils, such as the one that characterizes the present experimentation area, could increase the transfer of phosphorus from bones, especially from skeletonized extremities. Indeed, the macroscopic exam of the carcasses executed contextually to each exhumation supported this hypothesis, showing how the corpses were subjected to an initial skeletonization at 207 days of PBI (site A) and 235 days PBI (site B). The skeletonization was completed about 711 days PBI in site A and 726 days PBI in site B [18,20], in accordance with the analysis results. After that time, the skeletal remains stayed in the soil and continued to release phosphorus, maintaining its levels steadily high in the surrounding environment, at least until 924 days PBI (Figure 2 and Figure 3).
Nonetheless, even if the method used in this study cannot discriminate the source of the phosphorus (whether it be the corpse itself or the bacterial community normally resident in the soil), the two peaks of bioavailable phosphorus concentration suggests that the presence of cadaveric material into the soil has a huge impact on it. In addition, [13] assess that a decomposing carcass, which releases a large pulse of phosphorus into the surrounding soil, can contribute toward an increased microbial biomass, as soil microbes react to disturbance, becoming, in turn, indicators of soil chemical changes [40].
To deepen this matter and to detect the actual reason of the permanence of phosphorus in soil, even after almost three years, ultramicroscopic analysis of P2O5 in the sampling locations have been considered. By observing the development of the mean concentrations of phosphate through time (Figure 4 and Figure 5), it is possible to notice a consistency with the trends highlighted by Olsen’s bioavailable phosphorus analysis results. Indeed, both “plug” and “dust” samples show a double-peak curve, with the second one followed by a long period of anomalous concentration of the analyte, although in the graph derived by the analysis on the dust specimens, irregularities occurred.
The dust method should still be considered as valid since the graphics show two phases of increasing and decreasing amounts of P2O5 over time. This causes it to be possible to assume that even 924 days after burial, the carcasses, even when skeletonized, can still be a relevant source of phosphate in the soil. Moreover, “dust” and “plug” specimens show different absolute values (as expected) and relatively different trends (in particular for the “dust” specimens of area B). In this light, it is possible to assume that the “plug” specimens allow the correct quantitative characterization of the phosphatic impregnations, whereasthe “dust” specimens are suitable only as indices of presence/absence of such features to be used in preliminary investigations.
Regarding the phosphatic features detected through the SEM-EDS analysis of the specimens, they seem to originate from the impregnation by body fluids, resulting from the decomposition of the swine carcasses on the mineral and organic particles constituting the soil and sediment matrix. The impregnation process, which is comparable to the crystalline pedofeatures genesis in soils [41], seems to be independent from the soil matrix grainsize: indeed, the phosphatic coatings were recorded on sand grains up to 300 m size, as well phosphatic impregnations affecting soil microaggregates constituted of clay particles (i.e., <2 μm).
These kinds of phosphatic impregnation in soils, if observed and sampled at the bottom of a supposed clandestine grave, can thus be used as indicators of the former presence of a decomposing body in the overlying soil material even in the absence of the body itself. Unfortunately, until now, it is not yet possible to discriminate the origin of phosphate (i.e., human or animal) with this method. Furthermore, the experiment described here shows the existence of such phosphatic features only in the time interval ranging from 42 to 924 days post burial.
4.2. Environmental Control on the Persistence of Phosphorus
Considering the trend for Pav (Figure 2 and Figure 3), differences can be observed between site A and site B. In particular, the Pav values of the specimens collected in the open area’s pits show a higher rate of variability, compared to those collected in the woodland. The peaks of the Pav concentration are steeper in site A than in site B. This finding is not surprising, as the ecological balance of the woodland is typically more solid than the one found in open area characterized by dry grasslands [42]. Indeed, through the action of roots and the addiction of organic matter, vegetation cover can influence soil’s structural development [43], which in turn controls properties such as root penetration, aeration status, and water and solute retention and movement [44]. The major differences occur in the litter and A horizons. Specifically, grass soil tends to have a very thin litter layer and a thick friable horizon underneath it. On the contrary, under woodland the litter is usually thicker, while the A horizon presents fewer and larger roots and, consequently, greater compactness [43]. Furthermore, stability differences in deeper horizons are likely to be related to disparities in the type of organic matter added [45]: in the B horizon, the organic carbon content should be greater under woodland, a function of the greater rooting depth of trees, explaining a major aggregate stability at this site [43]. The main consequence is a reduced mobilization of a chemical element (such as phosphorus) compared to the one observed in grassland and, therefore, a lower variability of its concentration through time.
Moreover, in site A, the second peak of the Pav concentration is observed earlier than the one that characterized site B (396 vs. 726 days PBI) (Figure 2 and Figure 3). The dissimilar environmental conditions that characterize the two sites could have influenced the rate, extent, and nature of decomposition [46] that, in this study case, proceeds faster in the open area than in the woodland.
In addition, since the permanence of phosphorus seems to be due to phosphates and phosphatic features originating from the chemical disturbance of cadaveric material, the mean concentrations of P2O5 detected on the specimens’ surfaces (Figure 4 and Figure 5) has been considered for environmental observations. Excluding the “dust” specimens’ values, due to their lack of accuracy, the results of the ultramicroscopic analysis detected a higher mean concentration of phosphates in site B, with the only exceptions at 207–235 days (14 December 2009–25 January 2010; winter) and 711–726 days PBI (2–31 May 2011; spring). This condition seems to be justified by the rainfall data carried out collecting daily measurements from the Vigevano-Ponte Ticino SS494 weather station. Indeed, in the amount of time between the exhumations from area A and area B, there have been episodes of abundant rainfall, respectively, of 116.4 mm and 62.2 mm (Figure S1a–c).
Therefore, we could assess that the variation in the phosphorus content seems to be also depending on its persistency in the soil, which could be related to environmental (i.e., soil moisture) conditions, due to the leaching of phosphorus induced by the percolation of natural rainwater [47]. The late spring/summer are typically dry seasons in northern Italy, while the autumn is usually a wet season in northern Italy, although it was very dry at the end of the experimentation period. Nevertheless, these two episodes of abundant rainfall in spring and winter have been intense enough to influence the results of the analysis of samples of soil relative to 235 and 726 days PBI (Figure S1a,c).
Taking into account the already discussed and only qualitative significance of the “dust” specimens, it is clear that the higher values of the measured P2O5 always correspond to dryer seasons (i.e., summer and the last autumn of the experimentation), while lower values of P2O5 correspond to wetter season (i.e., the first autumn and winter of the experimentation).
Finally, it is important to underline that dependent and independent data, collected during the same sets of experiments [20], seem to indicate the same seasonal fluctuations, as for instance regarding some bulk routine geopedological analyses and of geo-electrical prospection anomalies.
Indeed, in recent studies, it has been discussed that soil moisture can be the dominant environmental parameter governing cadaver decomposition in soil [7]: the present experimentation seems to indicate that the moisture could also determine the fate of decomposition products in the soil.
5. Conclusions
In conclusion, the results of the present set of experiments demonstrate the importance of considering the soil as an archive of evidence able to show the environmental conditions that can be related to a criminal event. Specifically, the results may lead the way to tackle questions related to PBI in real cases of clandestine burials as well as assisting in the identification of graves from which the victim has been removed at a later time, as such.
In particular, this study shows how the persistence of phosphorus, in the form of available phosphorus or phosphatic features in soil, can be related to the presence of body fluids related to decomposition processes and, consequently, that the phosphorus concentration seems to be a good indicator for locating the decomposition of remains, even when using different methods to measure it. Above all, the application of the Olsen P method in a forensic context turned out to be a valid strategy to track the behavior of bioavailable phosphorus, meant as a cadaver-impacted soil chemical marker, over time.
On the contrary, the described phosphatic features, which SEM-EDS analysis can identify, can be detected in two kinds of samples in order to the produce specimens suitable for preliminary investigations (“dust”), which require quick results, or for more precise quantitative analyses (“plug”), which are rather consistent with bioavailable phosphorus determinations.
Moreover, the soil moisture conditions at the time of exhumation have been identified as the most effective environmental signal, recorded by the persistence of phosphorus, which is coherent with the control that soil moisture has over the decomposition processes concerning a body buried in soil.
A further step in understanding the variability could be induced by the different grain sizes of the impregnated soil particles (i.e., sand grain coatings versus impregnation on micropeds), a factor that must be tested more accurately in future work, as well as a more intensive sampling. In this light, further experiments are planned, both from the microscopic/ultramicroscopic and the geopedological/chemical point of view, to clarify the pathways of the described phosphorus precipitation and leaching: longer periods of burial, recurring seasons of exhumation, and different soil types. The present study has therefore been considered a valid starting point for the design of a new project, since it showed how the interaction between a decomposing body and the environment can be recorded in the form of biogeochemical anomalies in the soil.
Finally, geopedology is a science rich in variability with a great potential to reconstruct and solve various forensic cases. Many studies and experiments still need to be carried out to improve the knowledge of the specific processes, to provide correct answers to forensic and legal questions, and to reach a standardization for the processed analyses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geosciences13020024/s1, Figure S1: Rainfall graph showing the accumulated daily rainfall and “plug” specimens’ P2O5 mean concentration during the period of study: (a) from 15 May 2009 to 15 March 2010; (b) from 15 March 2010 to 15 March 2011; and (c) from 15 March 2011 to 15 December 2011.
Author Contributions
Conceptualization, S.I.E.E., C.C. and L.T.; formal analysis, S.I.E.E., R.C. and F.T.; investigation, S.I.E.E., C.C. and L.T.; resources, C.C. and L.T.; data curation, G.T. and A.M.; writing-original draft preparation, G.T. and A.M.; writing review and editing, G.T., A.M., C.C. and L.T.; supervision, C.C. and L.T.; project administration, C.C. and L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval received from the University of Milan Ethics Committee.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary material.
Acknowledgments
The authors would like to thank the Parco naturale lombardo della Valle del Ticino, Rizzi Agostino, and Pasquale Poppa. The authors want to thank three anonymous reviewers for their constructive remarks that improved the first version of the manuscript.
Conflicts of Interest
We all declare that there is no Conflict of Interest, and this article is neither sent nor published in any other journal.
References
- Somma, R.; Cascio, M.; Silvestro, M.; Torre, E. A GIS-based quantitative approach for the search of clandestine graves. Italy J. Forensic Sci. 2018, 63, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Somma, R.; Costa, N. Unraveling Crimes with Geology: As Geological and Geographical Evidence Related to Clandestine Graves May Assist the Judicial System. Geosciences 2022, 12, 339. [Google Scholar] [CrossRef]
- France, D.L.; Griffin, T.J.; Swanburg, J.G.; Lindemann, J.W.; Davenport, C.G.; Trammel, V. Multidisciplinary approach to the detection of clandestine graves. J. Forensic Sci. 1992, 37, 1445–1458. [Google Scholar] [CrossRef]
- Goldberg, P.; Macphail, R.I. Practical and Theoretical Geoarchaeology; Blackwell Publishing: Malden, MA, USA, 2006. [Google Scholar]
- Schultz, J.J.; Collins, M.E.; Falsetti, A.B. Sequential monitoring of burials containing small pig cadavers using ground penetrating radar. J. Forensic Sci. 2008, 53, 279–287. [Google Scholar] [CrossRef]
- Tagliabue, G.; Crespi, S.; Trombino, L. Il Suolo Come Contenitore di Evidenze: Le Geoscienze Applicate allo Studio delle Sepolture della Cripta della Ca’ Granda. In Sepolcreto della Ca’ Granda, un Tesoro Storico e Scientifico di Milano.; Mattia, M., Ed.; Ledizioni: Milano, Italy, 2021; pp. 167–174. [Google Scholar]
- Carter, D.O.; Tibbett, M.; Yellowlees, D. Moisture can be the dominant environmental parameter governing cadaver decomposition in soil. Forensic Sci. Int. 2010, 200, 60–66. [Google Scholar] [CrossRef]
- Turner, P.; Wiltshire, B.D. Experimental validation of forensic evidence: A study of the decomposition of buried pigs in a heavy clay soil. Forensic Sci. Int. 1999, 101, 112–113. [Google Scholar] [CrossRef]
- Carter, D.O.; Tibbett, M.; Yellowlees, D. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 2007, 94, 12–24. [Google Scholar] [CrossRef]
- Ross, S.L.; Cunningham, A.H. Time-since-death and bone weathering in a tropical environment. Forensic Sci. Int. 2011, 204, 126–133. [Google Scholar] [CrossRef]
- Zangarini, S.; Trombino, L.; Cattaneo, C. Micromorphological and ultramicroscopic aspects of buried remains: Time-dependent markers of decomposition and permanence in soil in experimental burial. Forensic Sci. Int. 2016, 263, 74–82. [Google Scholar] [CrossRef]
- Szelecz, I.; Koenig, I.; Seppey, C.V.; Le Bayon, R.C.; Mitchell, E.A. Soil chemistry changes beneath decomposing cadavers over a one-year period. Forensic Sci. Int. 2018, 286, 155–165. [Google Scholar] [CrossRef]
- Benninger, L.A.; Carter, D.O.; Forbes, S.L. The biochemical alteration of soil beneath a decomposing carcass. Forensic Sci. Int. 2008, 180, 70–75. [Google Scholar] [CrossRef]
- Tibbet, M.; Carter, D.O. Soil Analysis in Forensic Taphonomy. Chemical and Biological Effects of Buried Human Remains; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Stokes, K.L.; Forbes, S.L.; Benninger, L.A.; Carter, D.O.; Tibbett, M. Decomposition Studies Using Animal Models in Contrasting Environments: Evidence from Temporal Changes in Soil Chemistry and Microbial Activity. In Criminal and Environmental Soil Forensics; Springer: Dordrecht, The Netherlands, 2009; pp. 357–377. [Google Scholar]
- Caccianiga, M.; Bottacin, S.; Cattaneo, C. Vegetation dynamics as a tool for detecting clandestine graves. J. Forensic Sci. 2012, 57, 983–988. [Google Scholar] [CrossRef]
- Coyle, H.M.; Lee, C.L.; Lin, W.Y.; Lee, H.C.; Palmbach, T.M. Forensic botany: Using plant evidence to aid in forensic death investigation. Croat. Med. J. 2005, 46, 606–612. [Google Scholar]
- Poppa, P.; Trombino, L.; Cattaneo, C. L’individuazione e la Caratterizzazione dei siti di Occultamento di Resti Umani in Ambito Forense: Applicazioni Geopedologiche e Geoarcheologiche. Ph.D. Thesis, Università Degli Studi di Milano, Milano, Italy, 2011. [Google Scholar]
- Juerges, A.; Pringle, J.K.; Jervis, J.R.; Masters, P. Comparisons of magnetic and electrical resistivity surveys over simulated clandestine graves in contrasting burial environments. Near Surf. Geophys. 2010, 8, 529–539. [Google Scholar] [CrossRef]
- Ern, S.; Trombino, L.; Cattaneo, C. Scienze Naturali in Ambito Forense: L’apporto di Studi Geopedologici e Botanici. Ph.D. Thesis, Università Degli Studi di Milano, Milano, Italy, 2012. [Google Scholar]
- Vass, A.A.; Bass, W.B.; Wolf, J.B.; Foss, J.E.; Ammons, J.T. Time since death determinations of human cadavers using soil solution. J. Forensic Sci. 1992, 37, 1236–1253. [Google Scholar] [CrossRef]
- Hrapovic, L.; Rowe, R.K. Intrinsic degradation of volatile fatty acids in laboratory-compacted clayey soil. J. Contam. Hydrol. 2002, 58, 221–242. [Google Scholar] [CrossRef]
- Taborelli, A.; Grandi, M.A.; Cattaneo, C. Alterazioni Microscopiche Della Decomposizione: Implicazioni per la Medicina Legale. Ph.D. Thesis, Università Degli Studi di Milano, Milano, Italy, 2010. [Google Scholar]
- Fiedler, S.; Graw, M. Decomposition of buried corpses, with special reference to the formation of adipocere. Naturwissenschaften 2003, 90, 291–300. [Google Scholar] [CrossRef]
- Haskell, N.; Hall, R.; Cervenka, V.; Clark, M. On the Body: Insects’ Life Stage Presence and their Postmortem Artifacts. In Forensic Taphonomy: The Post Mortem Fate of Human Remains; Haglund, W., Sorg, M.H., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 415–448. [Google Scholar]
- Van Belle, L.E.; Carter, D.O.; Forb, S.L. Measurement of ninhydrin reactive nitrogen influx into gravesoil during aboveground and belowground carcass (Sus domesticus) decomposition. Forensic Sci. Int. 2009, 193, 37–41. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, 618–626. [Google Scholar] [CrossRef]
- Jaggers, K.A.; Rogers, T.L. The Effects of Soil Environment on Postmortem Interval: A Macroscopic Analysis. Forensic Sci. Int. 2009, 54, 1217–1222. [Google Scholar] [CrossRef]
- Ern, S.; Trombino, L.; Cattaneo, C. Micromorphological Aspects of Forensic Geoarchaeology: Ultramicroscopic Characterization of Phosphatic Impregnations on Soil Particles in Experimental Burials—Preliminary Results. In Proceedings of the 14th International Working Meeting on Soil Micromorphology, Lleida, Spain, 8–14 July 2012. [Google Scholar]
- Ente Regionale di Sviluppo Agricolo della Lombardia (ERSAL). I Suoli del Parco Ticino Settore Meridionale; Progetto Carta Pedologica, ERSAL: Milano, Italy, 1996. [Google Scholar]
- Soil Survey Staff, Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA (United States Department of Agriculture): Washington, DC, USA, 1999. [Google Scholar]
- Sims, J.T. Soil test phosphorus: Methods of phosphorus analysis for soils, sediments, residuals, and waters. South. Coop. Ser. Bull 2000, 396, 17–19. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular 939; U.S. Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Armigliato, A.; Valdrè, U. Microscopia Elettronica a Scansione e Microanalisi; Alfatest: Bologna, Italy, 1981. [Google Scholar]
- Dent, B.B.; Forbes, S.L.; Stuart, B.H. Review of human decomposition processes in soil. Environ. Geol. 2004, 45, 576–585. [Google Scholar] [CrossRef]
- Perrault, K.A.; Forbes, S.L. Elemental analysis of soil and vegetation surrounding decomposing human analogues. Can. Soc. Forensic Sci. J. 2016, 49, 138–151. [Google Scholar] [CrossRef]
- Fiedler, S.; Schneckenberger, K.; Graw, M. Characterization of Soils Containing Adipocere. Arch. Environ. Contam. Toxicol. 2004, 47, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Eidt, R.C. Detection and examination of anthrosols by phosphate analysis. Science 1977, 4311, 1327–1333. [Google Scholar] [CrossRef]
- Waldron, T. The Potential of Analysis of Chemical Constituents of Bone. In Death, Decay and Reconstruction: Approaches to Archaeology and Forensic Science; Boddington, I., Garland, A.N., Janaway, R., Eds.; Manchester University Press: Manchester, UK, 1987; pp. 149–159. [Google Scholar]
- Kerek, M.; Drijber, R.A.; Powers, W.L.; Shearman, R.C.; Gaussoin, R.E.; Streich, A.M. Accumulation of microbial biomass within particulate organic matter of aging golf greens. Agro. J. 2002, 94, 455–461. [Google Scholar] [CrossRef]
- Bullock, P.; Fedoroff, N.; Jongerius, A.; Stoops, G.; Tursina, T.; Babel, U. Handbook for Soil Thin Section Description; Waine Research Publications: Wolverhampton, UK, 1985. [Google Scholar]
- Monson, R.K. Ecology and the Environment; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Grieve, I.C. Some contrasts in soil development between grassland and deciduous woodland sites. J. Soil Sci. 1980, 31, 137–145. [Google Scholar] [CrossRef]
- Russell, E.W. Soil structure. Its maintenance and improvement. J. Soil Sci. 1971, 22, 137–151. [Google Scholar] [CrossRef]
- Greenland, D.J. Changes in the Nitrogen status and physical condition of soils under pastures, with special reference to the maintenance of the fertility of Australian soils used for growing wheat. Soils Fertil. 1971, 34, 237–251. [Google Scholar]
- Wilson, A.S.; Janaway, R.C.; Holland, A.D.; Dodson, H.I.; Baran, E.; Pollard, A.M.; Tobin, D.J. Modelling the buried human body environment in upland climes using three contrasting field sites. Forensic Sci. Int. 2007, 169, 6–18. [Google Scholar]
- Duchaufour, P. Pédologie: Sol, Végétation, Environnement. Abregés, 4th ed.; Masson: Paris, France, 1995. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).